Abstract
Integrative genomics helped characterize molecular heterogeneity in HCC, leading to targeted drug candidates for specific HCC subtypes. However, no consensus was achieved for genes and pathways commonly altered in HCC. Here we performed a meta-analysis of 15 independent datasets (n=784 human HCC) and identified a comprehensive signature consisting of 935 genes commonly deregulated in HCC as compared to the surrounding non-tumor tissue. In the HCC signature, up-regulated genes were linked to early genomic alterations in hepatocarcinogenesis, particularly gains of 1q and 8q. The HCC signature covered well-established cancer hallmarks, such as proliferation, metabolic reprogramming, and microenvironment remodeling, together with specific hallmarks associated with protein turnover and epigenetics. Subsequently, the HCC signature enabled us to assess the efficacy of signature-relevant drug candidates, including histone deacetylase inhibitors that specifically reduced the viability of six human HCC cell lines. Overall, this integrative genomics approach identified cancer hallmarks recurrently altered in human HCC that may be targeted by specific drugs. Combined therapies targeting common and subtype-specific cancer networks may represent a relevant therapeutic strategy in liver cancer.
Introduction
Liver carcinogenesis is a long process associated with multiple risk factors that contribute to HCC heterogeneity, e.g. viral hepatitis, alcohol abuse, metabolic disorders and obesity (1). Most HCC develop in the setting of chronic liver disease associated with cycles of tissue destruction and regeneration that result in the activation of numerous signaling pathways and the accumulation of genomic alterations. Lately, next-generation sequencing approaches highlighted the large spectrum of mutational processes underlying the development of HCC (2,3). Other factors such as cell plasticity and tumor microenvironment remodeling contribute to HCC heterogeneity, as well (4).
Over the last two decades gene expression studies in HCC have revealed the great diversity of transcriptional alterations occurring in liver carcinogenesis (5). By integrating gene expression profiles from various sources, a consensus has been achieved successfully and three main HCC subtypes were identified (6). However, translating these findings into individualized treatments is still a matter of debates. Surprisingly, the definition of recurrent transcriptional alterations in HCC has been largely neglected, so far. Failure to define a robust common transcriptional fingerprint in HCC may have resulted from technical variabilities and/or from the inherent HCC molecular heterogeneity. The later even raises the question about the existence of a substantial core expression signature in HCC (7). However, genomic characterization of HCC mouse models demonstrated that various oncogenic pathways could generate similar expression profiles at the tumor stage, suggesting that a common HCC signature probably exists in humans (8). This observation prompted us to perform a meta-analysis of publicly available human HCC datasets that were generated over a period of >10 years. Starting from raw microarray data and by using the same analysis algorithms to circumvent technical variabilities, our aim was to define a universal and comprehensive transcriptional signature in HCC and to determine whether this signature could be useful to identify clinically relevant drug candidates.
Materials and Methods
Analysis of microarray datasets
The meta-analysis was performed using gene expression datasets available from open databases, namely Gene Expression Omnibus (GEO) (www.ncbi.nlm.nih.gov/geo/) and ArrayExpress (www.ebi.ac.uk/arrayexpress/). Twenty-eight liver-oriented datasets were retrieved through a systematic review of the literature on PubMed and queries of the microarray databases using PubMed identifiers (PMID) or keywords associated with human liver carcinogenesis and large scale gene expression profiling, e.g. HCC, microarray and gene profiling (Supplementary Table 1). Before performing the analysis, all microarray platforms (n=19) were re-annotated using the Database for Annotation, Visualization and Integrated Discovery (DAVID) (https://david.ncifcrf.gov/) (9). In total, 24,085 non-redundant annotated genes were present at least in one out of 28 retrieved microarray datasets. Statistical analysis of microarray data was performed using R-based BRB-ArrayTools as previously described (10). Median-based normalization was applied and differentially expressed genes between the tumor and surrounding non-tumor tissues (ST) were identified by a 2-sample univariate t test (P<0.01) and a random variance model as described (10). Permutation P values for significant genes were computed on the basis of 10,000 random permutations. To define the core HCC gene signature only the genes up- and down-regulated in more than 50% datasets were retained. Bias in the distribution of chromosomal location of genes included in the core HCC signature was evaluated by Chi-square testing using the chromosomal location of the genes present on the integrated dataset as a background, i.e. 24,085 genes, see above. Clustering analysis was performed using Cluster and TreeView algorithm as previously described (10).
Data mining of the core HCC gene signature
Several tools dedicated to the discovery of specific enrichments for biological functions or canonical pathways were used, including Enrichr algorithm (http://amp.pharm.mssm.edu/Enrichr) and ingenuity pathway analysis (IPA). IPA was also used to examine the functional association between differentially expressed genes and to generate the highest significant gene networks using the IPA scoring system. Gene set enrichment analysis (GSEA) was performed by using the Java-tool developed at the Broad Institute (Cambridge, MA, USA, www.broadinstitute.org/gsea) as previously described (11). Connectivity map (cMap) algorithm was used to link gene expression signatures with putative therapeutic molecules (10,12). Briefly, from the permuted results cMap table, we focused on negative enrichments to retain only the perturbagens (i.e. molecules) that potentially reverse the expression of genes included in the core HCC signature. Only the perturbagens with a permuted p-value <0.003 and a number of replicate >5 were retained.
Cell culture
A panel of 6 liver cancer cell lines was purchased from ATCC (www.lgcstandards-atcc.org), including SNU-475 (ATCC® CRL-2236™, grade II–IV/V), SNU-449 (ATCC® CRL-2234™, grade II–III/IV), SNU-423 (ATCC® CRL-2238™, grade III/IV), SNU-387 (ATCC® CRL-2237, grade IV/V), HepG2/C3A (ATCC® CRL-10741™) and SK-HEP-1 (ATCC® HTB-52™). ATCC performed cell lines authentication by STR DNA profiling. The impact of selected molecules was evaluated within 6 months after receipt. Cells were grown in a RPMI-1640 medium supplemented with 100U/ml penicillin, 100μg/ml streptomycin and 10% fetal bovine serum. Cultures were performed at 37°C in a 5% CO2 atmosphere. Alpha-estradiol, LY294002, rapamycin, resveratrol, sorafenib and suberoylanilide hydroxamic Acid (SAHA, also known as vorinostat) were purchased from Santa Cruz Biotechnology (Heidelberg, Germany). Trichostatin A was purchased from Sigma-Aldrich (St. Louis, MO, USA). All molecules were solubilized in a dimethyl sulfoxide (DMSO) solution. The concentration of each molecule used to treat the cells was determined based on the detailed results table provided by cMap and a review of the literature. Cell viability was evaluated using a PrestoBlue® cell viability reagent (Invitrogen) after 48h and 72h of treatment with DMSO, trichostatin A (1μM), alpha-estradiol (1μM), vorinostat (50μM), sorafenib (2μM), rapamycin (2μM), LY294002 (50μM) and resveratrol (500μM). Independent culture experiments were performed (n=4 independent biological replicates; n=4 technical replicates for each biological replicate). The significance of differences in cell viability between experimental conditions was determined by a two-tailed non-parametric Mann-Whitney test. For the microarray experiments the concentrations were optimized to induce 50% cell mortality after a 72h drug exposure, in order to allow the extraction of nucleic acids from the remaining viable cells. Accordingly, cells were treated with trichostatin A (0.6μM), alpha-estradiol (1μM), vorinostat (3.7μM), sorafenib (0.65μM), rapamycin (2μM), LY294002 (45μM) and resveratrol (166μM).
Gene expression profiling
Total RNA was purified from SNU-423 cells at 50% confluence with a miRNAeasy kit (Qiagen, Valencia, CA, USA). Genome-wide expression profiling was performed using the low-input QuickAmp labeling kit and human SurePrint G3 8×60K pangenomic microarrays (Agilent Technologies, Santa Clara, CA, USA) as previously described (10). Starting from 150ng total RNA, amplification yield was 7.0±1.3μg cRNA and specific activity was 18.2±2.3pmol Cy3 per μg of cRNA. Gene expression data were processed using Feature Extraction and GeneSpring softwares (Agilent Technologies). Microarray data have been deposited in NCBI’s GEO and are accessible through GEO Series accession numbers GSE79246 and GSE85257.
Results
Generation of a compendium of gene expression profiles in human HCC
The initial step of the study was to assemble well-annotated human HCC gene expression profiles. From the main public databases (i.e. GEO and ArrayExpress), 28 liver-oriented datasets were retrieved including 1,657 HCC gene expression profiles from a total of 3,047 (Fig. 1A). A detailed characterization of HCC datasets is provided in the Supplementary Table 1. Out of these 28 datasets, 15 (MDS1 to MDS15) included both HCC and ST tissues and thus were relevant to derive a core HCC signature defined as a set of genes differentially expressed between HCC and ST. Importantly, the gene expression profiles were generated from various microarray platforms (i.e. from academic or industrial sources, including several updated contents). Consequently, variations in both the number and the nature of interrogated gene features between platforms greatly impede the integrative analysis of the datasets. To overcome this issue, all the platforms (n=19, Fig. 1A) were re-annotated by using the DAVID database (9). In total, 24,085 annotated genes were present at least in one microarray dataset (Fig. 1A).
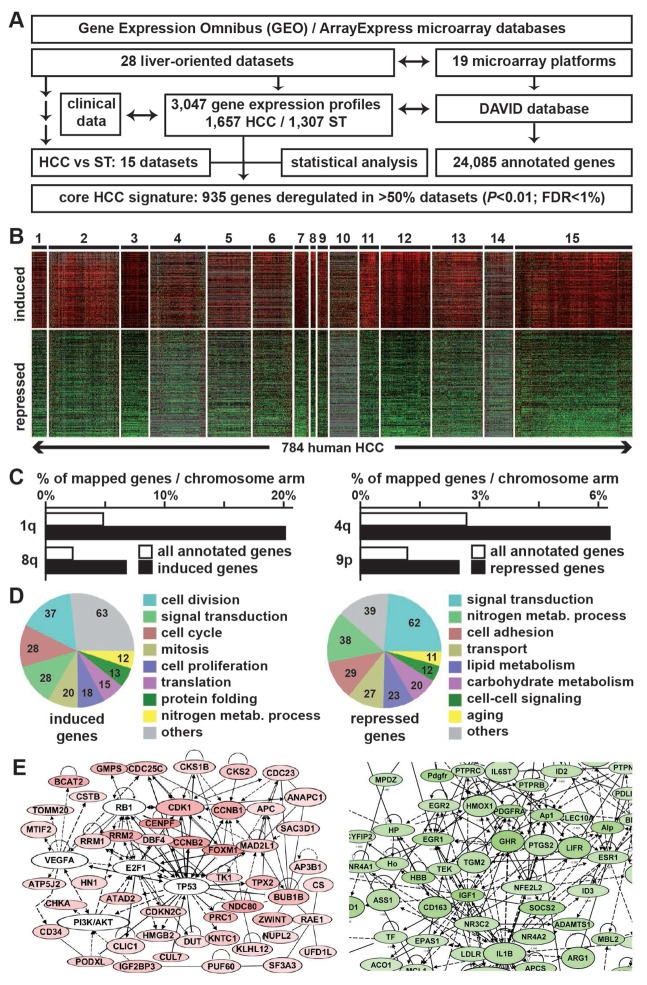
Figure 1.
Large scale meta-analysis of public genomic data identifies core transcriptional hallmarks in human HCC. A, analytical workflow of the meta-analysis that led to the identification of a core gene expression signature in human HCC (935-gene HCC signature). B, heat map of the 935-gene HCC signature. Induced and repressed genes in HCC as compared to the surrounding non-tumor tissue are represented in red and green respectively. Missing values are colored in grey. Numbers on the top represent the 15 HCC microarray datasets that were investigated to derive the signature. C, specific enrichment of induced (left) and repressed (right) genes of the 935-gene HCC signature on specific chromosomal arms. D, gene ontology analysis of induced (left) and repressed (right) genes of the 935-gene HCC signature highlighting functional cancer hallmarks. E, top gene networks identified by Ingenuity Pathway Analysis. Highlighted networks were associated with cell cycle and proliferation for induced genes (left) and with cellular metabolism for repressed genes (right). DAVID, database for annotation, visualization and integrated discovery; FDR, false discovery rate; HCC, hepatocellular carcinoma; ST, surrounding non-tumor tissue.
Identification of a comprehensive 935-gene HCC signature linked to recurrent early genomic alterations in liver carcinogenesis
By using the same normalization, filtration and statistical algorithms a set of genes significantly deregulated between HCC and ST tissues was identified (P<0.01; FDR<1%; n=15 datasets; Fig. 1A). The so-called universal core HCC signature consisted of 935 genes significantly deregulated in more than 50% HCC datasets and included 41% up-regulated genes (Fig. 1B and Supplementary Table 2). Clustering analysis based on the expression of these genes in 15 datasets revealed a clear transcriptional homogeneity in the investigated tumors (n=784 HCC, Fig. 1B). Validating the gene selection, the 935-gene HCC signature included well-known HCC biomarkers (e.g. GPC3, PEG10) or HCC suppressor genes (e.g. DLC1) (Supplementary Table 2). In addition, chromosomal mapping of genes included in the 935-gene HCC signature highlighted a bias in the distribution of up- and down-regulated genes at specific locations known to be frequently altered in HCC (Fig. 1C and Supplementary Table 3). Thus, up-regulated genes were significantly (P<0.001) enriched in 1q and 8q whose amplifications were previously reported as the earliest events that occur in human hepatocarcinogenesis (13,14). Similarly, down-regulated genes were enriched in loci frequently subjected to early deletions in HCC (e.g. 4q). These specific locations also correlated with the deregulation of HCC-associated genes (e.g. LAMC1 at 1q31, RAD21 at 8q24 or ALB at 4q13). Altogether, these observations demonstrated that transcriptional changes identified in the 935-gene HCC signature significantly correlated with early and recurrent structural genomic alterations linked to stepwise HCC carcinogenesis in human.
The 935-gene HCC signature covers well-established cancer hallmarks
Data mining of the 935-gene HCC signature (Fig. 1D–E and Supplementary Tables 4 and 5) identified gene networks that reflect a definite transcriptional reprogramming associated with previously described hallmarks of cancer cells, as exemplified thereafter (15).
Sustained proliferation and evasion to growth suppressors
Gene networks and gene ontology analysis clearly demonstrated that active cell proliferation is the most prominent feature within the 935-gene HCC signature (Fig. 1E). Thus, numerous cell cycling genes were up-regulated (Supplementary Table 2), including cyclins (e.g. CCNB1, CCNE2), cyclin dependent kinases and inhibitors (e.g. CDK1, CDK4, CDKN2A), cell cycle and cell division checkpoints (e.g. BUB1, MAD2L1, NDC80, RAN), together with well-established proliferation markers (e.g. PCNA, MKI67). Accordingly, numerous genes related to DNA replication were induced (e.g. CDC6, MCM2-4, RCF4). These observations coincided with the induction of pro-proliferative pathways and growth factors (e.g. ERBB3, MAPK6, YWHAH, FIBP) and the repression of negative feed-back regulators (e.g. PINK1, DUSP1).
Genome instability, oxidative stress and apoptosis resistance
A sustained proliferation phenotype generally associates with DNA damages, accumulation of mutations and genome instability, which ultimately lead to apoptosis (15). Interestingly, the 935-gene HCC signature highlighted gene deregulations that clearly sign mechanisms of DNA damages associated with DNA hyper-replication together with resistance to cell death (Supplementary Table 2). Thus, several genes essential for DNA double-strand break repair (e.g. RAD21, RUVBL2) and nucleotide excision repair (e.g. ERCC3, FEN1, OGG1) were induced. Notably, DNA glycosylase OGG1 is involved in the excision of 8-oxoguanine, a mutagenic base byproduct which occurs as a result of exposure to reactive oxygen species. Further implying oxidative stress in this process, key regulators of redox homeostasis were repressed in the 935-gene HCC signature (e.g. CTH, CBS, NFE2L2, PRDX4). Besides, several inducers of apoptosis were repressed (e.g. CRADD, DAPK1) while genes encoding proteins that prevent apoptotic cell death were induced (e.g. BIRC5, DAD1).
Metabolic reprogramming
Deregulation of metabolism-associated genes (e.g. lipid, carbohydrate and amino acid metabolisms) was a prominent feature in the 935-gene HCC signature, in agreement with metabolic changes observed in cancer cells during tumor onset and progression (16). This was particularly noticeable for down-regulated genes involved in liver specific metabolisms (Fig. 1D and Supplementary Tables 4 and 5), including those encoding acute phase plasma proteins (e.g. A2M, ALB, CP), components of complement and coagulation cascades (e.g. C5-9, CFB, F2) or detoxication enzymes (e.g. ADH1A, CYP2E1). While such enrichment may reflect a loss of hepatocyte differentiation accompanying tumor development, other deregulations affecting key enzymes of bioenergetics and biosynthesis may be directly linked to a specific reprogramming of cellular metabolism (Supplementary Table 2). As example, ACLY and CS were recurrently up-regulated in HCC. ACLY encodes ATP citrate lyase, the primary enzyme for the synthesis of acetyl-CoA, a major intermediate for biosynthetic pathways including lipogenesis. CS encodes the citrate synthase, a key enzyme in the tricarboxylic acid cycle that contributes to lipogenesis by enhancing the conversion of glucose to lipids. While lipogenesis was enhanced, genes encoding key components of lipid catabolism were repressed, including genes involved in the mitochondrial fatty acid beta-oxidation pathway (e.g. ACADM, ACADL, ECHS1). In addition, we observed a shift toward the down-regulation of genes involved in gluconeogenesis (e.g. PCK1, FBP1) that may contribute to enhance the rate of glycolysis in HCC cancer cells.
Induction of angiogenesis
Fueling proliferative and metabolically active tumor cells frequently correlates with an enhanced angiogenesis to support nutrient supply (15). Accordingly, the 935-gene HCC signature included several angiogenesis-related genes. As example, PLG encoding plasminogen was strongly repressed in almost all HCC (Supplementary Table 2). Of note, plasminogen is activated by proteolysis and converted to plasmin, an activator of matrix metalloproteases, and angiostatin, a potent inhibitor of angiogenesis. Similarly, AMOTL2 encoding an angiomotin like 2 protein was repressed. Angiomotin is known to mediate the inhibitory effect of angiostatin on tube formation. While these angiogenesis inhibitors were repressed, endothelial cell markers were induced (e.g. ESM1).
Microenvironment remodeling and invasion
In agreement with a loss of hepatocyte differentiation (see above), we observed a decreased expression of the epithelial marker E-cadherin (CDH1) in >70% datasets (Supplementary Table 2). Loss of E-cadherin is frequently observed in cancer and notably contributes to tumor progression by increasing proliferation and invasion. The 935-gene HCC signature was also significantly enriched in genes encoding extracellular matrix proteins (e.g. COL4A1, COL4A2, LAMC1). Induction of these genes frequently correlates with changes in the cellular microenvironment associated with liver fibrosis and tumor invasion, a process largely controlled by the transforming growth factor (TGF)-β pathway. Interestingly, SMAD2 that mediates TGFβ signals was either induced (80% datasets) or repressed, highlighting the functional duality of the TGFβ pathway, acting as a tumor promoting or a tumor suppressing factor in cancer, including HCC (17). SFRP1, acting as a negative modulator of the Wnt/β-catenin pathway frequently activated in HCC microenvironment, was repressed.
Avoiding immune destruction
Several immune-associated genes were identified and most of them were repressed (Supplementary Table 2), including activation markers of cytotoxic T lymphocytes and natural killer (NK) cells (e.g. CD69, KLRK1, GZMA). In addition, immunoregulatory mediators with chemotactic activity for competent immune cells were repressed (e.g. CCL19, CCL2/MCP1, CXCL12/SDF1).
Emerging hallmarks enriched in HCC
In addition to the well-characterized hallmarks of cancer cells described above, two more discrete but promising functional categories emerged from the analysis of the 935-gene HCC signature. These specific hallmarks were associated with protein turnover and epigenetics (Supplementary Table 2). In agreement with an active metabolic activity, we observed an increased expression of several translation-associated factors, including genes encoding ribosomal subunits (e.g. RPS5, RPL38) and translational machinery (e.g. EIF4G2). Unexpectedly, genes involved in protein ubiquitination (e.g. UBE2A, UBE2C) and degradation through the 26S proteasome pathway (e.g. PSMA1, PSMA6, PSMD2, PSMD4) were similarly overexpressed, evocative of a proteotoxic stress associated with a protein hyper-production and/or mysfolding (18). The second prominent promising HCC hallmark is the up-regulation of numerous genes acting at the epigenetic level (Supplementary Table 2). Thus, the 935-gene HCC signature included important regulators of chromatin assembly and remodeling (e.g. CHAF1A, HDAC1, HDAC5, HMGB2), components of the polycomb-repressive complex 2 (e.g. EZH2, SUZ12) that catalyzes the trimethylation of H3K27 (H3K27me3), and master regulators of microRNA processing (e.g. DROSHA).
The 935-gene HCC signature highlights drug candidates for systemic therapies
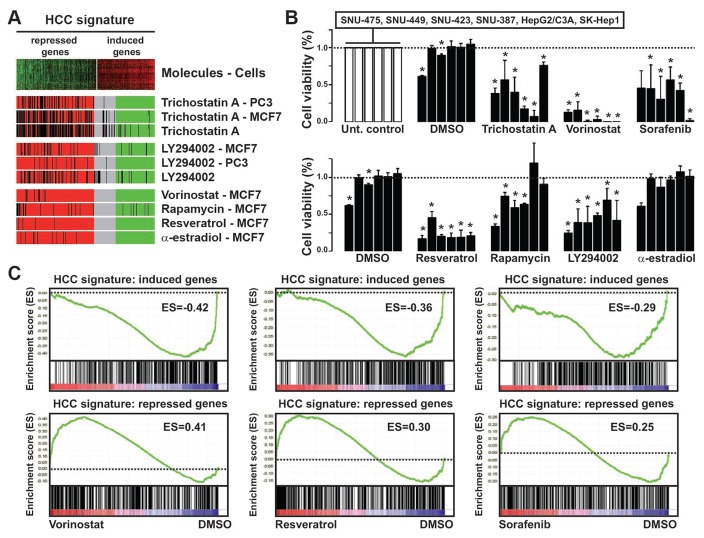
Next, the cMap algorithm was used to identify relevant drugs in HCC, as previously described (10). We selected drugs that generated gene expression profiles inversely correlated with the 935-gene HCC signature with the highest confidence (P<0.003). Six candidate molecules were identified, including two histone deacetylase (HDAC) inhibitors (trichostatin A and vorinostat), PI3K inhibitor LY294002, mTOR inhibitor sirolimus (also known as rapamycin), α-estradiol and resveratrol (Fig. 2A and Supplementary Table 6). The impact of these drugs as compared to sorafenib that is currently used for the treatment of advanced HCC (19) was evaluated on the viability of 6 HCC-derived cell lines. Remarkably, except for α-estradiol, all the drugs significantly (P<0.05) reduced cell viability (Fig. 2B). The SNU-423 cell line was used as a paradigm to test the drug-induced transcriptional reprogramming based on the positive response of this cell line to drug treatments. Thus, gene expression profiling combined with GSEA demonstrated that these drugs could at least partly reversed the core transcriptional programming of HCC cells, as exemplified by vorinostat, resveratrol and sorafenib (Fig. 2C). Indeed, up-regulated genes of the 935-gene HCC signature were enriched in the gene expression profiles of DMSO treated cells (i.e. negatively enriched in drug treated cells) while down-regulated genes were positively enriched in drug treated cells, evidencing a drug-induced transcriptional reprogramming (Fig. 2C). These results were further validated in SNU-387 and HepG2/C3A cell lines (Supplementary Figure 1). Finally, data mining of genes included in the core enrichment for each drug (Supplementary Table 7) demonstrated that the identified drugs are able to reverse the expression of numerous genes involved in the functional networks that operate to establish the hallmarks of HCC cancer cells.
Figure 2.
The 935-gene HCC signature highlights relevant drug candidates. A, connectivity map algorithm applied to the 935-gene HCC signature identified 5 candidate molecules: trichostatin A, LY294002, vorinostat, rapamycin, resveratrol and α-estradiol. The barview shows the enrichment of treatment instance ordered by their corresponding connectivity scores. All selected drug candidates exhibit a significant negative enrichment as regard to the 935-gene HCC signature. B, impact of identified drug candidates on the viability of 6 human HCC cell lines. Cell viability was determined after 72hrs of treatment with drugs at various concentrations (see methods, n=4 independent experiments). Each bar represents cell viability (mean ± SD) in the investigated cell lines (SNU-475, SNU-449, SNU-423, SNU-387, HepG2/C3A, SK-Hep-1, from left to right). A two-tailed non-parametric Mann-Whitney test was used for the comparison between experimental groups for each cell line (DMSO vs. untreated control and drug vs. DMSO). * denotes a P value <0.05. C, Gene set enrichment analysis (GSEA) of the induced genes (upper 3 panels) and the repressed genes (lower 3 panels) from the 935-gene HCC signature in the gene expression profiles of the SNU-423 cell line treated with DMSO or vorinostat (left 2 panels), resveratrol (middle 2 panels) and sorafenib (right 2 panels). Positive and negative enrichment scores (ES) were determined by GSEA. DMSO, dimethyl sulfoxide; HCC, hepatocellular carcinoma.
Discussion
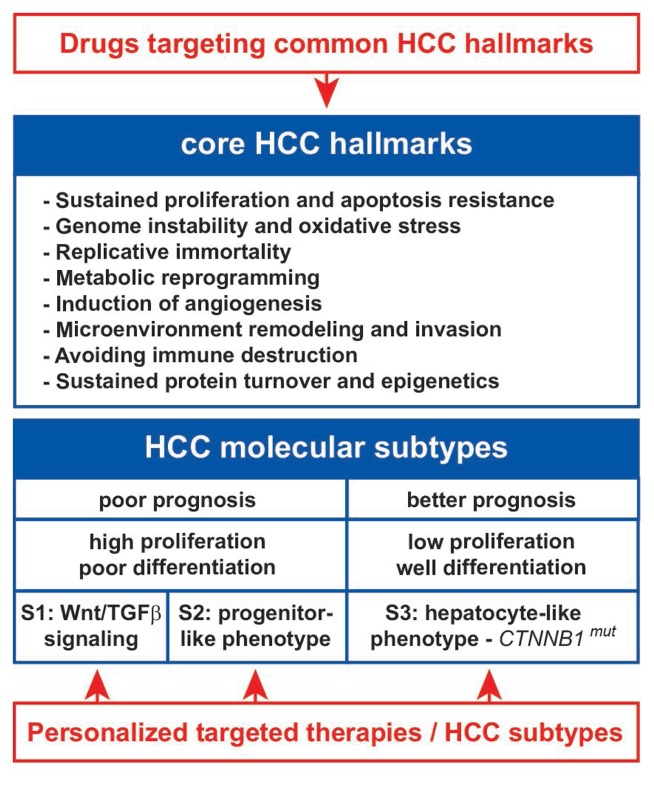
HCC is a deadly cancer worldwide, mainly due to late diagnosis and the absence of effective treatments for advanced stages of the disease. A strategy of HCC stratification into molecular subgroups has been developed worldwide with the objective to translate these knowledges into individualized treatments (6,20,21). Thus, Hoshida and colleagues reported the existence of three major HCC subtypes referred to as S1, S2, and S3 (6). These subtypes were associated with specific biological and clinical features (Fig. 3). S1 and S2 subtypes included aggressive HCC and were associated with an aberrant activation of the WNT signaling pathway by TGFβ (S1 subtype) or a progenitor-like phenotype associated with MYC and AKT activation (S2 subtype). S3 subtype included good prognosis HCC that exhibited a hepatocyte-like phenotype and CTNNB1 mutations (Fig. 3). The classification into molecular subtypes highlighted specific pathways for drug targeting, including TGFβ, WNT and AKT inhibitors. Conceptually, our study is slightly different as it hypotheses that optimized treatments should take into consideration not only the molecular alterations that occur in specific HCC subtypes but also those recurrently altered in all tumors (Fig. 3). However, the definition of core transcriptional hallmarks in HCC has never been really explored in details, so far. Our study fills this gap and demonstrates the existence of a substantial transcriptional homogeneity in HCC. Hence, we provide a robust, exhaustive and comprehensive signature of 935 genes commonly deregulated in human HCC from data generated in various laboratories and covering all the known etiologies. We hypothesized that analyzing multiple datasets with the same algorithms significantly increases the accuracy of the findings as compared to conclusions raised from single studies. Consistent with the specific objectives of Hoshida’s study (i.e. the definition of signatures for molecular HCC subtypes) and our study (i.e. the definition of a common transcriptional fingerprint for all HCC), there was almost no overlap between the genes included in the S1–3 signatures and our core HCC signature. Indeed, in the 935-gene HCC signature, only 3%, 2% and 13% genes overlapped with the S1, S2 and S3 signatures, respectively (Supplementary Figure 2). Besides, most of genes overlapping with the S3 signature were metabolism-associated genes that were shown to be relatively up-regulated in S3-HCC, as compared to poorly differentiated HCC of S1 and S2 subtypes (5,6). However, regardless of HCC subtypes, these genes were commonly repressed when compared to the surrounding non-tumor tissues, as observed in our core HCC signature. Each of the signatures (i.e. S1–3 and core) are then specific in term of constituting genes but could be applied to large HCC cohorts. Altogether, we believe that our signature constitutes a unique and specific fingerprint of recurrent and common transcriptional alterations in HCC.
Figure 3.
Therapeutic strategies integrating core and subtype-specific transcriptional hallmarks in human HCC. The meta-analysis presented in this study enhances our knowledge on the molecular characterization of HCC tumors and echoes previous studies focused on HCC stratification. Recently, a consensus has been achieved identifying 3 main HCC molecular subtypes (S1, S2 and S3) (6). S1 and S2 subtypes are associated with a poor prognosis and included highly proliferative and poorly differentiated tumors. S1 subtype is particularly associated with the activation of a pro-metastatic TGFβ signaling and the S2 subtype included tumors with a progenitor-like phenotype. S3 subtype is associated with a better prognosis, low proliferation and includes well-differentiated tumors that retained a hepatocyte-like phenotype. At the molecular level the S3 subtype is enriched in tumors that exhibit activating mutations in CTNNB1 gene encoding β-catenin. HCC stratification into homogeneous subtypes opened new avenues for personalized targeted therapies. The highlighting of core transcriptional hallmarks in human HCC suggests that efficient therapies should consider drugs targeting common HCC hallmarks together with drugs targeting specific HCC subtypes. CTNNB1mut, mutated beta-catenin gene; HCC, hepatocellular carcinoma; TGFβ, transforming growth factor beta.
One striking observation is that the 935-gene HCC signature covers almost all hallmark capabilities of cancer cells described previously (Fig. 3) (15). It is noteworthy that none of the genes included in the 935-gene HCC signature was directly related to replicative immortality. This cancer hallmark is largely controlled by telomerase activity. Actually, telomerase has been shown to be reactivated in >90% HCC, mostly due to TERT amplification and somatic mutations or HBV insertion in the TERT promoter (3). More than being only recurrent in HCC, genetic alterations in TERT represent early events in human hepatocarcinogenesis (22). Importantly, we show that the 935-gene HCC signature was associated with early chromosomal alterations described in human hepatocarcinogenesis (14). Thus, the signature should include relevant candidate biomarkers for early HCC diagnosis, including secreted biomarkers (e.g. SPINK1). In addition, identifying cell surface markers (e.g. ligands and/or receptors) over-expressed in most HCC may lead to new drug targets with high specificity. Accordingly, innovative nanoparticles may be formulated including specific peptides derived from the 935-gene HCC signature, in order to increase drug delivery while reducing drug side effects. Interestingly, the 935-gene HCC signature highlighted specific hallmarks associated with protein turnover (i.e. synthesis and proteasomal degradation) and epigenetics (Fig. 3). Over-activation of the ubiquitin-proteasome system has been reported in several cancers and proteasome inhibitors, e.g. bortezomib, provided promising results in treating hematological malignancies (23). In experimental models of HCC, the combination of sorafenib and bortezomib demonstrated synergistic anti-tumor effects through AKT inactivation (24).
Though cMap algorithm, drug candidates were identified and validated in several HCC cell lines, including resveratrol, HDAC inhibitors (HDACi) and PI3K/AKT/mTOR inhibitors, in agreement with the landscape of genetic alterations frequently observed in HCC (2). It is noteworthy that cMap results were mainly derived from MCF7 breast cancer cells (Supplementary Table 6) that may harbor a different spectrum of mutations than HCC cell lines. Interestingly, the comparison of genetic profiles though the Cancer Cell Line Encyclopedia project (www.broadinstitute.org/ccle) identified a mutation signature consisting in 16 genes mutated both in MCF7 and at least 3/6 HCC cell lines investigated. This signature included key cancer genes involved in EMT, tumor growth, metastasis and angiogenesis (e.g. AAK1, CDK11B, ILK, MAP3K1, MAP3K14, NCAM1, PRKDC, and VEGFC). One can hypothesize that the efficacy of the identified drugs may be related to these signaling pathways. Resveratrol and HDACi were shown to target multiple cancer hallmarks in preclinical models, including effects on growth inhibition, cell differentiation, angiogenesis and immune surveillance (25,26). At the molecular level resveratrol was reported to modulate cancer-related signaling pathways, including FAS/FASL, PI3K/AKT, NFkB, and WNT (26). HDACi-induced growth arrest has been linked to the induction of the cyclin-dependent kinase (CDK) inhibitors p21 and p27. HDACi-induced cell death involves caspase-dependent and caspase-independent pathways. HDACi also cause hyper-acetylation and inactivation of HSP90, leading to the degradation of proteins that require the chaperone function of HSP90, including some oncoproteins (25). HDACi can block tumor angiogenesis by inhibiting hypoxia inducible factors and expression of VEGF (27) and impair immune surveillance by reducing viability and effector functions of NK cells (28). In liver cancer, we showed that HDACi could interfere with tumor-stroma crosstalk (10) and loss of hepatocyte differentiation (29). In HCC, the results of a multicenter phase I/II study in patients with unresectable tumors demonstrated that HDAC inhibition with belinostat was well-tolerated and associated with tumor stabilization (30).
However, the picture is obviously not so simple given that besides sorafenib, most mono-therapies evaluated in phase III clinical trials failed to improve the survival of patients with advanced HCC (31). In addition, recent results of combined therapies, e.g. sorafenib associated with erlotinib or doxorubicin, failed to demonstrate meaningful clinical benefits (32,33). This raises questions about the optimal backbone not only for drug combinations (31,32) but also for combined treatment modalities, including surgery with adjuvant multi-drug chemotherapies and biotherapies, personalized radioembolization and immune-based therapies. Our comprehensive 935-gene HCC signature may help to resolve this issue by identifying combinations of treatments to target various signaling pathways altered in HCC. We believe that evaluating these strategies in combination with molecules targeting pathways deregulated in specific HCC subtypes may represent promising approaches, particularly in clinical trials where patients for each subtypes are selected based on the expression specific biomarkers.
Supplementary Material
Supplementary Figure 1. Reversion of the core transcriptional programming of HCC cells by selected drugs. In A and B, gene set enrichment analysis (GSEA) of the induced genes (upper panels) and the repressed genes (lower panels) from the 935-gene HCC signature in the gene expression profiles of the SNU-387 and HepG2/C3A cell lines. A, GSEA from the gene profiles of SNU-387 (left 2 panels) and HepG2/C3A (right 2 panels) cells treated with DMSO or vorinostat. B, GSEA from the gene profiles of HepG2/C3A cells treated with DMSO or Sorafenib (left 2 panels) or Resveratrol (right 2 panels). Positive and negative enrichment scores (ES) were determined by GSEA. DMSO, dimethyl sulfoxide; HCC, hepatocellular carcinoma.
Supplementary Figure 2. Four-way Venn diagram comparing gene sets included in S1, S2 and S3 HCC subtype signatures from Hoshida’s study [1] and our core HCC signature.
Supporting the specificity of the gene signatures in both Hoshida’s study and our study, there was almost no overlap between the S1–3 subtype signatures and our core HCC signature. Indeed, 82% genes included in our core HCC signature were unique while only 3%, 2% and 13% genes overlapped with the S1, S2 and S3 signatures, respectively. In addition, most of genes that overlap with the S3 signature were metabolism-associated genes that were shown to be relatively up-regulated in S3-HCC, as compared to poorly differentiated HCC of S1 and S2 subtypes [1]. However, regardless of HCC subtypes, most of these genes remain commonly repressed when compared to the surrounding non-tumor tissues, as observed in our core HCC signature. This comparison suggests that our core signature constitutes a unique and specific fingerprint of recurrent and common transcriptional alterations in HCC.
Supplementary Table 1: Characteristics of the 28 liver-oriented HCC microarray datasets (MDS).
Supplementary Table 2: Description of the core human HCC signature.
Supplementary Table 3: Enrichment of up- and down-regulated genes of the core human HCC signature in specific chromosome locations.
Supplementary Table 4: Ingenuity Pathway Analysis (IPA) of up- and down-regulated genes from the core human HCC signature.
Supplementary Table 4a: IPA of up-regulated genes
Supplementary Table 4b: IPA of down-regulated genes
Supplementary Table 5: Exhaustive data mining of up- and down-regulated genes of the core human HCC signature using Enrichr algorithm.
Supplementary Table 5a. Functional categories associated with gene transcription.
Supplementary Table 5b. Functional categories associated with signaling pathways.
Supplementary Table 5c. Functional categories associated with gene ontologies.
Supplementary Table 6: Connectivity map results of the core human HCC signature.
Supplementary Table 7: List of genes included in the core enrichment for each drug from the up- or down-regulated genes of the core human HCC signature.
Supplementary Table 7a: List of genes included in the core enrichment for each drug from the up-regulated genes of the core human HCC signature.
Supplementary Table 7b: List of genes included in the core enrichment for each drug from the down-regulated genes of the core human HCC signature.
Acknowledgments
Financial Support
This research was supported by Inserm and University of Rennes 1. C.C. is supported by grants from the National Cancer Institute (Cancéropôles Ile-de-France & Grand-Ouest), Ligue contre le cancer (CD35, 44, 49) and Association Française pour l’Etude du Foie, France.
The authors thank all the researchers who contribute to the genomic characterization of HCC over the last two decades allowing this meta-analysis to be performed. We thank Dr Snorri S. Thorgeirsson (NCI, Bethesda, USA) for critical reading of the manuscript.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors declare no potential conflicts of interest.
References
- 1.Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31(4):339–46. doi: 10.1038/ng0802-339. [DOI] [PubMed] [Google Scholar]
- 2.Schulze K, Imbeaud S, Letouze E, Alexandrov LB, Calderaro J, Rebouissou S, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47(5):505–11. doi: 10.1038/ng.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Totoki Y, Tatsuno K, Covington KR, Ueda H, Creighton CJ, Kato M, et al. Transancestry mutational landscape of hepatocellular carcinoma genomes. Nat Genet. 2014;46(12):1267–73. doi: 10.1038/ng.3126. [DOI] [PubMed] [Google Scholar]
- 4.Marquardt JU, Andersen JB, Thorgeirsson SS. Functional and genetic deconstruction of the cellular origin in liver cancer. Nat Rev Cancer. 2015;15(11):653–67. doi: 10.1038/nrc4017. [DOI] [PubMed] [Google Scholar]
- 5.Hoshida Y, Toffanin S, Lachenmayer A, Villanueva A, Minguez B, Llovet JM. Molecular classification and novel targets in hepatocellular carcinoma: recent advancements. Semin Liver Dis. 2010;30(1):35–51. doi: 10.1055/s-0030-1247131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoshida Y, Nijman SM, Kobayashi M, Chan JA, Brunet JP, Chiang DY, et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 2009;69(18):7385–92. doi: 10.1158/0008-5472.CAN-09-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Malenstein H, van Pelt J, Verslype C. Molecular classification of hepatocellular carcinoma anno 2011. Eur J Cancer. 2011;47(12):1789–97. doi: 10.1016/j.ejca.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 8.Coulouarn C, Gomez-Quiroz LE, Lee JS, Kaposi-Novak P, Conner EA, Goldina TA, et al. Oncogene-specific gene expression signatures at preneoplastic stage in mice define distinct mechanisms of hepatocarcinogenesis. Hepatology. 2006;44(4):1003–11. doi: 10.1002/hep.21293. [DOI] [PubMed] [Google Scholar]
- 9.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 10.Coulouarn C, Corlu A, Glaise D, Guenon I, Thorgeirsson SS, Clement B. Hepatocytestellate cell cross-talk in the liver engenders a permissive inflammatory microenvironment that drives progression in hepatocellular carcinoma. Cancer Res. 2012;72(10):2533–42. doi: 10.1158/0008-5472.CAN-11-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coulouarn C, Factor VM, Andersen JB, Durkin ME, Thorgeirsson SS. Loss of miR-122 expression in liver cancer correlates with suppression of the hepatic phenotype and gain of metastatic properties. Oncogene. 2009;28(40):3526–36. doi: 10.1038/onc.2009.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamb J, Crawford ED, Peck D, Modell JW, Blat IC, Wrobel MJ, et al. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science. 2006;313(5795):1929–35. doi: 10.1126/science.1132939. [DOI] [PubMed] [Google Scholar]
- 13.Longerich T, Mueller MM, Breuhahn K, Schirmacher P, Benner A, Heiss C. Oncogenetic tree modeling of human hepatocarcinogenesis. Int J Cancer. 2012;130(3):575–83. doi: 10.1002/ijc.26063. [DOI] [PubMed] [Google Scholar]
- 14.Midorikawa Y, Yamamoto S, Tsuji S, Kamimura N, Ishikawa S, Igarashi H, et al. Allelic imbalances and homozygous deletion on 8p23. 2 for stepwise progression of hepatocarcinogenesis. Hepatology. 2009;49(2):513–22. doi: 10.1002/hep.22698. [DOI] [PubMed] [Google Scholar]
- 15.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11(2):85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 17.Coulouarn C, Factor VM, Thorgeirsson SS. Transforming growth factor-beta gene expression signature in mouse hepatocytes predicts clinical outcome in human cancer. Hepatology. 2008;47(6):2059–67. doi: 10.1002/hep.22283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell. 2009;136(5):823–37. doi: 10.1016/j.cell.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.European Association For The Study Of The L, European Organisation For R, Treatment Of C. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–43. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Lee JS, Heo J, Libbrecht L, Chu IS, Kaposi-Novak P, Calvisi DF, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med. 2006;12(4):410–6. doi: 10.1038/nm1377. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136(3):1012–24. doi: 10.1053/j.gastro.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zucman-Rossi J, Villanueva A, Nault JC, Llovet JM. Genetic Landscape and Biomarkers of Hepatocellular Carcinoma. Gastroenterology. 2015;149(5):1226–39. e4. doi: 10.1053/j.gastro.2015.05.061. [DOI] [PubMed] [Google Scholar]
- 23.Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348(26):2609–17. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 24.Chen KF, Yu HC, Liu TH, Lee SS, Chen PJ, Cheng AL. Synergistic interactions between sorafenib and bortezomib in hepatocellular carcinoma involve PP2A-dependent Akt inactivation. J Hepatol. 2010;52(1):88–95. doi: 10.1016/j.jhep.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6(1):38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- 26.Shukla Y, Singh R. Resveratrol and cellular mechanisms of cancer prevention. Ann N Y Acad Sci. 2011;1215:1–8. doi: 10.1111/j.1749-6632.2010.05870.x. [DOI] [PubMed] [Google Scholar]
- 27.Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene. 2007;26(37):5541–52. doi: 10.1038/sj.onc.1210620. [DOI] [PubMed] [Google Scholar]
- 28.Rossi LE, Avila DE, Spallanzani RG, Ziblat A, Fuertes MB, Lapyckyj L, et al. Histone deacetylase inhibitors impair NK cell viability and effector functions through inhibition of activation and receptor expression. J Leukoc Biol. 2012;91(2):321–31. doi: 10.1189/jlb.0711339. [DOI] [PubMed] [Google Scholar]
- 29.Dubois-Pot-Schneider H, Fekir K, Coulouarn C, Glaise D, Aninat C, Jarnouen K, et al. Inflammatory cytokines promote the retrodifferentiation of tumor-derived hepatocyte-like cells to progenitor cells. Hepatology. 2014;60(6):2077–90. doi: 10.1002/hep.27353. [DOI] [PubMed] [Google Scholar]
- 30.Yeo W, Chung HC, Chan SL, Wang LZ, Lim R, Picus J, et al. Epigenetic therapy using belinostat for patients with unresectable hepatocellular carcinoma: a multicenter phase I/II study with biomarker and pharmacokinetic analysis of tumors from patients in the Mayo Phase II Consortium and the Cancer Therapeutics Research Group. J Clin Oncol. 2012;30(27):3361–7. doi: 10.1200/JCO.2011.41.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Llovet JM, Hernandez-Gea V. Hepatocellular carcinoma: reasons for phase III failure and novel perspectives on trial design. Clin Cancer Res. 2014;20(8):2072–9. doi: 10.1158/1078-0432.CCR-13-0547. [DOI] [PubMed] [Google Scholar]
- 32.Lencioni R, Llovet JM, Han G, Tak WY, Yang J, Guglielmi A, et al. Sorafenib or Placebo plus TACE with Doxorubicin-Eluting Beads for Intermediate-Stage HCC: Phase II, Randomized, Double-Blind SPACE Trial. J Hepatol. 2016 doi: 10.1016/j.jhep.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Zhu AX, Rosmorduc O, Evans TR, Ross PJ, Santoro A, Carrilho FJ, et al. SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015;33(6):559–66. doi: 10.1200/JCO.2013.53.7746. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Reversion of the core transcriptional programming of HCC cells by selected drugs. In A and B, gene set enrichment analysis (GSEA) of the induced genes (upper panels) and the repressed genes (lower panels) from the 935-gene HCC signature in the gene expression profiles of the SNU-387 and HepG2/C3A cell lines. A, GSEA from the gene profiles of SNU-387 (left 2 panels) and HepG2/C3A (right 2 panels) cells treated with DMSO or vorinostat. B, GSEA from the gene profiles of HepG2/C3A cells treated with DMSO or Sorafenib (left 2 panels) or Resveratrol (right 2 panels). Positive and negative enrichment scores (ES) were determined by GSEA. DMSO, dimethyl sulfoxide; HCC, hepatocellular carcinoma.
Supplementary Figure 2. Four-way Venn diagram comparing gene sets included in S1, S2 and S3 HCC subtype signatures from Hoshida’s study [1] and our core HCC signature.
Supporting the specificity of the gene signatures in both Hoshida’s study and our study, there was almost no overlap between the S1–3 subtype signatures and our core HCC signature. Indeed, 82% genes included in our core HCC signature were unique while only 3%, 2% and 13% genes overlapped with the S1, S2 and S3 signatures, respectively. In addition, most of genes that overlap with the S3 signature were metabolism-associated genes that were shown to be relatively up-regulated in S3-HCC, as compared to poorly differentiated HCC of S1 and S2 subtypes [1]. However, regardless of HCC subtypes, most of these genes remain commonly repressed when compared to the surrounding non-tumor tissues, as observed in our core HCC signature. This comparison suggests that our core signature constitutes a unique and specific fingerprint of recurrent and common transcriptional alterations in HCC.
Supplementary Table 1: Characteristics of the 28 liver-oriented HCC microarray datasets (MDS).
Supplementary Table 2: Description of the core human HCC signature.
Supplementary Table 3: Enrichment of up- and down-regulated genes of the core human HCC signature in specific chromosome locations.
Supplementary Table 4: Ingenuity Pathway Analysis (IPA) of up- and down-regulated genes from the core human HCC signature.
Supplementary Table 4a: IPA of up-regulated genes
Supplementary Table 4b: IPA of down-regulated genes
Supplementary Table 5: Exhaustive data mining of up- and down-regulated genes of the core human HCC signature using Enrichr algorithm.
Supplementary Table 5a. Functional categories associated with gene transcription.
Supplementary Table 5b. Functional categories associated with signaling pathways.
Supplementary Table 5c. Functional categories associated with gene ontologies.
Supplementary Table 6: Connectivity map results of the core human HCC signature.
Supplementary Table 7: List of genes included in the core enrichment for each drug from the up- or down-regulated genes of the core human HCC signature.
Supplementary Table 7a: List of genes included in the core enrichment for each drug from the up-regulated genes of the core human HCC signature.
Supplementary Table 7b: List of genes included in the core enrichment for each drug from the down-regulated genes of the core human HCC signature.