Abstract
The human microbiota is a complex ecosystem of diverse microorganisms consisting of bacteria, viruses, and fungi residing predominantly in epidermal and mucosal habitats across the body, such as skin, oral cavity, lung, intestine and vagina. These symbiotic communities in health, or dysbiotic communities in disease, display tremendous interaction with the local environment and systemic responses, playing a critical role in the host’s nutrition, immunity, metabolism and diseases including cancers. While the profiling of normal microbiota in healthy populations is useful and necessary, more recent studies have focused on the microbiota associated with disease, particularly cancers. In this paper, we review current evidence on the role of the human microbiota in four cancer types (colorectal cancer, head and neck cancer, pancreatic cancer, and lung cancer) proposed as affected by both the oral and gut microbiota, and provide a perspective on current gaps in the knowledge of the microbiota and cancer.
Introduction
In 2012 there were more than 14 million new cases of human cancer, threatening 8.2 million lives worldwide, making cancer one of the leading global causes of death (1). The global cancer burden is projected to rise as growing populations are exposed to risk factors for cancers. Data support that genetic susceptibility, environmental factors, microbial alterations and/or chronic inflammation all contribute to carcinogenesis (2). A number of epithelial cancers are now ascribed to single microbes that modify epithelial cell biology to yield cancer development (Table 1) (2–7). Only recently, however, have we started to appreciate the compelling evidence indicating the role of microbial communities and organization in carcinogenesis.
Table 1.
Single microbes cause various types of human cancers.
| Cancer Type | Single microbe | Ref. |
|---|---|---|
| Gastric Cancer | Helicobacter pylori | [2–4] |
| Liver Cancer | Hepatitis B virus, Hepatitis C virus | [5] |
| Biliary Tree Cancer | Clonorchis sinensis, Opisthorchis viverrini | [5] |
| Cervical Cancer | Human papillomavirus (HPV) | [2,3] |
| Head and Neck Cancer | HPV | [43, 45–48] |
| Urinary Bladder Cancer | Schistosoma hematobium | [5] |
| Lymphoma | Epstein-Barr virus | [6] |
| Merkel Cell Carcinoma | Merkel cell polyomavirus | [7] |
| Kaposi Sarcoma | Kaposi sarcoma-associated herpesvirus | [112] (113) |
The emergence of next generation sequencing technologies, including high-resolution 16S rRNA gene sequencing and metagenomic technologies, has enabled discoveries of the diversity of the microbial gene repertoire (8, 9). Based on the key features of the microbiome including microbial diversity, relative abundance and microbial gene richness (10, 11), increasing research aims to define the bacterial composition and function associated with various cancer types across the body. Compelling evidence suggests environmental and physiological factors including diet, smoking, alcohol consumption, obesity and chronic inflammation significantly and differentially modify the microbiota within diverse anatomic sites. These same factors are often linked to carcinogenesis, fostering studies to discern links between the microbiota and cancer development. One intriguing aspect of these emerging data is the putative link between the oral or gut microbiota and cancer biology.
Herein, we seek to give an overview on recent data revealing the relationship between microbial alterations and four types of cancers including colorectal cancer, head and neck cancer, pancreatic cancer, and lung cancer. In each of these cancers, the oral and/or the gut microbiota have been postulated to contribute to cancer pathogenesis. The detailed mechanisms underlying microbe-host interactions in carcinogenesis, usually derived from in vivo murine models, are beyond the scope of this review but have been discussed elsewhere (2, 3, 12). Instead, we will focus on summarizing the available human epidemiological data and discuss the next potential steps in translational research to understand the interaction between the microbiota and cancer.
The Gut Microbiota and Colon-Inhabiting Oral Microbes in Colorectal Cancer
Each year, 1.3 million new cases of colorectal cancer (CRC) occur, which threaten 694,000 lives equally divided among both men and women worldwide (1). In the United States, excluding gender-specific cancers, CRC remains the second leading cause of cancer-related deaths in men and women despite a significant decline in incidence rates, particularly in people over age 50 years, due to colonoscopy screening (1, 13). Strikingly, recent epidemiological data demonstrate altered CRC incidence patterns in the United States from 1974 to 2013, notably underscoring an increasing CRC risk in the population younger than age 50 years (14). This disturbing trend calls for new public awareness and research to delineate probable causes of age-specific CRC risk, particularly in the young.
CRC often takes more than 10 years to develop due to the accumulation of genetic mutations in colonic epithelial cells and sequential histopathological stages including aberrant crypt foci, polyps, adenomas and carcinomas (13, 15). Hereditary forms only account for 3–5% of all CRC cases, while more than 95% of CRC is sporadic and mainly occurs in people older than 50 years (13, 16). Besides genetic susceptibility, other risk factors include inflammatory colitis, high consumption of red and processed meat, obesity, smoking and excessive alcohol consumption (13). Strong evidence suggests these environmental and physiological factors significantly modify the colonic microbiota composition and function (2, 3), which has led to increasing interest in the role of the microbiota in colon carcinogenesis.
Gut Specific Pathogens and CRC
Based on our and others’ studies using murine models, individual bacterial species provoke colon tumorigenesis by means of both microbial factors and host immune responses (2, 3, 17). Enterotoxigenic Bacteroides fragilis (ETBF) is one of the most prevalent pathobionts detected in human CRC patients. The mechanisms by which ETBF induces murine colon tumorigenesis include the induction of reactive oxygen species to directly initiate DNA damage, as well as activation of Wnt signaling pathways via E-cadherin cleavage by B. fragilis enterotoxin (BFT), and induction of proinflammatory cytokine IL-17 pathways to promote cell survival and proliferation (18–20). Additionally, pks+ Escherichia coli (E.coli) (21) and Enterococcus faecalis (22) can putatively induce tumorigenesis by generating DNA mutagens such as the genotoxin colibactin, and superoxide and hydrogen peroxide, respectively. Although the tumorigenic potential of these specific species has been studied in depth with murine models, and each has been found in association with human CRC, these species have yet to be directly shown to induce CRC in humans which requires demonstrating, for example, that the host was exposed to the bacterium prior to the onset of disease. Thus we still lack an understanding of whether these bacteria or other microbes are causal in human CRC cases, and intensive and difficult to do longitudinal studies from early colonization to the development of cancer may help prove causality (23, 24).
Gut Bacterial Dysbiosis in CRC
In addition to the studies focusing on single pathogenic species, increasing studies have identified compositional shifts of the gut microbiota associated with CRC, which supports the hypothesis that altered microbial communities serve as contributors to CRC development (25). For example, several studies demonstrate an emergence of putative pathogenic bacteria coincidently with substantial commensal depletion in CRC patients (23, 24, 26). The pro-carcinogenic mechanisms by which bacterial dysbiosis contributes to CRC are speculated to reflect microbial-induced alterations in host metabolism and mucosal immune responses (2) (26) (27). However, studies on the gut microbiota profiling in human CRC have yielded diverse results regarding the patterns of bacterial communities enriched in tumor regions as previously reviewed (25). It remains uncertain whether bacterial dysbiosis is merely secondary to colon tumor development or a causative exposure prior to onset of colon carcinogenesis.
Colonic Biofilms in CRC
Bacterial biofilms are complex ecosystems composed of polymicrobial aggregates of diverse bacteria embedded in an extracellular polymeric matrix, mainly consisting of polysaccharides as well as proteins, nucleic acids and lipids (28). According to previous studies, colonic biofilms were defined as bacterial community invasion into the inner mucus layer spanning at least a linear distance of 200μm across the colon epithelial surface (23, 29). The association of colonic biofilms with human CRC was reported in 2014 when our lab discovered that colonic biofilms were a nearly universal feature of right-sided CRC and their paired normal mucosa obtained from the distant edge of surgical resections (23). Interestingly, right colon cancers were not uniquely susceptible to biofilm formation as approximately 17% (6/35) of left colon cancers also displayed similar biofilms. Even more surprisingly, 13% (15/120) of colonoscopy biopsies collected from healthy people also displayed biofilms, although the bacterial compositions of these biofilms were typically less dense and thinner than the exuberant structures on tumors in CRC patients. The colonic epithelial cells under the biofilms in the paired normal tissues of CRC patients or normal colon biopsies obtained from healthy individuals exhibited altered epithelial biology consistent with a pro-oncogenic state. These alterations featured reduced or redistributed E-cadherin, enhanced IL-6 production, activated Stat3 as well as increased epithelial cell proliferation (23).
Colonic biofilms associated with CRC are all polymicrobial entities. Thus, we propose that defining the bacterial organization and function of biofilms from CRC patients at the species and/or the strain level will provide new insights into mechanisms of microbiota-driven CRC. A collaborative study (26) supported the idea that colonic biofilms modified mucosal biology by demonstrating that bacterial biofilms altered the cancer or normal colonic mucosal metabolome via upregulation of polyamine metabolism, a possible promoter of colonic epithelial proliferation and cancer progression. Biofilm-associated enhancement of polyamine metabolites was associated with enriched Clostridia groups including Sporobacter, Peptostreptococcaceae and Ceilonellaceae, but reduced Bacteroidales (26). Nevertheless, neither the carcinogenic potential of colonic biofilms in humans, nor a clear causal role for any gut bacterium, virulence factor or community structure in human CRC has yet to be well defined. Prospective and longitudinal epidemiological studies are needed to determine the role of microbial communities in the initiation and progression of CRC. An impediment for conducting these studies is the predicted long course from onset to detection of carcinogenesis in humans.
Colon-Inhabiting Oral Microbes in CRC
The finding of Fusobacterium in about 30% of CRC cases by 16S rRNA sequencing (30) (31) (32) was unexpected and has initiated discussions of an association between the oral microbiota in the colon and CRC, a topic expanded upon by other authors (33) (34). The Fusobacterium genus, particularly Fusobacterium nucleatum (F. nucleatum), is more frequently identified in CRC cases compared with less consistent results in colorectal adenoma cases, suggesting that Fusobacterium may contribute to later progression instead of earlier initiation of colon carcinogenesis (30, 35, 36). Alternatively, increased multiplicity of colon adenomas in susceptible mouse models in some, but not all experiments, may reflect a capacity of F. nucleatum for tumor initiation (24, 37, 38). F. nucleatum appears to utilize several adherence factors such as FadA combined with a proclivity to invade tissue, disrupt cell-cell adhesion, activate Wnt cell proliferation signals and stimulate proinflammatory pathways to potentially enhance the accumulation of genetic mutations (24, 37, 39). In addition to Fusobacterium, other groups of oral bacteria such as Porphyromonas (36), Peptostreptococcus, Prevotella, Parvimonas, and Gemella genera are often found associated with the colon microbiome of patients with colon cancer. The carcinogenic potential and virulence factors of these genera are unknown. Furthermore, it remains to be validated whether these oral microbes detected in the colon represent the same species or strains that inhabit the oral cavity. Even if the oral bacteria residing in the colon originate from the oral cavity, we do not understand their mechanisms to adapt to the colon environment and the implications for colon carcinogenesis.
The Oral Microbiota and Head and Neck Cancers
Head and neck cancers refer to the malignancies of the mucous membranes lining the head and neck cavities, including the nasal cavity and paranasal sinuses, oral cavity, pharynx (nasopharynx, oropharynx and hypopharynx), and larynx (40). Ninety percent of head and neck cancers are squamous cell carcinomas (HNSCC) originating from squamous cells, among which oral SCC (OSCC) accounts for the most common malignant lesions (41). Tobacco and alcohol consumption are two major risk factors for OSCC and these risk factors appear to further modify the microbial composition of the oral cavity where a dense microbiota resides [41]. Increasing evidence suggests that periodontitis, poor oral hygiene, and oral microorganisms are associated with the development of head and neck cancers (40–43). Moreover, studies are beginning to define potential mechanisms by which the oral microbiota, combined with known OSCC risk factors, may drive oral carcinogenesis. For example, oral bacteria can transform alcohol to acetaldehyde, a mutagen promoting carcinogenesis of head and neck mucosa (3, 40, 44).
HNSCC includes multiple cancers originating from heterogeneous tissue in various anatomical sites, partly accounting for the variations in current sequencing results. Therefore, clinical studies linking the microbiota to HNSCC face complex challenges. Further, the salivary microbiome dramatically differs from that of the oral mucosa. Thus, future studies are needed to clarify the role of bacterial dysbiosis in head and neck cancers. In Table 2, we summarize available epidemiological studies seeking to characterize the oral bacterial features of human HNSSC cases and describe some of these data below.
Table 2.
Oral microbiota profiling in human head and neck cancers.
| Cancer type |
Study type and Population |
Microbiota analysis |
Main results | Comments |
|---|---|---|---|---|
| OSCC | Cross sectional study. Saliva samples from OSCC patients (45), and OSCC-free subjects (229). USA 2005 (56) | Checkerboard DNA-DNA hybridization with DNA probes of 40 strains. | Capnocytophaga gingivalis, Prevotella melaninogenica and Streptococcus mitis were elevated in OSCC saliva, which was found to be a predictor. | No antibiotic treatment in previous 3 months. Paired normal regions were not included. OSCC-free patients were not defined. |
| OSCC | Cross sectional study. Tumor resection and paired normal specimens from OSCC patients (10/10). UK 2007 (60) | FISH for universal bacteria. PCR, cloning and ABI Prism BigDye terminator cycle sequencing on 16S rDNA. | Distinct microbial composition between cancer and paired normal specimens. Most taxa isolated from within the tumor tissue represented saccharolytic and aciduric species. | Paired normal were included, but healthy controls were not. Patient details were not stated. |
| OSCC | Cross sectional study. Saliva samples from OSCC patients (3), and matched normal controls (2). USA 2011 (57) | 16S rDNA (V4, V5) PCR amplification, DGGE analysis and pyrosequencing. | Salivary microbiota in OSCC differs from normal controls. Fifteen unique phylotypes were present in all three OSCC subjects. | Very small sample size but included matched normal controls. Patient details were not provided. |
| OSCC | Cross sectional study. 10 OSCC patients. Tumor mucosae (10) and paired normal (10). USA 2012 (54). | 16S rDNA (V4, V5) PCR amplification and DGGE analysis. 16S rDNA cloning and Sanger sequencing. | Clonal analysis characterized 80 bacterial species/phylotypes. Some Streptococcus spp. are highly prevalent at tumor sites. | Healthy controls were not included. Patient details were not provided. |
| Oral cancer | Cross sectional study. Discovery cohort: oral cancer (5). Confirmation cohort: oral cancer (9), carcinoma in situ (1), pre-cancer (8), healthy controls (6). USA 2014 (42). | Discovery cohort: 16S rDNA (V4) amplicon pyrosequencing followed by validation using Illumina sequencing on oral mucosal swab DNA. | Abundance of Firmicutes (especially Streptococcus) and Actinobacteria (especially Rothia) was significantly decreased in cancer lesions and precancers. PCoA indicated 12 taxa separated most cancers from other samples. | Paired normal tissues and healthy controls were included and well defined. Tobacco and alcohol usage was documented. Histopathological types of oral cancer were not stated. |
| NPC | Longitudinal study: Saliva samples from NPC (3) and healthy controls (3). China 2014 (58). | 16S rDNA (V1–V3) amplicon pyrosequencing on salivary DNA. | PCoA: microbial separation was found not only between NPC patients and healthy controls, but also between pre- and post chemoradiation therapy. | First study to define oral microbiota in NPC patients. No antibiotic treatment in previous 1 month. |
| LSCC | Cross sectional study. Oral swab and tissue from LSCC patients (27); vocal cord polyps (28). China 2014 (53). | 16S rDNA (V1–V3) amplicon pyrosequencing on oral swab and tissue DNA. | Bacterial diversity significantly varied in different anatomical sites of throat, and between carcinoma and polyps. | No antibiotic treatment in previous 3 months. Paired normal were included, while healthy controls were not stated. |
| OSCC | Cross sectional study: OPSCC (11) OCSCC (6) and normal controls (25). Longitudinal study: follow up 11 patients every 3 months post treatment. USA 2016 (49). | 16S rDNA (V3–V5) amplicon pyrosequencing on salivary rinse DNA. | HNSCC patients had a significant loss in bacterial richness and diversity*. Genus Streptococcus, Dialister, and Veillonella were found to discriminate tumors from normal controls. | First report of microbiome associated with HNSCC subsites, HPV status, and surgical treatment. Paired normal was not included. Tobacco and alcohol usage were not stated. |
| HNSCC | Cross sectional study: Tumor resection and paired normal specimens from 121 HNSCC. USA 2017 (59). | 16S rDNA (V1–V3) amplicon pyrosequencing on mucosal DNA from different anatomic sites. | No significant difference in alpha diversity. Genus Actinomyces up to the phylum level were significantly depleted, while Parvimonas was increased in tumor relative to paired normal. | No healthy controls and antibiotic treatment was not stated. Multiple cancer types were enrolled without analysis of site-specific microbiota. |
HNSCC: Head and Neck Squamous Cell Carcinoma; OPSCC: Oropharyngeal Squamous Cell Carcinoma; O(C)SCC: Oral (Cavity) Squamous Cell Carcinoma; LSCC: Laryngeal Squamous Cell Carcinoma; NPC: nasopharyngeal; OTU: Operational Taxonomic Units; DGGE: Denaturing Gradient Gel Electrophoresis; PCoA: Principal Coordination Analysis; FISH: Fluorescent In Situ Hybridyzation; qPCR: real-time quantitative PCR; HOM database: Human Oral Microbiome database.
Neisseria and Aggregatibacter (Proteobacteria), Haemophillus (Firmicutes) and Leptotrichia (Fusobacteria) were significantly depleted in salivary microbiota of OSCC patients as compared with normal controls.
Specific Pathogens and OSCC
During the last decade, oral infection with human papillomavirus (HPV), particularly high-risk HPV type 16 (HPV-16), has been widely considered an independent risk factor inducing oropharyngeal squamous cell carcinoma (OPSCC) (43, 45–48). The mechanisms of HPV-driven OPSCC have been largely attributed to the overexpression of the protein p16 and oncogenes E6 and E7 (47). However, the tissue tropism of HPV to preferentially target oropharyngeal squamous cells is poorly understood. Recently, HPV infection was found to be coincident with the presence of select bacterial groups known to be associated with oral dysbiosis, namely, Gemellaceae and Leuconostoc (49). Another study (43) reported that poor oral health increases the risk of oral HPV infection and might contribute to subsequent HPV-related OPSCC. Therefore, characterizing microbial communities that favor oral HPV infection may shed light on studies about microbial biomarkers for HPV-related OPSCC.
Porphyromonas gingivalis and Fusobacterium nucleatum are two major oral pathobionts in periodontitis, and their molecular mechanisms to promote cell survival, proliferation and invasion have been studied in detail in periodontal diseases (39, 50, 51). Their carcinogenic potential in oral squamous cells had not been tested until one recent study reported that chronic infection of P. gingivalis or F. nucleatum in a murine model promoted the development of OSCC and stimulated an IL-6-STAT3 inflammatory cascade. However, the clinical relevance of these biological mechanisms in human OSCC cases remains to be investigated. No single pathogen can be detected in all OSCC patients, and in turn, specific pathogens like P. gingivalis or F. nucleatum can be also found in healthy subjects (52). So, a more comprehensive understanding about the composition and function of the oral microbiota in OSCC is needed to advance our knowledge of cancer-provoking microbial signatures. Bacterial strains in the same species may well induce different phenotypic traits. Culture and isolation of more clinical strains are needed to test pathogenic variations among clinical isolates in order to address the role of the microbiota in OSCC.
Oral Dysbiosis and OSCC
In healthy saliva and oral mucosa, the predominant bacterial phyla consist of Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Fusobacteria, TM7 and Spirochaete (10, 42, 53, 54). By microbiological culturing methods, some earlier studies described more enriched aerobic and anaerobic bacterial species at oral tumor sites compared to healthy controls (55), however, these studies could possibly be biased by failing to account for unculturable bacterial species. Using high-throughput sequencing approaches, a few studies have started to investigate the complexity and significant shifts of the oral microbiota in health and disease. A new etiologic framework has been proposed, namely, oral microbial variations, as communities of synergism and/or dysbiosis, account for the development of periodontal diseases, rather than only exogenous pathogens (52). Emerging studies suggest that cancer sites present a loss of richness and diversity of commensal species (49), and such microbial disturbances may enhance emergence of carcinogenic species or modulation of the inflammation status to indirectly promote cancer development. In general, the profiling of the salivary microbiota has been limited to clinical studies of small sample size with inconsistent results (56, 57). Limited current data suggest a remarkable segregation of the cancer microbiome from healthy controls (56–58), and some salivary species like Capnocytophaga gingivalis, Prevotella melaninogenica and Streptococcus mitis correlate with OSCC (56). Furthermore, growing attention is being placed on the mucosal microbiome by comparing tumor sites with normal tissue (54, 59). Dysbiosis at tumor sites in HNSCC seems to harbor a signature of markedly decreased abundance of the phylum Actinobacteria as compared with paired normal tissue (42, 53, 59). Additionally, one study showed that the phylum Firmicutes was also decreased at tumor sites (59). However, the results diverge across studies when analyzing the microbial composition at the genus or species level (42, 44, 54, 59, 60). Thus, longitudinal studies with larger sample size are needed to validate the etiological impact and diagnostic value of the microbial alterations.
Oral Biofilms and OSCC
Poor oral health, including periodontitis and poor oral hygiene, is an independent risk factor for oral cancers (40, 43, 61, 62), in which chronic inflammation and the oral microbiota are closely involved (41, 50, 63). Meanwhile, periodontitis has long been recognized as a pathogenic state of biofilm-induced inflammation (62, 64, 65). F. nucleatum and Porphyromonas spp. work synergistically to promote adhesion, aggregation and colonization during oral biofilm formation. Surprisingly, no studies thus far have characterized the relationship between microbial contributors to oral biofilms, also known as dental plaques, and oral SCC. If similar to colonic biofilms (e.g. promoting epithelial proliferation and DNA damage), oral biofilms may also change tissue biology to augment the initiation or progression of oral SCC and perhaps be a source to define the carcinogenic bacterial contributors. Studying the cause-and-effect relationship of biofilms and oral cancer is hampered by lack of experimental models of oral cancer and well-designed longitudinal epidemiological studies.
The Oral and Gut Microbiota in Pancreatic Cancer
Pancreatic ductal adenocarcinoma accounts for about 85% of pancreatic malignancy, however, the pancreas lacks an established microbiome. As one of the most aggressive and deadly cancer types, pancreatic cancer has an extremely poor prognosis with a five-year survival rate of about 5%, and with mortality increasing during the past decades in many countries worldwide (1, 66). Conventional risk factors of pancreatic cancer include smoking, genetic susceptibility, obesity, diabetes mellitus, alcohol and consumption of smoked or processed meat (67, 68). No recommended screening tests are available to date for the early detection of pancreatic cancer (66, 69), unveiling a clear need to which perhaps microbiota science can contribute.
The Oral Microbiota and Pancreatic Cancer
Recently, accumulating data suggest that oral dysbiosis is associated with increased risk of pancreatic cancer (Table 3), data supported by observations of a positive association between chronic periodontitis and pancreatic cancer (3, 70–72). The salivary microbiome from patients with pancreatic cancer has been profiled using the Human Oral Microbe Identification Microarray (HOMIM) (73). The overall alterations of oral microbial composition between patients with pancreatic cancer and healthy controls were predominantly attributed to the bacterial phyla of Firmicutes, Proteobacteria, CFB group bacteria (Cytophaga-Flavobacterium-Bacteroides group) and Actinobacteria. The combination of decreased Neisseria elongate (within Proteobacteria) and Streptococcus mitis (within Firmicutes) was suggested as a promising biomarker for the diagnosis of pancreatic cancer. However, the prevalence of these oral microbes in pancreatic cancer vs. healthy individuals remains uncertain as this study was limited to only 38 pancreatic cancer cases and 38 matched healthy controls.
Table 3.
Microbiota profiling in human pancreatic cancer and lung cancer.
| Cancer type |
Study type and Population |
Microbiota analysis |
Main results | Comments |
|---|---|---|---|---|
| Pancreatic Cancer | Cross sectional study. Discovery cohort: pancreatic cancer (10), matched healthy controls (10). Validation cohort: pancreatic cancer (28), matched healthy controls (28). USA 2012 (73). | Hybridization of 16S rDNA amplicons to HOMIM microarray. Validation qPCR on salivary DNA from independent samples. | Significant variation of salivary microflora between patients with pancreatic cancer and healthy controls. Neisseria elongata and Streptococcus mitis were validated and suggested to distinguish patients with pancreatic cancer. | First study to define oral microbiota variation associated with pancreatic cancer and propose oral microbiota as source for potential biomarkers of pancreatic disease. Antibiotic treatment was not stated. |
| Cross sectional study. Pancreatic cancer (8), healthy controls (22), and other diseases (78). USA 2015 (74). | 16S rDNA amplification with universal primers on salivary DNA followed by Illumina sequencing. | Pancreatic cancer: higher ratio of Leptotrichia to Porphyromonas in saliva. Possible lower abundance of Neisseria and Aggregatibacter. | Limited numbers of patients with pancreatic cancer and unmatched healthy controls. Antibiotic treatment prior to sampling was not stated. | |
| Prospective cross sectional study. Pancreatic adenocarcinoma (361), and matched healthy controls (371). USA 2016 (77). | 16S rDNA (V3, V4) amplicon pyrosequencing on DNA of oral wash samples. Taxonomic assignment using the HOM Database. | Pancreatic cancer associated with abundant Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans and decreased abundance of phylum Fusobacteria and its genus Leptotrichia. | Prospective case-control study with large sample size and matched controls, which tends to suggest oral microbiota might serve as a biomarker for pancreatic cancer. | |
| Lung Cancer | Cross sectional study. Lung cancer patients (87) undergoing lung resection. France 2012 (107). | qPCR targeting viruses and 16S rDNA of bacteria from BAL, lung tumors and healthy lungs. | Distal airways were bacteria-free by PCR. Herpesviridae positive by PCR might be related to higher risk for postoperative complication. | Standard IV prophylactic antibiotic treatment was given to all the patients. Paired normal controls were included. |
| Cross sectional study. Non-smoker lung cancer (8), healthy controls (8). China and USA 2014 (109). | 16S rDNA (V1, V2) amplicon pyrosequencing on DNA of sputum and buccal rinse sample. | Bacterial diversity in sputum of patients with lung cancer was distinct from controls. Granulicatella, Abiotrophia and Streptococcus were enriched in lung cancer cases. | Sputum was collected through cough without induction and oral microbiota contamination couldn’t be excluded. Antibiotic treatment prior sampling was not stated. | |
| Cross sectional study. Pulmonary carcinoma patients (9) undergoing lung resection. Japan 2014 (110). | Anaerobic culture of bacteria from intraoperative bronchial fluids. 16S rDNA PCR with universal primers followed by BigDye terminator cycle sequencing. | Bacteria in intraoperative bronchial fluids, dominated by Streptococcus, Veillonella, Gemella, and Porphyromonas, potentially derived from oral microbiota. | Used a novel bronchoscopic microsampling probe to obtain uncontaminated bronchial fluids. IV cefazolin was given prior to bronchoscopy. No control cases were included. | |
| Cross sectional study. Discovery cohort: Lung SCC (10), AC (10); healthy controls (10). Validation cohort: Lung SCC (13), AC (28); healthy controls (15). China 2015 (99). | 16S rDNA (V3, V6) amplicon Illumina sequencing on salivary DNA. qPCR validation targeting bacterial candidates discovered by sequencing. | Increased abundance of salivary Capnocytophaga and Veillonella may discriminate SCC and AC from healthy controls. The abundance of Neisseria was decreased in SCC and AC compared with healthy controls. | Patient details were not provided. Antibiotic treatment was not stated. |
qPCR: real-time quantitative PCR; HOMIM: Human Oral Microbiome Identification Microarray; BAL: Bronchoalveolar Lavage; AC: Adenocarcinoma; I.V.: Intravenous
Despite a number of studies investigating the association of oral microbes with pancreatic cancer cases, some inconsistencies have been reported. A study using the Human Oral Microbiome Identification Microarray found that the presence of Neisseria elongate and Streptococcus mitis was able to distinguish patients with pancreatic cancer from healthy subjects with 96.4% sensitivity and 82.1% specificity (73). Michaud et al. reported a positive association between P. gingivalis and pancreatic cancer (74), Torres et al. observed enrichment of F. leptotrichia and decreased P. gingivalis in pancreatic cancer cases (75). This discrepancy may be due to the fact that the Michaud et al. findings were based on antibodies to P. gingivalis and not necessarily the oral presence of the microbe as explored by Torres et al. Other researchers found that Fusobacterium species in the oral cavity were prevalent in 8.8% of patients with pancreatic cancers, which was not associated with any disease-related gene mutation or epigenetic alterations, but was independently associated with worse prognosis (76). Thus, additional evidence will be needed to validate the carcinogenic potential of bacterial dysbiosis or specific bacterial pathogens or pathobionts in pancreatic cancer. Similar to other cancer and microbiota research, studies to define bacterial strain(s) contributing to carcinogenesis could be essential to unravel reported inconsistencies as a single bacterial species can harbor pathogenic and nonpathogenic strains.
An earlier study suggested that exposure to Porphyromonas gingivalis may contribute to higher risk of pancreatic cancer due to identifying high serum antibodies against Porphyromonas gingivalis, a pathobiont associated with periodontal disease (74). A newly published prospective nested case-control study provided supportive evidence of this association. Analysis of 16S rRNA gene sequencing suggested that the presence of P. gingivalis and Aggregatibacter actinomycetemcomitans in the oral cavity correlates with pancreatic carcinogenesis (77). This large prospective cohort generated potential insights into the microbial associations with pancreatic cancer including 361 incident pancreatic adenocarcinoma cases during a follow-up period of 10 to 17 years. The oral wash specimens for microbiome sequencing were collected before disease progression, thereby suggesting that oral microbial variations could be a marker for pancreatic cancer and not a secondary finding reflective of the cancer microenvironment. Multiple environmental factors including diet, inflammatory status and altered epithelial function modify the microbial composition over time, however, to date, studies generally have failed to characterize the dynamic changes of the microbiota during the time line of carcinogenesis. The features of the oral microbiome may serve as a non-invasive biomarker for identification of people at high risk for pancreatic cancer initiation, progression or poor prognosis, though better characterization of the bacterial dysbiosis through disease course would be necessary to develop future clinical applications.
Despite emerging results suggesting an association between oral dysbiosis and pancreatic ductal adenocarcinoma (70, 73, 77), direct evidence is not yet available defining whether this oral dysbiosis is causative or merely a reactive effect of early distal cancer. No single species has been proven to directly induce or inhibit pancreatic carcinogenesis in experimental models or human epidemiological studies. One idea is that oral microbiota translocation may contribute to pancreas carcinogenesis. Some studies have shown that oral bacteria may migrate or colonize (78) to remote sites by swallowing or via the circulatory system (77, 79–81). However, it is unknown whether oral bacteria present in the pancreas belong to the same species/strains as those in the oral cavity. Systemic inflammation (74), bacterial components and metabolic products (82) are highly likely to be involved, but the specific tissue tropism, if microbiota translocation occurs, and the molecular mechanisms of microbiota-driven carcinogenesis at distal sites are thus far unexplored.
The gut microbiota and pancreatic cancer
Two meta-analysis studies have suggested that seropositivity for Helicobacter pylori (H. pylori), a common pathogen residing in the upper gastrointestinal tract, significantly increased the risk of pancreatic carcinogenesis (83, 84). In contrast, case-control studies (85, 86) and one population-based prospective cohort study (87) suggest, also using serology, that the association between H. pylori infection and pancreatic cancer is unconvincing. Although the upper gastrointestinal microbiota may link with pancreatic ductal carcinogenesis because of close anatomic location and/or ductal connections, no results demonstrate pancreas colonization by H. pylori or pancreatic inflammation being attributable to H. pylori infection in the upper gastrointestinal tract (88). Despite the fact that conventional risk factors for pancreatic cancer (e.g., obesity and diabetes) are known to be associated with gut dysbiosis, no data, as yet, define compositional shifts of the gut microbiota in patients with pancreatic cancer as compared with healthy controls.
The Oral and Lung Microbiota in Lung cancer
Lung cancer has been continuously ranked as the most common cancer type and the leading cause of cancer death worldwide. Smoking is a well-known risk factor for lung cancer due to its carcinogenic chemical components (89). Emerging studies suggest that multiple additional factors, including systemic inflammatory responses (90), infections with certain microorganisms (91–95) and periodontal diseases (33, 96, 97) contribute to lung cancer development.
The Oral Microbiota and Lung Cancer
Research during the past ten years has consistently shown that periodontal diseases, known to alter the oral microbiota, are associated with lung cancer risk (33, 96, 97), but most of the previous studies failed to exclude the role of confounding factors like smoking (97). One recent study (65) indicated that advanced periodontitis was associated with a 2.5-fold increase in lung cancer among people who never smoked. Periodontitis-related lung cancer has been proposed to result from inflammatory immune responses initiated in the oral cavity that modify the respiratory epithelium and promote carcinogenesis (96), but the responsive immune cell populations and cytokines involved in the biological mechanisms remain undetermined. Limited preliminary data propose a few transcriptomic salivary biomarkers, relating to growth regulation and tumor suppressor genes, as possibly useful to discriminate lung cancer from health (98), but prospective longitudinal data are needed. While oral pathogens or pathobionts have been proposed to colonize the lung contributing to lung carcinogenesis, empiric data are lacking. Very limited 16S rRNA gene sequencing results of salivary microbiome suggest an elevated abundance of Capnocytophaga and Veillonella together with a reduced number of Neisseria (99). Current data are insufficient to conclude that oral microbial variations contribute to lung cancer.
The Lung Microbiota and Lung Cancer
There is interest in exploring if various factors beyond smoking may be associated with or, in fact, contribute to lung carcinogenesis. Various studies suggest lung cancer development is fostered, in part, by certain specific microorganisms including Chlamydia pneumoniae (91, 92), human immunodeficiency virus (100–103), HPV (68, 104, 105), Pneumoccocus (94), and mycobacteria. However, this is an area of active debate given that current studies are limited by small sample size, observational study designs and inconsistent results (90, 105, 106). The lower respiratory tract was considered microorganism free (107) until the use of 16S rRNA gene sequencing to detect the bacterial community in bronchoalveolar lavage (BAL) fluid or expectorated sputum (Table 3) (108). One preliminary study demonstrated that sputum dysbiosis associated with lung cancer correlates with an increased relative abundance of Granulicatella, Abiotrophia and Streptococcus (109). A Japanese group reported that Streptococcus, Veillonella, Gemella, and Porphyromonas identified in intraoperative bronchial fluids might derive from the oral microbiota (110). However, the clinical significance of these bacterial groups is unknown and it is further unknown if the contamination of BAL fluid or sputum with the microbiota in the upper respiratory tract and oral cavity contributes to these results.
Summary
Despite having witnessed substantial advance of technologies and knowledge to begin to understand cancer as being attributable to the microbiota, we should recognize the limitations and challenges in pursuing causality of the bacterial microbiota in various cancer types as summarized in Table 4. Interestingly, identical cancer states can correlate with diverse dysbiosis and/or multiple microbial species that have carcinogenic potential. Overall, three hypotheses are proposed to delineate the role of the microbiota in cancer etiology including: (1) the role of individual pathogens; (2) the role of polymicrobial communities; and (3) the interactions between specific microbes with a microbial community (2).
Table 4.
Areas for development to assist in establishing causality between the microbiota and cancer development.
Concepts:
|
Methodology:
|
Limitations of current studies:
|
Future perspectives:
|
The first hypothesis reflects Koch’s postulates (111) that can be traced back to the late nineteenth century. Koch’s postulates state three steps to define a pathogen: (1) a microbe must be purified from every diseased individual and under circumstances that account for the pathological changes; (2) the microbe of interest does not exist in health or any other diseases; and (3) the microbe can be isolated in pure culture and is capable of reproducing the disease in an independent susceptible subject. These principles have long served as a framework to define the role of individual microbes in disease pathogenesis. While microbes as displayed in Table 1 have been considered cancer-causing microbes, pathobionts, such as those discussed in this review would not qualify. This limitation of the classic Koch’s postulates has been discussed (Fredrick and Relman) and, thus, we now need to consider in our analyses for putative disease causation microbial communities or specific microbes that induce disease under certain conditions (113).
In contrast, the second model describes mechanisms by which microbial communities, and not individual species, drive carcinogenesis. This model hypothesizes that interactions among microbial communities and the host, involving microbial genotoxins, carcinogenic metabolites and/or pro-inflammatory cascades, underlie bacterial-related cancer development (2). Advances in the technologies of next generation sequencing, transcriptomic, proteomic and/or metabolomic analyses, are being used to define the links between the microbiota and carcinogenesis. Microbial dysbiosis in the context of various cancers has been increasingly characterized; however, limitations exist including lack of evidence on species/strain, small sample size and inconsistent findings among studies with variable criteria. Although microbiological culturing methods could be one tool used to validate the promising results from sequencing analyses, the technical difficulties in recovering and culturing many anaerobes or other fastidious organisms from clinical specimens hinder the discovery of novel cancer-provoking bacteria in dysbiotic communities. Further, to date, pursuit of the contributions of other microbes (e.g., viruses, fungi) is more limited.
Last but not least, the third proposed model for microbial-induced carcinogenesis suggests individual microbe-community interaction(s) that influence cancer initiation or progression. For example, colonization by a specific pathogen or pathobiont could cause disruption of the microbiota organization, yielding dysbiosis that alone could be carcinogenic or, in turn, render the host vulnerable to carcinogenic pathogens. As we reviewed in this paper, oral HPV infection and biofilm formation are associated with bacterial dysbiosis that correlates with oral or colon cancers respectively. However, knowledge about the compositional features and mechanisms of microbial interactions is limited (114) and additional work is needed to understand the biological impact and clinical relevance. It must be remembered that microbial communities are dynamic, with the microbiota expected to evolve during the chronic course of cancer progression.
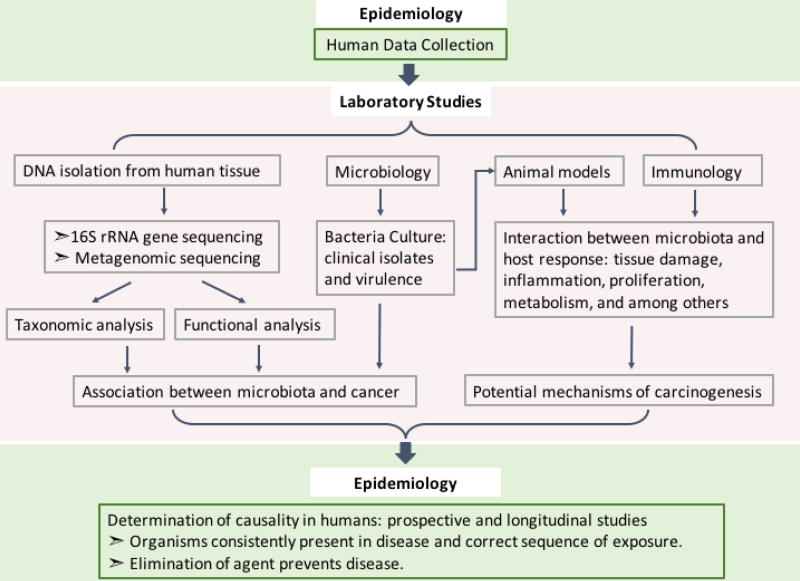
In keeping these three hypotheses in mind, the identification of a single carcinogenic microbe, such as ETBF in murine models, must be investigated under conditions that would prove or disprove the other two hypotheses. Despite the discovery of specific microbes and their associated cancers (Table 1), the diversity of the microbiota does not always afford for one microbe to be associated with a particular disease. Considering the work flow described in Fig. 1, future studies should aim to uncover pro-carcinogenic communities and the co-occurrence of specific microbes. Once identified these communities can be used in murine models (including gnotobiotic mice) of cancer development. Additionally, if specific microbes are found to be causative without the presence of other microbes, investigation into their carcinogenic potential in the presence of commensals should be explored. Thus, carefully designed studies are needed to define whether the microbiota can serve as a clinically useful, robust indicator of early carcinogenesis and, therefore, help address the burden, morbidity and mortality of diverse cancers (Fig 1).
Fig.1. Framework for studying etiology of microbiota-driven carcinogenesis.
To better understand the impact of the microbiota on carcinogenesis in humans, assembly of adequately sized human populations for study is needed. DNA from the human tissue or other samples can then be extracted and sequenced for taxonomic and functional analyses of the microbiota. In parallel, culturing of isolated microbes, studies in animal models, as well as profiling of the immune responses to potential carcinogenic microbes will allow for the discovery of microbiota-associated mechanisms driving carcinogenesis. From what is discovered in the laboratory, prospective and longitudinal human studies will be required to confirm the causal effects of the microbiota on human cancers. Critical study approaches include, for example, studies to confirm that the person is exposed to the microbe of interest prior to disease onset, and approaches to prevent disease such as through vaccination against the implicated microbe.
Acknowledgments
We acknowledge the support of the Bloomberg-Kimmel Cancer Immunotherapy Institute, the Johns Hopkins Department of Medicine and NIH grants NCI R01CA196845 (to C.L.S). J.C is supported by the Department of Molecular Microbiology and Immunology, Bloomberg School of Public Health) as an international student.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. (Reviewed in May 2017) http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
- 2.Sears CL, Garrett WS. Microbes, microbiota, and colon cancer. Cell Host Microbe. 2014;15(3):317–328. doi: 10.1016/j.chom.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13(11):800–812. doi: 10.1038/nrc3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peek RM, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2(1):28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 5.Sears CL, Pardoll DM. Perspective: alpha-bugs, their microbial partners, and the link to colon cancer. J Infect Dis. 2011;203(3):306–311. doi: 10.1093/jinfdis/jiq061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hjalgrim H, Askling J, Rostgaard K, et al. Characteristics of Hodgkin's lymphoma after infectious mononucleosis. N Engl J Med. 2003;349(14):1324–1332. doi: 10.1056/NEJMoa023141. [DOI] [PubMed] [Google Scholar]
- 7.Nghiem PT, Bhatia S, Lipson EJ, et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N Engl J Med. 2016;374(26):2542–2552. doi: 10.1056/NEJMoa1603702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakravorty S, Helb D, Burday M, et al. A detailed analysis of 16S ribosomal RNA gene segments for the diagnosis of pathogenic bacteria. J Microbiol Methods. 2007;69(2):330–339. doi: 10.1016/j.mimet.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langille MG, Zaneveld J, Caporaso JG, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31(9):814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drewes JL, Housseau F, Sears CL. Sporadic colorectal cancer: microbial contributors to disease prevention, development and therapy. Br J Cancer. 2016;115(3):273–280. doi: 10.1038/bjc.2016.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383(9927):1490–1502. doi: 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- 14.Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. J Natl Cancer Inst. 2017;109(8) doi: 10.1093/jnci/djw322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fodde R, Smits R, Clevers H. APC, signal transduction and genetic instability in colorectal cancer. Nat Rev Cancer. 2001;1(1):55–67. doi: 10.1038/35094067. [DOI] [PubMed] [Google Scholar]
- 16.Jasperson KW, Tuohy TM, Neklason DW, et al. Hereditary and familial colon cancer. Gastroenterology. 2010;138(6):2044–2058. doi: 10.1053/j.gastro.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gagliani NHB, Huber S, Elinav E, Flavell R. The Fire Within: Microbes Inflame Tumors. Cell. 2014;157:776–783. doi: 10.1016/j.cell.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Thiele Orberg E, Fan H, Tam AJ, et al. The myeloid immune signature of enterotoxigenic Bacteroides fragilis-induced murine colon tumorigenesis. Mucosal Immunol. 2016 doi: 10.1038/mi.2016.53. Epub 2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu S, Rhee KJ, Albesiano E, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15(9):1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodwin AC, Destefano Shields CE, Wu S, et al. Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc Natl Acad Sci U S A. 2011;108(37):15354–15359. doi: 10.1073/pnas.1010203108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arthur JC, Perez-Chanona E, Muhlbauer M, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338(6103):120–123. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huycke MM, Abrams V, Moore DR. Enterococcus faecalis produces extracellular superoxide and hydrogen peroxide that damages colonic epithelial cell DNA. Carcinogenesis. 2002;23(3):529–536. doi: 10.1093/carcin/23.3.529. [DOI] [PubMed] [Google Scholar]
- 23.Dejea CM, Wick EC, Hechenbleikner EM, et al. Microbiota organization is a distinct feature of proximal colorectal cancers. Proc Natl Acad Sci U S A. 2014;111(51):18321–18326. doi: 10.1073/pnas.1406199111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kostic AD, Chun E, Robertson L, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14(2):207–215. doi: 10.1016/j.chom.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dejea C, Wick E, Sears CL. Bacterial oncogenesis in the colon. Future Microbiol. 2013;8(4):445–460. doi: 10.2217/fmb.13.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson CH, Dejea CM, Edler D, et al. Metabolism links bacterial biofilms and colon carcinogenesis. Cell Metab. 2015;21(6):891–897. doi: 10.1016/j.cmet.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12(10):661–672. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- 28.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2(2):95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 29.Swidsinski A, Weber J, Loening-Baucke V, et al. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J Clin Microbiol. 2005;43(7):3380–3389. doi: 10.1128/JCM.43.7.3380-3389.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahn J, Sinha R, Pei Z, et al. Human gut microbiome and risk for colorectal cancer. J Natl Cancer Inst. 2013;105(24):1907–1911. doi: 10.1093/jnci/djt300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kostic AD, Gevers D, Pedamallu CS, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22(2):292–298. doi: 10.1101/gr.126573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castellarin M, Warren RL, Freeman JD, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22(2):299–306. doi: 10.1101/gr.126516.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vogtmann E, Goedert JJ. Epidemiologic studies of the human microbiome and cancer. Br J Cancer. 2016;114(3):237–242. doi: 10.1038/bjc.2015.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flynn KJ, Baxter NT, Schloss PD. Metabolic and Community Synergy of Oral Bacteria in Colorectal Cancer. mSphere. 2016;1(3) doi: 10.1128/mSphere.00102-16. Epub 00102-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng Q, Liang S, Jia H, et al. Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nat Commun. 2015;6:6528. doi: 10.1038/ncomms7528. [DOI] [PubMed] [Google Scholar]
- 36.Zackular JP, Rogers MA, Ruffin MTt, et al. The human gut microbiome as a screening tool for colorectal cancer. Cancer Prev Res (Phila) 2014;7(11):1112–1121. doi: 10.1158/1940-6207.CAPR-14-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Y, Weng W, Peng J, et al. Fusobacterium nucleatum Increases Proliferation of Colorectal Cancer Cells and Tumor Development in Mice by Activating Toll-Like Receptor 4 Signaling to Nuclear Factor-kappaB, and Up-regulating Expression of MicroRNA-21. Gastroenterology. 2017;152(4):851–866. e824. doi: 10.1053/j.gastro.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomkovich S, Yang Y, Winglee K, et al. Locoregional Effects of Microbiota in a Preclinical Model of Colon Carcinogenesis. Cancer Res. 2017;77(10):2620–2632. doi: 10.1158/0008-5472.CAN-16-3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rubinstein MR, Wang X, Liu W, et al. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14(2):195–206. doi: 10.1016/j.chom.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galvao-Moreira LV, da Cruz MC. Oral microbiome, periodontitis and risk of head and neck cancer. Oral Oncol. 2016;53:17–19. doi: 10.1016/j.oraloncology.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 41.Chi AC, Day TA, Neville BW. Oral cavity and oropharyngeal squamous cell carcinoma--an update. CA Cancer J Clin. 2015;65(5):401–421. doi: 10.3322/caac.21293. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt BL, Kuczynski J, Bhattacharya A, et al. Changes in abundance of oral microbiota associated with oral cancer. PLoS One. 2014;9(6):e98741. doi: 10.1371/journal.pone.0098741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Printz C. Poor oral health tied to oral HPV infection. Cancer. 2013;119(24):4215. doi: 10.1002/cncr.28488. [DOI] [PubMed] [Google Scholar]
- 44.Homann N, Jousimies-Somer H, Jokelainen K, et al. High acetaldehyde levels in saliva after ethanol consumption: methodological aspects and pathogenetic implications. Carcinogenesis. 1997;18(9):1739–1743. doi: 10.1093/carcin/18.9.1739. [DOI] [PubMed] [Google Scholar]
- 45.Ma Y, Madupu R, Karaoz U, et al. Human papillomavirus community in healthy persons, defined by metagenomics analysis of human microbiome project shotgun sequencing data sets. J Virol. 2014;88(9):4786–4797. doi: 10.1128/JVI.00093-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tezal M, Scannapieco FA, Wactawski-Wende J, et al. Local inflammation and human papillomavirus status of head and neck cancers. Arch Otolaryngol Head Neck Surg. 2012;138(7):669–675. doi: 10.1001/archoto.2012.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mirghani H, Amen F, Moreau F, et al. Do high-risk human papillomaviruses cause oral cavity squamous cell carcinoma? Oral Oncol. 2015;51(3):229–236. doi: 10.1016/j.oraloncology.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 48.D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 49.Guerrero-Preston R, Godoy-Vitorino F, Jedlicka A, et al. 16S rRNA amplicon sequencing identifies microbiota associated with oral cancer, human papilloma virus infection and surgical treatment. Oncotarget. 2016;7(32):51320–51334. doi: 10.18632/oncotarget.9710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Inaba H, Sugita H, Kuboniwa M, et al. Porphyromonas gingivalis promotes invasion of oral squamous cell carcinoma through induction of proMMP9 and its activation. Cell Microbiol. 2014;16(1):131–145. doi: 10.1111/cmi.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mao S, Park Y, Hasegawa Y, et al. Intrinsic apoptotic pathways of gingival epithelial cells modulated by Porphyromonas gingivalis. Cell Microbiol. 2007;9(8):1997–2007. doi: 10.1111/j.1462-5822.2007.00931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hajishengallis G, Lamont RJ. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol. 2012;27(6):409–419. doi: 10.1111/j.2041-1014.2012.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gong H, Shi Y, Zhou X, et al. Microbiota in the Throat and Risk Factors for Laryngeal Carcinoma. Appl Environ Microbiol. 2014;80(23):7356–7363. doi: 10.1128/AEM.02329-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pushalkar S, Ji X, Li Y, et al. Comparison of oral microbiota in tumor and non-tumor tissues of patients with oral squamous cell carcinoma. BMC Microbiol. 2012;12:144. doi: 10.1186/1471-2180-12-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Byakodi R, Krishnappa R, Keluskar V, et al. The microbial flora associated with oral carcinomas. Quintessence Int. 2011;42(9):e118–123. [PubMed] [Google Scholar]
- 56.Mager DL, Haffajee AD, Devlin PM, et al. The salivary microbiota as a diagnostic indicator of oral cancer: a descriptive, non-randomized study of cancer-free and oral squamous cell carcinoma subjects. J Transl Med. 2005;3:27. doi: 10.1186/1479-5876-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pushalkar S, Mane SP, Ji X, et al. Microbial diversity in saliva of oral squamous cell carcinoma. FEMS Immunol Med Microbiol. 2011;61(3):269–277. doi: 10.1111/j.1574-695X.2010.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu Y, Teng F, Huang S, et al. Changes of saliva microbiota in nasopharyngeal carcinoma patients under chemoradiation therapy. Arch Oral Biol. 2014;59(2):176–186. doi: 10.1016/j.archoralbio.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 59.Wang H, Funchain P, Bebek G, et al. Microbiomic differences in tumor and paired-normal tissue in head and neck squamous cell carcinomas. Genome Med. 2017;9(1):14. doi: 10.1186/s13073-017-0405-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hooper SJ, Crean SJ, Fardy MJ, et al. A molecular analysis of the bacteria present within oral squamous cell carcinoma. J Med Microbiol. 2007;56(Pt 12):1651–1659. doi: 10.1099/jmm.0.46918-0. [DOI] [PubMed] [Google Scholar]
- 61.Meurman JH, Uittamo J. Oral micro-organisms in the etiology of cancer. Acta Odontol Scand. 2008;66(6):321–326. doi: 10.1080/00016350802446527. [DOI] [PubMed] [Google Scholar]
- 62.Kim J, Amar S. Periodontal disease and systemic conditions: a bidirectional relationship. Odontology. 2006;94(1):10–21. doi: 10.1007/s10266-006-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kerr AR. The oral microbiome and cancer. J Dent Hyg. 2015;89(Suppl 1):20–23. [PubMed] [Google Scholar]
- 64.Ahn J, Segers S, Hayes RB. Periodontal disease, Porphyromonas gingivalis serum antibody levels and orodigestive cancer mortality. Carcinogenesis. 2012;33(5):1055–1058. doi: 10.1093/carcin/bgs112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Michaud DS, Kelsey KT, Papathanasiou E, et al. Periodontal disease and risk of all cancers among male never smokers: an updated analysis of the Health Professionals Follow-up Study. Ann Oncol. 2016;27(5):941–947. doi: 10.1093/annonc/mdw028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol. 2016;22(44):9694–9705. doi: 10.3748/wjg.v22.i44.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bosetti C, Bertuccio P, Negri E, et al. Pancreatic cancer: overview of descriptive epidemiology. Mol Carcinog. 2012;51(1):3–13. doi: 10.1002/mc.20785. [DOI] [PubMed] [Google Scholar]
- 68.Li D, Xie K, Wolff R, et al. Pancreatic cancer. Lancet. 2004;363(9414):1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 69.Shin EJ, Canto MI. Pancreatic cancer screening. Gastroenterol Clin North Am. 2012;41(1):143–157. doi: 10.1016/j.gtc.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Michaud DS, Joshipura K, Giovannucci E, et al. A prospective study of periodontal disease and pancreatic cancer in US male health professionals. J Natl Cancer Inst. 2007;99(2):171–175. doi: 10.1093/jnci/djk021. [DOI] [PubMed] [Google Scholar]
- 71.Otomo-Corgel J, Pucher JJ, Rethman MP, et al. State of the science: chronic periodontitis and systemic health. J Evid Based Dent Pract. 2012;12(3 Suppl):20–28. doi: 10.1016/S1532-3382(12)70006-4. [DOI] [PubMed] [Google Scholar]
- 72.Hujoel PP, Drangsholt M, Spiekerman C, et al. An exploration of the periodontitis-cancer association. Ann Epidemiol. 2003;13(5):312–316. doi: 10.1016/s1047-2797(02)00425-8. [DOI] [PubMed] [Google Scholar]
- 73.Farrell JJ, Zhang L, Zhou H, et al. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut. 2012;61(4):582–588. doi: 10.1136/gutjnl-2011-300784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Torres PJ, Fletcher EM, Gibbons SM, et al. Characterization of the salivary microbiome in patients with pancreatic cancer. Peer J. 2015;3:e1373. doi: 10.7717/peerj.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Michaud DS, Izard J, Wilhelm-Benartzi CS, et al. Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut. 2013;62(12):1764–1770. doi: 10.1136/gutjnl-2012-303006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mitsuhashi K, Nosho K, Sukawa Y, et al. Association of Fusobacterium species in pancreatic cancer tissues with molecular features and prognosis. Oncotarget. 2015;6(9):7209–7220. doi: 10.18632/oncotarget.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fan X, Alekseyenko AV, Wu J, et al. Human oral microbiome and prospective risk for pancreatic cancer: a population-based nested case-control study. Gut. 2016 doi: 10.1136/gutjnl-2016-312580. Epub 2016-312580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Swidsinski A, Schlien P, Pernthaler A, et al. Bacterial biofilm within diseased pancreatic and biliary tracts. Gut. 2005;54(3):388–395. doi: 10.1136/gut.2004.043059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Crasta K, Daly CG, Mitchell D, et al. Bacteraemia due to dental flossing. J Clin Periodontol. 2009;36(4):323–332. doi: 10.1111/j.1600-051X.2008.01372.x. [DOI] [PubMed] [Google Scholar]
- 80.Lockhart PB, Brennan MT, Sasser HC, et al. Bacteremia associated with toothbrushing and dental extraction. Circulation. 2008;117(24):3118–3125. doi: 10.1161/CIRCULATIONAHA.107.758524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ahn J, Chen CY, Hayes RB. Oral microbiome and oral and gastrointestinal cancer risk. Cancer Causes Control. 2012;23(3):399–404. doi: 10.1007/s10552-011-9892-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ochi A, Nguyen AH, Bedrosian AS, et al. MyD88 inhibition amplifies dendritic cell capacity to promote pancreatic carcinogenesis via Th2 cells. J Exp Med. 2012;209(9):1671–1687. doi: 10.1084/jem.20111706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guo Y, Liu W, Wu J. Helicobacter pylori infection and pancreatic cancer risk: A meta-analysis. J Cancer Res Ther. 2016;12(Supplement):C229–C232. doi: 10.4103/0973-1482.200744. [DOI] [PubMed] [Google Scholar]
- 84.Trikudanathan G, Philip A, Dasanu CA, et al. Association between Helicobacter pylori infection and pancreatic cancer. A cumulative meta-analysis. JOP. 2011;12(1):26–31. [PubMed] [Google Scholar]
- 85.Lindkvist B, Johansen D, Borgstrom A, et al. A prospective study of Helicobacter pylori in relation to the risk for pancreatic cancer. BMC Cancer. 2008;8:321. doi: 10.1186/1471-2407-8-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu G, Murphy G, Michel A, et al. Seropositivity to Helicobacter pylori and risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2013;22(12):2416–2419. doi: 10.1158/1055-9965.EPI-13-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen XZ, Schottker B, Castro FA, et al. Association of helicobacter pylori infection and chronic atrophic gastritis with risk of colonic, pancreatic and gastric cancer: A ten-year follow-up of the ESTHER cohort study. Oncotarget. 2016;7(13):17182–17193. doi: 10.18632/oncotarget.7946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang C, Li J. Pathogenic Microorganisms and Pancreatic Cancer. Gastrointest Tumors. 2015;2(1):41–47. doi: 10.1159/000380896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hecht SS. Lung carcinogenesis by tobacco smoke. Int J Cancer. 2012;131(12):2724–2732. doi: 10.1002/ijc.27816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gomes M, Teixeira AL, Coelho A, et al. The role of inflammation in lung cancer. Adv Exp Med Biol. 2014;816:1–23. doi: 10.1007/978-3-0348-0837-8_1. [DOI] [PubMed] [Google Scholar]
- 91.Hua-Feng X, Yue-Ming W, Hong L, et al. A meta-analysis of the association between Chlamydia pneumoniae infection and lung cancer risk. Indian J Cancer. 2015;52(Suppl 2):e112–115. doi: 10.4103/0019-509X.172506. [DOI] [PubMed] [Google Scholar]
- 92.Zhan P, Suo LJ, Qian Q, et al. Chlamydia pneumoniae infection and lung cancer risk: a meta-analysis. Eur J Cancer. 2011;47(5):742–747. doi: 10.1016/j.ejca.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 93.Lin FC, Huang JY, Tsai SC, et al. The association between human papillomavirus infection and female lung cancer: A population-based cohort study. Medicine (Baltimore) 2016;95(23):e3856. doi: 10.1097/MD.0000000000003856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin TY, Huang WY, Lin JC, et al. Increased lung cancer risk among patients with pneumococcal pneumonia: a nationwide population-based cohort study. Lung. 2014;192(1):159–165. doi: 10.1007/s00408-013-9523-z. [DOI] [PubMed] [Google Scholar]
- 95.Winstone TA, Man SF, Hull M, et al. Epidemic of lung cancer in patients with HIV infection. Chest. 2013;143(2):305–314. doi: 10.1378/chest.12-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zeng XT, Xia LY, Zhang YG, et al. Periodontal Disease and Incident Lung Cancer Risk: A Meta-Analysis of Cohort Studies. J Periodontol. 2016;87(10):1158–1164. doi: 10.1902/jop.2016.150597. [DOI] [PubMed] [Google Scholar]
- 97.Michaud DS, Liu Y, Meyer M, et al. Periodontal disease, tooth loss, and cancer risk in male health professionals: a prospective cohort study. Lancet Oncol. 2008;9(6):550–558. doi: 10.1016/S1470-2045(08)70106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang L, Xiao H, Zhou H, et al. Development of transcriptomic biomarker signature in human saliva to detect lung cancer. Cell Mol Life Sci. 2012;69(19):3341–3350. doi: 10.1007/s00018-012-1027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yan X, Yang M, Liu J, et al. Discovery and validation of potential bacterial biomarkers for lung cancer. Am J Cancer Res. 2015;5(10):3111–3122. [PMC free article] [PubMed] [Google Scholar]
- 100.Liu J, Kandasamy S, Zhang J, et al. Prebiotic effects of diet supplemented with the cultivated red seaweed Chondrus crispus or with fructo-oligo-saccharide on host immunity, colonic microbiota and gut microbial metabolites. BMC Complement Altern Med. 2015;15:279. doi: 10.1186/s12906-015-0802-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lavole A, Toper C, Belmont L, et al. Lung cancer and HIV infection. Rev Mal Respir. 2014;31(2):133–141. doi: 10.1016/j.rmr.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 102.Hessol NA, Martinez-Maza O, Levine AM, et al. Lung cancer incidence and survival among HIV-infected and uninfected women and men. AIDS. 2015;29(10):1183–1193. doi: 10.1097/QAD.0000000000000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shcherba M, Shuter J, Haigentz M., Jr Current questions in HIV-associated lung cancer. Curr Opin Oncol. 2013;25(5):511–517. doi: 10.1097/CCO.0b013e328363dfdb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhai K, Ding J, Shi HZ. HPV and lung cancer risk: a meta-analysis. J Clin Virol. 2015;63:84–90. doi: 10.1016/j.jcv.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 105.Anantharaman D, Gheit T, Waterboer T, et al. No causal association identified for human papillomavirus infections in lung cancer. Cancer Res. 2014;74(13):3525–3534. doi: 10.1158/0008-5472.CAN-13-3548. [DOI] [PubMed] [Google Scholar]
- 106.Brenner DR, McLaughlin JR, Hung RJ. Previous lung diseases and lung cancer risk: a systematic review and meta-analysis. PLoS One. 2011;6(3):e17479. doi: 10.1371/journal.pone.0017479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.D'Journo XB, Bittar F, Trousse D, et al. Molecular detection of microorganisms in distal airways of patients undergoing lung cancer surgery. Ann Thorac Surg. 2012;93(2):413–422. doi: 10.1016/j.athoracsur.2011.09.049. [DOI] [PubMed] [Google Scholar]
- 108.Dickson RP, Erb-Downward JR, Huffnagle GB. The role of the bacterial microbiome in lung disease. Expert Rev Respir Med. 2013;7(3):245–257. doi: 10.1586/ers.13.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hosgood HD, Sapkota AR, Rothman N, et al. The potential role of lung microbiota in lung cancer attributed to household coal burning exposures. Environ Mol Mutagen. 2014;55(8):643–651. doi: 10.1002/em.21878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hasegawa A, Sato T, Hoshikawa Y, et al. Detection and identification of oral anaerobes in intraoperative bronchial fluids of patients with pulmonary carcinoma. Microbiol Immunol. 2014;58(7):375–381. doi: 10.1111/1348-0421.12157. [DOI] [PubMed] [Google Scholar]
- 111.Evans AS. Causation and disease: the Henle-Koch postulates revisited. Yale J Biol Med. 1976;49(2):175–195. [PMC free article] [PubMed] [Google Scholar]
- 112.Byrd AL, Segre JA. Infectious disease. Adapting Koch's postulates. Science. 2016;351:224–226. doi: 10.1126/science.aad6753. [DOI] [PubMed] [Google Scholar]
- 113.Dittmer DP, Damania B. Kaposi sarcoma-associated herpesvirus: immunobiology, oncogenesis, and therapy. The Journal of clinical investigation. 2016;126:3165–3175. doi: 10.1172/JCI84418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Virgin HW. The virome in mammalian physiology and disease. Cell. 2014;157:142–150. doi: 10.1016/j.cell.2014.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]