Abstract
Seleno-short-chain chitosan (SSCC) is a synthesized chitosan derivative. In this study, antitumor activity and underlying mechanism of SSCC on human non-small-cell lung cancer A549 cells were investigated in vitro. The MTT assay showed that SSCC could inhibit cell viability in a dose- and time-dependent manner, and 200 μg/ml SSCC exhibited significantly toxic effects on A549 cells. The cell cycle assay showed that SSCC triggered S phase cell cycle arrest in a dose- and time-dependent manner, which was related to a downregulation of S phase associated cyclin A. The DAPI staining and Annexin V-FITC/PI double staining identified that the SSCC could induce A549 cells apoptosis. Further studies found that SSCC led to the generation of reactive oxygen species (ROS) and the disruption of mitochondrial membrane potential (MMP) by DCFH-DA and Rhodamin 123 staining, respectively. Meanwhile, free radical scavengers N-acetyl-l-cysteine (NAC) pretreatment confirmed that SSCC-induced A549 cells apoptosis was associated with ROS generation. Furthermore, real-time PCR and western blot assay showed that SSCC up-regulated Bax and down-regulated Bcl-2, subsequently incited the release of cytochrome c from mitochondria to cytoplasm, activated the increase of cleaved-caspase 3 and finally induced A549 cells apoptosis in vitro. In general, the present study demonstrated that SSCC induced A549 cells apoptosis via ROS-mediated mitochondrial apoptosis pathway.
Keywords: Seleno-short-chain chitosan, A549 cells, ROS, Mitochondrial apoptosis pathway
Introduction
Lung cancer is one of the most common malignant carcinomas in the world. Annually, about 1.8 million lung cancers are diagnosed and 1.6 million patients die from lung cancer worldwide. According to the cell type, lung cancer is categorized into non-small-cell lung cancer and small-cell lung cancer. Non-small-cell lung cancer (NSCLC) accounts for more than 80% of all lung cancers. Surgery, chemotherapy and radiotherapy are the most widely-used therapies for NSCLC, however, they are not always helpful and the clinic effects are unsatisfying (Sui et al. 2016). Currently, although chemotherapy has been regarded as one of the most effective therapies and some new antitumor drugs have been developed, lung cancer still has a low cure rate (Lin et al. 2016). Increasing researches showed that chemotherapeutic compounds have toxic effects on normal cells and might lead to multiple organ damage, and further deteriorate life quality of lung cancer patients (Rukkijakan et al. 2016). Therefore, it is urgent to search for novel and effective antitumor compounds with low toxicity for patients.
Apoptosis is programmed cell death process that consists of a cascade of molecular events in stimulated cells. The extrinsic death receptor pathway and the intrinsic mitochondrial pathway are two major apoptosis pathways (Lee and Hong 2010). Reactive oxygen species (ROS) is a family of active molecules containing superoxide anion radical, hydrogen peroxide, singlet oxygen and hydroxyl radical, which are mainly produced by mitochondria. Low levels of ROS act as a physiological regulator for the proliferation and differentiation of normal cells. However, excess intracellular ROS could cause oxidative damage to lipids, DNA and proteins via apoptosis (Mieyal et al. 2008). In detail, in apoptotic cells, over-expression of intracellular ROS damages respiratory chain and inhibits mitochondrial electron transport chain, which leads to the transition of mitochondrial permeability and the loss of mitochondrial membrane potential (MMP) (Zhou et al. 2013). Subsequently, mitochondrial permeability transition pores (PTP) open and cytochrome c (Cyt c) is released from mitochondria into cytosol. Finally, caspase-cascade system is activated and thereby induces tumor cell apoptosis (Hua et al. 2015). Recently, numerous researches showed that many antitumor compounds targeting ROS metabolism could induce tumor cell apoptosis and might develop into potential antitumor drugs (Deepagan et al. 2016).
Selenium is an essential trace element that plays an important role in the therapy of hypercholesterolemia, cardiovascular disease and cancers. Epidemiological data showed that both organic and inorganic forms of Se are able to induce tumor cells apoptosis via several mechanisms, such as oxidative stress, cell cycle arrest or the activation of caspase enzymes (Dong et al. 2002; Lee et al. 2005; Stewart et al. 1999). Especially, selenium polysaccharides have gained wide attention due to the effectively biological activity and low toxicity on patients. Selenide mannan, SeGLP and Ch-SeNPs have been reported for antitumor activities in Hep G2 cells (Decker et al. 2001; Estevez et al. 2014; Yang et al. 1992). Accordingly, SeASP2 also displayed obvious anti-proliferation activity to three tumor cell lines including Hep G2, A549 and Hela cells (Wang et al. 2016). Chitosan is a polysaccharide and is derived from partial deacetylation of chitin, which is composed of β-(1-4)-linked D-glucosamine and N-acetyl glucosamine subunits. Owing to unique polymeric cationic characteristic, good biocompatibility and low toxicity, chitosan has been widely applied in food and pharmaceutics (Anal et al. 2006). Therefore, combination of selenium and chitosan will develop into a potential chemopreventive compound for oncotherapy. Our previous study showed that seleno-short-chain chitosan is a synthesized chitosan derivative with the molecular weight distribution of 5000–10,000 Da and chemically modified by introducing more stable selenic acid groups (–SeO3 −) to –OH or –NH2 groups. It was also observed that seleno-short-chain chitosan could suppress suspended human leukemia K562 cells growth in vitro (Liu et al. 2008). However, it still unknown whether seleno-short-chain chitosan has an antitumor activity on adherent non-small-cell lung cancer A549 in vitro. The present study aimed to explore the cytotoxicity of seleno-short-chain chitosan on A549 cells in vitro and to investigate the possible apoptosis mechanism.
Materials and methods
Reagents
Seleno-short-chain chitosan (SSCC), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and dimethyl sulfoxide (DMSO) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). FITC Annexin V/PI apoptosis detection kit was obtained from Keygen Biotech (Nanjing, China). RT-PCR Kit and SYBR® Premix Ex Taq TM II Kit were purchased from Takara Bio Co. (Dalian, China). 4,6-diamidino-2-phenylindole (DAPI), Reactive oxygen species (ROS), N-acetyl-l-cysteine (NAC), Rhodamin 123 assay kit, PIPA and cytosolic protein lysis buffer, Bradford Protein Assay Kit, Enhanced chemiluminescence (ECL) kit and Trizol reagent were purchased from Solarbio Science & Technology Co, Ltd (Beijing, China). The antibodies specific to β-actin, cyclin A, cyclin-dependent kinase CDK2, Bax, Bcl-2, cytochrome c and caspase-3 were obtained from Tianjin Sungene Biotech Co. (Tianjin, China). The other chemical regents were of analytical grade.
Synthesis of seleno-short-chain chitosan
Seleno-short-chain chitosan (SSCC) was synthesized as described previous (Liu et al. 2008). Briefly, 10 g chitosan was dissolved with acetic acid solution (100 ml, 5%), and was degraded with 3% hydrogen peroxide. Then the supernatants of chitosan were harvested by centrifugation (200 rpm/4 g, 12 h). SeO2 and chitosan solution (1:10, w/v) were mixed and reacted in the condition of vacuum (45 °C, 24 h). After the synthesis, the reaction mixture was dialyzed using a 5 kDa cutoff dialysis membrane for 24 h at room temperature and precipitated by ethanol to get SSCC (Patent Number CN1600793A).
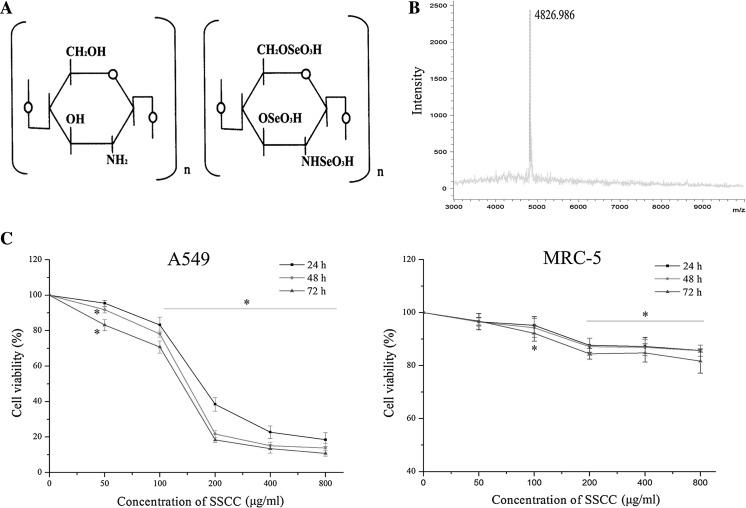
In the present study, 20 mg/ml SSCC solution and saturated 2,5-Dihydroxy benzoic acid (DHB) matrix solution (1:1, v/v) were mixed. The mixture (2 μl) was spotted to stainless steel target and allowed to dry. The molecular weight of SSCC was detected by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) (Bruker Daltonics Inc, Billerica, MA, USA). The chemical structure and molecular weight distribution of SSCC are shown in Fig. 1.
Fig. 1.
Toxic effects of SSCC on A549 cells and normal lung MRC-5 cells. a Chemical structure of chitosan and seleno-short-chain chitosan (SSCC). b The mass spectrum analysis of SSCC. c A549 cells and normal lung MRC-5 cells were treated with SSCC (50–800 μg/ml) for 24–72 h. Cell viability was determined by MTT assay. *p < 0.05 compared to control group was considered as statistically significant difference
Cell culture
A549 cells and normal lung MRC-5 cells (Tianjin Medical University, Tianjin, China) were cultured in RPMI 1640 medium containing 10% (v/v) heat inactivated fetal bovine serum (FBS) (Thermo Scientific Hyclone, Shanghai, China), 100 U/ml penicillin and 100 μg/ml streptomycin (Gibco, Burlington, ON, Canada). The cells were kept at 37 °C in a humidified atmosphere containing 5% CO2.
Cell viability assay
The effect of SSCC on cell viability was analyzed by MTT method. The cells were seeded in 96-well plates at a density of 5 × 103/well and incubated for 24 h. Then cells were exposed to different concentrations of SSCC for 24–72 h, respectively. 20 μl MTT solution (5 mg/ml) was added and incubated for additional 4 h at 37 °C. The precipitated formazan was dissolved with 150 μl DMSO and the absorbance was measured at 570 nm using an ELISA reader (Model 680; Bio-Rad, Hercules, CA, USA). Cell viability was calculated using the following formula: cell viability (%) = b/a × 100%, where a and b stand for the absorbance of the control and treatment cells, respectively (Lin et al. 2016).
Cell cycle assay
Cell cycle was detected by PI staining. After incubation for 24 h, A549 cells were exposed to different concentrations of SSCC (100, 200 and 400 μg/ml) for different time. Then the cells were washed with PBS and fixed with cold 70% ethanol (stored at −20 °C) overnight. Next, A549 cells were incubated with RNase A (0.1 mg/ml) at 37 °C for 30 min. Finally, they were stained with PI (50 μg/ml) in dark at 37 °C for 30 min. The percentages of the stained cells in each phase were measured by flow cytometry (BD FACSCalibur, San Jose, CA, USA), and analyzed by ModFit LT software.
Morphological changes of cell nucleus assay
The morphological changes of typical apoptosis cell nucleus were examined by DAPI staining. After incubation for 24 h, A549 cells were treated with 200 μg/ml SSCC for 24–72 h at 37 °C. Then the cells were washed with PBS and fixed with 4% paraformaldehyde for 10 min. The fixed cells were stained with DAPI (1.5 μg/ml) in the dark at room temperature for 15 min and washed with PBS. Finally, the apoptotic cells were observed by inverted fluorescence microscope (Nikon, Tokyo, Japan).
Cell apoptosis assay
The apoptosis rate of A549 cells was detected using FITC Annexin V/PI apoptosis detection kit. After incubation for 24 h, the cells were exposed to 200 μg/ml SSCC for 24–72 h, then harvested and washed with PBS. Next, the cells were resuspended in annexinV-binding buffer and incubated with annexinV/PI in dark for 10 min according to the manufacture’s instructions. The levels of apoptotic cells were measured by flow cytometry (BD FACSCalibur), and analyzed by CellQuest Pro. Software. NAC was used in this process as a free radical scavenger. A549 cells were pretreatment with 5 mM NAC (Hua et al. 2015) for 12 h and then the cells were treated with 200 μg/ml SSCC for 60 h. Cell apoptosis was inhibited and the percentages of apoptotic cells were also measured in the same conditions.
Generation of intracellular ROS assay
The generation of intracellular ROS was measured by DCFH-DA staining. After incubation in 6-well plates for 24 h, A549 cells were treated with 200 μg/ml SSCC for 24–72 h. Then cells were resuspended in PBS containing 10 µM DCFH-DA at 37 °C for 30 min. Finally, cells were analyzed with flow cytometry. In the ROS inhibitor group, cells were pretreated with 5 mM NAC for 12 h following 200 μg/ml SSCC for 60 h, and the levels of ROS were measured in the same condition.
Mitochondrial membrane potential assay
The mitochondrial membrane potential (MMP) was determined by Rhodamin 123 staining. A549 cells were seeded into 6-well plates for 24 h and treated with 200 μg/ml SSCC for 24–72 h, then harvested and washed with PBS. Next, cells were incubated with 5 mg/ml Rhodamin 123 for 30 min in the dark at 37 °C. The MMP was examined with flow cytometry and analyzed by Cell Quest software (BD, USA). In the ROS inhibitor group, cells were pretreated with 5 mM NAC for 12 h following 200 μg/ml SSCC for 60 h and the disruption of MMP was measured in the same condition.
Real-time PCR assay
To explore apoptosis mechanism, the mRNA levels of Bax and Bcl-2 were detected by real-time PCR. A549 cells were treated with 200 μg/ml SSCC for 24–72 h, and total RNA was extracted using Trizol reagent. The RNA concentration was determined using BioPhotometer plus of Eppendorf Company (Hamburg, Germany). The reverse transcript of RNA to cDNA was synthesized by using Bio-rad MyCycler Thermal Cycler according to RT-PCR Kit (TaKaRa, Dalian, China). The primers sequences were summarized in Table 1. To obtain the relative quantitative values for gene expression, GAPDH was used as an internal control. Real-time PCR was performed using qTOWER 2.2 of Analytik Jena Company (Jena, Germany) according to SYBR® Premix Ex Taq TM II Kit (Takara, Dalian, China). The quantitative calculation was performed by the 2−ΔΔCt method.
Table 1.
List of primers for Real time PCR
| Gene name | Primer sequences (5′–3′) | Orientation |
|---|---|---|
| GADPH | GGAGCGAGATCCCTCCAAAAT GGCTGTTGTCATACTTCTCATGG |
Forward Reverse |
| Bax | GCGAGTGTCTCAAGCGCATC CCAGTTGAAGTTGCCGTCAGAA |
Forward Reverse |
| Bcl-2 | TCGCCCTGTGGATGACTGAG CAGAGTCTTCAGAGACAGCCAGGA |
Forward Reverse |
Western blot assay
The expression of proteins related to mitochondria apoptosis was detected by western blot. In brief, after treatment with 200 μg/ml SSCC for 24–72 h, A549 cells were harvested and lysed using PIPA or cytosolic protein lysis buffer. Concentration of proteins was quantified using Bradford Protein Assay Kit. The supernatants proteins were mixed with 1× reducing electrophoresis loading buffer and boiled for 5 min. Protein samples (50–70 μg) were separated by 12% SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membrane by semi-dry blotting. Membranes were blocked with 5% bovine serum albumin for 2 h at room temperature and incubated with primary antibody overnight at 4 °C. Then membranes were further incubated with the corresponding horseradish peroxidase (HRP)-conjugated secondary antibody for 1.5 h at room temperature. Protein bands were examined using chemiluminescence (ECL) reagent and exposed to X-ray photographic films in a darkroom. Then the bands were visualized with Quantity One software.
Statistical analysis
All experiments were performed at least three times. Experimental data were presented as mean ± standard deviation. Significant differences were established through analysis of variance (ANOVA) and mean comparisons were achieved by Duncan’s multiple range test. Data analysis was evaluated using SPSS software (SPSS Inc., Chicago, IL, USA). *p < 0.05 was considered statistically significant difference.
Results
Cytotoxicity of SSCC on A549 cells
The chemical structure of seleno-short-chain chitosan (SSCC) is showed in Fig. 1a. The matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) assay (Fig. 1b) showed that the molecular weight of SSCC was about 4826.986 Da. In order to explore toxic effects of SSCC on A549 cells and normal lung MRC-5 cells in vitro, cell viability was examined by MTT method. The results (Fig. 1c) showed that SSCC reduced A549 cells viability in a dose- and time-dependent manner (p < 0.05). Meanwhile, it was noted that 200 μg/ml SSCC caused remarkable cytotoxicity on A549 cells. However, normal lung MRC-5 cells were observed to survive at the highest concentration of 800 μg/ml SSCC (p < 0.05). The data showed that toxic effects of SSCC on A549 cell were a slow process, and inhibition rate could attain 89.25% after incubation of 72 h. Thus, 200 μg/ml SSCC was selected for the next investigations.
SSCC induced S phase cell cycle arrest in A549 cells
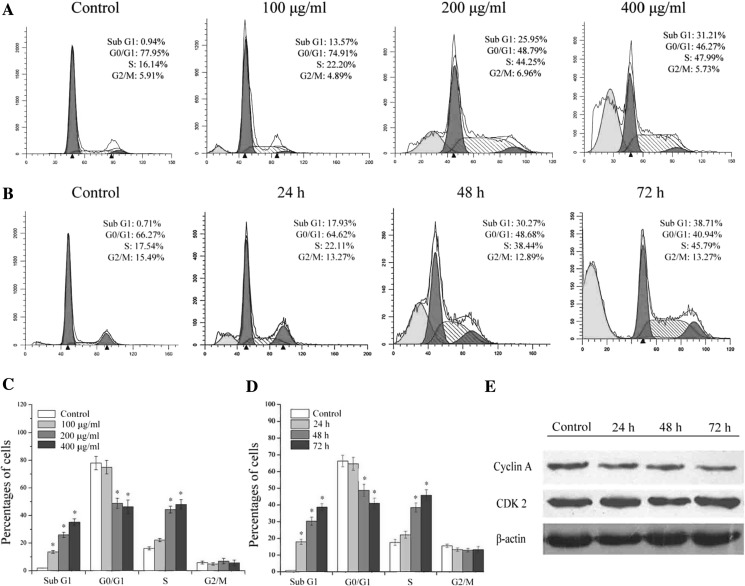
To further investigate the effect of SSCC on cell cycle progression, the number of cells in each phase of cell cycle was detected by flow cytometry. A549 cells were treated with different concentrations of SSCC (100, 200 and 400 μg/ml) for 48 h. The result (Fig. 2a, c) showed that the percentages of cells in S phase significantly increased from 16.14 to 47.99% in a dose-dependent manner, with a concomitant decrease in the G0/G1 phase from 77.95 to 46.27% (p < 0.05). Moreover, cells were treated with 200 μg/ml SSCC for 24–72 h. The result (Fig. 2b, d) showed the number of cells in S phase increased from 17.54 to 45.79% in a time-dependent manner, with a concomitant decrease in the G0/G1 phase from 66.27 to 40.94% (p < 0.05). These data indicated that SSCC could induce S phase cell cycle arrest in a dose- and time-dependent manner. The percentages of Sub G1 cells represent the levels of apoptotic cells (Shu et al. 2014), our data showed that SSCC elevated the numbers of apoptotic nuclei and induced A549 cells apoptosis in vitro. Furthermore, the protein levels of S phase related cyclin A and cyclin-dependent kinase CDK2 were determined by western blot. The result (Fig. 2e) showed that SSCC treatment caused a downregulation of S phase associated cyclin A, while the expression of CDK2 was not affected. These data demonstrated that SSCC-induced suppression on A549 cells was possibly mediated by slowing down cell cycle progress in S phase.
Fig. 2.
The effect of SSCC on A549 cells cell cycle distribution. a A549 cells were exposed to different concentrations of SSCC (100, 200, 400 μg/ml) for 48 h and then stained with PI. The number of cells was analyzed by flow cytometry. b A549 cells were treated with 200 μg/ml SSCC for 24–72 h and then the number of cells was analyzed by flow cytometry. c Columns represent the percentages of corresponding cell cycle phase after treatment with different concentrations of SSCC for 48 h. d Columns represent the percentages of corresponding cell cycle phase after treatment with 200 μg/ml SSCC for 24–72 h. e The protein levels of cyclin A and cyclin-dependent kinase CDK2 were analyzed by western blot. *p < 0.05 compared to control group was considered as statistically significant difference
SSCC induced apoptosis in A549 cells
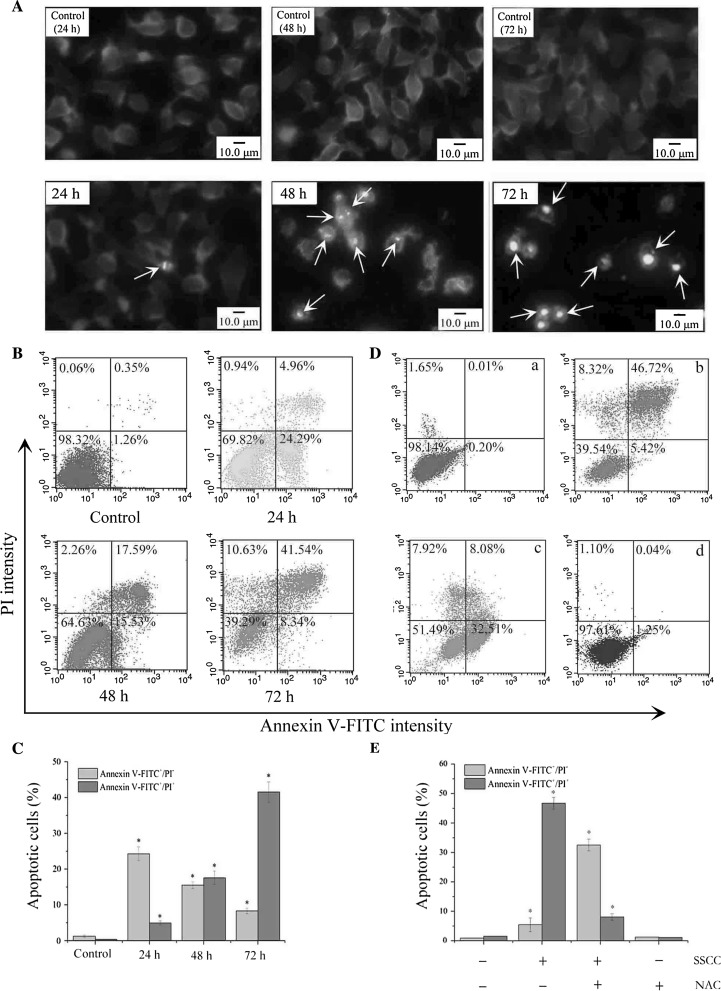
Cell morphology changes and chromatin condensation of cell nucleus are crucial characteristics in the apoptosis process of various cells. The changes of cell nucleus in SSCC-treated A549 cells were detected by DAPI staining. The results (Fig. 3a) showed that the untreated cells were stained equally with blue fluorescence, which indicated the steady chromatin distribution in nucleus. Moreover, it was observed that the numbers of cells were increased in a time-dependent manner. In contrast, SSCC-treated A549 cells emitted bright fluorescence due to chromatin congregation and nucleus shrinkage. As pointed to by the arrows, with the increase of incubation time, cell nucleus in the treatment group became disintegrated and formed many nucleus fragments.
Fig. 3.
SSCC induced apoptosis of A549 cells. a Morphological changes of A549 cells were detected by DAPI staining (magnification, ×40). Cells were exposed to 200 μg/ml SSCC for 24–72 h. The white arrows in SSCC treatment groups represent apoptotic nuclear fragments. b Apoptosis rate of A549 cells was detected by Annexin V-FITC/PI double staining. Cells were treated with 200 μg/ml SSCC for 24–72 h. c Columns represent the percentages of apoptotic cells after treatment with 200 μg/ml SSCC for 24–72 h. The light grey bars represent the persentages of “Annexin V-FITC+/PI-”, and the dark grey bars represent the percentages of “Annexin V-FITC+/PI+”. d NAC (free radical scavenger) inhibited SSCC-induced A549 cells apoptosis. The cells were treated with 200 μg/ml SSCC for 72 h in the presence or absence of NAC, and apoptotic cells were examined by flow cytometry. a Control group; b Treatment with 200 μg/ml SSCC; c Treatment with 5 mM NAC for 12 h followed by treatment with 200 μg/ml SSCC for 60 h; d Treatment with 5 mM NAC. e Columns represent the percentages of apoptotic cells in the presence or absence of NAC. The light grey bars represent the persentages of “Annexin V-FITC+/PI-”, and the dark grey bars represent the percentages of “Annexin V-FITC+/PI+”
The externalization of phosphatidylserine as one of apoptotic hallmarks was examined by Annexin V-FITC/PI double staining. The result (Fig. 3b, c) showed that untreated cells displayed low or negative staining with both Annexin V and PI, which indicated the presence of a large number of viable cells. When treatment with 200 μg/ml SSCC for 24–72 h, the result showed the progression of cells from early to late apoptosis. The total Annexin V-positive cells (%) significantly increased from 1.61 to 29.25, 33.12, and 49.88% with the increase of incubation time of 24–72 h (p < 0.05). NAC is usually used as a free radical scavenger. Pretreatment with 5 mM NAC for 12 h blocked effectively the cytotoxicity of SSCC on A549 cells. As shown in Fig. 3d, e, it was observed that numerous late apoptotic cells transformed into viable cells or early apoptotic cells, and the percentages of apoptotic cells decreased from 52.14 to 40.59%.
Effects of SSCC on ROS generation and mitochondrial membrane potential (MMP)
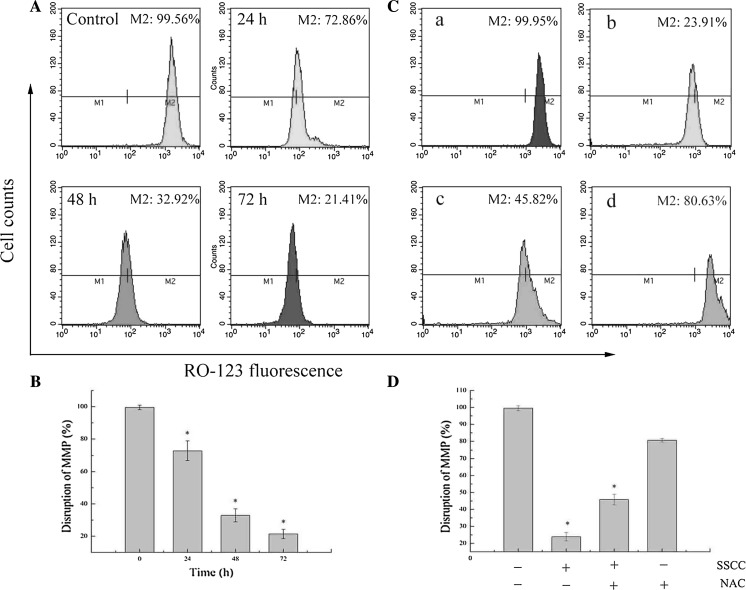
Researches showed that the disruption of MMP is always associated with mitochondria apoptosis pathway (Zhang et al. 2012). In order to explore the possible mechanism of SSCC-treated A549 cells apoptosis, the disruption of MMP were analyzed by Rhodamine 123 staining. The results (Fig. 4a, b) showed that fluorescence intensity of Rhodamine 123 significantly decreased from 99.56 to 72.86, 32.92 and 21.41% in a time-dependent manner after treatment with 200 μg/ml SSCC (p < 0.05). Meanwhile, it was observed that NAC inhibited SSCC-induced loss of MMP and the percentages of MMP increased from 23.91 to 45.82% after pretreatment with 5 mM NAC for 12 h (p < 0.05) (Fig. 4c, d). These data indicate that mitochondria apoptosis pathway was involved in SSCC-induced apoptosis in A549 cells.
Fig. 4.
Mitochondrial membrane potential (MMP) disruption in A549 cells was examined by Rhodamine 123 staining and analyzed using flow cytometry. a A549 cells were treated with 200 μg/ml SSCC for 24–72 h. b Columns represent the percentages of MMP disruption after treatment with 200 μg/ml SSCC for 24–72 h. c NAC (free radical scavenger) inhibited SSCC-induced loss of MMP. The cells were treated with 200 μg/ml SSCC for 72 h in the presence or absence of NAC, and MMP was analyzed using flow cytometry. a Control group; b Treatment with 200 μg/ml SSCC; c Treatment with 5 mM NAC for 12 h followed by treatment with 200 μg/ml SSCC for 60 h; d Treatment with 5 mM NAC. d Columns represent the percentages of MMP disruption in the presence or absence of NAC
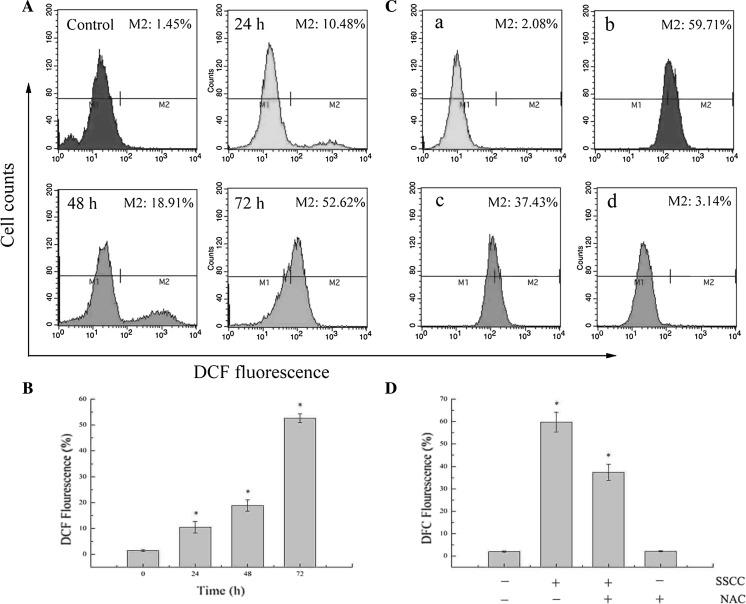
The generation of intracellular ROS and depletion of glutathione (GSH) are usually related to the disruption of MMP and eventually induce cell apoptosis (Chan et al. 2015). To investigate the effect of SSCC on intracellular ROS of A549 cells, the generation of ROS was analyzed by DCFH-DA staining. The results (Fig. 5a, b) showed that SSCC induced ROS generation in a time-dependent manner. After treatment with 200 μg/ml SSCC for 24–72 h, the levels of ROS increased from 1.45 to 10.48, 18.91 and 52.62% (p < 0.05). Mean while, NAC decreased the production of SSCC-induced ROS. The data (Fig. 5c, d) showed that the intracellular ROS declined from 59.71 to 37.43% after pretreatment with 5 mM NAC for 12 h (p < 0.05). These results further confirmed that SSCC-induced apoptosis in A549 cells was triggered by high levels of intracellular ROS.
Fig. 5.
ROS generation in A549 cells was examined by DCFH-DA staining and analyzed using flow cytometry. a Cells were exposed to 200 μg/ml SSCC for 24–72 h. b Columns represent the levels of intracellular ROS after treatment with 200 μg/ml SSCC for 24–72 h. c NAC (free radical scavenger) inhibited SSCC-induced generation of ROS. The cells were treated with 200 μg/ml SSCC for 72 h in the presence or absence of NAC, and intracellular ROS was analyzed using flow cytometry. a Control group; b Treatment with 200 μg/ml SSCC; c Treatment with 5 mM NAC for 12 h followed by treatment with 200 μg/ml SSCC for 60 h; d Treatment with 5 mM NAC. d Columns represent the levels of intracellular ROS in the presence or absence of NAC
Effects of SSCC on apoptosis-related regulators involved in mitochondrial pathway
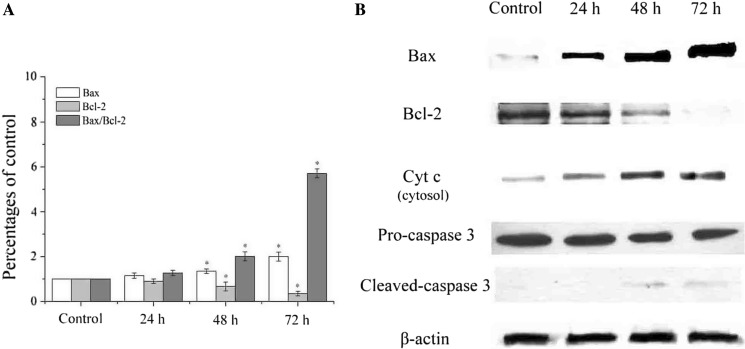
To explore the molecular mechanism of SSCC-induced A549 cells apoptosis, the mRNA levels of Bax and Bcl-2 were measured by real-time PCR. As shown in Fig. 6a, compared with control group, the mRNA level of Bax significantly increased, while the mRNA level of Bcl-2 decreased, which led to a time-dependent up-regulation of Bax/Bcl-2 ratio in SSCC-treated A549 cells (p < 0.05). To further verify the mitochondrial apoptosis mechanism, the protein levels of Bax, Bcl-2, Cyt c, pro-caspase 3 and cleaved-caspase 3 were measured by western blot. The result (Fig. 6b) showed that SSCC increased the protein levels of Bax, Cyt c, cleaved-caspase 3 and decreased the expression of Bcl-2. These data indicated that antitumor activity of SSCC on A549 cells was performed through ROS-mediated mitochondrial apoptosis pathway.
Fig. 6.
The effects of SSCC on apoptosis-related regulators involved in mitochondrial pathway in A549 cells. a The effects of SSCC on mRNA levels of Bax, Bcl-2 and Bax/Bcl-2 were detected by real-time PCR. b The effects of SSCC on protein levels of Bax, Bcl-2, Cyt c, pro-caspase 3 and cleaved-caspase 3 were detected by western blot
Discussion
Proliferation inhibition and apoptosis induction of tumor cells are effective ways to prevent cell growth and eliminate tumors in vivo and in vitro. Apoptosis is an evolutionarily conserved program for cell self-destruction that occurs under a variety of physiological and pathological conditions, such as chemical reagents, radiation, free radicals, and virus infection (Ma et al. 2014). In the present study, a synthesized seleno-short-chain chitosan (SSCC) with the molecular weight of 4826.986 Da was explored due to its prominent antitumor activity and low toxic effects on normal cell. MTT assay showed SSCC could inhibit non-small-cell lung cancer A549 cells viability in vitro in a dose- and time-dependent manner. Moreover, 200 μg/ml SSCC caused remarkable growth inhibition and the inhibition rate attained about 89.25% after treatment for 72 h. In contrast, SSCC exhibited low cytotoxicity on normal lung MRC-5 cells in vitro, which supported the application of SSCC in the clinic lung cancer treatment. The result was in agreement with our previous study that SSCC suppressed the growth of suspended human leukemia K562 cells in vitro and exhibited few toxic effect on normal mouse embryonic fibroblasts NIH3T3. However, the most effective inhibition concentration of SSCC on K562 cells was 100 μg/ml, which may be associated with the type of tumor cells (Liu et al. 2008).
Apoptosis induction of tumor cells could be triggered by cell cycle arrest. Numerous researches showed that many antitumor drugs could block cell cycle at a specific checkpoint and thereby induce cell apoptosis (Chang et al. 2013; Zhang et al. 2015). Sub G1 represents the percentages of tumor cell apoptosis nuclei and it is used to detect the number of apoptotic cells (Shu et al. 2014). In this study, SSCC increased the percentages of apoptotic cells and arrested A549 cell cycle in S phase in a dose- and time-dependent manner, which indicated that SSCC-treated A549 cells underwent an apoptosis process and cell cycle arrest in S phase was one of the mechanisms of growth inhibition. A number of reports showed that cell cycle progression is tightly regulated through complex network of cell cycle regulatory molecules. Cyclin A and cyclin-dependent kinase CDK2 protein regulate the progression of cell cycle in S phase (Su et al. 2015), our result showed that SSCC down-regulated the expression of cyclin A, however, there was no significant effect on the expression of CDK2. These data further demonstrated that SSCC triggered S phase cell cycle arrest of A549 cells in vitro. With the occurrence of apoptosis, some morphological features including chromatin condensation, DNA fragmentation, and formation of apoptotic body in A549 cells were also observed by DAPI staining. Furthermore, the translocation of phosphatidylserine from the inner to the outer leaflet of the plasma membrane is a crucial process in apoptotic cells (Jiang et al. 2016). To calculate the percentages of apoptotic cells, annexinV-FITC/PI staining was thus performed. The result showed that the total Annexin V-positive cells contained early and late apoptotic cells significantly increased in a time-dependent manner and the apoptosis rate of A549 cells could attain 49.88% after treatment with 200 μg/ml SSCC for 72 h. Similarly, according to the research of Hua et al. (2015), 40 μM cepharanthine induced A549 cells apoptosis and the apoptosis rate could reach 44.87% in vitro. In addition, Zhang et al. (2016) found that CSTMP, a tetramethylpyrazine analogue, displayed potential cytotoxicity on A549 cells and the percentages of apoptotic cells reached about 35% after treatment with 150 μM CSTMP. Moreover, in this study, NAC as a free radical scavenger blocked the toxic effects of SSCC on A549 cells in vitro. These findings indicated that SSCC triggered toxic impacts on A549 cells through the generation of ROS.
ROS are recognized as mediators of apoptosis signaling pathway. High levels of ROS are able to induce DNA damage, genomic instability and cell apoptosis. Mounting researches have demonstrated that ROS could lead to apoptosis in many different cell types (Simon et al. 2000). In this work, we found that SSCC significantly increased ROS levels and decreased MMP distribution in a time-dependent manner. Additionally, pretreatment with the free radical scavenger NAC distinctly attenuated the generation of ROS and the loss of MMP, and further weakened A549 cells apoptosis, which identified that the SSCC-induced A549 cells apoptosis was associated with generation of ROS.
The Bcl-2 family is composed of crucial regulatory proteins involved in the mitochondrial apoptosis pathway. Members of this family such as Bax and Bcl-2 are involved in complex interactions with each other to determine cell apoptosis. Bcl-2 inhibits the release of Cyt c from mitochondria to the cytosol and promote cell growth, while Bax opens the mitochondrial permeability transition pores (PTP), leading to the disruption of MMP and further resulting in the release of Cyt c (Danial and Korsmeyer 2004; Tedeschi 1980). When the ratio of Bax/Bcl-2 increased, the protective effects of Bcl-2 on the mitochondrial membrane are blocked and mitochondrial membrane permeability enhanced, allowing Cyt c leaking into cytosol and triggering cell apoptosis (Ma et al. 2014). In the current study, a significant time-dependent up-regulation of Bax and a down-regulation of Bcl-2 were observed in SSCC-treated A549 cells by real-time PCR and western blot, which led to an elevation of Bax/Bcl-2 ratio and the release of Cyt c from mitochondria to cytosol. Caspase 3 is one of the most common apoptosis executioners, which is responsible for the majority of apoptosis processes and usually exists in form of an inactive pro-enzyme. Once the released Cyt c is combined with cytosolic apoptosis protease activating factor (Apaf-1) and caspase 9, the cleaved-caspase 3 is activated and eventually leads to cell death (Renner et al. 2003; Wu et al. 2005). Our data showed that protein level of cleaved-caspase 3 is elevated in a time-dependent manner, which suggested A549 cells have undergone irreversible apoptosis. In a word, these findings indicated that SSCC-induced A549 cells apoptosis was associated to the ROS-mediated mitochondrial apoptosis pathway. The results were in agreement with other selenium compounds which induce tumor cells apoptosis through the mitochondrial apoptosis pathway (Fernandes and Gandin 2015; He et al. 2013; Sarada et al. 2008).
The present work demonstrated that SSCC-induced apoptosis in human non-small-cell lung cancer A549 cells was activated through the ROS-regulated mitochondrial pathway in vitro. These findings provide a new insight for further clinic application of SSCC as a potential antitumor drug. In a follow-up study, we will explore the toxic effects of SSCC on A549 cells in vivo.
References
- Anal AK, Stevens WF, Remunan-Lopez C. Ionotropic cross-linked chitosan microspheres for controlled release of ampicillin. Int J Pharm. 2006;312:166–173. doi: 10.1016/j.ijpharm.2006.01.043. [DOI] [PubMed] [Google Scholar]
- Chan CK, Supriady H, Goh BH, Kadir HA. Elephantopus scaber induces apoptosis through ROS-dependent mitochondrial signaling pathway in HCT116 human colorectal carcinoma cells. J Ethnopharmacol. 2015;168:291–304. doi: 10.1016/j.jep.2015.03.072. [DOI] [PubMed] [Google Scholar]
- Chang CC, Hung CM, Yang YR, Lee MJ, Hsu YC. Sulforaphane induced cell cycle arrest in the G2/M phase via the blockade of cyclin B1/CDC2 in human ovarian cancer cells. J Ovarian Res. 2013;6:41. doi: 10.1186/1757-2215-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/S0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Decker C, Bianchi C, Decker D, Morel F. Photoinitiated polymerization of vinyl ether-based systems. Prog Org Coat. 2001;42:253–266. doi: 10.1016/S0300-9440(01)00203-X. [DOI] [Google Scholar]
- Deepagan VG, Kwon S, You DG, Nguyen VQ, Um W, Ko H, Lee H, Jo DG, Kang YM, Park JH. In situ diselenide-crosslinked polymeric micelles for ROS-mediated anticancer drug delivery. Biomaterials. 2016;103:56–66. doi: 10.1016/j.biomaterials.2016.06.044. [DOI] [PubMed] [Google Scholar]
- Dong BY, Ganther HE, Stewart C. Ip C: identification of molecular targets associated with selenium-induced growth inhibition in human breast cells using cDNA microarrays. Cancer Res. 2002;62:708–714. [PubMed] [Google Scholar]
- Estevez H, Garcia-Lidon JC, Luque-Garcia JL, Camara C. Effects of chitosan-stabilized selenium nanoparticles on cell proliferation, apoptosis and cell cycle pattern in HepG2 cells: comparison with other selenospecies. Colloid Surf B. 2014;122:184–193. doi: 10.1016/j.colsurfb.2014.06.062. [DOI] [PubMed] [Google Scholar]
- Fernandes AP, Gandin V. Selenium compounds as therapeutic agents in cancer. BBA-Gen Subj. 2015;1850:1642–1660. doi: 10.1016/j.bbagen.2014.10.008. [DOI] [PubMed] [Google Scholar]
- He N, Shi X, Zhao Y, Tian L, Wang D, Yang X. Inhibitory effects and molecular mechanisms of selenium-containing tea polysaccharides on human breast cancer MCF-7 cells. J Agric Food Chem. 2013;61:579–588. doi: 10.1021/jf3036929. [DOI] [PubMed] [Google Scholar]
- Hua P, Sun M, Zhang G, Zhang Y, Tian X, Li X, Zhang X. Cepharanthine induces apoptosis through reactive oxygen species and mitochondrial dysfunction in human non-small-cell lung cancer cells. Biochem Biophys Res Commun. 2015;460:136–142. doi: 10.1016/j.bbrc.2015.02.131. [DOI] [PubMed] [Google Scholar]
- Jiang G, Liu J, Ren B, Tang Y, Owusu L, Li M, Zhang J, Liu L, Li W. Anti-tumor effects of osthole on ovarian cancer cells in vitro. J Ethnopharmacol. 2016;193:368–376. doi: 10.1016/j.jep.2016.08.045. [DOI] [PubMed] [Google Scholar]
- Lee JS, Hong EK. Hericium erinaceus enhances doxorubicin-induced apoptosis in human hepatocellular carcinoma cells. Cancer Lett. 2010;297:144–154. doi: 10.1016/j.canlet.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Lee SO, Nadiminty N, Wu XX, Lou W, Dong Y, Ip C, Onate SA, Gao AC. Selenium disrupts estrogen signaling by altering estrogen receptor expression and ligand binding in human breast cancer cells. Cancer Res. 2005;65:3487–3492. doi: 10.1158/0008-5472.CAN-04-3267. [DOI] [PubMed] [Google Scholar]
- Lin M, Tang S, Zhang C, Chen H, Huang W, Liu Y, Zhang J. Euphorbia factor L2 induces apoptosis in A549 cells through the mitochondrial pathway. Acta Pharmaceutica Sinica B. 2016 doi: 10.1016/j.apsb.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Song W, Cao D, Liu X, Jia Y. Growth inhibition and apoptosis of human leukemia K562 cells induced by seleno-short-chain chitosan. Method find Exp Clin. 2008;30:181–186. doi: 10.1358/mf.2008.30.3.1213209. [DOI] [PubMed] [Google Scholar]
- Ma WD, Zou YP, Wang P, Yao XH, Sun Y, Duan MH, Fu YJ, Yu B. Chimaphilin induces apoptosis in human breast cancer MCF-7 cells through a ROS-mediated mitochondrial pathway. Food Chem Toxicol. 2014;70:1–8. doi: 10.1016/j.fct.2014.04.014. [DOI] [PubMed] [Google Scholar]
- Mieyal JJ, Gallogly MM, Qanungo S, Sabens EA, Shelton MD. Molecular mechanisms and clinical implications of reversible protein S-Glutathionylation. Antioxid Redox Sign. 2008;10:1941–1987. doi: 10.1089/ars.2008.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner K, Amberger A, Konwalinka G, Kofler R, Gnaiger E. Changes of mitochondrial respiration, mitochondrial content and cell size after induction of apoptosis in leukemia cells. BBA-Mol Cell Res. 2003;1642:115–123. doi: 10.1016/s0167-4889(03)00105-8. [DOI] [PubMed] [Google Scholar]
- Rukkijakan T, Ngiwsara L, Lirdprapamongkol K, Svasti J, Phetrak N, Chuawong P. A synthetic 2,3-diarylindole induces cell death via apoptosis and autophagy in A549 lung cancer cells. Bioorg Med Chem Lett. 2016;26:2119–2123. doi: 10.1016/j.bmcl.2016.03.079. [DOI] [PubMed] [Google Scholar]
- Sarada SKS, Himadri P, Ruma D, Sharma SK, Pauline T, Mrinalini Selenium protects the hypoxia induced apoptosis in neuroblastoma cells through upregulation of Bcl-2. Brain Res. 2008;1209:29–39. doi: 10.1016/j.brainres.2008.02.041. [DOI] [PubMed] [Google Scholar]
- Shu G, Yang J, Zhao W, Xu C, Hong Z, Mei Z, Yang X. Kurarinol induces hepatocellular carcinoma cell apoptosis through suppressing cellular signal transducer and activator of transcription 3 signaling. Toxicol Appl Pharmacol. 2014;281:157–165. doi: 10.1016/j.taap.2014.06.021. [DOI] [PubMed] [Google Scholar]
- Simon HU, Hajyehia A, Levischaffer SF. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–418. doi: 10.1023/A:1009616228304. [DOI] [PubMed] [Google Scholar]
- Stewart MS, Spallholz JE, Neldner KH, Pence BC. Selenium compounds have disparate abilities to impose oxidative stress and induce apoptosis. Free Radic Biol Med. 1999;26:42–48. doi: 10.1016/S0891-5849(98)00147-6. [DOI] [PubMed] [Google Scholar]
- Su JY, Luo X, Zhang XJ. Immunosupressive activity of pogostone on T cells: blocking proliferation via S phase arrest. Int Immunopharmacol. 2015;26:328–337. doi: 10.1016/j.intimp.2015.04.019. [DOI] [PubMed] [Google Scholar]
- Sui Y, Li S, Shi P, Wu Y, Li Y, Chen W, Huang L, Yao H, Lin X. Ethyl acetate extract from Selaginella doederleinii Hieron inhibits the growth of human lung cancer cells A549 via caspase-dependent apoptosis pathway. J Ethnopharmacol. 2016;190:261–271. doi: 10.1016/j.jep.2016.06.029. [DOI] [PubMed] [Google Scholar]
- Tedeschi H. The mitochondrial membrane potential. Biol Rev. 1980;55:171–206. doi: 10.1111/j.1469-185X.1980.tb00692.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Li Q, Bao A, Liu X, Zeng J, Yang X, Yao J, Zhang J, Lei Z. Synthesis of selenium-containing Artemisia sphaerocephala polysaccharides: solution conformation and anti-tumor activities in vitro. Carbohydr Polym. 2016;152:70–78. doi: 10.1016/j.carbpol.2016.06.090. [DOI] [PubMed] [Google Scholar]
- Wu CC, Chan ML, Chen WY, Tsai CY, Chang FR, Wu YC. Pristimerin induces caspase-dependent apoptosis in MDA-MB-231 cells via direct effects on mitochondria. Mol Cancer Ther. 2005;4:1277–1285. doi: 10.1158/1535-7163.MCT-05-0027. [DOI] [PubMed] [Google Scholar]
- Yang M, Wang K, Gao L, Han YT, Lu JF, Zou TT. Exploration for a natural selenium supplement-characterization and bioactivities of Se-containing polysaccharide from garlic. J Chin Pharm Sci. 1992;1:28–32. [Google Scholar]
- Zhang CZ, Zhang H, Yun J, Chen GG, Lai PBS. Dihydroartemisinin exhibits antitumor activity toward hepatocellular carcinoma in vitro and in vivo. Biochem Pharmacol. 2012;83:1278–1289. doi: 10.1016/j.bcp.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Zhang CG, Huang JC, Liu T, Li XY. Anticancer effects of bishydroxycoumarin are mediated through apoptosis induction, cell migration inhibition and cell cycle arrest in human glioma cells. Original Artic. 2015;20:1592–1600. [PubMed] [Google Scholar]
- Zhang J, Liang Y, Lin Y, Liu Y, You Y, Yin W. IRE1α-TRAF2-ASK1 pathway is involved in CSTMP-induced apoptosis and ER stress in human non-small cell lung cancer A549 cells. Biomed Pharmacother. 2016;82:281–289. doi: 10.1016/j.biopha.2016.04.050. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Lu N, Zhang H, Wei L, Tao L, Dai Q, Zhao L, Lin B, Ding Q, Guo Q. HQS-3, a newly synthesized flavonoid, possesses potent anti-tumor effect in vivo and in vitro. Eur J Pharm Sci. 2013;49:649–658. doi: 10.1016/j.ejps.2013.04.016. [DOI] [PubMed] [Google Scholar]