Abstract
Objective
To compare odds of survival to hospital discharge among pediatric out-of-hospital cardiac arrest (OHCA) patients receiving either bag-valve-mask ventilation (BVM), supraglottic airway (SGA) or endotracheal intubation (ETI), after adjusting for the propensity to receive a given airway intervention.
Methods
Retrospective cohort study using the Cardiac Arrest Registry to Enhance Survival (CARES) database from January 1 2013– December 31 2015. The CARES registry includes data on cardiac arrests from 17 statewide registries and approximately 55 additional US cities. We included patients less than18 years of age who suffered a non-traumatic OHCA and received a resuscitation attempt by Emergency Medical Services (EMS). The key exposure was the airway management strategy (BVM, ETI, or SGA). The primary outcome was survival to hospital discharge.
Results
Of the 3,793 OHCA cases included from 405 EMS agencies, 1724 cases were analyzed after limiting the analysis to EMS agencies that used all 3 devices. Of the 1724, 781 (45.3%) were treated with BVM only, 727 (42.2%) ETI, and 215 (12.5%) SGA. Overall, 20.7% had ROSC and 10.9% survived to hospital discharge. After using a propensity score analysis, the odds ratio for survival to hospital discharge for ETI compared to BVM was 0.39 (95%CI 0.26–0.59) and for SGA compared to BVM was 0.32 (95% CI 0.12–0.84). These relationships were robust to the sensitivity analyses including complete case, EMS-agency matched, and age-stratified.
Conclusions
BVM was associated with higher survival to hospital discharge compared to ETI and SGA. A large randomized clinical trial is needed to confirm these findings.
Introduction
Cardiac arrest is a rare but devastating problem in children. The estimated incidence among all patients less than 18 years of age is 8–20 per 100,000 person years, and in infants is 78 per 100,000 person years.1,2 Survival from pediatric out-of-hospital cardiac arrest (P-OHCA) has typically been estimated between 6–12% and, when compared to adults, survival is lower in infants and higher in adolescents.1,3–5
Over the last decade, survival among adults suffering an OHCA from all rhythms has increased significantly, concurrent with emphasis on bystander CPR, high quality CPR, and early defibrillation.6 Unfortunately, outcomes from P-OHCA have not improved over that same time period.5 Pediatric out-of-hospital airway management is controversial, and the single controlled trial conducted to date, published in the year 2000, found no benefit to endotracheal intubation (ETI) compared to bag-valve-mask ventilation (BVM), with ETI trending towards harm in some groups.7 However, despite these findings, ETI remains a common method of airway management in pediatric OHCA.8 ETI is rarely implemented by individual paramedics in pediatric patients necessitating ongoing training (usually simulation) for skill maintenance.9 This lack of patient exposure may raise the risk of harm and complications associated with pediatric ETI in EMS. A recent in-hospital study of airway management in pediatric cardiac arrest found that BVM was associated with significantly higher odds of survival compared to ETI, indicating that ETI may be inferior for initial cardiac arrest management even under more controlled circumstances.10 Overall, the literature exploring the association between airway management (BVM, ETI and supraglottic airway [SGA] devices) and survival from P-OHCA is extremely limited.
The objective of this study was to measure the association between airway management strategy (BVM vs ETI vs SGA) and survival to hospital discharge in P-OHCA after adjusting for the propensity to receive each of the airway management alternatives. We hypothesized that advanced airway management (ETI or SGA) would be associated with poorer survival in P-OHCA compared to airway management with BVM.
Methods
Study Design
This was a retrospective cohort study using the Cardiac Arrest Registry to Enhance Survival (CARES) data from January 1 2013– December 31 2015. At the end of 2015, the CARES registry included 17 statewide registries and 55 communities in 23 additional states covering a catchment area of over 100 million people (all ages) in the United States. The database includes 405 Emergency Medical Services (EMS) agencies that had at least one pediatric arrest entered into the database during the study period. There were 649 unique hospitals that received the patients included in this study. Data is entered into CARES from emergency call centers, EMS providers, and hospitals. The CARES database includes support for quality control cleaning and error checking of the data since it was established in 2004. The details of quality control have been previously published.11
Study Participants
We included all CARES patients less than 18 years of age with non-traumatic out-of-hospital cardiac arrest for whom resuscitation was attempted by EMS. Children who have obvious signs of death such as rigor mortis, or who have a “do not resuscitate” order are generally not recorded in CARES and were thus excluded from the study. We included arrests of all etiologies other than trauma.
Primary Exposure and Variables
The exposure of interest in this study was the final airway management strategy used during EMS care of the patient. Options for airway management in CARES include ETI, SGA devices, or BVM. The database indicates if an advanced airway was successfully placed or not. If an advanced airway was successful placed, the types of airways are then categorized as: endotracheal tube, Combitube, Laryngeal Mask Airway, King Airway, or other. In cardiac arrest scenarios, ventilations are initially delivered with BVM. After using BVM to start resuscitation, providers may subsequently place an ETI or SGA. Each agency has a specific protocol that dictates how advanced airways are used in pediatric arrests, and if they are used, often specifies in what situations each is indicated. For this analysis, the “final” airway device was the successfully placed advanced airway (SGA or ETI), or BMV if an advanced airway was not successfully placed. There is generally no reason to remove an advanced airway once it has been successfully placed, so we believe it is appropriate to consider this the “final” airway used in the scenario. This “final” airway strategy has been previously used in studies analyzing CARES.12 Since the individual EMS agency protocols are a significant determinant of which airway is used, we included the EMS agency as a variable in developing the propensity score. Further, only EMS agencies where all three airway strategies were available options were included for analysis. We also conducted a sensitivity analysis that was EMS-agency matched.
Table 1 displays the variables included in the analysis. Variables were categorized by whether or not they were known prior to choice of airway management strategy for the purposes of a propensity score analysis. For medical history, the predetermined medical history variables in CARES include presence of heart disease, diabetes, cancer, hypertension, renal disease, respiratory disease, hyperlipidemia, or stroke. We adjusted for any condition with greater than 1% prevalence in the cohort as a separate variable. All other conditions were combined into an “other conditions” group. Sustained ROSC was defined as ROSC for 20 consecutive minutes or ROSC at conclusion of EMS care.
Table 1.
Patient, response, arrest and intervention characteristics included in analysis
| Patient Variables | Agency and Response Variables | Arrest Variables | Intervention Variables | |
|---|---|---|---|---|
| Variables known during choice of airway management strategy | Age | Medical History | Time to EMS arrival | Presenting Rhythm |
| Gender | Unique agency ID | Hour of the call | Etiology of Cardiac Arrest | |
| Race | Location | Arrest witness status | Bystander CPR | |
| Type of bystander CPR (compressions, ventilations)* | ||||
| Use of AED | ||||
| Variables not known during choice of airway management strategy | Transport time | Death in the field | Hypothermia in the field | |
| Time on scene | Sustained ROSC in the field | Epinephrine administered | ||
| When did sustained ROSC occur?* | Other drugs administered | |||
| Emergency Department Outcome | Vascular access* (IV or IO) | |||
| Hospital Outcome | ||||
| Neurologic Status at Discharge (PCPC) |
indicates optional variables in CARES. ROSC — return of spontaneous circulation. PCPC — Pediatric Cerebral Performance Score. IV — intravenous line. IO — intraosseous line.
Outcomes
The primary outcome in the study was survival to hospital discharge. Secondary outcomes include survival to hospital discharge with Pediatric Cerebral Performance Category (PCPC) of 1 or 2, indicating a patient who recovers to normal or mild disability (possible range 1–5, with 5 being vegetative state or coma); survival to hospital admission; and sustained ROSC.
Analysis
To minimize bias, preserve the sampling design of CARES, and maximize power, we used multiple imputation to handle missing values. Please see the eMethods in the online supplement for a full description of the imputation methods, including the SAS code used. The use of multiple imputation for handling missing out-of-hospital data has previously been validated.13–15 We used flexible chains regression models for multiple imputation using SAS-callable IVEware (IVEware, University of Michigan, Ann Arbor, MI) with generation of 10 imputed data sets.14,15 Missingness for key variables for the analysis includes: airway technique was missing in 18.4% of cases, first rhythm in 0.1%, neurologic outcome in 1.8%, survival to hospital discharge (survival/location of death) in 1.5%, sustained ROSC in <0.1%. Arrest witness status was known for all cases.
To account for potential differences in the probability of EMS providers using one airway strategy successfully over another (BVM, ETI and SGA), we conducted a propensity score analysis based on variables likely to be consistently available before the time of airway intervention (Table 1). We excluded drugs and vascular access since it is possible that these interventions are performed after airway management. The method of inverse probability of treatment weighting (IPTW) was used to correct for imbalances in patient characteristics between the three airway groups.16 This method was used given its unique ability to compare three interventions. Please see the eMethods in the online supplement for the R code used to generate the propensity scores. As opposed to randomized studies, the assignment of a treatment in an observational study may be related to observed patient characteristics (confounding by indication). For example, a provider may choose a particular airway management technique based on the perceived severity of illness or age of the child, and this could introduce bias into the analysis. The use of a propensity score method can help minimize this bias by balancing the observed patient characteristics between the treatment groups.17,18
A requirement of a propensity score analysis is that all observations are eligible for all treatments. Therefore, we limited the data to patients from EMS agencies that had at least one patient with each airway. Propensity scores and weights were generated using the R package ‘twang’ for R 3.1.1 (Vienna, Austria)19 The weights generated by IPTW are used to balance the covariates between treatment groups by increasing the weight of certain individuals in the dataset.17 All covariates were assessed for adequate overlap across the three airway interventions (e.g. did patients of all ages receive all airways?).
Using the weights generated via IPTW, logistic regression was performed using SAS-callable IVEWare. Please see the eMethods in the online supplement for the SAS code used to conduct the regression analysis. After propensity score weighting, all variables representing factors known by the EMS team prior to determining airway management interventions were well balanced across the airway groups except age and first rhythm (Table 2). We recognized age and first rhythm were critical confounders so we also adjusted for them in the final model. In addition, we included all variables that had imbalance indicated by a standardized difference >0.20 (threshold set prior to analysis). The logistic regression results from all 10 imputed datasets were examined for consistency, then combined using Rubin’s rules to account for variance within and between imputed datasets.20 A p-value of 0.05 was the cutoff for statistical significance, and all tests were two-sided.
Table 2.
Standardized Difference of Patient Characteristics between Airway Choice, Before and After Propensity Score Weighting
| Prior to propensity score analysis | After propensity score analysis | |||||
|---|---|---|---|---|---|---|
| BVM v ETI | BVM v SGA | ETI v SGA | BVM v ETI | BVM v SGA | ETI v SGA | |
| Age, mean(SE)* | −13.4% | −61.3% | −43.7% | −7.0% | −18.3% | −12.9% |
| Female, n(%)* | 2.6% | 7.7% | 5.1% | −0.3% | 1.0% | 1.2% |
| Witness and Bystander CPR, n(%) | ||||||
| Bystander witnessed* | −5.9% | −28.7% | −22.7% | −1.8% | −8.2% | −6.4% |
| EMS Witnessed* | −2.8% | −19.6% | −16.9% | −1.6% | −5.6% | −4.0% |
| Unwitnessed* | 6.9% | 37.7% | 30.7% | 2.4% | 10.5% | 8.0% |
| Bystander Compressions* | −2.5% | 2.3% | 4.7% | −3.5% | −0.2% | 3.3% |
| Bystander Ventilations* | 5.6% | 21.3% | 15.7% | 2.6% | 10.3% | 7.7% |
| Time variables in minutes, mean(SE) | ||||||
| Time to EMS arrival* | −2.6% | −8.9% | −5.7% | −1.9% | −8.2% | −6.8% |
| EMS Scene Time | −35.1% | −68.0% | −27.5% | −39.5% | −39.3% | −10.4% |
| Transport Time | −9.7% | −29.4% | −16.4% | −14.0% | −26.2% | −16.7% |
| Location of arrest, n(%) | ||||||
| Healthcare* | 0.7% | 2.1% | 1.4% | 2.6% | 8.7% | 6.2% |
| Public Building* | −4.6% | −16.7% | −12.2% | −1.1% | −5.8% | −4.7% |
| Street/Highway* | 3.6% | −11.9% | −15.3% | 3.9% | −4.8% | −8.6% |
| Place of Recreation* | −3.8% | −22.8% | −19.4% | 0.1% | 1.8% | 1.6% |
| Home* | 6.0% | 31.0% | 25.0% | −0.3% | 3.2% | 3.5% |
| Other* | −9.3% | −9.3% | 0.0% | −7.0% | −5.7% | 1.3% |
| Initial rhythm, n(%) | ||||||
| Shockable* | 0.9% | −38.2% | −39.1% | 1.8% | −7.4% | −9.2% |
| PEA* | 0.9% | −4.6% | −5.5% | 1.1% | −4.8% | −6.0% |
| Asystole* | −11.3% | 22.4% | 33.9% | −6.3% | −5.3% | 1.0% |
| Non-airway resuscitation, n(%) | ||||||
| Epinephrine given | −65.2% | −61.8% | 3.4% | −59.1% | −57.6% | 1.4% |
| Defibrillation | −5.8% | −48.8% | −43.1% | −1.6% | −10.9% | −9.4% |
= Variables consistently known prior to airway management.
Pre-planned sensitivity analyses included: 1) complete-case analysis excluding all patients with missing outcomes or missing continuous variables, with missing categorical data included in a new “unknown” category; 2) stratified analyses of patients greater than versus less than 1 year of age; 3) propensity score analysis matched by EMS agency; and 4) an analysis assessing the possibility of unmeasured confounders. To assess for unmeasured confounders we used a previously described method used in an analysis of airway management during in-hospital pediatric cardiac arrest.10,21
Results
Patient Characteristics
Of the 3,793 P-OHCA cases included from 405 EMS agencies during the study period, 1,724 cases were analyzed after limiting to agencies that used all 3 devices. Of the 1,724, 781 (45.3%) were treated with BVM only, 727 (42.2%) received an ETI, and 215 (12.5%) were treated with a SGA. The median number of cases submitted by each EMS agency was 3 (minimum 1, maximum 195, IQR 1–9). Patient characteristics are described in Table 3. Overall, 786 (20.7%) patients had sustained ROSC, 430 (11.3%) survived to hospital discharge, and 331 (8.7%) survived to discharge with a PCPC score of 1 or 2. The overall survival to discharge for the entire cohort of patients (n=3,793) treated with BVM was 14%, with ETI was 8.9%, and with SGA was 9%. In the cohort restricted to agencies where all three devices were used (n=1,724) the survival in those treated with BVM was 14.1%, with ETI was 7.0%, and SGA was 10.2%.
Table 3.
Characteristics of Patients Included in the Primary Analysis
| Overall N=3793 |
Primary Propensity Analysis (n=1724) |
BVM only (n=781, 45.3%) |
ETI (n=727, 42.2%) |
SGA (n=215, 12.5%) |
||||
|---|---|---|---|---|---|---|---|---|
| Age, mean(SE) | 3.76 (0.09) | 3.69 (0.13) | 2.25 (0.15) | 3.78 (0.20) | 8.59 (0.45) | |||
| Female, n(%) | 1513 (39.9%) | 713 (41.4%) | 331 (42.4%) | 299 (41.1%) | 83 (38.6%) | |||
| Witness and Bystander CPR, n(%) | ||||||||
| Bystander witnessed | 860 (22.7%) | 379 (22%) | 152 (19.4%) | 159 (21.8%) | 69 (31.8%) | |||
| EMS Witnessed | 184 (4.9%) | 94 (5.5%) | 36 (4.6%) | 38 (5.2%) | 21 (9.6%) | |||
| Unwitnessed | 2749 (72.5%) | 1251 (72.6%) | 594 (76.0%) | 531 (73.0%) | 126 (58.6%) | |||
| Bystander Compressions | 1578 (41.6%) | 677 (39.3%) | 304 (38.9%) | 292 (40.1%) | 81 (37.8%) | |||
| Bystander Ventilations | 913 (24.1%) | 312 (18.1%) | 156 (20.0%) | 129 (17.8%) | 26 (12.2%) | |||
| Time variables in minutes, mean(SE) | ||||||||
| Time to EMS arrival | 8.11 (0.15) | 7.84 (0.14) | 7.60 (0.17) | 7.91 (0.20) | 8.51 (0.35) | |||
| EMS Scene Time | 19.4 (0.46) | 19.97 (0.43) | 15.27 (0.52) | 22.63 (0.65) | 28.04 (1.39) | |||
| Transport Time | 11.31 (0.29) | 11.78 (0.32) | 10.55 (0.29) | 12.18 (0.47) | 14.92 (1.00) | |||
| Location of arrest, n(%) | ||||||||
| Healthcare | 92 (2.4%) | 38 (2.2%) | 18 (2.3%) | 16 (2.2%) | 4 (2.0%) | |||
| Public Building | 220 (5.8%) | 92 (5.3%) | 34 (4.4%) | 39 (5.4%) | 18 (8.5%) | |||
| Street/Highway | 107 (2.8%) | 39 (2.3%) | 17 (2.2%) | 13 (1.7%) | 9 (4.3%) | |||
| Place of Recreation | 141 (3.7%) | 54 (3.1%) | 18 (2.3%) | 21 (2.9%) | 15 (7.1%) | |||
| Home | 3194 (84.2%) | 1484 (86.1%) | 690 (88.4%) | 628 (86.4%) | 165 (76.8%) | |||
| Other | 39 (1%) | 17 (1%) | 4 (0.5%) | 10 (1.4%) | 3 (1.4%) | |||
| Initial rhythm, n(%) | ||||||||
| Shockable | 271 (7.1%) | 108 (6.3%) | 38 (4.9%) | 35 (4.7%) | 35 (16.5%) | |||
| PEA | 489 (12.9%) | 226 (13.1%) | 102 (13.0%) | 93 (12.7%) | 31 (14.6%) | |||
| Asystole | 2619 (69%) | 1173 (68.1%) | 525 (67.2%) | 527 (72.4%) | 121 (56.4%) | |||
| Non-airway resuscitation, n(%) | ||||||||
| Epinephrine given | 2831 (74.6%) | 1314 (76.2%) | 482 (61.7%) | 644 (88.5%) | 188 (87.4%) | |||
| Defibrillation | 669 (17.6%) | 198 (11.5%) | 66 (8.5%) | 74 (10.2%) | 57 (26.5%) | |||
| Outcomes, n(%) | ||||||||
| Sustained ROSC | 786 (20.7%) | 346 (20.1%) | 141 (18.0%) | 148 (20.3%) | 58 (27.0%) | |||
| Survival to hospital admission | 1019 (26.9%) | 455 (26.4%) | 201 (25.7%) | 185 (25.4%) | 69 (32.2%) | |||
| Survival to hospital discharge | 430 (11.3%) | 183 (10.6%) | 110 (14.1%) | 51 (7.0%) | 22 (10.2%) | |||
| Survival to discharge PCPC=1 or 2 | 331 (8.7%) | 136 (7.9%) | 89 (11.4%) | 34 (4.7%) | 13 (6.0%) | |||
N within each airway varies +/− 1 observation due to rounding error.
Main results
For the propensity score analysis, BVM was associated with improved odds of survival to discharge compared to both ETI and SGA with an odds ratio for ETI compared to BVM of 0.39 (95% CI 0.26–0.59) and for SGA compared to BVM of 0.32 (95% CI 0.12–0.84). Table 4 shows the results of the final analysis of survival to discharge as well as sustained ROSC and neurologically intact survival. In general, the findings for survival to discharge with PCPC 1 or 2 and survival to discharge overall were similar. The airway choice was not significantly associated with sustained ROSC with the primary analysis finding odds ratios of 1.13 (95% CI 0.85–1.51) for ETI compared to BVM and 1.24 (95% CI 0.69–2.25) for SGA compared to BVM.
Table 4.
Results of Primary and Sensitivity Analyses
| Survival to Discharge OR (95%CI) |
PCPC=1 or 2 OR (95%CI) |
Sustained ROSC OR (95%CI) |
|
|---|---|---|---|
| Original IPTW analysis | |||
| ETI vs. BVM | 0.39 (0.26–0.59) | 0.30 (0.18–0.50) | 1.13 (0.85–1.51) |
| SGA vs. BVM | 0.32 (0.12–0.84) | 0.14 (0.05–0.39) | 1.24 (0.69–2.25) |
| Complete Case Analysis | |||
| ETI vs. BVM | 0.50 (0.37–0.67) | 0.43 (0.31–0.60) | 1.08 (0.86–1.36) |
| SGA vs. BVM | 0.30 (0.21–0.42) | 0.10 (0.06–0.17) | 1.13 (0.89–1.42) |
| Age stratified analysis | |||
| ≤1 year old | |||
| ETI vs. BVM | 0.35 (0.15–0.77) | 0.30 (0.11–0.83) | 1.00(0.62–1.63) |
| SGA vs. BVM | 0.21 (0.01–7.04) | 0.06 (0.00–*) | 2.24 (0.83–6.05) |
| >1 year old | |||
| ETI vs. BVM | 0.42 (0.23–0.74) | 0.32 (0.16–0.63) | 1.22 (0.78–1.91) |
| SGA vs. BVM | 0.31 (0.14–0.68) | 0.17 (0.06–0.46) | 0.80 (0.46–1.41) |
| EMS agency matched analysis | |||
| ETI vs. BVM | 0.30 (0.14–0.65) | 0.17 (0.06–0.50) | 1.08 (0.63–1.83) |
| SGA vs. BVM | 0.22 (0.10–0.51) | 0.10 (0.03–0.31) | 0.92 (0.51–1.67) |
Due to the small sample size for SGA in the complete case analysis, the 95% confidence interval upper limit approaches infinity. Other covariates included in the analysis include age and first recorded rhythm (shockable, asystole, PEA) for the primary and EMS agency matched analyses; age, race, who applied the AED, and the time of day of the call broken down by hour for the complete case analysis; age, first recorded rhythm and race for ≤1 year old; and age, presumed arrest etiology, and who first defibrillated for >1 year old. OR = Adjusted Odds Ratio, CI = Confidence Interval.
The results of the pre-planned sensitivity analyses are included in Table 4. The complete case analysis was limited to 1091 cases and was nearly identical to the analysis using the 10 imputed datasets. In general, the findings of all sensitivity analyses were consistent with the primary analysis. The results of the sensitivity analysis that explored the potential effect of unmeasured confounders suggest that unmeasured confounders are unlikely to be masking a positive association or no association between ETI or SGA and survival compared to BVM (Figure 1). For an unmeasured confounder to completely mask the observed results (OR 0.32), it would require a prevalence of 80% in the unexposed (BVM) group and 20% in the exposed (ETI) group, with an odds ratio of 4–5, which is relatively high in comparison to the odds ratios in the measured confounders. The existence of such a confounder is unlikely.
Figure 1. Effect of an Unmeasured Confounder.
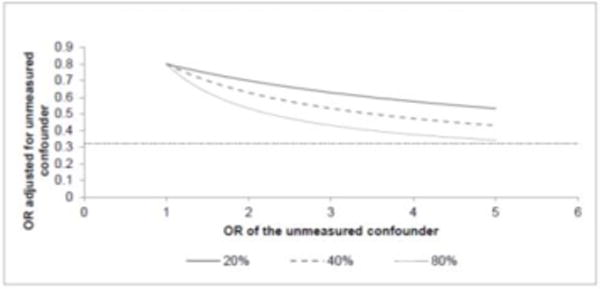
The reference line is at 0.32 which is the observed odds ratio from the primary analysis (unadjusted for any unmeasured confounders) for survival to hospital discharge with good neurological outcome. The three lines represent the prevalence of the unmeasured confounder in the BVM (unexposed) group. For this graph, the ratio of the prevalence in the BVM versus the ETI (exposed) group is 4 to 1 (e.g. for the solid line- the prevalence of the unmeasured confounder is 20% in BVM group and 5% in the ETI group). For an unmeasured confounder to result in the finding of an odds ratio of 0.32, the unmeasured confounder would need to be 80% in the BVM group, 20% in the ETI group and would have an odds ratio of at least 5.0 for survival to hospital discharge with good neurological outcome. OR=Odds ratio.
Discussion
In this study, we compared various airway management strategies for EMS-treated P-OHCA using a large cardiac arrest registry. We found that in pediatric patients who suffered a non-traumatic OHCA, advanced airway management techniques (both ETI and SGA) were associated with decreased survival to hospital discharge compared to BVM, after accounting for the probability of having each airway intervention performed. This finding was consistent across the majority of sensitivity analyses and the outcome of neurologically intact survival. A unique aspect of our study is that we included SGA devices, previously studied in the specific setting of P-OHCA. Children managed with a SGA had somewhat poorer outcomes overall. This could be because SGAs are used as a backup and are a marker for difficult airway management. This study uses a registry of cardiac arrest patients and is thus a pragmatic comparison of airway management strategies based on actual field performance of these techniques with their inherent benefits and limitations in the EMS environment.
While ETI is the gold standard for airway management in the in-hospital environment, its use remains common for pediatric patients in the relatively uncontrolled out-of-hospital setting where the providers, training, equipment, environment, and system have different constraints. Several studies have suggested intubation during cardiac arrest is associated with either unimproved or decreased survival.12,22,7 Similar to this study, a recently published retrospective study of in-hospital pediatric cardiac arrests found decreased survival with ETI compared to BVM with a risk ratio of 0.89.10 Several ongoing randomized trials are currently evaluating ETI vs. SGA in adult OHCA.23
There are several potential mechanisms through which advanced airway management could negatively impact survival from P-OHCA. First, having an advanced airway in place may contribute to inadvertent hyperventilation. In BVM, the mask seal takes place at the face rather than at the glottis or trachea, increasing the likelihood of air leak which may protect against excessive ventilation. Hyperventilation following an arrest may reduce cardiac output by increasing intrathoracic pressure, thereby reducing right ventricular filling and coronary blood flow.24 Hyperventilation may also have negative effects on brain perfusion by causing cerebral vasoconstriction, lowering the mean arterial pressure, and increasing intracranial pressure.25,26. We did not find an association between advanced airway use and ROSC which may support the concept that the negative effect of the advanced airway is primarily on the brain. Second, and perhaps more important, airway management procedures may cause interruptions in CPR and delays in epinephrine administration as well as other potentially important interventions.27,28 Additional mechanisms linking advanced airway management and worse outcomes have also been described.29 These mechanistic pathways suggest intubation itself, even when performed under optimal circumstances, may be detrimental to survival from pediatric OHCA. This is supported by the findings of a recent study where ETI was associated with decreased survival for in-hospital pediatric arrests where circumstances are relatively controlled.10
Pediatric endotracheal intubation is a complex and uncommonly performed procedure in prehospital care, requiring ongoing skill maintenance.9 Previous studies have found that pediatric intubation errors are common.30,31 Errors and other complications during intubation may contribute to the reduced likelihood of survival in children treated with advanced airways. Our finding of worse survival with SGAs, which are often used as a rescue device after ETI failure, may also support this mechanistic hypothesis. The effect size of the findings of our study are much larger than that in the recent in-hospital arrest study, which found odds ratios of 0.89 for survival with ETI compared to BVM.10 One potential explanation for our larger effect size is that both the physiologic mechanisms and the aforementioned complexities associated with out-of-hospital management contribute to poorer outcomes associated with ETI in P-OHCA.
Limitations
This was an observational study using a registry, and although we used propensity score matching to control for confounding and conducted several sensitivity analyses to examine the robustness of our findings, it is possible that unmeasured confounders still exist. Observational studies may also over-estimate effect sizes, which is likely given the large odds ratios observed in this study. In some cases, patients may achieve ROSC very quickly, and there may not be time to place an advanced airway. This could introduce bias into the study in favor of BVM. However, patients with very early recovery would be expected to have sustained field ROSC (>20 minutes or at termination of EMS care), and we did not find any significant association between airway technique on this outcome. In CARES, advanced airway interventions are only recorded if the procedure was “successful”, so multiple airways may have been attempted in the same patient. Finally, CARES only includes EMS treated arrests, so the true denominator of all P-OHCA cases is unknown. Regional differences in initiating treatment versus declaring death in the field could introduce bias.
Conclusions
In this US-based registry study, BVM is associated with improved survival (compared to ETI and SGA) in non-traumatic P-OHCA. Based on these findings, it may be reasonable for agencies to adopt a primary BVM strategy for P-OHCA. A large multicenter randomized trial is needed to confirm these findings.
Supplementary Material
Acknowledgments
We would like to acknowledge the CARES participating sites: https://mycares.net/sitepages/map.jsp)
Funding
This work is funded by the National Heart Lung and Blood Institute grant number 5K12HL108974-04
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions:
BM developed the CARES project and was critical in acquisition of the data. MH, AL, MD, RF, DY, DZ, and CN conceived the data analysis plan. AL conducted the statistical analysis with critical input from MH, RF, CE, MD, DY, and CN. All authors contributed substantially to the interpretation of the data analysis. MH and AL drafted the work. All authors revised the work critically and approved the final version to be published.
Conflicts of interest
The authors declare they have no conflicts of interest
References
- 1.Atkins DL, Everson-Stewart S, Sears GK, et al. Epidemiology and Outcomes From Out-of-Hospital Cardiac Arrest in Children The Resuscitation Outcomes Consortium Epistry—Cardiac Arrest. Circulation. 2009;119(11):1484–1491. doi: 10.1161/CIRCULATIONAHA.108.802678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sirbaugh PE, Pepe PE, Shook JE, et al. A prospective, population-based study of the demographics, epidemiology, management, and outcome of out-of-hospital pediatric cardiopulmonary arrest. Ann Emerg Med. 1999;33(2):174–184. doi: 10.1016/s0196-0644(99)70391-4. [DOI] [PubMed] [Google Scholar]
- 3.Donoghue AJ, Nadkarni V, Berg RA, et al. Out-of-Hospital Pediatric Cardiac Arrest: An Epidemiologic Review and Assessment of Current Knowledge. Ann Emerg Med. 2005;46(6):512–522. doi: 10.1016/j.annemergmed.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 4.Kuisma M, Suominen P, Korpela R. Paediatric out-of-hospital cardiac arrests — epidemiology and outcome. Resuscitation. 1995;30(2):141–150. doi: 10.1016/0300-9572(95)00888-Z. [DOI] [PubMed] [Google Scholar]
- 5.Jayaram N, McNally B, Tang F, Chan PS. Survival After Out-of-Hospital Cardiac Arrest in Children. J Am Heart Assoc. 2015;4(10):e002122. doi: 10.1161/JAHA.115.002122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daya MR, Schmicker RH, Zive DM, et al. Out-of-hospital cardiac arrest survival improving over time: Results from the Resuscitation Outcomes Consortium (ROC) Resuscitation. 2015;91:108–115. doi: 10.1016/j.resuscitation.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gausche M, Lewis RJ, Stratton SJ, et al. Effect of out-of-hospital pediatric endotracheal intubation on survival and neurological outcome: a controlled clinical trial. JAMA J Am Med Assoc. 2000;283(6):783–790. doi: 10.1001/jama.283.6.783. [DOI] [PubMed] [Google Scholar]
- 8.Hansen M, Lambert W, Guise J-M, Warden CR, Mann NC, Wang H. Out-of-hospital pediatric airway management in the United States. Resuscitation. 2015;90:104–110. doi: 10.1016/j.resuscitation.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babl FE, Vinci RJ, Bauchner H, Mottley L. Pediatric pre-hospital advanced life support care in an urban setting. Pediatr Emerg Care. 2001;17(1):5–9. doi: 10.1097/00006565-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Andersen LW, Raymond TT, Berg RA, et al. Association Between Tracheal Intubation During Pediatric In-Hospital Cardiac Arrest and Survival. JAMA. 2016;316(17):1786–1797. doi: 10.1001/jama.2016.14486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNally B, Stokes A, Crouch A, Kellermann AL, CARES Surveillance Group CARES: Cardiac Arrest Registry to Enhance Survival. Ann Emerg Med. 2009;54(5):674–683 e2. doi: 10.1016/j.annemergmed.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 12.McMullan J, Gerecht R, Bonomo J, et al. Airway management and out-of-hospital cardiac arrest outcome in the CARES registry. Resuscitation. 2014;85(5):617–622. doi: 10.1016/j.resuscitation.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Newgard C, Malveau S, Staudenmayer K, et al. Evaluating the Use of Existing Data Sources, Probabilistic Linkage, and Multiple Imputation to Build Population-based Injury Databases Across Phases of Trauma Care. Acad Emerg Med. 2012;19(4):469–480. doi: 10.1111/j.1553-2712.2012.01324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newgard CD. The validity of using multiple imputation for missing out-of-hospital data in a state trauma registry. Acad Emerg Med Off J Soc Acad Emerg Med. 2006;13(3):314–324. doi: 10.1197/j.aem.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Newgard CD, Haukoos JS. Advanced statistics: missing data in clinical research–part 2: multiple imputation. Acad Emerg Med Off J Soc Acad Emerg Med. 2007;14(7):669–678. doi: 10.1197/j.aem.2006.11.038. [DOI] [PubMed] [Google Scholar]
- 16.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deb S, Austin PC, Tu JV, et al. A Review of Propensity-Score Methods and Their Use in Cardiovascular Research. Can J Cardiol. 2016;32(2):259–265. doi: 10.1016/j.cjca.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 18.McCaffrey DF, Ridgeway G, Morral AR. Propensity Score Estimation With Boosted Regression for Evaluating Causal Effects in Observational Studies. Psychol Methods. 2004;9(4):403–425. doi: 10.1037/1082-989X.9.4.403. [DOI] [PubMed] [Google Scholar]
- 19.Ridgeway G, McCaffrey DF, Morral AR, Burgette LF, Griffin BA. Toolkit for Weighting and Analysis of Nonequivalent Groups. http://www.rand.org/pubs/tools/TL136z1.html. Published 2014. Accessed February 22, 2017.
- 20.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: Wiley; 1987. [Google Scholar]
- 21.Lin DY, Psaty BM, Kronmal RA. Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics. 1998;54(3):948–963. [PubMed] [Google Scholar]
- 22.Hasegawa K, Hiraide A, Chang Y, Brown DFM. Association of prehospital advanced airway management with neurologic outcome and survival in patients with out-of-hospital cardiac arrest. JAMA. 2013;309(3):257–266. doi: 10.1001/jama.2012.187612. [DOI] [PubMed] [Google Scholar]
- 23.Wang HE, Prince DK, Stephens SW, et al. Design and implementation of the Resuscitation Outcomes Consortium Pragmatic Airway Resuscitation Trial (PART) Resuscitation. 2016;101:57–64. doi: 10.1016/j.resuscitation.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aufderheide TP, Sigurdsson G, Pirrallo RG, et al. Hyperventilation-induced hypotension during cardiopulmonary resuscitation. Circulation. 2004;109(16):1960–1965. doi: 10.1161/01.CIR.0000126594.79136.61. [DOI] [PubMed] [Google Scholar]
- 25.Bouzat P, Suys T, Sala N, Oddo M. Effect of moderate hyperventilation and induced hypertension on cerebral tissue oxygenation after cardiac arrest and therapeutic hypothermia. Resuscitation. 2013;84(11):1540–1545. doi: 10.1016/j.resuscitation.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Yannopoulos D, McKnite SH, Metzger A, Lurie KG. Intrathoracic pressure regulation for intracranial pressure management in normovolemic and hypovolemic pigs. Crit Care Med. 2006;34(12 Suppl):S495–S500. doi: 10.1097/01.CCM.0000246082.10422.7E. [DOI] [PubMed] [Google Scholar]
- 27.Andersen LW, Berg KM, Saindon BZ, et al. Time to Epinephrine and Survival After Pediatric In-Hospital Cardiac Arrest. JAMA. 2015;314(8):802–810. doi: 10.1001/jama.2015.9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang HE, Simeone SJ, Weaver MD, Callaway CW. Interruptions in Cardiopulmonary Resuscitation From Paramedic Endotracheal Intubation. Ann Emerg Med. 2009;54(5):645–652.e1. doi: 10.1016/j.annemergmed.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 29.Benoit JL, Prince DK, Wang HE. Mechanisms Linking Advanced Airway Management and Cardiac Arrest Outcomes. Resuscitation. 2015;93:124–127. doi: 10.1016/j.resuscitation.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ehrlich PF, Seidman PS, Atallah O, Haque A, Helmkamp J. Endotracheal intubations in rural pediatric trauma patients. J Pediatr Surg. 2004;39(9):1376–1380. doi: 10.1016/j.jpedsurg.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Hansen M, Meckler G, Lambert W, et al. Patient safety events in out-of-hospital paediatric airway management: a medical record review by the CSI-EMS. BMJ Open. 2016;6(11):e012259. doi: 10.1136/bmjopen-2016-012259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


