Abstract
Camellia sinensis (Cs) is a plant which is rich in polyphenols and has antioxidant, antiinflammatory, antimutagenic, anticarcinogenic and antibacterial activities. In this study, two different methanol extracts (Cs-I and Cs-II) were prepared from the leaf of C. sinensis in order to investigate the wound healing and anticancer activities. Total phenolic content and antioxidant activity of the extracts were determined. Wound healing effects of Cs extracts were evaluated by using Masson’s Trichrome Tecnique on NIH3T3 fibroblast cells. Cytotoxic and apoptotic effects of the extracts were determined by MTT and AnnexinV-PI assays on U2OS osteosarcoma cells. Total phenolic contents and antioxidant activities of the extracts were almost the same. The highest concentration (60 µg/mL) of the extracts showed significant cytotoxic and apoptotic effects on U2OS cells. Especially, the highest apoptotic effect was determined with 60 µg/mL Cs-I extract. Significant wound healing potential on NIH3T3 fibroblast cells were determined especially with low extract concentrations (0.5, 1 and 5 µg/mL), while high extract concentrations showed significant anticancer effects. As a result, two Cs leaf extracts exhibited important apoptotic properties and both have wound healing potential. However, the Cs-I extract was found more effective on apoptotic osteosarcoma cell death and has an increased wound healing potential than the Cs-II extract.
Keywords: Antioxidant, Wound healing, Camellia sinensis, Osteosarcoma, Anticancer
Introduction
Camellia sinensis (Cs) (L.) Kuntze (Theaceae) (tea) is being used in Far Eastern countries for many years and in time has spread to Europe, America and also other parts of the world which is one of the most widely consumed beverages, second only to water (Weisburger 1997). Because of the bioactive substances in the composition of tea, it is attracting the attention of many researchers and studies examining the effects of tea on health are conducted extensively (Weisburger 1997; Chen and Dou 2008; Kelly 2007; Sharangi 2009). Green tea is a powerful antioxidant because of the flavonoid content and also the anti-inflammatory, antioxidant, antimutagenic, anticarcinogenic, antiangiogenic, apoptotic, anti-arteriosclerotic, antidiabetic, antibacterial, antiviral and antiaging effects of green tea have been demonstrated by numerous studies; in which green tea inhibited the formation and development of many diseases (Chen and Dou 2008; Brown 1999; Tosun and Karadeniz 2005; Fisunoğlu and Besler 2008; Hsu et al. 2005; Sahin and Özdemir 2006). Epidemiological studies showed that, along with healthy eating habits, consuming 5–6 cups of tea a day will help to reduce the risk of chronic diseases because of the polyphenols in the tea (Fisunoğlu and Besler 2008).
Cancer is one of the most important health problems of nowadays. According to the results of many experimental studies, green tea is described as a protector against many types of cancer including particularly colon, stomach, esophagus and lung cancers, and also breast, skin and prostate cancers (Sharangi 2009; Inoue et al. 2001; Katiyar et al. 2000). The protective effects of green tea and catechin composition against cancer are explained by mechanisms such as; inhibition of cell proliferation, cell cycle arrest, reduction of the release of cytokines, transcription factor receptors suppression and mitotic stimulation, mutagenicity and genotoxicity prevention, free radical scavenging, activation of detoxification enzymes, inducing apoptosis of cancer cells and angiogenesis inhibition (Sahin and Özdemir 2006; Koo and Cho 2004).
Wound healing is a result of an extremely complex biochemical and cellular chain of events that occur in the organism, and it is a tissue response that brings the injured tissue to the normal status and also the defense mechanism of the body created against these injuries. Many herbal extract involving Cs are used for the herbal treatment of wound healing until today (Nursal et al. 1999). The wound healing effects of green tea were previously demonstrated with studies performed on laboratory animals (Hsu et al. 2005,2003; Fu et al. 2000; Kapoor et al. 2004; Kim et al. 2008; Zhang et al. 2006).
In this study, two different methanol extracts of Cs leaves collected from Trabzon/Sürmene located in the Black Sea region of Turkey were prepared and the effects of these extracts were investigated in different cell culture experimental models. U2OS osteosarcoma cells for evaluating the anticarcinogenic activity of extracts and NIH3T3 fibroblasts cells for the wound healing potential were used.
Materials and methods
Plant material and extractions
In this study, we have collected green tea leaves from Sürmene Town of Trabzon City, is the located in the Eastern Black Sea region of TURKEY. Two different methanol extracts were prepared from the leaves of Cs. Extraction procedures were described in detail below as described elsewhere in details (Oztürk et al. 2009).
Wet and fresh Cs leaves were steamed for 3 min at 100–110 °C and then quickly cooled at 20 °C. Subsequently, drying process was performed at 60–70 °C for 15–20 min. Dried Cs leaves were crumbled into small particles and used for extraction. Total weight of dry Cs was scaled and put into cartridges made from filter paper and macerated in methanol for one night. Next day, the extraction of the sample was performed in a Soxhlet apparatus. This extract was named as Cs methanol extract-I (Cs-I) (Oztürk et al. 2009).
A portion of fresh Cs leaves were dried in the dark at room temperature and crumbled. Total weight of dry Cs was scaled and macerated in methanol for one night. Then, extract was prepared as described above and this extract was named as Cs methanol extract-II (Cs-II) (Oztürk et al. 2009).
Determination of total phenolic content (Folin–Ciocalteu method)
The total phenolic contents of the extracts were determined by spectrophotometrically at 750 nm based on the absorbance measurement of the extracts as equivalent to gallic acid as described previously (Folin and Ciocalteu 1928; Dikmen et al. 2011a). Briefly, 0.5 mL of extract solutions (0.5 mg/mL) were placed into test tubes, then 2.5 mL of Folin–Ciocalteu reagent solution (10% in water) and 7.5 mL of Na2CO3 (20% in water) solution were added. The reaction mixture allowed to stand at room temperature in the dark for 2 h and absorbance was measured at 750 nm. Quantification of total polyphenols in the extracts were made using gallic acid to establish a calibration curve. Total phenolic content was expressed as gallic acid equivalents (GAE) per gram of dry weight of the plant extract. The results were expressed as average of three measurements.
β-Carotene-linoleic acid assay
A stock solution of β-carotene linoleic acid mixture was prepared as follows: 3 mg of β-carotene was dissolved in chloroform and mixed with 40 mg linoleic acid and 400 mg Tween 80. Chloroform was gently removed by using a rotary evaporator. Then, 100 mL of distilled water, saturated with oxygen, was added slowly to the residue and the solution was vigorously agitated to form a stable emulsion. To an aliquot of 3 mL of this emulsion, 0.2 mL of sample solutions (0.6 mg/mL concentration) was added and each sample solution was transferred to a 96-well microplate. Absorbance was measured at 490 nm after incubation for every 15 min until 180 min at 45 °C using a microplate reader. Butylated hydroxytoluene (BHT) was used as a reference synthetic antioxidant. An equal amount of methanol was used as control. All determinations were performed in triplicate and results were averaged. The inhibition percentages were calculated (Shahsavari et al. 2008; Agar et al. 2015).
Free radical scavenging activity using DPPH· method
DPPH· (2,2-diphenyl-l-picrylhydrazyl) assay was used as a rapid spectroscopic method to provide an evaluation of antioxidant activity due to scavenging free radicals. Being a stable free radical with purple color, DPPH· is reduced into the yellow colored diphenylpicryl hydrazine. Free radical scavenging effects of Cs extracts on DPPH· were estimated according to the previously described method with some modifications (Sanchez-Moreno et al. 1998). Methanol solution (0.1 mL) containing different sample concentrations was added to 3 mL of freshly prepared 0.05 mM DPPH· in methanol. The mixture was shaken and kept at room temperature for 30 min protected from the light. The absorbance of the resulting solution was measured spectrophotometrically at 517 nm. The measurement of radical scavenging activity was performed in triplicate and the activity of the tested extracts expressed as inhibition (%) against DPPH·. It was calculated as follows: Inhibition (%) = [(A control − A sample)/A control] × 100 (Sanchez-Moreno et al. 1998).
Antitumor effects
The culture of U2OS cells
U2OS (ATCC HTB-96, ATCC, Manassas, VA, USA) human osteosarcoma cells were grown in RPMI-1640 medium supplemented with 2 mM l-glutamine and 10% fetal bovine serum, 1% penicillin/streptomycin at 37 °C in a humidified incubator with 5% CO2. Cs extracts were dissolved in DMSO as a stock solution and the stock solution was diluted to the required concentrations in cell culture medium. The same concentration of DMSO at 2 μL/mL was used for the solvent control group. There was no significant difference between the medium controls and solvent controls (p > 0.05), and results of experimental groups were compared with solvent control group.
Cell viability assay
The viability of the cells were assessed by MTT [(3,4,5-dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide] assay, which is based on the reduction of MTT by the mitochondrial dehydrogenase of intact cells to a purple formazan product. Yellow MTT is reduced to purple formazan in the mitochondria of living cells. This reduction takes place only when mitochondrial reductase enzymes are active, and therefore, the conversion can be directly related to the number of viable (living) cells (Mosmann 1983; Dikmen et al. 2011a).
U2OS cells were seeded into 96-well culture plates at densities of 3 × 103 cells per well. After 24 h, they were treated with the 5, 10, 20, 40 and 60 μg/mL concentrations of Cs methanol extract for 24 h. After 24 h incubation, 10 μl of MTT solution (5 μg/mL) was added to each well of 96-well plate and incubated for 3 h at 37 °C. After incubation, the purple MTT-formazan crystals were dissolved by adding 100 μl DMSO. The absorbance of the samples were measured with an ELISA reader (OD540 nm, EL800 BioTek). In the experiment, each group was performed in 8 wells. The data are mean values from three different experiments. MTT reductions were used to estimate cell viability at the end of the assay (Dikmen et al. 2011a, b).
Detection of apoptosis
The ApopNexin™ FITC Apoptosis Detection Kit (Millipore, Billerica, MA, USA, APT750) was used to detect apoptosis as described by the manufacturer. Briefly, U2OS cells were seeded in 6-well plates (5x105/well) and treated with extract concentrations (5, 10, 20, 40 and 60 μg/mL) for 24 h. After the treatment, cell suspensions were centrifuged at 1200 rpm for 5 min and the pellets were washed twice with 3 mL of cold phosphate-buffered saline (PBS). The cells were resuspended in 100 µL binding buffer and stained with 3 µL Annexin V-FITC solution and 3 µL propidium iodide (PI) solution for 20 min at room temperature in the dark. Then the samples were diluted with 400 µL of 1× binding buffer and processed for data acquisition and analysed on a Becton–Dickinson FACS Aria flow cytometer using FACSDiva Version 6.1.1. Software. At least 10,000 cells were analyzed per sample. The fraction of cell populations in different quadrants were analyzed using quadrant statistics. The X-and Y-axes indicate the fluorescence of annexin-V (green) and PI (red), respectively. Quadrant settings were based on the control. It was possible to detect and quantitatively compare the percentages of gated populations in all of the four regions delineated. Four distinct phenotypes were distinguishable: viable [(Annexin-V−/PI−) lower left quadrant, Q3], early apoptotic [(Annexin-V+/PI−) lower right quadrant, Q4], late apoptotic (Annexin-V +/PI +) upper right quadrant, Q2), and necrotic/damaged cells [(Annexin-V−/PI+) upper left quadrant, Q1] (Dikmen et al. 2011b).
Determination of wound healing potencial
The culture of NIH3T3 mouse fibroblast cells
In the present study, the NIH3T3 mouse fibroblast cells (ATCC number CRL-1658) were grown in DMEM medium supplemented with 2 mM l-glutamine and 10% fetal bovine serum, 1% penicillin/streptomycin at 37 °C in a humidified incubator with 5% CO2. The extract of Centella asiatica (TECA, which was obtained from Bayer Chemical Industry, Istanbul, Turkey), which has wound healing effect, was used as positive control. TECA and Cs extracts were dissolved in DMSO as a stock solution and the stock solution was diluted to the required concentrations in medium.
Cell viability assay
In order to constitute an in vitro wound healing model, non-cytotoxic concentrations of the methanol extracts were determined on NIH3T3 mouse fibroblast cells with MTT cell viability assay as described briefly (Dikmen et al. 2011b). NIH3T3 cells were seeded into 96-well culture plates at densities of 3.103 cells per well. After 24 h, they were treated with the extract concentrations (0.5, 1, 5, 10 μg/mL) and TECA for 24 h. Then, MTT was added and after 3 h, the purple MTT-formazan crystals were dissolved by DMSO. The absorbance of the samples were measured with ELISA (OD540 nm, EL800 BioTek). MTT reductions were used to estimate cell viability at the end of the assay.
Treatment of NIH3T3 mouse fibroblast cells
Fibroblasts, which are the important cellular components of the dermis regulating the skin physiology, are typically quiescent cells. In the proliferative phase of wound healing, fibroblasts generate various components of the extracellular matrix such as collagen. Re-epithelialization occurring in this phase also involves proliferation and migration of fibroblasts cells (Agar et al. 2015; Chen et al. 2014; Guo and Dipietro 2010). Therefore, wound healing process has been successfully applied in fibroblast cell culture in order to developing new compounds (Maquart et al. 1990).
NIH-3T3 cells were seeded at a density of 5 × 105 and cell monolayers were grown on coverslips. A total of 70–80% confluent cells were treated with Cs extract concentrations (0.5, 1, 5 and 10 μg/mL) and C. asiatica (TECA) for 24 h in medium. After then, cells were stained with Masson’s Trichrome technique and examined under a light microscope in the light of previous studies (Dikmen et al. 2011c; Korkmaz et al. 2000; Öztürk et al. 2007). Cell proliferation, morphological changes and collagen production in the fibroblasts were evaluated to approach possible wound repair mechanisms. Results were evaluated in comparison to solvent control group, as it was used as a vehicle to dissolve TECA and the extracts. Morphometric analysis performed in this study showed that there were no significant differences between the medium control groups and solvent controls (p > 0.05). In addition, the DMSO concentration used here (0.2% v/v) was found to induce no significant negative effects on cultured NIH3T3 cells.
Cell staining procedure
Staining of cultured NIH3T3 fibroblasts were conducted according to Masson’s Trichrome technique, as described previously (Agar et al. 2015; Dikmen et al. 2011c; Korkmaz et al. 2000). Cell monolayers grown on cover slips were fixed in 70% ethanol solution for 15 min, and rehydrated in distilled water for 5–10 min. In order to stain the nuclei of fibroblasts, cover slips were allowed to stand for 10 min in Harris’s Hematoxylin solution. They were differentiated with acid–alcohol solution and washed with tap water. Cover slips were, then, immersed in acid fuchsin solution for 10 min and rinsed in distilled water. They were treated with 1.0% phosphomolybdic acid solution for 5 min. Cover slips were put in methylene blue solution for 5 min and rinsed in distilled water. Subsequently, they were treated with acetic acid solution (glacial acetic acid:distilled water 1:99, v/v) for 2 min and dried using 70% (v/v) ethanol solution. Entellan was dropped on a microscope slide and cover slip was mounted on it for the morphometric analysis of NIH3T3 fibroblasts under microscope.
Morphometric analysis of cultured NIH3T3 fibroblasts
Morphometric analysis was carried out according to previously described procedure (Dikmen et al. 2011c; Korkmaz et al. 2000; Öztürk et al. 2007). After staining, fibroblast cells grown on cover slips were examined under a light microscope (Olympus, Tokyo, Japan) using 400× magnification. Visible areas with these magnifications were calculated by using a Neubauer slide. Fusiform, polygonal, round and vacuole containing fibroblasts whose morphological shapes reflect functional states of the cells, were expressed as a percentage of the total cell number. Fibroblasts in polygonal shape were an experimental measure for the migration, while fusiform fibroblasts were accepted as quiescent cells. Vacuole-containing cells were a measure for aging of cell population, while round fibroblasts were the cells which loose their viabilities excluding the dye. Total cell numbers and number of cells entering mitosis were used as a parameter of proliferation of fibroblast cells. The number of collagen granules in a cell was also counted in at least 10 cells of five individual experiments.
Statistical analysis
The data were expressed as a mean ± standard error (SEM) and analysed statistically using one-way ANOVA. When ANOVA showed significant differences between groups, Tukey’s post hoc test was used to determine the specific pairs of groups showing statistically significant differences. A p value of less than 0.05 was considered as a statistical significance. (p < 0.05*, p < 0.01**, p < 0.001***). p > 0.05 was non-significant. Also, apoptotic results (Annexin V/PI) were evaluated by flow cytometer using FACSDiva Version 6.1.1. Software and the apoptotic cells were determined as the percentage of cells.
Results
Determination of total phenolic content and antioxidant activity
Table 1 shows the total phenolic content and antioxidant activity of Cs extracts employed in this study. The content of total phenols was expressed as gallic acid equivalents (mg GAE/g extract). The extraction yields of Cs-I and Cs-II were 36.75 (wt/wt) and 21.60%, and total phenolic contents were 360.70 and 357.81 mg of gallic acid equivalents per gram of extracts (dry weight), respectively. Free radical scavenging potential of the extracts were also tested by the DPPH· method in comparison with that of a synthetic antioxidant, BHT. Percentage inhibition value is a parameter widely used to measure the free radical scavenging activity. That is, a higher percentage inhibition value corresponds to a higher antioxidant activity. These data indicate that two extracts had the same activity as BHT. Cs methanol extracts showed the highest DPPH radical scavenging ability (EC50 = 47.30 ± 0.95 and 51.36 ± 0.98 μg/mL, p > 0.05 vs. BHT), which was similar to that of the reference compound BHT (EC50 = 51.08 ± 0.67 μg/mL). The potential of the extracts to inhibit lipid peroxidation were also evaluated in β-carotene-linoleic acid assay (Shahsavari et al. 2008; Agar et al. 2015). There was no significant differences between the extracts in this assay (p > 0.05). The decrease in β-carotene absorbance in Cs extracts and well-known antioxidant BHT, which was used as standard, are shown in Table 1. In this test system, it was obvious that Cs-II and Cs-I oxidized most rapidly with % inhibition values of 67.77 ± 2.35 and 71.07 ± 2.06, after BHT (87.11 ± 2.06), respectively. Two Cs extracts showed the same antioxidant activities according to the results of DPPH radical scavenging and β-carotene-linoleic acid assay (Table 1).
Table 1.
Total phenolic content and antioxidant activity of C. sinensis extracts
| Extracts | Total phenolic content | DPPH· scavenging assay | β-carotene-linoleic acid assay |
|---|---|---|---|
| mg GAE/g extracta | EC50 (μg/mL) | AA (%)b | |
| Cs-I | 360.70 ± 3.64 | 47.30 ± 0.95 | 71.07 ± 2.06 |
| Cs-II | 357.81 ± 2.17 | 51.36 ± 0.98 | 67.77 ± 2.35 |
| BHT | – | 51.08 ± 0.67 | 87.11 ± 2.06 |
Results are represented as mean ± standard deviation (n = 3), p > 0.05
AA antioxidant activity
aGallic acid equivalent
bInhibition (%) = 100 × [1−(A0s − A180s)/(A0c − A180c)]
Antitumor effects of extracts on U2OS cells
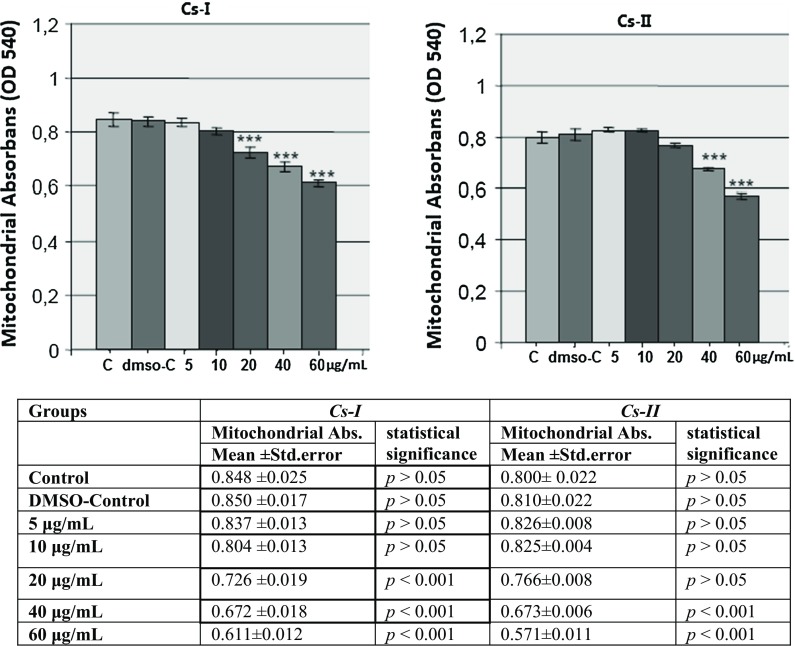
In this study, the effects of both two Cs extracts on cell viability were investigated with MTT assay according to mitochondrial actvity. U2OS cells were incubated with various concentrations of both extracts and depending on the increasing concentrations, decrease in cell viability was determined. 20, 40 and 60 µg/mL of Cs-I extract and 40 and 60 µg/mL of Cs-II extract concentrations significantly decreased the mitochondrial activity according to the control group (p < 0.001). According to MTT assay results, especially 40 and 60 µg/mL of extract concentrations caused cytotoxic effects on U2OS cells and decreased the cell viability significantly which is an important finding (Fig. 1).
Fig. 1.
Effects of Cs extracts on U2OS cell viability for 24 h. Cell viability was determined by MTT assay. Mitochondrial absorbance values are expressed as mean ± standard error of three independent experiments (n = 8), p > 0.05, non significant; p < 0.05*, p < 0.01** and p < 0.001*** were considered to be significant compared to DMSO-Control group
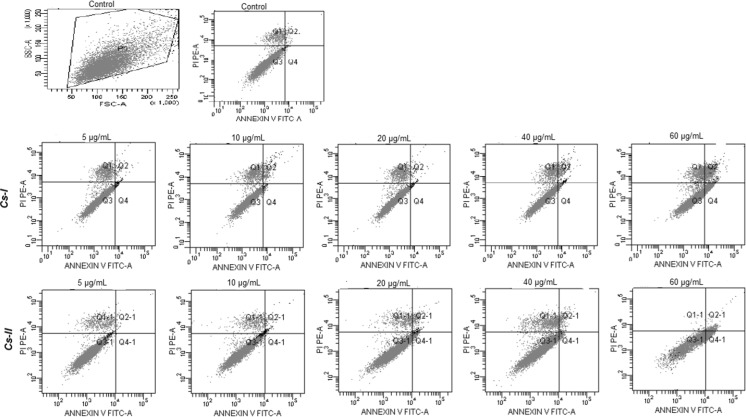
In parallel to the MTT results, especially 60 µg/mL of Cs-I and Cs-II extract concentrations caused significant apoptotic effects, which were determined by annexin-PI flow cytometer analysis (Fig. 2). With 40 µg/mL Cs-I concentration apoptotic cell ratio was 7.7% and necrotic cell ratio was 6.1%, while with 60 µg/mL concentration, it was 26.3 and 8.6%; with 40 µg/mL Cs-II concentration, apoptotic cell ratio was 4.2% and necrotic cell ratio was 9.3%, while with 60 µg/mL concentration, it was 18.9 and 0.4%, respectively (Table 2). Especially with 60 µg/mL Cs-I extract concentration, cell viability was determined as 65.1% and the apoptotic cell ratio was 26.3%, demonstrating that Cs-I extract has important anticancer effects of U2OS cells.
Fig. 2.
Typical quadrant analysis of annexin V-FITC/propidium iodide flow cytometry of U2OS cells treated with Cs extracts for 24 h
Table 2.
Percents of typical quadrant analysis of annexin V FITC/propidium iodide flow cytometry of U2OS cells treated with extracts
| Groups | Viable cells % (Q3) | Apoptotic cells % (Q2 + Q4) | Necrotic cells % (Q1) | |
|---|---|---|---|---|
| DMSO-control (2 µL/mL) | 96.6 | 0.1 | 3.4 | |
| Cs-I(µg/mL) | ||||
| 5 | 88.0 | 1.7 | 10.3 | |
| 10 | 89.7 | 3.2 | 7.0 | |
| 20 | 90.5 | 2.1 | 7.3 | |
| 40 | 86.2 | 7.7 | 6.1 | |
| 60 | 65.1 | 26.3 | 8.6 | |
| Cs-II(µg/mL) | ||||
| 5 | 93.7 | 0.3 | 6.0 | |
| 10 | 93.5 | 1.4 | 5.1 | |
| 20 | 90.0 | 5.2 | 4.8 | |
| 40 | 86.5 | 4.2 | 9.3 | |
| 60 | 80.7 | 18.9 | 0.4 | |
At least 10.000 cells were analyzed per sample, and quadrant analysis was performed. The proportion (%) of cell number is shown in each quadrant. Q1, necrotic cells; Q2 + Q4, apoptotic cells (late and early); Q3, viable cells
Determination of wound healing potency
Wound healing effects of the extracts were investigated on NIH3T3 fibroblast cells. Firstly, non-toxic concentrations of the extracts were obtained with MTT assay for wound healing analysis. In 0.5–10 µg/mL concentration range for both TECA and two methanol extracts, NIH3T3 cell viability did not decrease; moreover, with 0.5 and 5 µg/mL concentrations, cell proliferation increased according to the control group (Table 3). Afterwards, the wound healing effects of these concentrations were investigated morphometrically on fibroblast cells. After 24 h incubation period, total 3T3 cell numbers were increased with all Cs-I, Cs-II and TECA (used as positive control) concentrations in comparison to the control (DMSO solvent control) group. In parallel to this finding, the number of cells entering mitosis were increased with 10 µg/mL of TECA and Cs extract concentrations according to the solvent control group. Especially, the number of cells entering mitosis was 5.4 in the control group; while it was 8.70 with 0.5 µg/mL TECA, 8.40 and 9.00 with 5 and 10 µg/mL Cs-I concentrations, 8.80 and 7.60 with 0.5 and 1 µg/mL with Cs-II concentrations, respectively. These results show that both Cs extracts have mitosis inducing effects (Fig. 3; Table 4). Numbers of fusiform cells were decreased with TECA and both extract concentrations (p < 0.001), numbers of polygonal cells were increased significantly (p < 0.001; p < 0.01) in comparison to the control group. Especially with 0.5 and 1 µg/mL Cs-I concentrations, polygonal cell numbers were determined as 73.24 and 75.21, respectively (p < 0.001) (Table 5). Depending on the cell aging, the number of vacuolated and round cells were increased with TECA and high concentrations of the extracts. Round cell numbers were increased statistically significant with 5 and 10 µg/mL Cs-I concentrations (p < 0.001) (Table 6). Maximum increase in vacuolated cell numbers was obtained with 10 µg/mL TECA concentration (p < 0.001). One of the most important markers of wound healing effect is the increased collagen synthesis. In parallel to collagen synthesis increase, collagen granule numbers in the cell were increased at 0.5 µg/mL of TECA and at 0.5–10 µg/mL of Cs extract concentrations (Table 6). Consequently, both Cs methanol extracts showed important anticancer and wound healing effects in this study. 60 µg/mL concentration of both extracts showed significant cytotoxic and apoptotic effects on U2OS cells. Especially the highest apoptotic effect was obtained with 60 µg/mL Cs-I concentration. Taking together the increase in the mitotic and polygonal cell numbers of NIH3T3 fibroblast cells and also collagen granules, and wound healing effects obtained at low concentrations of both extracts (0.5, 1 and 5 µg/mL) with respect to according to the control group and TECA show that these extracts have wound healing potential at low concentrations.
Table 3.
Effects of Cs methanol extracts on NIH3T3 cell viability
| Groups | Cs-I | Cs-II | TECA |
|---|---|---|---|
| Mean ± SEr | Mean ± SE | Mean ± SE | |
| Control | 0.897 ± 0.021 | 0.889 ± 0.013 | 0.825 ± 0.023 |
| DMSO-control | 0.884 ± 0.010 | 0.885 ± 0.020 | 0.824 ± 0.010 |
| 0.5 μg/mL | 0.934 ± 0.017 | 0.924 ± 0.015 | 0.827 ± 0.008 |
| 1 μg/mL | 0.956 ± 0.051 | 0.901 ± 0.012 | 0.830 ± 0.004 |
| 5 μg/mL | 0.885 ± 0.005 | 0.864 ± 0.009 | 0.827 ± 0.005 |
| 10 μg/mL | 0.821 ± 0.013 | 0.869 ± 0.005 | 0.812 ± 0.006 |
| Statistical significance | p > 0.05 | ||
Cell viability was determined by MTT assay. Mitochondrial absorbance values are expressed as mean ± standard error of three independent experiments (n = 8), p > 0.05; non significant compared to DMSO-Control group. TECA: (Centella asiatica extract)
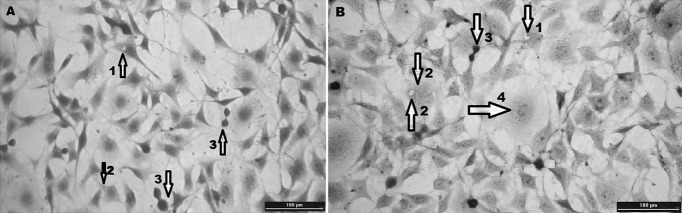
Fig. 3.
The images of stained NIH3T3 fibroblasts using Masson’s Trichrome technique (Magnification ×400; Scale bar 100 µm). DMSO-Control (a) and Cs-I extract (b). Arrows indicate collagen granules (1) in the cell and vacuole containing cells (2), fibroblast proliferating by mitosis (3) and rounding cells (4)
Table 4.
Effects of Cs extracts and TECA on the total number of NIH3T3 fibroblasts and on the number of proliferating (mitosis) NIH3T3 fibroblasts (mean fibroblast densities ± SEM are given as cells/mm2, n = 5 in each case, objective ×40)
| Groups | Total number of cells | Number of proliferating (mitosis) cells |
|---|---|---|
| DMSO-control (2 μl/mL) | 81.00 ± 2.81 | 5.40 ± 0.45 |
| TECA (C. asiatica extract) | ||
| 0.5 (μg/mL) | 99.80 ± 2.03p<0.001 | 8.70 ± 0.68p<0.01 |
| 1 (μg/mL) | 106.40 ± 0.92p<0.001 | 6.50 ± 0.45 |
| 5 (μg/mL) | 106.20 ± 0.58p<0.001 | 6.20 ± 0.46 |
| 10 (μg/mL) | 99.00 ± 1.34p<0.001 | 5.20 ± 0.32 |
| Cs-I | ||
| 0.5 (μg/mL) | 96.80 ± 3.65p<0.01 | 7.80 ± 0.69 |
| 1 (μg/mL) | 98.60 ± 2.63p<0.01 | 8.40 ± 0.63p<0.05 |
| 5 (μg/mL) | 94.20 ± 2.95p<0.05 | 9.00 ± 0.55p<0.001 |
| 10 (μg/mL) | 87.80 ± 2.59 | 3.50 ± 0.40 |
| Cs-II | ||
| 0.5 (μg/mL) | 103.40 ± 2.83p<0.001 | 8.80 ± 0.84p<0.01 |
| 1 (μg/mL) | 96.00 ± 2.04p<0.001 | 7.60 ± 0.60 |
| 5 (μg/mL) | 100.00 ± 2.77p<0.001 | 6.70 ± 0.53 |
| 10 (μg/mL) | 103.20 ± 3.62p<0.001 | 4.80 ± 0.46 |
* p < 0.05; ** p < 0.01 and *** p < 0.001 were considered to be significant compared to DMSO-control
Table 5.
Effects of Cs extracts and TECA on percentages of NIH3T3 fibroblasts in fusiform and polygonal shape
| Groups | Fusiform cells (%) | Polygonal cells (%) |
|---|---|---|
| DMSO-control (2 μl/mL) | 30.86 ± 1.12 | 55.73 ± 1.85 |
| TECA (C. Asiatica) | ||
| 0.5 (μg/mL) | 17.88 ± 0.85p<0.001 | 69.12 ± 0.52p<0.001 |
| 1 (μg/mL) | 17.10 ± 0.94p<0.001 | 65.79 ± 1.31p<0.001 |
| 5 (μg/mL) | 19.20 ± 0.83p<0.001 | 62.72 ± 1.64 |
| 10 (μg/mL) | 13.75 ± 1.11p<0.001 | 65.26 ± 0.97p<0.01 |
| Cs-I | ||
| 0.5 (μg/mL) | 12.85 ± 1.95p<0.001 | 73.24 ± 2.05p<0.001 |
| 1 (μg/mL) | 13.91 ± 1.32p<0.001 | 75.21 ± 1.70p<0.001 |
| 5 (μg/mL) | 19.73 ± 0.87p<0.001 | 63.54 ± 0.63p<0.05 |
| 10 (μg/mL) | 19.12 ± 0.84p<0.001 | 61.33 ± 1.42 |
| Cs-II | ||
| 0.5 (μg/mL) | 17.72 ± 1.37p<0.001 | 69.50 ± 1.41p<0.001 |
| 1 (μg/mL) | 20.21 ± 0.62p<0.001 | 67.35 ± 1.36p<0.001 |
| 5 (μg/mL) | 16.13 ± 1.25p<0.001 | 68.69 ± 1.68p<0.001 |
| 10 (μg/mL) | 18.00 ± 1.49p<0.001 | 66.38 ± 1.83p<0.001 |
(Data are mean ± SEM are given as cells/mm2, n = 5 in each case, objective X40)
Statistical significance * p < 0.05; ** p < 0.01 and *** p < 0.001 were considered to be significant compared to DMSO-control group
Table 6.
Effects of Cs extracts and TECA on percentages of NIH3T3 fibroblasts in round shape and vacuole containing cells and the number of collagen granules in NIH3T3 fibroblasts
| Groups | Round cells (%) | Vacuole containing cells (%) | Number of granules (%) |
|---|---|---|---|
| DMSO-Control (2 μl/mL) | 2.20 ± 0.43 | 11.18 ± 1.12 | 1.60 ± 0.13 |
| TECA (C. Asiatica) | |||
| 0.5 μg/mL | 1.78 ± 0.34 | 11.20 ± 0.75 | 1.70 ± 0.19 |
| 1 μg/mL | 2.07 ± 0.36 | 15.03 ± 0.81 | 1.55 ± 0.18 |
| 5 μg/mL | 2.82 ± 0.29 | 15.24 ± 0.91 | 1.50 ± 0.15 |
| 10 μg/mL | 3.62 ± 0.21 | 17.35 ± 0.45p<0.01 | 1.30 ± 0.10 |
| Cs-I | |||
| 0.5 μg/mL | 4.40 ± 0.50 | 9.59 ± 0.79 | 1.65 ± 0.1 |
| 1 μg/mL | 2.05 ± 0.34 | 8.81 ± 1.09 | 1.70 ± 0.19 |
| 5 μg/mL | 4.91 ± 0.35p<0.05 | 11.80 ± 0.96 | 1.50 ± 0.13 |
| 10 μg/mL | 7.46 ± 0.55p<0.001 | 12.07 ± 0.57 | 1.47 ± 0.15 |
| Cs-II | |||
| 0.5 μg/mL | 2.68 ± 0.29 | 10.08 ± 0.92 | 2.25 ± 0.20 |
| 1 μg/mL | 1.66 ± 0.25 | 10.76 ± 1.07 | 1.75 ± 0.14 |
| 5 μg/mL | 3.00 ± 0.31 | 12.16 ± 1.39 | 1.60 ± 0.15 |
| 10 μg/mL | 2.87 ± 0.37 | 12.73 ± 0.59 | 1.65 ± 0.13 |
(Data are mean ± SEM are given as cells/mm2, round shape and vacuole containing cells, n = 5 in each case; the number of collagen granules, n = 10 in each case)
Statistical significance * p < 0.05; ** p < 0.01 and *** p < 0.001 were considered to be significant compared to DMSO-Control group
Discussion and conclusion
Mostly methanol is used for extraction various polar compounds but certain groups of non polar compounds are fairly soluble in methanol if not readily soluble. Therefore methanol is commonly used for extraction of bioactive compounds. Phenolic compounds in tea have been found to be efficient free-radical scavengers (Higdon and Frei 2003).Various extraction methods affect the extraction yield and antioxidant activity of tea extracts. Some studies on the extraction conditions of active antioxidant components have been reported (Pan et al. 2003). Enzymatic browning is a common phenomenon observed upon harvesting, cutting, bruising or storage of fruits and vegetables. The enzyme responsible for this browning is polyphenol oxidase (PPO), a copper containing enzyme that is also present in tea leaves (Halder et al. 1998). The three major classes of teas are known as green, black and oolong. If the enzymes are allowed to act, they turn the green leaf black in much the same way that a freshly cut apple blackens. If the enzymes in the leaf are inactivated by heat, an in blanching, then the leaf remains green. If a partial oxidation is allowed to occur by delayed heating, then an intermediate tea of the oolong type is obtained (Potter and Hotchkiss 1998). The purpose of this work was to investigate the effects of two methanol extraction methods on the antioxidant activity and associated with anticancer and wound healing effects. Unlike extract from Cs-II, in the Cs-I extraction, the leaves of fresh C. sinensis were steamed at 100–110 °C for 3 min to inactivate all oxidation enzymes, mainly the polyphenol oxidase enzyme.
Plants rich in polyphenolic compounds are widely used for wound healing as well as anti-aging effects and in the treatment of some diseases. The total content of polyphenols in tea leaves vary from approximately 20–40%, depending on the subspecies of the plant and geographic location (Stephen 2005; Eric et al. 2011). In this study, the extraction yields of Cs-I and Cs-II were 36.75% (wt/wt) and 21.60%, and total phenolic contents were 360.70 and 357.81 mg of gallic acid equivalents per gram of extracts (dry weight), respectively. Also, Cs methanol extracts showed the highest DPPH radical scavenging ability similar to BHT. According to β-carotene-linoleic acid assay, Cs-I and Cs-II oxidized most rapidly with % inhibition values of 67.77 ± 2.35 and 71.07 ± 2.06, respectively. Furthermore, both Cs extracts showed the same antioxidant activities according to the results of DPPH radical scavenging and β-carotene-linoleic acid assay. Khokhar and Magnusdottir (2002) reported higher phenolic content levels for green tea (65.8–106.2 mg/g). Also, the level of total phenols in different teas are very variable and that comparison of data on different teas can be insufficient to conclude that a certain type of tea is rich or poor in total phenols (Khokhar and Magnusdottir 2002). Tea polyphenols are well-known for their antioxidant properties. Studies have shown that the strong antioxidant properties of green tea are attributed to catechins of Epigallocatechin gallate (EGCG) and Epigallocatechin (EGC) (Nanjo et al. 1996; Horžic et al. 2009; Chan et al. 2007). Although green tea has higher total phenolic content and free radical scavenging activity (Chan et al. 2010).
According to the results of experimental studies using in vitro cells and in vivo animal models, green tea protects against many cancer types, such as prostate, breast and skin cancer, and especially against colon, stomach, esophageal and lung cancer (Nihal et al. 2005). However, effects of green tea on bone cancer have not been studied extensively. In this study, anti-cancer effects of Cs methanol extracts on U2OS osteosarcoma cells were determined. In particular, with increasing concentrations of the extracts, the U2OS cell viabilty decreased.
At the end of 24 h of incubation, mitochondrial activity significantly decreased compared to the control group with higher concentrations of the extracts. The cytotoxic effects and apoptotic effects were increased in U2OS cells depending on the increasing concentrations. The maximum apoptotic effect was observed with 60 μg/mL extract concentration. Green tea was reported to have anti-carcinogenic and antioxidant effects in many studies conducted during previous years. Ahmad et al. (1997) stated that EGCG had an apoptotic effect only on malignant tumor cells. In the study by Nihal et al. (2005) EGCG caused significantly induction of cell cycle arrest and apoptosis of melanoma cells (Nihal et al. 2005).
Yang et al. (1998) stated that different tea polyphenols caused inhibitory effects of tea extracts on cell growth. It was reported that the green tea polyphenols inhibited in the A431 epidermoid carcinoma cells (Lin et al. 1999) and in human PC-9 lung cancer cells (Fujiki et al. 2000) Scientists who have aimed to protect the skin more effectively using herbal compounds have suggested that green tea could be used in cutaneous diseases and wounds due to its polyphenolic ingredient. Moreover, it was reported that the strong antioxidant polyphenols of green tea destroyed ROS, inhibited COX-2, phase-1 and-2 enzymes, inflammation-dependent pathways, suppressed the caspase-3-mediated apoptosis pathway in tumor cells (Hsu et al. 2005).
As far as we know, this is the first study that investigated the in vitro wound healing effect of Cs extracts using Masson’s Trichrome staining method on NIH3T3 cells. In general, there are reports of animal tests in the literature about the wound healing mechanism of green tea and polyphenols. In parallel with the results, in studies carried out with experimental animals, green tea has been reported to have a wound healing effect (Kapoor et al. 2004; Hajiaghaalipour et al. 2013). Also, it was reported that topical application to rats of the tea extract at a high concentration (200 mg/mL) significantly increased the fibroblast growth, collagen synthesis, and thus the healing process by accelerating the rate of wound healing (Hajiaghaalipour et al. 2013). Kapoor et al. (2004) investigated the healing effects of epigallocatechin gallate on cut wounds of rats. In a study by Kim et al. (2008) investigating the effect of epigallocatechin gallate in diabetic rat wounds, the authors reported that induction of wound contraction, re-epithelization, angiogenesis and granulation tissue organization were related to epigallocatechin gallate’s myofibroblast triggering activity. Hsu et al. (2005) demonstrated that green tea has been reported to aid the skin healing process by stimulating old keratinocytes and inhibiting apoptosis. In another study by Hsu et al. (2003), it was reported that green tea polyphenols stimulated the regeneration of keratinocytes in the aging skin. It was explained that green tea polyphenols stimulate keratinocyte differentiation in the basal layer of the epidermis and concluded that green tea components could be beneficial for wound healing, skin regeneration and some cutaneous diseases. Fu et al. (2000) reported that green tea polyphenols increased keratinocyte proliferation in rat keratinocytes. They reported that there was a connection between the protective effects of green tea polyphenols and the increasing effect of the lipid peroxidation product, the increasing effect of glutathione peroxidase levels, and the antioxidant properties. In another study, green tea extract was reported to significantly inhibit collagen production (Zhang et al. 2006). When taking into consideration the fact that polyphenol amount may vary depending on environmental factors such as genetic structure, climate, temperature, light, nutrition and age of the leaf and the effect of catechin groups may differ with their amount in tea, it is possible to find different results on the wound healing effect of green tea (Tosun and Karadeniz 2005). Especially polygonal cell numbers indicate that the migration ability of cells have increased. Migration is very important for wound healing and it has been reported in previous studies that cells should be polygonal and not fusiform for migration and cell movements. Based on the results of this study and the previous in vivo studies, we can state that Cs extracts have positive effects on wound healing.
As known, Cs has anti-oxidant and anti-inflammatory properties. These properties may explain the correlation between its significant anticancer effects and wound healing potential. In this study, important wound healing, apoptotic and cytotoxic effects of two different Cs methanol extracts were determined. Especially, wound healing effects on NIH3T3 fibroblast cells of low concentrations of the Cs extracts were found to be more effective. Cs polyphenols have been shown to possess cancer preventive effects in many cell lines. However, effects of green tea on bone cancer have not been studied extensively. Here, we found that Cs methanol extracts have an important anti-proliferative potential on U2OS osteosarcoma cells. Cytotoxic and apoptotic effects on U2OS cells were significantly increased depending on the extract concentrations. According to our data, especially Cs-I extract showed further apoptotic effects and important wound healing potential.
Acknowledgements
This study is a part of master’s thesis of Sinem Er, which was conducted under the advisor Miriş Dikmen. All authors have read and approved the final version of the manuscript. Thanks for help in preparing extracts and antioxidant analysis to Prof. Dr. Nilgün Öztürk. The U2OS cell line was obtained from Prof. Dr. Hülya Sivas and Prof. Dr. Zerrin İncesu, and TECA (C. asiatica extract) from Bayer (Turkey). The flow cytometer analysis of the study was performed at Anadolu University Medicinal Plants, Drugs and Scientific Research Center.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Agar OT, Dikmen M, Ozturk N, Yilmaz MA, Temel H, Turkmenoglu FP. Comparative studies on phenolic composition, antioxidant, wound healing and cytotoxic activities of selected Achillea L. species growing in Turkey. Molecules. 2015;20:17976–18000. doi: 10.3390/molecules201017976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad N, Feyes DK, Nieminen AL, Agarwal R, Mukhtar H. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J Natl Cancer Inst. 1997;89(24):1881–1886. doi: 10.1093/jnci/89.24.1881. [DOI] [PubMed] [Google Scholar]
- Brown MD. Green tea (Camellia sinensis) extract and its possible role in the prevention of cancer. Altern Med Rev. 1999;4:5. [PubMed] [Google Scholar]
- Chan EW, Lim YY, Chew YL. Antioxidant activity of Camellia sinensis leaves and tea from a lowland plantation in Malaysia. Food Chem. 2007;102:1214–1222. doi: 10.1016/j.foodchem.2006.07.009. [DOI] [Google Scholar]
- Chan EW, Lim YY, Chong KL, Tan JBL, Wong SK. Antioxidant properties of tropical and temperate herbal teas. J Food Compos Anal. 2010;23:185–189. doi: 10.1016/j.jfca.2009.10.002. [DOI] [Google Scholar]
- Chen D, Dou QP. Tea polyphenols and their roles in cancer prevention and chemotherapy. Int J Mol Sci. 2008;9:1196–1206. doi: 10.3390/ijms9071196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YS, Lee SM, Lin YJ, Chiang SH, Lin CC. Effects of danshensu and salvianolic Acid B from Salvia miltiorrhiza Bunge (Lamiaceae) on cell proliferation and collagen and melanin production. Molecules. 2014;19:2029–2041. doi: 10.3390/molecules19022029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikmen M, Ozturk N, Ozturk Y. The antioxidant potency of Punica granatum L. Fruit peel reduces cell proliferation and induces apoptosis on breast cancer. J Med Food. 2011;14:1638–1646. doi: 10.1089/jmf.2011.0062. [DOI] [PubMed] [Google Scholar]
- Dikmen M, Canturk Z, Ozturk Y, Tunalı Y. Investigation of the apoptotic effect of curcumin in human leukemia HL-60 cells by using flow cytometry. Cancer Biother Radiopharm. 2011;25:749–755. doi: 10.1089/cbr.2010.0822. [DOI] [PubMed] [Google Scholar]
- Dikmen M, Öztürk Y, Sagratini G, Ricciutelli M, Vittori S, Maggi F. Evaluation of the wound healing potentials of two subspecies of Hypericum perforatum on cultured NIH3T3 fibroblasts. Phytother Res. 2011;25:208–214. doi: 10.1002/ptr.3243. [DOI] [PubMed] [Google Scholar]
- Eric WC, Chan EYS, Pei PT, Yon PL. Antioxidant and antibacterial properties of green, black, and herbal teas of Camellia sinensis. Pharmacognosy Res. 2011;3:266–272. doi: 10.4103/0974-8490.89748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisunoğlu M, Besler HT (2008) Çay ve Sağlık İlişkisi. Hacettepe Üniversitesi, Sağlık Bilimleri Fakültesi Beslenme ve Diyatetik Bölümü, Ankara. Sağlık Bakanlığı Yayın, 727:18
- Folin O, Ciocalteu V. On tyrosine and tryptophane determinations in proteins. J Biol Chem. 1928;73:627–650. [Google Scholar]
- Fu YC, Jin XP, Wei SM. The effects on cell growth of tea polyphenols acting as a strong anti-peroxidatant and an inhibitor of apoptosis in primary cultured rat skin cells. Biomed Environ Sci. 2000;13:170–179. [PubMed] [Google Scholar]
- Fujiki H, Suganuma M, Okabe S. A new concept of tumor promotion by tumor necrosis factor-α and cancer preventive agents (-)-epigallocatechin gallate and gren tea. Cancer Detect Prev. 2000;24:91–99. [PubMed] [Google Scholar]
- Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajiaghaalipour F, Kanthimathi MS, Abdulla MA, Sanusi J (2013) The effect of Camellia sinensis on wound healing potential in an animal model. Evid Based Complement Alternat Med 386734 [DOI] [PMC free article] [PubMed]
- Halder J, Tamuli P, Bhaduri AN. Isolation and characterization of polyphenol oxidase from Indian tea leaf (Camellia sinensis) J Nutr Biochem. 1998;9:75–80. doi: 10.1016/S0955-2863(97)00170-8. [DOI] [Google Scholar]
- Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- Horžić D, Komes D, Belscak A, Ganic KK, Ivekovic D, Karlovic D. The composition of polyphenols and methylxanthines in teas and herbal infusions. Food Chem. 2009;115:441–448. doi: 10.1016/j.foodchem.2008.12.022. [DOI] [Google Scholar]
- Hsu S, Bollag WB, Lewıs J, Huang Q, Sıngh B, Sharawy M, Yamamoto T, Schuster G. Green tea polyphenols induce differentiation and proliferation in epidermal keratinocytes. J Pharmacol Exp Ther. 2003;306:29–34. doi: 10.1124/jpet.103.049734. [DOI] [PubMed] [Google Scholar]
- Hsu S, Yamamoto T, Borke J, Walsh DS, Singh B, Rao S. Green tea polyphenol induced epithelial cell terminal differentiation is associated with coordinated expression of p57/KIP2 and caspase 14. J Pharmacol Exp Ther. 2005;312:884–890. doi: 10.1124/jpet.104.076075. [DOI] [PubMed] [Google Scholar]
- Inoue M, Tajima K, Mizutani M. Regular consumption of green tea and the risk of breast cancer recurrence, follow-up study from the Hospital-based Epidemiologic Research Program at Aichi Cancer Center (HERPACC), Japan. Cancer Lett. 2001;167:175–182. doi: 10.1016/S0304-3835(01)00486-4. [DOI] [PubMed] [Google Scholar]
- Kapoor M, Howard R, Hall I, Appleton I. Effects of epicatechin gallate on wound healing and scar formation in a full thickness incisional wound healing model in rats. Am J Pathol. 2004;165:1. doi: 10.1016/S0002-9440(10)63297-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katiyar SK, Ahmad N, Mukhtar H. Green tea and skin. Arch Dermatol. 2000;136:989–994. doi: 10.1001/archderm.136.8.989. [DOI] [PubMed] [Google Scholar]
- Kelly GS. Centella asiatica. Altern Med Rev. 2007;12:69–72. [PubMed] [Google Scholar]
- Khokhar S, Magnusdottır SGM. Total phenol, catechin, and caffeine contents of teas commonly consumed in the United Kingdom. J Agric Food Chem. 2002;50:565–570. doi: 10.1021/jf010153l. [DOI] [PubMed] [Google Scholar]
- Kim H, Kawazoe T, Han DW, Matsumara K, Suzuki S, Tsutsumi S, Hyon SH. Enhanced wound healing by an epigallocatechin gallate-incorporated collagen sponge in diabetic mice. Wound Rep Reg. 2008;16:714–720. doi: 10.1111/j.1524-475X.2008.00422.x. [DOI] [PubMed] [Google Scholar]
- Koo MWL, Cho CH. Pharmacological effects of green tea on the gastrointestinal system. Eur J Pharmacol. 2004;500:177–185. doi: 10.1016/j.ejphar.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Korkmaz S, Zeytinoglu H, Zeytinoglu M, Aydın S, Öztürk Y, Baser KHC. Testing the wound-healing activity in t15 fibroblast culture: a morphometric analysis. Altern Lab Anim. 2000;28:41–51. doi: 10.1177/026119290002800107. [DOI] [PubMed] [Google Scholar]
- Lin JK, Liang YC, Lin-Shiau SY. Cancer chemoprevention by tea polyphenols through mitotic signal transduction blockade. Biochem Pharmacol. 1999;58:911–915. doi: 10.1016/S0006-2952(99)00112-4. [DOI] [PubMed] [Google Scholar]
- Maquart FX, Bellon G, Gillery P, Wegrowski Y, Borel JP. Stimulation of collagen synthesis in fibroblast cultures by triterpene extracted from Centella asiatica. Connect Tissue Res. 1990;24:107–120. doi: 10.3109/03008209009152427. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nanjo F, Goto K, Seto R, Suzuki M, Sakai M, Hara Y. Scavenging effects of tea catechins and their derivatives on 1,1-diphenyl-2-picrylhydrazyl radical. Free Radic Biol Med. 1996;21:895–902. doi: 10.1016/0891-5849(96)00237-7. [DOI] [PubMed] [Google Scholar]
- Nihal M, Ahmad N, Mukhtar H, Wood GS. Anti-proliferative and proapoptotic effects of (-)-epigallocatechin-3-gallate on human melanoma: possible implications for the chemoprevention of melanoma. Int J Cancer. 2005;114:513–521. doi: 10.1002/ijc.20785. [DOI] [PubMed] [Google Scholar]
- Nursal TZ, Baykal A, Hamaloğlu E. Yaşlılarda Yara İyileşmesi: fark var mı? Turk J Geriatr. 1999;2:29–32. [Google Scholar]
- Oztürk N, Tunçel M, Potoğlu-Erkara İ. Phenolic compounds and antioxidant activities of some Hypericum ssp. : a comparative study with H. Perforatum. Pharm Biol. 2009;47:120–127. doi: 10.1080/13880200802437073. [DOI] [Google Scholar]
- Öztürk N, Korkmaz S, Öztürk Y. Wound-healing activity of St. John’s Wort (Hypericum perforatum L.) on chicken embryonic fibroblasts. J Ethnopharmacol. 2007;111:33–39. doi: 10.1016/j.jep.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Pan X, Niu G, Liu H. Microwave-assisted extraction of tea polyphenols and tea caffeine from green tea leaves. Chem Eng Process. 2003;42:129–133. doi: 10.1016/S0255-2701(02)00037-5. [DOI] [Google Scholar]
- Potter NN, Hotchkiss J. Leafe processing. Kluwer Academic/Plenum: Food Science; 1998. p. 460. [Google Scholar]
- Sahin H, Özdemir F. Yeşil Çayın Sağlık Üzerine Etkisi, Türkiye 9. Bolu: Gıda Kongresi; 2006. pp. 219–222. [Google Scholar]
- Sanchez-Moreno C, Larrauri J, Saura-Calixto F. A procedure to measure the antiradical efficiency of polyphenols. J Sci Food Agric. 1998;76:270–276. doi: 10.1002/(SICI)1097-0010(199802)76:2<270::AID-JSFA945>3.0.CO;2-9. [DOI] [Google Scholar]
- Shahsavari N, Barzegar M, Sahari MA, Naghdibadi H. Antioxidant activity and chemical characterization of essential oil of Bunium persicum. Plant Foods Hum Nutr. 2008;63:183–188. doi: 10.1007/s11130-008-0091-y. [DOI] [PubMed] [Google Scholar]
- Sharangi AB. Medicinal and therapeutic potentialities of tea (Camellia sinensis L.) Food Res Int. 2009;42:529–535. doi: 10.1016/j.foodres.2009.01.007. [DOI] [Google Scholar]
- Stephen H. Green tea and the skin. J Am Acad Dermatol. 2005;52:1049–1059. doi: 10.1016/j.jaad.2004.12.044. [DOI] [PubMed] [Google Scholar]
- Tosun İ, Karadeniz B. Çay ve Çay Fenoliklerinin Antioksidan Aktivitesi. OMÜ Zir Fak. Dergisi. 2005;20:78–83. [Google Scholar]
- Weisburger JH. Tea and health: a historical perspective. Cancer Lett. 1997;114:315–317. doi: 10.1016/S0304-3835(97)04691-0. [DOI] [PubMed] [Google Scholar]
- Yang GY, Liao J, Kim K, Yurkow EJ, Yang CS. Inhibition of growth and induction of apoptosis in human cancer cell lines by tea polyphenols. Carcinogenesis. 1998;19:611–616. doi: 10.1093/carcin/19.4.611. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Kelly AP, Wang L, French SW, Tang X, Duong HS, Messadi DV, Le AD. Green tea extract and (-)-epigallocatechin-3-gallate inhibit mast cell-stimulated type I collagen expression in keloid fibroblasts via blocking pi-3 k/akt signaling pathways. J Investig Dermatol. 2006;126:2607–2613. doi: 10.1038/sj.jid.5700472. [DOI] [PubMed] [Google Scholar]