Abstract
Hepatoma cells are a candidate cell source for bio-artificial livers. However, they exhibit reduced liver functions compared with primary hepatocytes. In our previous study, genetically engineered mouse hepatoma cells were created by transduction with vectors mediating inducible overexpression of eight liver-enriched transcription factors. Upon the induction of the liver-enriched transcription factors transduced, the cells expressed both phenotypic and genotypic liver functions at high levels. In the present study, we performed three-dimensional culture of these cells using macroporous gelatin beads. When immobilized on the macroporous gelatin beads, these cells exhibited further enhancement in liver functionality, including increased albumin secretion, ammonia removal and cytochrome P450 activity. The levels of these functions were significantly enhanced compared to monolayer culture. The method is simple and scalable, and provides highly functional cells that can be used in basic and applied fields of hepatic research.
Keywords: Hepatoma cells, Cell immobilization, Macroporous gelatin beads, Liver function
Introduction
The liver is the largest and one of the most vital organs, responsible for important metabolic functions and eliminating toxic substances from the human body (Allen et al. 2001). In cases of liver failure, both acute and chronic, the only known curative procedure is liver transplantation. While survival rates after transplantation have increased over the years, the shortage of donor organs has become a considerable problem in the medical world. Alternatives to whole liver transplantation are being investigated, in the hope of alleviating donor organ shortages. Some of these alternatives include cell transplantation, implants, transgenic xenotransplantation, and bio-artificial liver devices (Chamuleau et al. 2005). Bio-artificial liver devices (BALs) are generally extracorporeal support systems that incorporate functional hepatic cells into bioreactors. The choice of cellular components in BALs is an important issue, as hepatic cells should undertake a wide array of functions. The bioreactor design also needs careful consideration, to provide for efficient transport of nutrients, an environment that allows for cell–cell interaction, and to be scalable to an effective size for therapy.
Primary hepatocytes are considered the ideal cellular component for BALs, but like donor organs, are in limited supply. Additionally, current protocols are unable to maintain the high level of metabolic function of primary human hepatocytes for more than few hours (Kidambi et al. 2009). Primary hepatocytes can be passaged when exposed to certain small-molecules, but current methods do not allow serial passage or long-term storage (Shan et al. 2013). This inability to be cultured for long periods has led to the investigation of alternative cell sources. The differentiation of hepatic cells from induced pluripotent stem cells and embryonic stem cells has made significant progress in recent years, but the resulting cells tend to have low metabolic and detoxification capabilities (Si-Tayeb et al. 2010). Hepatoma cells, or cells derived from liver carcinoma, are considered to be alternative cell sources. Hepatoma cell lines, such as HepG2 and Huh7, are readily available and simple to culture. However, such cell lines generally have low expression and function of cytochrome P450, which is responsible for drug metabolism. In a previous study (Yamamoto et al. 2012), we used overexpression of liver-enriched transcription factors (LETFs) to develop genetically engineered hepatoma cells that exhibit inducible liver functions, named Hepa/8F5 cells. The genes encoding the eight chosen LETFs, hepatocyte nuclear factor (HNF)-1α, HNF-1β, HNF-3β (FOXA2), HNF-4α, HNF-6, CCAAT/enhancer binding protein (C/EBP)-α, C/EBP-β and C/EBP-γ, were transduced into this hepatoma cell line as drug-inducible expression cassettes. The hepatic functionality of Hepa/8F5 cells was further enhanced by culturing them as spheroids. However, the creation and culture of spheroids takes considerable time, and is difficult to scale up to the cell mass required for BALs. This would limit the available number of spheroids, and their liver function capabilities. In this study, three-dimensional structures were formed by cells attached to the surface of macro-porous gelatin beads. This allowed for increased expression of liver functions in a manageable time frame, and could be easily scaled up.
Materials and methods
Cell culture
Hepa/8F5 cells were grown in high-glucose Dulbecco’s modified Eagle’s medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal bovine serum, 0.1 mg/mL streptomycin sulfate and 100 U/mL penicillin G potassium (Wako Pure Chemical Industries, Osaka, Japan). Cells were cultured at 37 °C in a 5% (v/v) CO2 humidified incubator.
Macroporous gelatin bead preparation
Commercially available macroporous gelatin beads (Cultispher-S; GE Healthcare Bio-sciences, Pittsburgh, PA, USA) were used for immobilization of Hepa/8F5 cells. Gelatin beads were prepared by rehydrating 0.5 g of Cultispher-S powder with 25 mL of phosphate-buffered saline (PBS). The beads were then autoclaved at 121 °C for 20 min, which allowed the beads to form a precipitate. These beads were then transferred to a 50-mL centrifuge tube containing 50 mL PBS (0.01 g/mL). The resulting concentration of beads was approximately 5525 beads/mL. In 24-well plates, beads concentration per well was approximately 276 beads/mL per well. The beads were stored at 4 °C before use in cell culture.
Attachment of cells to beads
Hepa/8F5 cells were statically cultured in monolayer for 3 days in 10 mL medium with doxycycline (Dox; 0.1 µg/mL) in 100-mm cell culture dishes (Thermo Fisher Scientific, Rochester, NY, USA). Rehydrated macroporous gelatin beads were suspended with Hepa/8F5 cells in a 50-mL centrifuge tube with 10 mL cell medium. The tube was immersed in a constant-temperature bath of 37 °C with gentle stirring every 30 min, to ensure cell adhesion to the beads. After 3 h of culture, 24-well low-attachment tissue culture plates (Corning, NY, USA) were seeded with the suspension of cells and beads (approximately 276 beads/mL), and subjected to several days of culture.
Observation of cells on beads
Cells immobilized on beads were cultured in the presence of Dox (0.1 µg/mL). On days 1, 6 and 11, beads were observed under a BZ-9000 fluorescence microscope (Keyence, Osaka, Japan). Living cells display a green fluorescence because their transgene cassettes drive expression of enhanced green fluorescent protein (EGFP). For observation of sections of cell-immobilized beads, beads were washed with PBS, fixed in 4% paraformaldehyde and embedded in paraffin. Bead sections were prepared by slicing at a thickness of 4 µm, and staining with hematoxylin and eosin (H&E). The sections were observed under a BZ-9000 microscope (Keyence, Tokyo, Japan).
Liver function analyses
Cells immobilized on beads were cultured in the presence of Dox (0.1 µg/mL). On the final day of culture, the medium was collected as a sample and used to measure liver functions, including albumin secretion, ammonia removal and cytochrome P450 (CYP3A) activity, as previously described (Yamamoto et al. 2012). These measurement values were normalized to the numbers of cells present, which were calculated from the DNA content in the constructs. The amounts of DNA in the beads were evaluated from lysates of cell-bearing beads prepared by sonication, using a commercially available DNA measurement kit (QuantiFluor dsDNA system; Promega, Madison, WI, USA).
Results
Cell attachment to macroporous gelatin beads
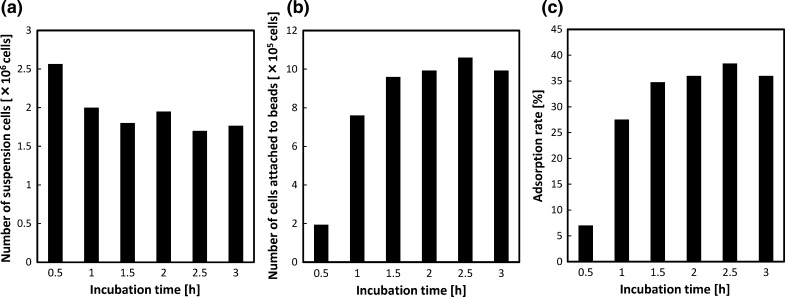
To quantify the adsorption of Hepa/8F5 cells to beads, they were incubated together at a ratio of approximately 100 cells per bead. Samples were removed at 0.5 h intervals, and the number of cells suspended in the medium and immobilized on beads was measured by quantifying the amounts of DNA from lysates of cell-bearing beads prepared by sonication, or from cells suspended in media (Fig. 1a, b). The maximum adsorption of cells to beads was 38.4% after 2.5 h incubation (Fig. 1c). However, the difference in cell attachment between 2 h and 3 h incubation was not significant. Therefore, for further experiments, cells were immobilized on beads by incubating them together for 3 h.
Fig. 1.
Adsorption of Hepa/8F5 cells onto macroporous gelatin beads. a Number of cells remaining in suspension following incubation with the beads for various times. b Number of cells attached to the beads after incubation. c Proportion of adsorbed cells at various time points
Cell seeding density and functionalization on beads
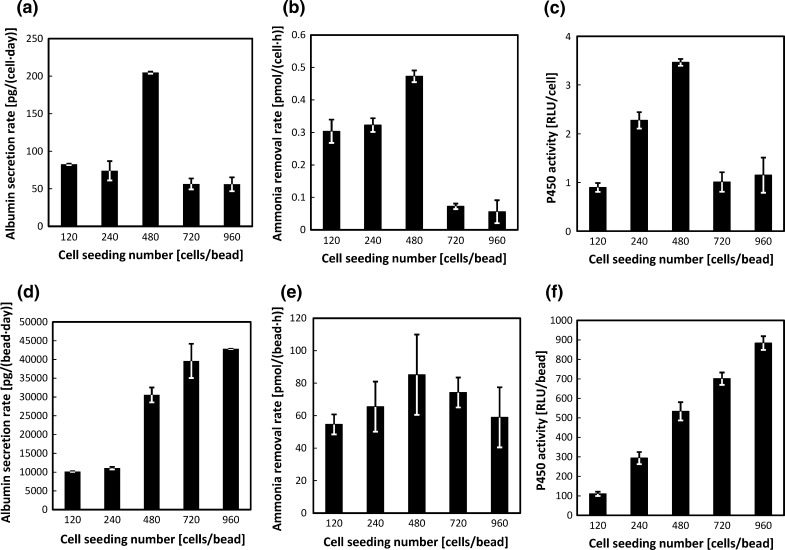
Next, we determined the optimal seeding density of cells per bead. Beads and cells were incubated together for 3 h at ratios of 120, 240, 480, 720 or 960 cells per bead. The resulting Hepa/8F5 cell-bearing beads were cultured for 5 days in the presence of Dox, to induce transgene expression. On day 5, average total cell numbers for cell ratios of 120, 240, 480, 720 and 960 were 3.4 × 104 cells, 4.0 × 104 cells, 3.2 × 104 cells, 2.0 × 105 cells and 2.2 × 105 cells, respectively. Their liver functions were then measured. A seeding density of 480 cells/bead produced the highest levels of albumin secretion, ammonia removal, and CYP3A activity, as measured per cell (Fig. 2a–c). However, a seeding density of 960 cells/bead tended to produce higher levels of albumin secretion and CYP3A activity per bead (Fig. 2d, f). In contrast, the ammonia removal rate per bead was significantly greater for the 480 cells/bead samples (Fig. 2e). These results demonstrate that greater cell numbers enhanced detoxification and albumin production functions on a per bead basis, but reduced these functions on a per cell basis. The functional activities measured per cell compared with per bead showed that a seeding density of 480 cells/bead was optimal for enhancing the liver functionality of Hepa/8F5 cells.
Fig. 2.
Liver function analyses of Hepa/8F5 cell-bearing macroporous gelatin beads seeded with cells at different densities. Hepa/8F5 cells were seeded at ratios of 120, 240, 480, 720 or 960 cells per bead and cultured in the presence of Dox for 5 days. They were then analyzed for albumin secretion rates per cell (a), and per bead (d), for ammonia removal rates per cell (b), and per bead (e), and for CYP3A activity per cell (c), and per bead (f). The data shown are the means ± SD (n = 3)
Long-term culture and expression of liver functions
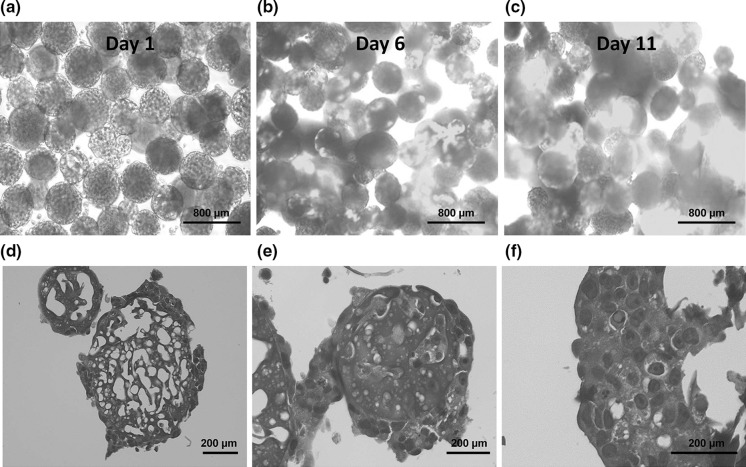
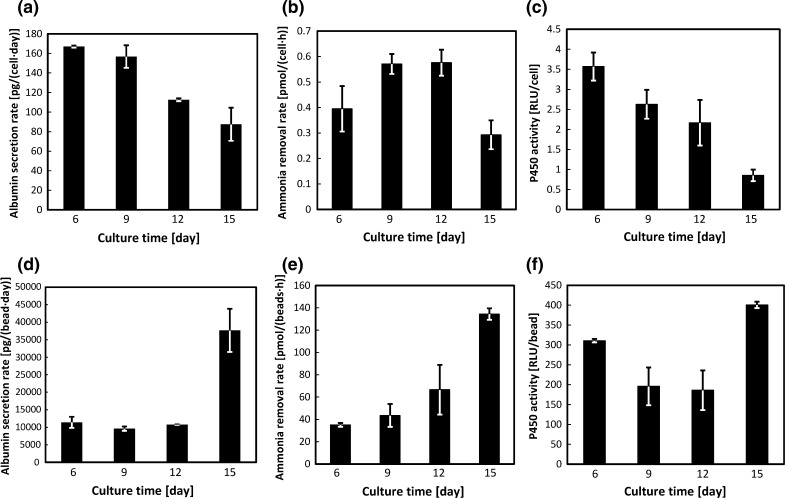
To test the longevity of their high expression of liver functions, cell-bearing beads were cultured in the presence of Dox and then harvested for functional analyses. Culture time was 15 continuous days with samples being harvested at day 6, 9, 12 and 15. Beads were observed to monitor cell necrosis (Fig. 3a–c). Judging from EGFP expression of bead-immobilized cells and later significant liver function per cell amount, the viability of bead-immobilized cells was relatively high. H&E staining of bead-immobilized cells taken at each time point also confirmed the development of tissue-like structures on beads. Large beads tended to be partially covered or encapsulated with cells (Fig. 3d). Smaller beads tended to have more cells able to penetrate the pores as well as embedded on the surface (Fig. 3e). The cells, which were successfully attached on beads, tended to form tissue-like structures (Fig. 3f). Average total cell numbers for day 6, 9, 12 and 15 were 2.1 × 104 cells, 1.7 × 104cells, 2.6 × 104 cells and 1.4 × 105 cells, respectively. Samples were then analyzed for albumin secretion, ammonia removal and CYP3A activity. The levels of liver functions per bead were maintained or enhanced over the 15 day-culture period. Sample measurements per bead showed that all functions had increased significantly at day 15 compared with day 6 (Fig. 4d–f). However, the levels of liver functions per cell gradually decreased with increasing culture time (Fig. 4a–c). Since cell density should increase with culture time, these results confirm the trend seen in the cell seeding density experiment, that beads with high cell density exhibited enhanced expression of liver functions, while the expression level per cell decreased.
Fig. 3.
Observation of Hepa/8F5 cells immobilized on macroporous gelatin beads during long-term culture. Cells were seeded at a density of 480 cells/bead at day 0 and cultured for 15 days in the presence of Dox. Cell-immobilized beads were observed using a fluorescence microscope on day 1 (a), 6 (b) and 11 (c) of culture. Scale bars are set to 800 µm. Thin-sections of cell-immobilized beads on day 11 were prepared and stained with H&E, and cells on the beads were observed (d–f). d presents a large and e presents a small bead. d A large bead covered with cells. e A small bead both covered and embedded with cells. f Formation of tissue-like structure on a bead. Scale bars are set to 200 µm
Fig. 4.
Liver function analyses of Hepa/8F5 cell-bearing macroporous gelatin beads during long term culture. Cells were seeded at a density of 480 cells/bead on day 0 and cultured for 15 days in the presence of Dox. They were then analyzed for albumin secretion rates per cell (a), and per bead (d), for ammonia removals rates per cell (b), and per bead (e), and for CYP3A activity per cell (c), and per bead (f). The data shown are the means ± SD (n = 3)
Discussion
In the liver, LETFs regulate genes related to liver function, and are generally expressed at high levels (Nagaki and Moriwaki 2008). In a previous study, we successfully established Hepa/8F5 cells, in which liver functions are enhanced when overexpression of eight LETF genes is induced by Dox. When Hepa/8F5 cells were cultured as spheroids in the presence of Dox for 5 days, higher levels of liver functions were seen compared with cells cultured as a monolayer (unpublished data). However, spontaneously formed spheroids took considerable time to produce, and it is not easy to prepare a large number of spheroids while controlling their size to avoid cell necrosis in their cores.
Therefore, in this study, we implemented three-dimensional culture of Hepa/8F5 cells by immobilizing them on macroporous gelatin beads. Hepatocytes and hepatoma cells have been previously reported to exhibit high levels of liver functions when maintained using three-dimensional culture techniques (Hamamoto et al. 1998; Chang and Hughes-Fulford 2009; Shan et al. 2013). Indeed, the Hepa/8F5 cells immobilized on beads in our present study showed increased albumin production, ammonia removal and cytochrome P450 activity. In comparison, monolayer culture exhibited a lower value of 99.9 ± 4.8 pg/(cell·day) albumin secretion, 0.147 ± 0.014 pmol/(cell·h) ammonia removal and 2.69 ± 0.08 RLU/cell CYP450 activity, on average (Table 1). These important liver functions reached levels comparable with those in spheroid cultures of the same cells (unpublished data). The enhanced liver functionality is likely due to the increased cell-to-cell interaction that takes place in cell aggregates (Fig. 3f), which does not occur in monolayer cultures.
Table 1.
Comparative representation of liver functions for Hepa/8F5 [Dox+] cultured in monolayer or with macroporous gelatin beads
| Cell type | Culture | Albumin secretion rate [pg/(cell·day)] | Ammonia removal rate [pmol/(cell·h)] | P450 (CYP3A) activity [RLU/cell] |
|---|---|---|---|---|
| Hepa/8F5 [Dox+] | Monolayer | 99.9 ± 4.8 | 0.147 ± 0.014 | 2.69 ± 0.08 |
| Beads | 107 ± 1 | 0.395 ± 0.089 | 3.57 ± 0.35 |
The data shown are the means ± SD (n = 3)
On the other hand, Hepa/8F5 cells seeded on gelatin beads at the highest density were unable to function as efficiently as those seeded at lower densities. This could be attributable to the poor surface area to volume ratio for highly cell-dense beads, with cells in the core of the bead producing more ammonia than can be removed. Furthermore, the reduced levels of liver functions per cell with increasing culture time could be due to unsuccessful induction of the cells with Dox, as the beads become over-confluent with cells. Thus, there appears to be an optimal cell density within the beads for maximizing the liver functionality of Hepa/8F5 cells.
Hepa/8F5 cells immobilized on beads could maintain high liver functionality for almost two weeks, and this culture system is easy to scale up as needed. To our knowledge, no studies outside of our own have successfully produced genetically modified hepatoma cells that inducibly express high liver functionality. This approach of combining genetic modification of hepatoma cells with an optimized culture system to induce high liver functionality could be applied to human hepatoma cell lines. Such genetically engineered hepatoma cells could be a new resource for hepatology research, and for the construction of novel bio-artificial liver systems.
Acknowledgements
This work was financially supported in part by Grants-in-Aid for Scientific Research (Nos. 23650289 and 26560216) from the Japan Society for the Promotion of Science (JSPS).
References
- Allen JW, Hassanein T, Bhatia SN. Advances in bioartificial liver devices. Hepatology. 2001;43:447–455. doi: 10.1053/jhep.2001.26753. [DOI] [PubMed] [Google Scholar]
- Chamuleau RA, Deurholt T, Hoekstra R. Which are the right cells to be used in the bioartificial liver? Metab Brain Dis. 2005;20:327–335. doi: 10.1007/s11011-005-7914-4. [DOI] [PubMed] [Google Scholar]
- Chang TT, Hughes-Fulford M. Monolayer and spheroid culture of human liver hepatocellular carcinoma cell line cells demonstrate distinct global gene expression patterns and functional phenotypes. Tissue Eng A. 2009;15:559–567. doi: 10.1089/ten.tea.2007.0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamamoto R, Yamada K, Kamihira M, Ijima S. Differentiation and proliferation of primary rat hepatocytes cultured as spheroids. J Biochem. 1998;124:972–979. doi: 10.1093/oxfordjournals.jbchem.a022215. [DOI] [PubMed] [Google Scholar]
- Kidambi S, Yarmush RS, Novik E, Chao P, Yarmush ML, Nahmias Y. Oxygen-mediated enhancement of primary hepatocyte metabolism, functional polarization, gene expression, and drug clearance. Proc Natl Acad Sci USA. 2009;106:15714–15719. doi: 10.1073/pnas.0906820106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaki M, Morikawa H. Transcription factor HNF and hepatocyte differentiation. Hepatol Res. 2008;38:961–969. doi: 10.1111/j.1872-034X.2008.00367.x. [DOI] [PubMed] [Google Scholar]
- Shan J, Schwartz RE, Ross NT, Logan DJ, Thomas D, Duncan SA, North TE, Goessling W, Carpenter AE, Bhatia SN. Identification of small molecules for human hepatocyte expansion and iPS differentiation. Nat Chem Biol. 2013;9:514–520. doi: 10.1038/nchembio.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si-Tayeb K, Noto FK, Nagaoka M, Li J, Battle MA, Duris C, North PE, Dalton S, Duncan SA. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2010;51:297–305. doi: 10.1002/hep.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Kawabe Y, Ito A, Kamihira M. Enhanced liver functions in mouse hepatoma cells by induced overexpression of liver-enriched transcription factors. Biochem Eng J. 2012;60:67–73. doi: 10.1016/j.bej.2011.10.004. [DOI] [Google Scholar]