Abstract
Objective
To determine if arterial oxygen and carbon dioxide abnormalities in the first 24 h after return of spontaneous circulation (ROSC) are associated with increased mortality in adult out-of-hospital cardiac arrest (OHCA).
Methods
We used data from the Resuscitation Outcomes Consortium (ROC), including adult OHCA with sustained ROSC ≥1 h after Emergency Department arrival and at least one arterial blood gas (ABG) measurement. Among ABGs measured during the first 24 h of hospitalization, we identified the presence of hyperoxemia (PaO2 ≥ 300 mmHg), hypoxemia (PaO2 < 60 mmHg), hypercarbia (PaCO2 > 50 mmHg) and hypocarbia (PaCO2 < 30 mmHg). We evaluated the associations between oxygen and carbon dioxide abnormalities and hospital mortality, adjusting for confounders.
Results
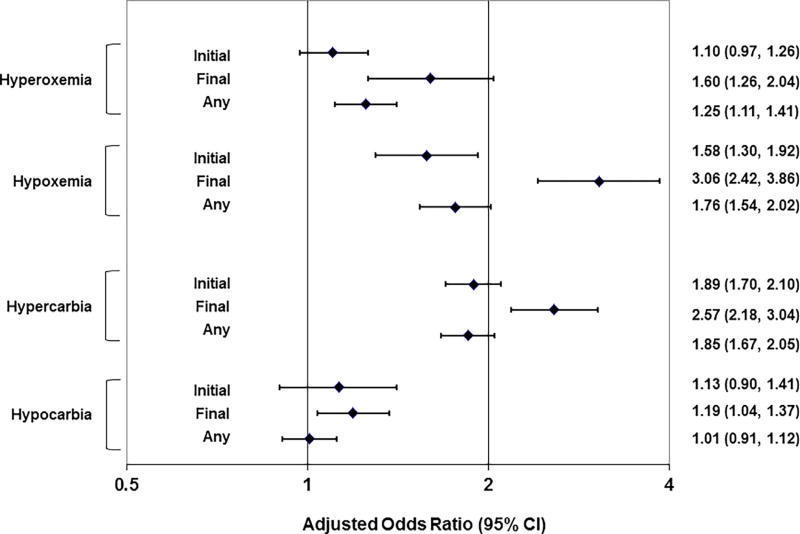
Among 9186 OHCA included in the analysis, hospital mortality was 67.3%. Hyperoxemia, hypoxemia, hypercarbia, and hypocarbia occurred in 26.5%, 19.0%, 51.0% and 30.6%, respectively. Initial hyperoxemia only was not associated with hospital mortality (adjusted OR 1.10; 95% CI: 0.97–1.26). However, final and any hyperoxemia (1.25; 1.11–1.41) were associated with increased hospital mortality. Initial (1.58; 1.30–1.92), final (3.06; 2.42–3.86) and any (1.76; 1.54–2.02) hypoxemia (PaO2 < 60 mmHg) were associated with increased hospital mortality. Initial (1.89; 1.70–2.10); final (2.57; 2.18–3.04) and any (1.85; 1.67–2.05) hypercarbia (PaCO2 > 50 mmHg) were associated with increased hospital mortality. Initial (1.13; 0.90–1.41), final (1.19; 1.04–1.37) and any (1.01; 0.91–1.12) hypocarbia (PaCO2 < 30 mmHg) were not associated with hospital mortality.
Conclusions
In the first 24 h after ROSC, abnormal post-arrest oxygen and carbon dioxide tensions are associated with increased out of-hospital cardiac arrest mortality.
Keywords: Cardiopulmonary arrest, Post-arrest care, Hyperoxemia, Hypoxemia, Hypercarbia, Hypocarbia
Introduction
Out-of-hospital cardiac arrest (OHCA) is a major public health problem affecting over 300,000 persons in the United States each year [1]. International consensus recommendations underscore the importance of post-arrest intensive care in facilitating OHCA survival [2]. Post-arrest hyperoxemia has been associated with a range of deleterious effects, including inhibition of mitochondrial function, free-radical formation, oxidative stress and acidosis, worsened myocardial contractility and brain injury [3–8]. In brain injured patients, alterations in carbon dioxide tension may adversely impact cerebral blood flow and perfusion [6, 7, 9].
While observational studies suggest associations between hyperoxemia, hypoxemia, hypercarbia and hypocarbia and cardiac arrest survival, these research efforts have important limitations [10–22]. While most of these studies were based upon measurements in the intensive care unit, the majority of OHCA patients receive initial post-arrest care in the Emergency Department (ED), where early oxygen and carbon dioxide tension may be most impactful. Prior studies included a heterogeneous mix of in-hospital and out-of-hospital cardiac arrests, used varying approaches to define oxygen and carbon dioxide tension measurements, and arrived at different conclusions regarding associations with survival [22].
There have been few Emergency Department studies of oxygen and carbon dioxide tension in the period immediately following return of spontaneous circulation (ROSC). In this study, we sought to determine the association of 24-h post-ROSC oxygen and carbon dioxide tension with OHCA mortality in the national Resuscitation Outcomes Consortium (ROC).
Methods
Design
We analyzed prospectively collected OHCA data from the ROC Epistry – Cardiac Arrest (“Epistry”) [23]. ROC clinical centers collected OHCA data in conformance with United States Department of Health and Human Services regulations for the protection of human subjects and provisions of the Canadian Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans. Additional reviews and approvals were provided by the Institutional Review Boards and research ethics boards for each community.
Study setting
ROC is a multicenter clinical trial network designed to conduct out-of-hospital interventional and clinical research in cardiac arrest and traumatic injury. Participating regional coordinating centers included Birmingham, AL; Dallas, TX; Milwaukee, WI; Pittsburgh, PA; Portland, OR; San Diego, CA; Seattle/King County, WA; British Columbia, Canada; Ottawa and the Ontario Prehospital Advanced Life Support study communities, Ontario, Canada; and Toronto and adjacent regions, Ontario, Canada. A data coordinating center was based in Seattle. Over 264 emergency medical services (EMS) agencies and 287 receiving hospitals participated in the ROC Epistry – Cardiac Arrest [23].
Data source
The ROC Epistry – Cardiac Arrest is a registry of consecutive cardiac arrests at participating ROC sites [23, 24]. Using dispatch logs, EMS patient care records, defibrillator files, and hospital and public death records, study personnel at each site determined clinical details of each OHCA, including prehospital response, patient demographics, clinical information, prehospital interventions, prehospital disposition, hospital information and outcomes. Data collection and reporting methods adhered to Utstein standards [25].
Selection of subjects
From the study period April 5, 2011–July 31, 2015, we included all adult (18 years old), EMS-treated non-traumatic OHCA achieving return of spontaneous circulation and surviving 1 h in the receiving Emergency Department. We further limited the analysis to patients receiving 1 arterial blood gas measurement (ABG) within the first 24 h of hospitalization. We excluded children (age <18 years), OHCA due to blunt, burn, or penetrating trauma, patients pronounced dead in the field, patients surviving <1 h in the Emergency Department, and cases where the time and date of death were not known.
Exposures
The primary exposures were hyperoxemia (PaO2 300 mmHg), hypoxemia (PaO2 < 60 mmHg), hypercarbia (PaCO2 > 50 mmHg) and hypocarbia (PaCO2 < 30 mmHg), defined by thresholds used in prior studies of oxygen and carbon dioxide tension [14, 21]. We identified the presence of these abnormalities using ABG measurements obtained during the first 24 h of hospitalization, regardless of the patient’s location in the hospital.
ROC Epistry protocols included collection of all ABG values during the first 24 h of hospitalization, a period intended to encompass ED and initial intensive care unit care. We did not include ABG measurements collected >24 h after hospital arrival. In the data analysis, we assessed the presence of each oxygen and carbon dioxide abnormality based upon the initial (first), final (last) or any ABG measurement during the first 24 h of hospitalization. ABG measurements were obtained according to local protocols. ROC protocols did not dictate the timing or frequency of ABG measurements.
Outcomes
The primary outcome was hospital death. Study personnel determined the primary outcome from review of hospital records.
Data analysis
We determined the characteristics of oxygen and carbon dioxide tension measurements among included subjects, including the number and patterns of ABG measurements, and the frequency of hyperoxemia, hypoxemia, hypercarbia and hypocarbia.
Using multivariable logistic regression, we fit a series of models assessing the associations between oxygen and carbon dioxide tension measurements and OHCA hospital death. We fit hospital death as the dependent variable of each model. We then fit a series of 12 separate a priori planned multivariable models reflecting a) each oxygen or carbon dioxide abnormality, and b) its presence in the initial, final or any ABG measurement. For example, for the “initial hyperoxemia” model, we classified the patient as “initial hyperoxemia present” if the initial PaO2 was 300 mmHg; we considered all other patients as “initial hyperoxemia absent.” For the “any hyperoxemia” model, we classified the patient as “any hyperoxemia present” if any PaO2 was 300 mmHg. We adjusted each of the models for patient age and sex, witnessed arrest status, provision of bystander CPR, initial ECG rhythm (shockable [ventricular fibrillation and pulseless ventricular tachycardia] vs. non-shockable [pulseless electrical activity and asystole]) and ROC clinical site.
Based upon observations in the initial analysis, we devised anumber of post hoc sensitivity analyses to test the robustness of the results. Because different types of oxygen tension abnormalities may be simultaneously present in the same patient, we fit a model examining the joint presence of any hyper- and any hypoxemia. We similarly fit a model examining the joint presence of any hyper- and any hypocarbia. Oxygen and carbon dioxide tensions may interact. Over the observation period of 24 h, oxygen and carbon dioxide tensions may also concurrently exist. To account for these possibilities, we fit a model assessing the joint presence of any hyperoxemia, hypoxemia, hypercarbia and hypocarbia.
To differentiate if very early oxygen and carbon dioxide tension measurements were harmful, we repeated the primary analysis limited to the first ABG measurements obtained during the first 2 h of Emergency Department care. Finally, using all available ABGs, we fit a model characterizing initial oxygen and carbon dioxide tension measurements on categorical scales. We adjusted all of these models for age, sex, witnessed arrest, bystander CPR, initial ECG rhythm and ROC clinical site.
Because of uncertainty in the nature and timing of clinician actions in response to observed ABG measurements, the best strategies for determining the duration of ABG values were not clear. We therefore opted not to include timing or duration of oxygen or carbon dioxide measurements in the analyses. Because of the large number of comparisons, we similarly refrained from incorporating additional models using continuous measures of oxygen and carbon dioxide. We conducted all analyses using R, version 3.1.1.
Results
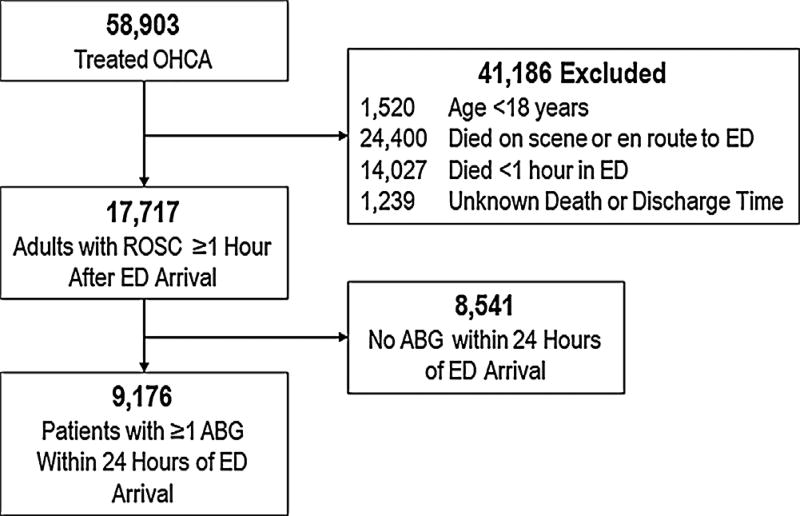
During the study period April 5, 2011 through July 31, 2015, there were 58,903 EMS-treated OHCA (Fig. 1). After excluding patients <18 years old, those who died on scene or en route to ED, those who survived <1 h in the ED, and those receiving no ABG measurements in the first 24 h of hospitalization, there were 9176 patients remaining in the analysis.
Fig. 1.
Study population. Analysis included OHCA = Out-of-hospital cardiac arrest. ROSC = Return of spontaneous circulation. ED = Emergency Department. ABG = Arterial blood gas.
Compared with those who did not receive ABG measurements, patients receiving ABG measurements were more likely to have received bystander CPR, present with VF/VT, and more likely to receive an advanced airway in the field (Table 1). Mortality was similar among patients with and without ABG measurements (67.3% vs 67.1% respectively).
Table 1.
Characteristics of post-arrest patients with and without arterial blood gas (ABG) measurements. ROSC = Return of spontaneous circulation.
| Characteristic | ABG Measured N = 9176 |
No ABG N = 8541 |
|---|---|---|
| Age, years – median (IQR) | 64 (53, 75) | 66 (54, 78) |
| Male, n (%) | 5914 (64.5%) | 5172 (60.6%) |
| Witnessed arrest, n (%) | 5784 (63.0%) | 5982 (70.0%) |
| Bystander CPR, n (%) | 8319 (90.7%) | 5922 (69.3%) |
| Initial rhythm, n (%) | ||
| Ventricular Fibrillation/Tachycardia | 3966 (43.6%) | 2717 (32.7%) |
| Pulseless Electrical Activity | 2112 (23.2%) | 2646 (31.9%) |
| Asystole | 2406 (26.4%) | 2209 (26.6%) |
| Other | 616 (6.8%) | 733 (8.8%) |
| Advanced airway in field, n (%) | 7739 (84.3%) | 6042 (70.7%) |
| Prehospital return of spontaneous circulation, n (%) | 7966 (86.8%) | 7133 (83.5%) |
| 911 to return of spontaneous circulation, minutes | ||
| Median (IQR) | 21.7 (16.1, 28.0) | 21.8 (15.4, 29.3) |
| >20 min, n (%) | 4481 (48.8%) | 3877 (45.4%) |
| Emergency Department length of stay, hours | ||
| Hours – Median (IQR) | 2.2 (1.2, 3.4) | 2.2 (1.3, 3.7) |
| >2 h – n (%) | 5005 (54.5%) | 2310 (27.0%) |
| Number of ABGs available | ||
| 1 | 1692 (18.4%) | N/A |
| 2 | 1428 (15.6%) | N/A |
| 3 | 1528 (16.7%) | N/A |
| 4 | 1321 (14.4%) | N/A |
| >4 | 3207 (34.9%) | N/A |
| Any ABG <4 h from ED arrival, n (%) | 7910 (86.2%) | N/A |
| Therapeutic hypothermia in field or ED, n (%) | 3569 (38.9%) | 1361 (15.9%) |
| Vasopressors in field, n (%) | 271 (3.0%) | 363 (4.3%) |
| Hospital Death | 6172 (67.3%) | 5728 (67.1%) |
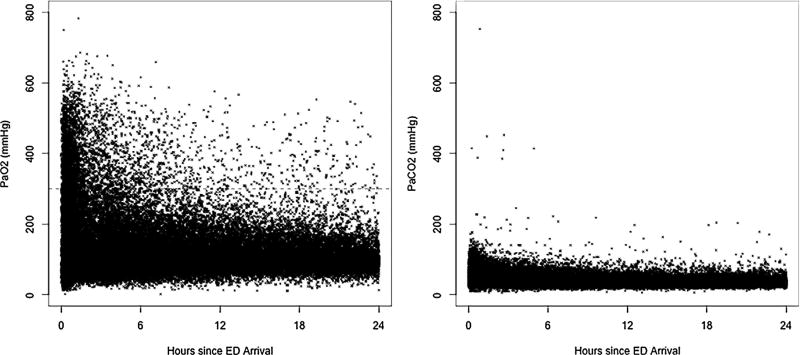
Among patients included in the analysis, there were a total of 35,576 ABG measurements. Patients received a median of 3 ABG measurements (IQR 2, 5; range 1, 23) (Table 2). Initial ABG measurements were obtained a mean of 2 h after ED arrival. ABG measurements frequently exhibited acidosis, hyperoxemia and hypercarbia. The prevalence of hyperoxemia (PaO2 ≥ 300 mmHg) and hypoxemia (PaO2 < 60 mmHg) were 26.5% and 18.9%, respectively (Table 2, Fig. 2). The prevalence of hypercarbia (PaCO2 > 50 mmHg) and hypocarbia (PaCO2 < 30 mmHg) were 51.0% and 30.6%, respectively (Table 2, Fig. 2).
Table 2.
Characteristics of post-cardiac arrest arterial blood gas measurements.
| Characteristic | Measure |
|---|---|
| Number of ABG measurements – N (%) | |
| 1 | 1692 (18.4%) |
| 2 | 1428 (15.6%) |
| 3 | 1528 (16.7%) |
| 4 | 1321 (14.4%) |
| ≥5 | 3207 (34.9%) |
| Median (IQR); min, max | 3 (2, 5); 1, 23 |
| First ABG measurement – Mean (SD); 25th, 75th percentile | |
| Time (Hours after ED arrival) | 2.0 (2.8); 0.4, 2.4 |
| pH | 7.16 (0.19); 7.04, 7.29 |
| PaCO2 | 53.0 (21.9); 39.0, 62.0 |
| PaO2 | 186.0 (129.0); 85.0, 260.0 |
| Hyperoxemia – N (%) | |
| Initial Hyperoxemia (Initial PaO2 ≥ 300 mmHg) | 1751 (19.1%) |
| Final Hyperoxemia (Last PaO2 ≥ 300 mmHg) | 549 (6.0%) |
| Any Hyperoxemia (Any PaO2 ≥ 300 mmHg) | 2431 (26.5%) |
| Hypoxemia – N (%) | |
| Initial Hypoxemia (Initial PaO2 < 60) | 740 (8.1%) |
| Final Hypoxemia (Last PaO2 < 60) | 701 (7.6%) |
| Any Hypoxemia (Any PaO2 < 60) | 1736 (18.9%) |
| Hypercarbia – N (%) | |
| Initial Hypercarbia (Initial PaCO2 > 50 mmHg) | 3988 (43.5%) |
| Final Hypercarbia (Last PaCO2 > 50 mmHg) | 1633 (17.8%) |
| Any Hypercarbia (Any PaCO2 > 50 mmHg) | 4681 (51.0%) |
| Hypocarbia – N (%) | |
| Initial Hypocarbia (Initial PaCO2 < 30) | 544 (5.9%) |
| Final Hypocarbia (Last PaCO2 < 30) | 1499 (16.3%) |
| Any Hypocarbia (Any PaCO2 < 30) | 2808 (30.6%) |
First 24 h of hospitalization.
Limited to patients with ABG performed. ABG = arterial blood gas. ED = Emergency Department.
Fig. 2.
Arterial partial pressure of oxygen (left panel) and carbon dioxide (right panel) vs. time of arterial blood gas sample. Includes 35,576 ABGs among 9176 patients with surviving at least 1 h in the Emergency Department. ABG = arterial blood gas. Hatched line depicts PaO2 = 300 mmHg.
Overall hospital mortality was 67.3%. Hospital mortality was higher for patients with any oxygen or carbon dioxide tension abnormalities (Appendix 1). After multivariable adjustment, the presence of initial hyperoxemia was not associated with hospital mortality (Fig. 3). However, the presence of final and any hyperoxemia were associated with increased hospital mortality. The presence of initial, final and any hypoxemia were associated with increased hospital mortality. Similarly, initial, final and any hypercarbia were associated with increased hospital mortality. While final hypocarbia was associated with increased hospital mortality, initial and any hypocarbia were not.
Fig. 3.
Adjusted associations between oxygen and carbon dioxide abnormalities and death. Whiskers depict results of 12 separate multivariable models, each with an individual oxygen or carbon dioxide abnormality as the key exposure. Each of the 12 models adjusted for age, sex, witnessed arrest, bystander CPR, initial ECG rhythm and ROC clinical site..
We conducted a number of post hoc sensitivity analyses to test the robustness of the results (Appendices 2–6). We first assessed the simultaneous presence of different oxygen tension abnormalities; the presence of hyperoxemia only, hypoxemia only, or both hyperoxemia and hypoxemia were associated with increased hospital mortality (Appendix 2). We observed similar associations for hypercarbia and hypocarbia. When modeled on categorical bases, initial and final oxygen and carbon dioxide tension measurements were associated with increased hospital mortality (Appendix 3). When examining the joint presence of all oxygen and carbon dioxide tension abnormalities, any hyperoxemia, any hypoxemia, and any hypercarbia were associated with increased hospital mortality; any hypocarbia was not associated with hospital mortality (Appendix 4). When considering only the first ABG obtained during the first two hours in the ED, the presence of initial hypoxemia, initial hypercarbia and initial hypocarbia were associated with increased hospital mortality; initial hyperoxemia was not associated with hospital mortality (Appendix 5). When considering all available ABGs with oxygen and carbon dioxide modeled on a categorical basis, the presence of initial hypoxemia, hyperoxemia (including PaO2 200–299 mmHg), hypercarbia, and hypocarbia were all associated with increased death (Appendix 6).
Discussion
In this study of over 9000 adult OHCA patients in the North American ROC network, abnormal oxygen and carbon dioxide tension measurements in the first 24-h after ROSC were associated with increased hospital mortality. While our primary analysis suggested that these adverse associations were limited to certain oxygen and carbon dioxide tension measurements, our complementary sensitivity analyses suggest that harm may be associated with any of the observed entities. Our study has important distinctions compared with prior efforts. We used a large heterogeneous data set encompassing over 9000 patients. Our analysis focused on OHCA only rather than a mix of out-of-hospital and in-hospital arrests. Most importantly we focused on the first 24-h of hospitalization, providing some of the first insights linking early ED oxygen and carbon dioxide control with OHCA outcomes.
Numerous studies post-arrest oxygen and carbon dioxide tension have arrived at varying conclusions [11, 13–15, 17–20]. In a study of 6326 intensive care unit patients at 120 hospitals, Kilgannon, et al. found that initial hyperoxemia was associated with increased hospital mortality [21]. Among 12,108 ICU patients in New Zealand, Bellomo, et al. observed associations between hyperoxemia and hospital mortality, but these relatioships were not as strong on sensitivity analyses [10]. In a study of 5258 cardiac arrest patients admitted to 82 ICUs in the Netherlands, Helmermorst et al. observed increased hospital mortality from all oxygen and carbon dioxide aberrancies [12]. Among 409 patients admitted to 21 ICUs in Finland, Vaahersalo, et al. found no association between oxygen and carbon dioxide tensions and 12-month neurologic outcomes. While the Vaahersalo study accounted for the duration of abnormal oxygen and carbon dioxide exposures, only 6% were exposed to PaO2 > 300 mmHg [16]. In a meta-analysis of 14 studies, C.H. Wang, et al. noted that although hyperoxemia was associated with increased hospital mortality, the results were heterogeneous and inconsistent in subgroup and sensitivity analyses [22].
These results highlight the importance of oxygen and carbon dioxide control in the initial post-ROSC period. However, the best practices and technology for managing oxygen and carbon dioxide remain unclear. In a pilot intensive care unit study, Eastwood, et al. suggested that manual titration of oxygen in post-arrest patient is feasible and safe [11]. However, in the prehospital HOT OR NOT study, 7 of 8 patients in the titrated FiO2 arm experienced hypoxemia (SaO2 < 88%) [26]. While exhaled end-tidal carbon dioxide (ETCO2) tension has been used to guide resuscitation, post-arrest patients often exhibit poor cardiac output and have a large alveolar dead space (wasted ventilation), impacting the correlation between ETCO2 and PaCO2 [16, 17, 27]. Until new technology is developed to aid oxygen and carbon dioxide titration, clinicians will likely need to resort to frequent protocolized ABG measurement and ventilator adjustment.
The associations observed in this analysis varied with modeling strategy, with the primary analysis suggesting only limited harm from initial hyperoxemia and hypocarbia but the sensitivity analyses suggesting harm from any oxygen or carbon dioxide tension measurements. Furthermore, while our analysis showed associations between oxygen and carbon dioxide aberrancies and OHCA outcomes, our observed relationships may also represent surrogate markers of clinical care. For example, final hypoxemia or hyperoxemia may reflect less attentive overall mechanical ventilation management. Until clarified by further prospective study, we believe that most pragmatic approaches are to 1) apply close and frequent ABG monitoring and 2) avoid all significant oxygen and carbon dioxide abnormalities in post-arrest patients.
This study was limited to OHCA patients surviving at least one hour after ROSC. Half of the study population did not have ABG measurements available during the first 24 h of hospitalization, but the reasons for ABG omission are unknown. Among potential factors, ABG missingness could have been due to premature death (-e.g., patient death prior to obtaining first ABG measurement), variations in clinical practices, or the applied data procurement and abstraction methods. Missing ABG values could also reflect situations where the patient rapidly regained consciousness without requiring prolonged ventilation. We note that there were many similarities between cases with and without ABG values – particularly mortality. Thus, we suspect that biases due to ABG missingness are likely limited and not systematic.
While we focused on the first 24 h of hospitalization, oxygen and carbon dioxide abnormalities may persist in subsequent ICU care. Because ABG measurements and clinical reactions were not protocolized, it was not possible to impute and model the duration of observed oxygen and carbon dioxide tension measurements. However, given the strong associations with the observed point measurements, different results with time varying models are unlikely. Protocolized ABG collection and ventilator adjustment would be necessary to determine the time-dependent nature of oxygen and carbon dioxide exposures. We chose to categorize oxygen and carbon dioxide tension into three categories based upon previous studies. It is possible that oxygen and carbon dioxide tension measurements may be markers of severe critical illness; more aggressive attempts to normalize these values may not necessarily improve outcomes.
We had no information on ventilation parameters (-e.g., pH, respiratory rate, tidal volume, fraction of inspired oxygen) or clinical actions in response to ABG measurements. Our observations reflect post-ROSC care and cannot shed light on the manner of ventilation or oxygenation during out-of-hospital EMS care or the first hour of Emergency Department care. The prospective study of post-arrest ventilation (with protocolized ABG measurements and detailed characterization of ventilatory practices and adjustments) is an important area for future study.
We did not account for hospital characteristics or processes of in hospital care such as provision of therapeutic hypothermia or percutaneous coronary intervention. We studied hospital survival as the primary outcome, not neurologically intact survival; some experts believe that the pathophysiological basis of oxygen and carbon dioxide harm is through brain injury [5–7].
Conclusion
In the first 24 h after return of spontaneous circulation, post-arrest oxygen and carbon dioxide tension abnormalities are associated with increased out of-hospital cardiac arrest mortality. Strategies to control post-arrest oxygen and carbon dioxide tensions may potentially improve clinical outcomes.
Supplementary Material
Acknowledgments
Financial support
The Resuscitation Outcomes Consortium was supported by a series of cooperative agreements to nine regional clinical centers and one Data Coordinating Center (5U01 HL077863-University of Washington Data Coordinating Center, HL077866-Medical College of Wisconsin, HL077867-University of Washington, HL077871-University of Pittsburgh, HL077872-St. Michael’s Hospital, HL077873-Oregon Health and Science University, HL077881-University of Alabama at Birmingham, HL077885-Ottawa Health Research Institute, HL077887-University of Texas SW Medical Center/Dallas, HL077908-University of California San Diego) from the National Heart, Lung and Blood Institute in partnership with the National Institute of Neurological Disorders and Stroke, U.S. Army Medical Research & Material Command, The Canadian Institutes of Health Research (CIHR) – Institute of Circulatory and Respiratory Health, Defence Research and Development Canada and the Heart, Stroke Foundation of Canada and the American Heart Association. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung and Blood Institute or the National Institutes of Health.
Abbreviations
- ROSC
return of spontaneous circulation
- OHCA
out-of-hospital cardiac arrest
- ABG
arterial blood gas
- ED
Emergency Department
- ROC
Resuscitation Outcomes Consortium
- CPR
Cardiopulmonary Resuscitation
Footnotes
A Spanish translated version of the abstract of this article appears as Appendix in the final online version at http://dx.doi.org/10.1016/j.resuscitation.2017.08.244.
Presented at: American Heart Association Resuscitation Science Symposium, New Orleans, Louisiana, November 2016; National Association of EMS Physicians Annual Meeting, New Orleans, Louisiana, January 2017.
Conflicts of interest
The authors declare no conflicts of interest.
Authorship
HEW designed the study. All authors contributed to the collection of data. DKP and SM carried out the analysis. HEW and DKP drafted the manuscript, and all author contributed to its critical review and revision. HEW accepts overall responsibility for the paper.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.resuscitation.2017.08.244.
References
- 1.Nichol G, Thomas E, Callaway CW, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300(12):1423–31. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Callaway CW, Donnino MW, Fink EL, et al. Part 8: post-cardiac arrest care: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 Suppl. 2):S465–82. doi: 10.1161/CIR.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Idris AH, Staples ED, O’Brien DJ, et al. Effect of ventilation on acid-base balance and oxygenation in low blood-flow states. Crit Care Med. 1994;22(11):1827–34. [PubMed] [Google Scholar]
- 4.Angelos MG, Yeh ST, Aune SE. Post-cardiac arrest hyperoxia and mitochondrial function. Resuscitation. 2011;82(Suppl. 2):S48–51. doi: 10.1016/S0300-9572(11)70151-4. [DOI] [PubMed] [Google Scholar]
- 5.Richards EM, Fiskum G, Rosenthal RE, Hopkins I, McKenna MC. Hyperoxic reperfusion after global ischemia decreases hippocampal energy metabolism. Stroke. 2007;38(5):1578–84. doi: 10.1161/STROKEAHA.106.473967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vereczki V, Martin E, Rosenthal RE, Hof PR, Hoffman GE, Fiskum G. Normoxic resuscitation after cardiac arrest protects against hippocampal oxidative stress, metabolic dysfunction, and neuronal death. J Cereb Blood Flow Metab. 2006;26(6):821–35. doi: 10.1038/sj.jcbfm.9600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hazelton JL, Balan I, Elmer GI, et al. Hyperoxic reperfusion after global cerebral ischemia promotes inflammation and long-term hippocampal neuronal death. J Neurotrauma. 2010;27(4):753–62. doi: 10.1089/neu.2009.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trzeciak S, Rivers EP. Emergency department overcrowding in the United States: an emerging threat to patient safety and public health. Emerg Med J. 2003;20(5):402–5. doi: 10.1136/emj.20.5.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muizelaar JP, Marmarou A, Ward JD, et al. Adverse effects of prolonged hyper-ventilation in patients with severe head injury: a randomized clinical trial. J Neurosurg. 1991;75(5):731–9. doi: 10.3171/jns.1991.75.5.0731. [DOI] [PubMed] [Google Scholar]
- 10.Bellomo R, Bailey M, Eastwood GM, et al. Arterial hyperoxia and in-hospital mortality after resuscitation from cardiac arrest. Crit Care. 2011;15(2):R90. doi: 10.1186/cc10090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eastwood GM, Schneider AG, Suzuki S, et al. Targeted therapeutic mild hypercapnia after cardiac arrest: a phase II multi-centre randomised controlled trial(the CCC trial) Resuscitation. 2016;104:83–90. doi: 10.1016/j.resuscitation.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 12.Helmerhorst HJ, Roos-Blom MJ, van Westerloo DJ, Abu-Hanna A, de Keizer NF, de Jonge E. Associations of arterial carbon dioxide and arterial oxygen concentrations with hospital mortality after resuscitation from cardiac arrest. Crit Care. 2015;19:348. doi: 10.1186/s13054-015-1067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider AG, Eastwood GM, Bellomo R, et al. Arterial carbon dioxide tension and outcome in patients admitted to the intensive care unit after cardiac arrest. Resuscitation. 2013;84(7):927–34. doi: 10.1016/j.resuscitation.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Roberts BW, Kilgannon JH, Chansky ME, Mittal N, Wooden J, Trzeciak S. Association between post resuscitation partial pressure of arterial carbon dioxide and neurological outcome in patients with post-cardiac arrest syndrome. Circulation. 2013;127(21):2107–13. doi: 10.1161/CIRCULATIONAHA.112.000168. [DOI] [PubMed] [Google Scholar]
- 15.Falkenbach P, Kamarainen A, Makela A, et al. Incidence of iatrogenic dyscarbia during mild therapeutic hypothermia after successful resuscitation from out-of-hospital cardiac arrest. Resuscitation. 2009;80(9):990–3. doi: 10.1016/j.resuscitation.2009.04.044. [DOI] [PubMed] [Google Scholar]
- 16.Vaahersalo J, Bendel S, Reinikainen M, et al. Arterial blood gas tensions after resuscitation from out-of-hospital cardiac arrest: associations with long-term neurologic outcome. Crit Care Med. 2014;42(6):1463–70. doi: 10.1097/CCM.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 17.Janz DR, Hollenbeck RD, Pollock JS, McPherson JA, Rice TW. Hyperoxia is associated with increased mortality in patients treated with mild therapeutic hypothermia after sudden cardiac arrest. Crit Care Med. 2012;40(12):3135–9. doi: 10.1097/CCM.0b013e3182656976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuisma M, Boyd J, Voipio V, Alaspaa A, Roine RO, Rosenberg P. Comparison of 30 and the 100% inspired oxygen concentrations during early post-resuscitation period: a randomised controlled pilot study. Resuscitation. 2006;69(2):199–206. doi: 10.1016/j.resuscitation.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Elmer J, Scutella M, Pullalarevu R, et al. The association between hyperoxia and patient outcomes after cardiac arrest: analysis of a high-resolution database. Intensive Care Med. 2015;41(1):49–57. doi: 10.1007/s00134-014-3555-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kilgannon JH, Jones AE, Parrillo JE, et al. Relationship between supranormal oxygen tension and outcome after resuscitation from cardiac arrest. Circulation. 2011;123(23):2717–22. doi: 10.1161/CIRCULATIONAHA.110.001016. [DOI] [PubMed] [Google Scholar]
- 21.Kilgannon JH, Jones AE, Shapiro NI, et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303(21):2165–71. doi: 10.1001/jama.2010.707. [DOI] [PubMed] [Google Scholar]
- 22.Wang CH, Chang WT, Huang CH, et al. The effect of hyperoxia on survival following adult cardiac arrest: a systematic review and meta-analysis of observational studies. Resuscitation. 2014;85(9):1142–8. doi: 10.1016/j.resuscitation.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 23.Morrison LJ, Nichol G, Rea TD, et al. Rationale, development and implementation of the resuscitation outcomes consortium epistry-cardiac arrest. Resuscitation. 2008;78(2):161–9. doi: 10.1016/j.resuscitation.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis DP, Garberson LA, Andrusiek DL, et al. A descriptive analysis of Emergency Medical Service Systems participating in the Resuscitation Outcomes Consortium (ROC) network. Prehosp Emerg Care. 2007;11(4):369–82. doi: 10.1080/10903120701537147. [DOI] [PubMed] [Google Scholar]
- 25.Cummins RO, Chamberlain DA, Abramson NS, et al. Recommended guidelines for uniform reporting of data from out-of-hospital cardiac arrest: the Utstein Style. A statement for health professionals from a task force of the American Heart Association, the European Resuscitation Council, the Heart and Stroke Foundation of Canada, and the Australian Resuscitation Council. Circulation. 1991;84(2):960–75. doi: 10.1161/01.cir.84.2.960. [DOI] [PubMed] [Google Scholar]
- 26.Young P, Bailey M, Bellomo R, et al. HyperOxic Therapy OR NormOxic Therapy after out-of-hospital cardiac arrest (HOT OR NOT): a randomised controlled feasibility trial. Resuscitation. 2014;85(12):1686–91. doi: 10.1016/j.resuscitation.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Gudipati CV, Weil MH, Bisera J, Deshmukh HG, Rackow EC. Expired carbon dioxide: a noninvasive monitor of cardiopulmonary resuscitation. Circulation. 1988;77(1):234–9. doi: 10.1161/01.cir.77.1.234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.