Abstract
Pig-to-human xenotransplantation offers a potential bridge to the growing disparity between patients with end-stage organ failure and graft availability. Early studies attempting to overcome cross-species barriers demonstrated robust humoral immune responses to discordant xenoantigens. Recent advances have led to highly efficient and targeted genomic editing, drastically altering the playing field towards rapid production of less immunogenic porcine tissues and even the discussion of human xenotransplantation trials. However, as these humoral immune barriers to cross-species transplantation are overcome with advanced transgenics, cellular immunity to these novel xenografts remains an outstanding issue. Therefore, understanding and optimizing immunomodulation will be paramount for successful clinical xenotransplantation. Costimulation blockade agents have been introduced in xenotransplantation research in 2000 with anti-CD154mAb. Most recently, prolonged survival has been achieved in solid organ (kidney xenograft survival > 400 days with anti-CD154mAb, heart xenograft survival > 900 days, and liver xenograft survival 29 days with anti-CD40mAb) and islet xenotransplantation (>600 days with anti-CD154mAb) with the use of these potent experimental agents. As the development of novel genetic modifications and costimulation blocking agents converges, we review their impact thus far on preclinical xenotransplantation and the potential for future application.
1. Introduction
Organ transplantation remains the definitive treatment for patients suffering from end-stage organ failure. Unfortunately, this treatment remains severely limited due to the critical shortage of suitable allografts for transplantation [1, 2]. The use of genetically engineered pigs as a supplemental source of tissues or organs offers a promising answer to this dilemma [3]. Pig-to-human xenotransplantation has been pursued for more than a century; however, early studies demonstrated substantial barriers to clinical application in the form of hyperacute rejection, acute humoral xenograft rejection (AHXR), and thrombosis [4, 5].
The modern era of xenotransplantation was stimulated by the identification of the Gal α(1,3) Gal (Gal) porcine epitope and its role in early rejection [6–8]. The subsequent advent of α1,3-galactosyltransferase gene knockout (GTKO) pigs eliminated a major barrier to xenotransplantation by negating the role of high percentage of human xenoreactive antibodies [9, 10]. However, residual preformed human antibodies to GTKO pig antigens suggested additional major barriers (i.e., anti-non-Gal antibodies), which would hinder progress towards clinical application. Nevertheless, this remains a major breakthrough as the identification of Gal and production of GTKO pigs demonstrated the potential of reducing porcine antigenicity through genetic modification.
The initial production of GTKO animals was performed through a tedious process of homologous recombination; however, recent advances in gene editing have dramatically sped the pace of xenotransplantation research (Table 1) [9, 11–13] setting the stage for highly efficient and rapid porcine genetic modification. Recently, the role of genetically engineered pigs has been reviewed, and this role effectively negates the human anti-pig humoral response to the threshold where hyperacute rejection and AHXR are no longer expected [9, 12–14]. In this climate of reduced humoral xenoantigenicity, an appraisal of pharmacologic strategies that will modulate the human cell-mediated response to porcine xenografts is increasingly relevant.
Table 1.
Timeline for application of evolving techniques for genetic engineering of pigs employed in xenotransplantation.
| Year | Technique |
|---|---|
| 1992 | Microinjection of randomly integrating transgenes |
| 2000 | Somatic cell nuclear transfer (SCNT) |
| 2002 | Homologous recombination |
| 2011 | Zinc finger nucleases (ZFNs) |
| 2013 | Transcription activator-like effector nucleases (TALENs) |
| 2014 | CRISPR/Cas9 |
CRISPR/Cas9: clustered randomly interspaced short palindromic repeats and the associated protein 9 (table adopted from Cooper et al.) [9].
The cell-mediated response in allotransplantation is addressed with an effective pharmacologic armamentarium, mainly with calcineurin or mTOR inhibitors [15, 16]. Today, one of the most active frontiers in immunology and transplantation research is T cell costimulation signal modification. Much work over the past decade has defined costimulation signals, which regulate T cell activation and immune tolerance [17]. Although most of these agents are still experimental and early in the development pathway, preclinical studies utilizing experimental costimulation blockade agents have demonstrated prolonged engraftment of both solid organ and islet xenografts [18–25]. The approval of LEA29Y (belatacept) as a CTLA4-Ig protein for use in renal allotransplantation brought costimulation blockade to the clinic in the early 2000s [26, 27]. This was made possible after promising results from belatacept administration in preclinical nonhuman primate studies [28, 29].
In the last decade, researchers have increasingly utilized pig-to-nonhuman primate xenotransplantation models to study novel xenograft modification and novel costimulatory immunosuppression strategies in parallel. As discussions of pig-to-human xenotransplantation trials are underway [30, 31], we herein provide an overview of costimulation pathways, the current standing of clinical and preclinical development of these agents, and the preclinical data regarding their use in xenotransplantation.
2. T Cell Regulation through Costimulation Pathways
The adaptive immune system generates targeted responses first through (i) T cells identifying the antigen of interest and (ii) supplementary stimuli in the form of costimulation to induce antigen-specific T cell proliferation. Without these adjunct signals, T cells become anergic or undergo apoptosis and thus the response against that antigen is abrogated [32]. In this way, costimulation pathways support the role of T cell receptors (TCRs)—major histocompatibility complex (MHC) interaction by providing T cell the context of the antigen. Secondary and tertiary signals driven by cell surface costimulation molecules and soluble cytokines, respectively, determine the parameters of T cell activation [33]. Cytokines produced by the antigen-presenting cell (APC) and the T cell itself further propagate this activation cascade to induce a robust T cell response. Conventional immunosuppression works to abrogate the TCR and cytokine-induced signaling pathways preventing T cell activation [15, 16]. However, their lack of specificity to T cell mechanisms has led to well-recognized adverse side effects.
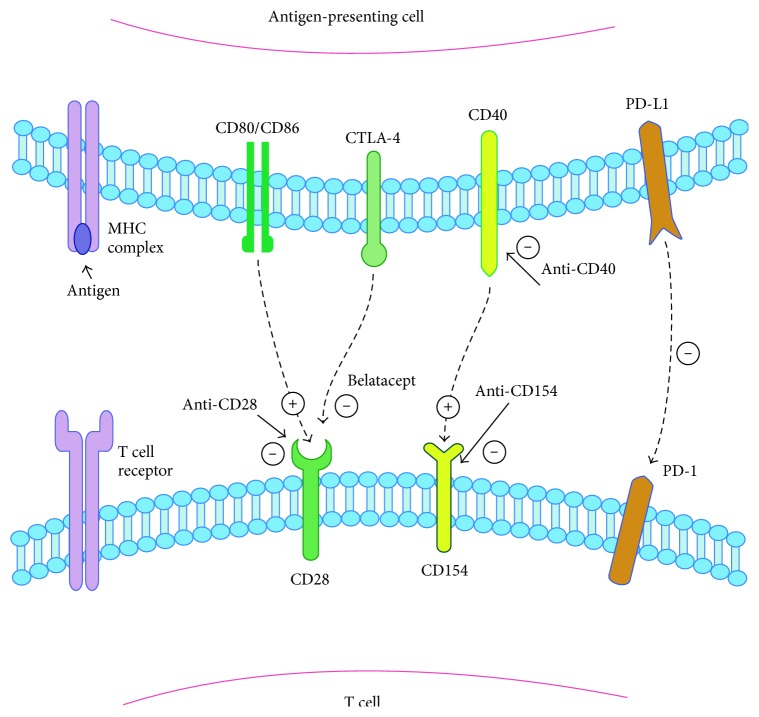
Costimulation pathways for T cell activation occur through a unique subset of cell surface markers, which are highly specific for the immune system and thus provide a target for immune modulators. Figure 1 depicts the most commonly studied costimulation signals for potential use in transplant applications. The interaction of CD28 with CD80/CD86 has been the best defined. CD28 is highly expressed on naïve T cells. During TCR engagement with an APC, binding of CD28 to CD80/CD86 results activation and proliferation of the T cell. A feedback mechanism occurs at this juncture by which CD28 is then downregulated and the T cell increases expression of CTLA4-Ig. This molecule binds CD80/CD86 with much higher affinity than CD28 and produces an inhibitory signal as a highly evolved feedback mechanism [34].
Figure 1.
Costimulation pathways in T cell regulation. Upon MHC-antigen interaction with the TCR, costimulation pathways can augment or suppress the activation of the T cell. From left to right, CD28 is activated by CD80/CD86; however, after T cell activation, CTLA-4 is upregulated and with higher affinity than CD80/CD86 and binds to CD28 inhibiting the signal. CTLA-4Ig and belatacept work by taking advantage of their higher affinity to CD28 over CD80/CD86 and thereby block CD80/CD86 activation of CD28. CD154 and CD40 are other potent activators of T cells; monoclonal antibodies against either of these surface proteins have potential for application in transplant immunosuppression. PD-1 is expressed on T cells, and interaction with PD-1 Ligand (PD-L1) produces a suppressive signal to the T cell.
Another increasingly significant costimulation pathway is the CD40/CD154 (CD40 ligand) interaction, which has been shown to be a potent stimulator of T and B cell activation through conventional APC interactions and also through interactions with innate immune cells and endothelium [35–38]. The inducible T cell costimulator (ICOS) molecule (CD278) has more recently been discovered to play an important role in T cell activation and differentiation as well as T and B cell interactions [39].
These costimulation pathways play a significant role during antigen recognition and T cell activation. Activated T cells rely on a specialized repertoire of surface proteins that assist in migration, adhesion, and interactions across the immunologic synapse to facilitate their effector function [40]. Lymphocyte function-associated antigen 1 (LFA1) is a well-studied molecule known to assist in immune cell endothelial attachment and migration and is recognized to play an important role in the stabilization of the immunologic synapse during antigen recognition and effector function (Figure 1) [41–43]. CD2 is more constitutively expressed on memory T cells, and interaction with LFA-3 is thought to not only have migration functions but also act as an activator of the potent memory T cell proliferation and response [40].
3. T Cell Costimulation in Organ Allotransplantation
Costimulation blockade has been extensively studied in preclinical allotransplantation models [41, 44–50]. Their relevance to xenotransplantation and xenoimmunity requires a thorough understanding of the salient findings from this growing body of research. One of the initial costimulation blockade agents was CTLA4-Ig, a protein that binds CD80/CD86 thus preventing CD28 costimulation and T cell activation. Preclinical data for CD40/CD154 blockade using anti-CD154 mAb also emerged in parallel with promising results. For example, an earlier study utilizing CTLA4-Ig and an anti-CD154 mAb (5C8 molecule) demonstrated synergistic prolongation of allograft survival in a nonhuman primate model, which continued even after withdrawal of immunosuppression [44]. Blockade of CD40/CD154 signaling pathway also was able to prolong graft survival in both renal and islet allotransplantation in nonhuman primates [44, 46, 51]. In these studies, the combination of both CTLA4-Ig and CD40 blockade appeared to prevent donor-specific antibody formation.
Memory T cells have been implicated in belatacept-resistant rejection; therefore, adjuvant therapy targeting memory T cell-specific features has been studied [40, 52]. An initial study of the LFA-3Ig molecule (alefacept) in vitro demonstrated suppression of alloreactive memory T cells, which were not suppressed by belatacept alone [45, 53]. Studies in nonhuman primates, however, demonstrated minimal benefit with an increased incidence of infectious complications [45, 47, 48, 53]. Based on early data, clinical use of the LFA-1 inhibitor, efalizumab, demonstrated some benefit in islet transplantation based on early data [42]. The use of LFA-1 inhibitor in combination with costimulation blockade also appeared to further prolong graft survival in islet allotransplantation [54]. LFA-1 exists in two forms: a commonly expressed, low-affinity form and a transient, high-affinity form, expressed only during activation. A recent study examined the use of more specific LFA-1 inhibitors (leukotoxin A and AL-579); targeting the high-affinity form of LFA-1 also did not demonstrate additional benefit in a renal transplant model [43]. Despite these data and the clinical potential, both alefacept and efalizumab were removed from the market by their manufacturers precluding further clinical study. A study using ICOS blockade with belatacept did not demonstrate any visible benefit to the combination of the two [50].
Costimulation blockade in clinical transplantation was first successfully introduced with the use of belatacept, a CTLA4-Ig molecule with higher affinity for B7 [26]. The initial BENEFIT trials demonstrated similar efficacy of belatacept-based regimens versus calcineurin inhibitors with an improved side effect profile [55–58]. However, a higher number of patients experienced an early severe rejection, which led to hesitation by many clinicians for widespread use [59]. Most of these rejection episodes were medically reversible which led to similar graft survival rates. The sparing of renal function demonstrated a potential benefit in long-term graft survival. Interestingly, patients who were on belatacept therapy also lacked significant production of donor-specific antibodies [29]. Further investigation into belatacept-resistant rejection demonstrated specific subsets of memory T cells that were present in patients who were not responsive to belatacept [40, 52, 60–62]. Alternative regimens incorporating belatacept in addition to conventional agents have shown promise [63–65], and further study to risk stratify these patients to individualize and introduce adjuvant therapy is ongoing.
Phase I clinical trials of a CD154 inhibitor demonstrated increased thrombotic phenomena not identified in preclinical testing and thus prevented clinical approval [66, 67] (as was subsequently demonstrated in xenotransplantation [68]). As preclinical data in allotransplant models appeared promising, newer agents to inhibit the CD40/CD154 and CD28/CD80/CD86 interaction and other costimulatory pathways are in the pipeline [69–72] but will need to complete their drug development cycle prior to consideration for human xenotransplant trials.
4. Costimulation Blockade in Xenotransplantation
The past two decades have been marked by great advances in the field of xenotransplantation with unprecedented graft survival times seen in preclinical models [1, 5, 13]. Tables 2, 3, and 4 summarize selected studies in solid organ (heart, kidney, and liver) and islet xenotransplantation with a specific use of anti-CD154mAb (Table 2), anti-CD40mAb (Table 3), or CTLA4-Ig (Table 4) between 2000 (the first use of costimulation blockade in xenotransplantation) to 2017. Continued development and improvement upon immunosuppressive regimens and the introduction of novel experimental agents appear to have contributed to this progress. Studies from the early part of the previous decade showed that induction therapy followed by high-dose conventional combination maintenance regimens was generally (but not uniformly) sufficient to sustain life-supporting pig grafts in nonhuman primates [73]. Conventional immunosuppressive therapy included agents such as cyclophosphamide, cyclosporine, mycophenolate mofetil, methylprednisone, and prednisolone (Tables 2, 3, and 4).
Table 2.
Selected studies using anti-CD154mAb in pig-to-nonhuman primate xenotransplantation.
| First author (year) | Donor pig | Recipient NHP | Immunosuppressive regimen | Longest survival (days) |
|---|---|---|---|---|
| Heart xenotransplantation, heterotopic | ||||
| Buhler (2000) [86] | WT | Baboon | TBI, TI, splenectomy, IA, ATG, CVF, CSA, or anti-CD154mAb, MMF +/− pig stem cells | N.A |
| Houser (2004) [87] | CD55 | Baboon | ATG, anti-CD2mAb, TI, CVF, anti-CD154mAb, MMF, CS | 139 |
| Dor (2005) [88] | GTKO | Baboon | ATG, anti-CD154mAb, MMF, CS | 179 |
| Kuwaki (2005) [89] | GTKO | Baboon | ATG, anti-CD2mAb, TI, CVF, anti-CD154mAb | 179 |
| Wu (2005) [90] | CD46 | Baboon | ATG, anti-CD154mAb, +/− anti-CD20mAb +/− CTLA4-Fc | 11 |
| Wu (2007) [91] | CD46 | Baboon | ATG, anti-CD154mAb, GAS194 or TPC, +/− IA | 36 |
| Ezzelarab (2009) [92] | GTKO | Baboon | ATG, CVF, anti-CD154mAb, MMF, CS | 56 |
| Mohiuddin (2012) [93] | GTKO.CD46 | Baboon | ATG, anti-CD20mAb, anti-CD154mAb, MMF, CS | 236 |
| Kim (2013) [94] | GTKO | Cynomolgus | ATG, anti-CD20mAb, anti-CD154mAb, tacrolimus, CS | 24 |
| Ezzelarab (2015) [95] | GTKO | Baboon | ATG, anti-CD154mAb, MMF | 56 |
| Iwase (2015) [96] | GTKO.CD46.TBM | Baboon | ATG, anti-CD20mAb, anti-CD154mAb, MMF, CS | 52 |
| Kidney xenotransplantation | ||||
| Buhler (2000) [86] | WT | Baboon | TBI, TI, splenectomy, IA, ATG, CVF, CSA, or anti-CD154mAb, MMF +/− pig stem cells | N.A |
| Buhler (2001) [97] | CD55 | Baboon | TBI, TI, splenectomy, IA, ATG, CVF, anti-CD154mAb, MMF, CS | 29 |
| Barth (2003) [98] | CD55 | Baboon | Thymokidneys, anti-CD2mAb, ATG, anti-CD154mAb, CyP, CVF, MMF, CS | 229 |
| Gollackner (2003) [99] | CD55 | Baboon | TI, splenectomy, IA, ATG, anti-CD154mAb, CyP, CVF, MMF, CS | 13 |
| Knosalla (2003) [100] | CD55 | Baboon | TI, splenectomy, IA, ATG, anti-CD154mAb, CyP, CVF, MMF, CS | 29 |
| Yamada (2005) [75] | GTKO | Baboon | Vascularized thymic lobe, WBI, anti-CD2mAb, anti-CD154mAb, MMF, CS, CVF | 68 |
| Shimizu (2005) [101] | CD55 | Baboon | Thymokidneys, splenectomy, IA, anti-CD3mAb, ATG, anti-CD154mAb, CyP, CVF, MMF | 30 |
| Griesemer (2009) [102] | GTKO | Baboon | Thymectomy, splenectomy, TBI, ATG, anti-CD2mAb, anti-CD154mAb, tacrolimus, MMF, anti-CD20mAb | 83 |
| Lin (2010) [103] | GTKO.CD46 | Baboon | ATG, antiCD154mAb, MMF, CVF, CS | 16 |
| Nishimura (2011) [104] | GTKO | Baboon | Thymokidney, thymectomy, splenectomy, anti-CD3, antiCD2mAb, ATG, anti-CD20mAb, tacrolimus, MMF, anti-CD154mAb | 15 |
| Ezzelarab (2015) [95] | GTKO | Baboon | ATG, anti-CD154mAb, MMF | 10 |
| Higginbotham (2015) [22] | GTKO.CD55 | Rhesus | Anti-CD4, anti-CD8, anti-CD154mAb, MMF, CS | 310 |
| Kim (2017) [76] | GTKO.CD55 | Rhesus | Anti-CD4, anti-CD8, anti-CD154mAb, MMF, CS | 405 |
| Liver xenotransplantation | ||||
| Kim (2002) [105] | GTKO | Baboon | ATG, LoCD2b, CVF, anti-CD154mAb, azathioprine, tacrolimus, CS | 9 |
| Navarro-Alvarez (2016) [106] | GTKO | Baboon | ATG, LoCD2b, CVF, anti-CD154mAb, tacrolimus, CS | 6 |
| Islet xenotransplantation | ||||
| Buhler (2002) [18] | WT | Baboon | Splenectomy, IA, TBI, ATG, CVF, anti-CD154mAb, CSA, MMF, CS | 28 |
| Hering (2006) [107] | WT | Cynomolgus | Anti-CD25mAb, FTY720, rapamycin, anti-CD154mAb | 187 |
| Cardona (2006) [108] | WT | Rhesus | Anti-CD25mAb, anti-CD154mAb, CTLA4-Ig | >260 |
| Rood (2007) [109] | GTKO | Cynomolgus | ATG, CVF, anti-CD154mAb, MMF, tacrolimus | >58 |
| Casu (2008) [110] | WT | Cynomolgus | ATG, anti-CD154mAb, MMF | >60 |
| van der Windt (2009) [19] | CD46 | Cynomolgus | ATG, anti-CD154mAb, MMF | 396 |
| Thompson (2011) [20] | GTKO | Rhesus | Anti-CD154mAb, anti-LFA1mAb, MMF, belatacept | 249 |
| Bottino (2014) [111] | GTKO.CD46. TFPI.CTLA4Ig.CD39 | Cynomolgus | ATG, MMF, anti-CD154mAb, CS | 365 |
| Shin (2015) [112] | WT | Rhesus | Anti-CD154mAb, ATG, rapamycin, CVF, adalimumab | >603 |
ATG: antithymocyte globulin; CS: corticosteroids; CSA: cyclosporine A; CVF: cobra venom factor; CyP: cyclophosphamide; NHP: nonhuman primate; TBI: total body irradiation; TI: thymus irradiation; mAb: monoclonal antibody; MMF: mycophenolate mofetil; mAb: monoclonal antibody; GTKO: α1,3-galactosyltransferase gene knockout; GAS914: a soluble glycoconjugate comprising Gal on poly-L-lysine backbone; N.A: not applicable; TBM: thrombomodulin; TPC: an aGal-polyethylene glycol polymer conjugate; WT: wild-type.
Table 3.
Selected studies using anti-CD40mAb in pig-to-nonhuman primate xenotransplantation.
| First author (year) | Donor pig | Recipient NHP | Immunosuppressive regimen | Longest survival (days) |
|---|---|---|---|---|
| Heart xenotransplantation, heterotopic | ||||
| Iwase (2015) [96] | GTKO.CD46.TBM | Baboon | ATG, belatacept, anti-CD40mAb, tacrolimus, MMF, CS | 130 |
| Mohiuddin (2016) [78] | GTKO.CD46.TBM | Baboon | ATG, anti-CD20mAb, anti-CD40mAb, CS | >900 |
| Kidney xenotransplantation | ||||
| Iwase (2015) [23] | GTKO.CD46.CD55 TBM.EPCR.CD39 | Baboon | ATG, anti-CD20mAb, anti-CD40mAb, rapamycin, tocilizumab, etanercept | 136 |
| Liver xenotransplantation | ||||
| Shah (2017) [24] | GTKO | Baboon | ATG, anti-CD40mAb, tacrolimus, CVF, CS | 29 |
| Islet xenotransplantation | ||||
| Thompson (2011) [21] | WT | Rhesus | Anti-CD25mAb, anti-CD40mAb, rapamycin, belatacept | 203 |
NHP: nonhuman primate; WT: wild-type; ATG: antithymocyte globulin; CVF: cobra venom factor; MMF: mycophenolate mofetil; mAb: monoclonal antibody; CS: corticosteroids; GTKO: α1,3-galactosyltransferase gene knockout; TBM: thrombomodulin; EPCR: endothelial cell protein C receptor.
Table 4.
Selected studies using CTLA4-Ig in pig-to-nonhuman primate xenotransplantation.
| First author (year) | Donor pig | Recipient NHP | Immunosuppressive regimen | Longest survival (days) |
|---|---|---|---|---|
| Heart xenotransplantation, heterotopic | ||||
| Iwase (2015) [96] | GTKO.CD46.CD55 | Baboon | ATG, anti-CD20mAb, abatacept, MMF, CS | 23 |
| Iwase (2015) [96] | GTKO.CD46.TBM | Baboon | ATG, belatacept, anti-CD40mAb, tacrolimus, MMF, CS | 130 |
| Liver xenotransplantation | ||||
| Shah (2017) [24] | GTKO | Baboon | ATG, belatacept, tacrolimus, CVF, CS | 25 |
| Islet xenotransplantation | ||||
| Cordona (2006) [108] | WT | Rhesus | Anti-CD25mAb, anti-CD154mAb, CTLA4-Ig | >260 |
| Hecht (2009) [113] | Fetal pancreatic fragments | Cynomolgus | Anti-CD25mAb, anti-CD154mAb, FTY720, rapamycin, CTLA4-Ig | 380 |
| Thompson (2011) [21] | WT | Rhesus | Anti-CD25mAb, anti-CD40mAb, rapamycin, belatacept | 203 |
| Thompson (2011) [20] | GTKO | Rhesus | Anti-CD154mAb, anti-LFA1mAb, MMF, belatacept | 249 |
| Thompson (2012) [79] | WT | Rhesus | MMF, belatacept, alefacept, anti-LFA1mAb, tacrolimus | 114 |
| Graham (2013) [114] | WT | Cynomolgus | Anti-CD25mAb, abatacept, tacrolimus, rapamycin | >180 |
NHP: nonhuman primate; WT: wild-type; ATG: anti-thymocyte globulin; CVF: cobra venom factor; MMF: mycophenolate mofetil; mAb: monoclonal antibody; CS: corticosteroids; GTKO: α1,3-galactosyltransferase gene knockout; TBM: thrombomodulin.
In 2000, Buhler et al. introduced the concept of costimulation blockade to the field of xenotransplantation [74]. Using a murine anti-human CD154mAb, they attempted to induce immune tolerance in nonhuman primates to transplanted pig peripheral blood mononuclear cells (PBMCs). More preclinical studies followed in both solid organ and islet xenotransplantation (Table 2) and increased markedly in the following decades. The most studied costimulatory modifiers within xenotransplantation have included anti-CD154mAb (Table 2), anti-CD40mAb (Table 3), and the CD28/B7 pathway (including CTLA4-Ig proteins abatacept and belatacept, as well as anti-CD28mAb, Table 4). Anti-CD154mAb therapy significantly prolongated porcine renal xenograft survival in nonhuman primates, with recent data demonstrating survival up to 405 days [22, 75, 76]. Unfortunately, this therapy is unlikely to be available for clinical xenotransplantation trials in the near future due to the agent's known thrombogenic properties [66–68]. High avidity CTLA4-Ig (belatacept) through interrupting the CD28/B7 pathway may be insufficient as monotherapy for xenograft maintenance [77]. Anti CD40mAb-based regimens have contributed to some of the longest reported xenograft survivals of pig heart and livers [24, 78]. Adhesion blockade with LFA-1 has also been utilized in a model of xenogenic islet transplantation, but with minimal benefit [79]. Further study continues in preclinical models to identify the most effective combination of costimulation blockade for xenotransplantation.
5. Costimulation Blockade and Genetic Modification of the Pig
Moving in parallel with this growing interest in xenotransplant costimulatory modification, genome-editing strategies aimed at costimulation pathways has also gained momentum. Xenotransplantation offers the unique potential to incorporate modifiers of the host immune response within the graft expression profile itself. To date, genetically modified pigs have been produced that alter the expression of endogenous porcine CTLA-4-Ig [80], or LEA29Y [81], or express human CD39 [82], or a human dominant-negative mutant class II transactivator [83]. Exhibiting variable successes, these approaches incorporate inhibitory regulation of the host costimulation interactions within the graft itself with the goal of facilitating suppression of host immune tolerance to the xenograft with less pharmacologic intervention than is required for allografts.
Regarding islet xenotransplantation, to date, five independent groups have reported survival of pig islets (genetically engineered or wild-type) for more than 3 months after transplantation into the liver of a nonhuman primate [19, 84]. Four groups utilized anti-CD154mAb-based immunosuppressive therapy (Table 2). Due to the likely unavailability of this agent, the Emory group has tried novel strategies with other clinically applicable or potentially clinically applicable medications such as basiliximab (anti-CD25mAb), LFA-1 blockade, and anti-CD40mAb (Table 3), in combination with belatacept.
Although several of these genetic strategies have provided promising results, the majority of gene-modification models are aimed at xenoantigen removal, complement regulation, or thromboregulatory properties of the xenograft. Indeed, these advances in genome-editing techniques have catalyzed a recent influx of novel and unique genetic backgrounds to the field of xenotransplantation. This rapid development raises a significant experimental issue; both novel genomic strategies and experimental immunosuppression strategies warrant individual appraisals. In the absence of a unified approach to gene modification within xenotransplantation, a cohesive appraisal of costimulatory intervention is challenging. The heterogeneity of genetic background thus prevents an effective stratification of costimulation blockade strategies for xenotransplantation. At present, a combination of graft modifications and exogenous immunosuppressive therapy to the host will be necessary to promote clinical application of xenotransplantation [1, 3, 13, 84, 85]. A standardized approach to testing genetic modification in combination with novel immunosuppressive agents will ideally bring clarity to the optimal combinations.
6. Conclusions
Currently published preclinical data demonstrate that immunosuppressive therapy, typically incorporating costimulation blockade agents, is required for successful engraftment of porcine tissues, even those with considerable genetic modification [9]. This convergence of experimental therapies in the preclinical setting presents a predicament when considering clinical xenotransplantation trials [31]. It is as yet uncertain whether conventional immunosuppressive agents may be effective enough to facilitate engraftment and maintenance of genetically modified (“humanized”) porcine organs or tissues. Furthermore, many of the immunosuppressive agents currently being tested in nonhuman primate models are not yet approved for clinical use. More rigorous testing of novel genetically modified pigs with minimal and/or more clinically relevant immunosuppression is warranted. However, the potential of costimulation blockade in xenotransplantation holds great promise for future use. Although genome-edited pig xenografts will certainly minimize the need for novel immunosuppressive agents, the increasing depth of our costimulation blockade library will benefit the future of allotransplantation and xenotransplantation alike.
Acknowledgments
The work on xenotransplantation in the Xenotransplantation Research Laboratory at Indiana University has been supported by internal funds of the Department of Surgery. The work on xenotransplantation at the University of Alabama at Birmingham is supported in part by NIH NIAID U19 Grant AI090959.
Abbreviations
- APC:
Antigen-presenting cells
- AHXR:
Acute humoral xenograft rejection
- Gal:
Gal α(1,3) Gal
- GTKO:
α1,3-Galactosyltransferase gene knockout
- LFA:
Lymphocyte function-associated antigen
- mAb:
Monoclonal antibody
- MHC:
Major histocompatibility complex.
Conflicts of Interest
None of the authors has a conflict of interest.
Authors' Contributions
This manuscript has been revised and approved by all authors.
References
- 1.Ekser B., Ezzelarab M., Hara H., et al. Clinical xenotransplantation: the next medical revolution? Lancet. 2012;379(9816):672–683. doi: 10.1016/S0140-6736(11)61091-X. [DOI] [PubMed] [Google Scholar]
- 2.Ekser B., Cooper D. K., Tector A. J. The need for xenotransplantation as a source of organs and cells for clinical transplantation. International Journal of Surgery. 2015;23(Part B):199–204. doi: 10.1016/j.ijsu.2015.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ekser B., Tector A. J., Cooper D. K. Progress toward clinical xenotransplantation. International Journal of Surgery. 2015;23(Part B):197–198. doi: 10.1016/j.ijsu.2015.08.036. [DOI] [PubMed] [Google Scholar]
- 4.Cooper D. K., Ekser B., Tector A. J. Immunobiological barriers to xenotransplantation. International Journal of Surgery. 2015;23(Part B):211–216. doi: 10.1016/j.ijsu.2015.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper D. K., Ezzelarab M. B., Hara H., et al. The pathobiology of pig-to-primate xenotransplantation: a historical review. Xenotransplantation. 2016;23(2):83–105. doi: 10.1111/xen.12219. [DOI] [PubMed] [Google Scholar]
- 6.Cooper D. K. Depletion of natural antibodies in non-human primates--a step towards successful discordant xenografting in humans. Clinical Transplantation. 1992;6(3, Part 1):178–183. [PubMed] [Google Scholar]
- 7.Cooper D. K., Good A. H., Koren E., et al. Identification of α-galactosyl and other carbohydrate epitopes that are bound by human anti-pig antibodies: relevance to discordant xenografting in man. Transplant Immunology. 1993;1(3):198–205. doi: 10.1016/0966-3274(93)90047-c. [DOI] [PubMed] [Google Scholar]
- 8.Good A. H., Cooper D. K., Malcolm A. J., et al. Identification of carbohydrate structures that bind human antiporcine antibodies: implications for discordant xenografting in humans. Transplantation Proceedings. 1992;24(2):559–562. [PubMed] [Google Scholar]
- 9.Cooper D. K., Ekser B., Ramsoondar J., Phelps C., Ayares D. The role of genetically engineered pigs in xenotransplantation research. The Journal of Pathology. 2016;238(2):288–299. doi: 10.1002/path.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phelps C. J., Koike C., Vaught T. D., et al. Production of α1,3-galactosyltransferase-deficient pigs. Science. 2003;299(5605):411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler J. R., Ladowski J. M., Martens G. R., Tector M., Tector A. J. Recent advances in genome editing and creation of genetically modified pigs. International Journal of Surgery. 2015;23(Part B):217–222. doi: 10.1016/j.ijsu.2015.07.684. [DOI] [PubMed] [Google Scholar]
- 12.Cooper D. K., Hara H., Ezzelarab M., et al. The potential of genetically-engineered pigs in providing an alternative source of organs and cells for transplantation. Journal of Biomedical Research. 2013;27(4):249–253. doi: 10.7555/jbr.27.20130063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butler J. R., Tector A. J. CRISPR genome-editing: a medical revolution. The Journal of Thoracic and Cardiovascular Surgery. 2017;153(2):488–491. doi: 10.1016/j.jtcvs.2016.08.067. [DOI] [PubMed] [Google Scholar]
- 14.Butler J. R., Martens G. R., Estrada J. L., et al. Silencing porcine genes significantly reduces human-anti-pig cytotoxicity profiles: an alternative to direct complement regulation. Transgenic Research. 2016;25(5):751–759. doi: 10.1007/s11248-016-9958-0. [DOI] [PubMed] [Google Scholar]
- 15.Halloran P. F. Immunosuppressive drugs for kidney transplantation. The New England Journal of Medicine. 2004;351(26):2715–2729. doi: 10.1056/nejmra033540. [DOI] [PubMed] [Google Scholar]
- 16.Wiseman A. C. Immunosuppressive medications. Clinical Journal of the American Society of Nephrology. 2016;11(2):332–343. doi: 10.2215/cjn.08570814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ford M. L., Adams A. B., Pearson T. C. Targeting co-stimulatory pathways: transplantation and autoimmunity. Nature Reviews Nephrology. 2014;10(1):14–24. doi: 10.1038/nrneph.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buhler L., Deng S., O'Neil J., et al. Adult porcine islet transplantation in baboons treated with conventional immunosuppression or a non-myeloablative regimen and CD154 blockade. Xenotransplantation. 2002;9(1):3–13. doi: 10.1034/j.1399-3089.2002.1o044.x. [DOI] [PubMed] [Google Scholar]
- 19.van der Windt D. J., Bottino R., Casu A., et al. Long-term controlled normoglycemia in diabetic non-human primates after transplantation with hCD46 transgenic porcine islets. American Journal of Transplantation. 2009;9(12):2716–2726. doi: 10.1111/j.1600-6143.2009.02850.x. [DOI] [PubMed] [Google Scholar]
- 20.Thompson P., Badell I. R., Lowe M., et al. Islet xenotransplantation using gal-deficient neonatal donors improves engraftment and function. American Journal of Transplantation. 2011;11(12):2593–2602. doi: 10.1111/j.1600-6143.2011.03720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson P., Cardona K., Russell M., et al. CD40-specific costimulation blockade enhances neonatal porcine islet survival in nonhuman primates. American Journal of Transplantation. 2011;11(5):947–957. doi: 10.1111/j.1600-6143.2011.03509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higginbotham L., Mathews D., Breeden C. A., et al. Pre-transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model. Xenotransplantation. 2015;22(3):221–230. doi: 10.1111/xen.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwase H., Liu H., Wijkstrom M., et al. Pig kidney graft survival in a baboon for 136 days: longest life-supporting organ graft survival to date. Xenotransplantation. 2015;22(4):302–309. doi: 10.1111/xen.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah J. A., Patel M. S., Elias N., et al. Prolonged survival following pig-to-primate liver xenotransplantation utilizing exogenous coagulation factors and costimulation blockade. American Journal of Transplantation. 2017;17(8):2178–2185. doi: 10.1111/ajt.14341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwase H., Hara H., Ezzelarab M., et al. Immunological and physiological observations in baboons with life-supporting genetically engineered pig kidney grafts. Xenotransplantation. 2017;24(2, article e12293) doi: 10.1111/xen.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wekerle T., Grinyo J. M. Belatacept: from rational design to clinical application. Transplant International. 2012;25(2):139–150. doi: 10.1111/j.1432-2277.2011.01386.x. [DOI] [PubMed] [Google Scholar]
- 27.Esensten J. H., Helou Y. A., Chopra G., Weiss A., Bluestone J. A. CD28 costimulation: from mechanism to therapy. Immunity. 2016;44(5):973–988. doi: 10.1016/j.immuni.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larsen C. P., Pearson T. C., Adams A. B., et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. American Journal of Transplantation. 2005;5(3):443–453. doi: 10.1111/j.1600-6143.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 29.Vincenti F., Larsen C., Durrbach A., et al. Costimulation blockade with belatacept in renal transplantation. The New England Journal of Medicine. 2005;353(8):770–781. doi: 10.1056/nejmoa050085. [DOI] [PubMed] [Google Scholar]
- 30.Hering B. J., Cooper D. K., Cozzi E., et al. The International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes-- executive summary. Xenotransplantation. 2009;16(4):196–202. doi: 10.1111/j.1399-3089.2009.00547.x. [DOI] [PubMed] [Google Scholar]
- 31.Cooper D. K. C., Wijkstrom M., Hariharan S., et al. Selection of patients for initial clinical trials of solid organ xenotransplantation. Transplantation. 2017;101(7):1551–1558. doi: 10.1097/tp.0000000000001582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharpe A. H., Abbas A. K. T-cell costimulation—biology, therapeutic potential, and challenges. New England Journal of Medicine. 2006;355(10):973–975. doi: 10.1056/nejmp068087. [DOI] [PubMed] [Google Scholar]
- 33.Sharpe A. H. Mechanisms of costimulation. Immunological Reviews. 2009;229(1):5–11. doi: 10.1111/j.1600-065x.2009.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mou D., Espinosa J., Lo D. J., Kirk A. D. CD28 negative T cells: is their loss our gain? American Journal of Transplantation. 2014;14(11):2460–2466. doi: 10.1111/ajt.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medzhitov R., Janeway C. A., Jr. Innate immune recognition and control of adaptive immune responses. Seminars in Immunology. 1998;10(5):351–353. doi: 10.1006/smim.1998.0136. [DOI] [PubMed] [Google Scholar]
- 36.Sprague D. L., Sowa J. M., Elzey B. D., Ratliff T. L. The role of platelet CD154 in the modulation in adaptive immunity. Immunologic Research. 2007;39(1–3):185–193. doi: 10.1007/s12026-007-0074-3. [DOI] [PubMed] [Google Scholar]
- 37.Ma D. Y., Clark E. A. The role of CD40 and CD154/CD40L in dendritic cells. Seminars in Immunology. 2009;21(5):265–272. doi: 10.1016/j.smim.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cerutti A., Puga I., Cols M. Innate control of B cell responses. Trends in Immunology. 2011;32(5):202–211. doi: 10.1016/j.it.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wikenheiser D. J., Stumhofer J. S. ICOS co-stimulation: friend or foe? Frontiers in Immunology. 2016;7:p. 304. doi: 10.3389/fimmu.2016.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Espinosa J. R., Samy K. P., Kirk A. D. Memory T cells in organ transplantation: progress and challenges. Nature Reviews Nephrology. 2016;12(6):339–347. doi: 10.1038/nrneph.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson D. J., Lo D. J., Leopardi F., et al. Anti-LFA-1 therapy in a nonhuman primate renal transplant model of costimulation blockade resistant rejection. American Journal of Transplantation. 2016;16(5):1456–1464. doi: 10.1111/ajt.13628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nicolls M. R., Gill R. G. LFA-1 (CD11a) as a therapeutic target. American Journal of Transplantation. 2006;6(1):27–36. doi: 10.1111/j.1600-6143.2005.01158.x. [DOI] [PubMed] [Google Scholar]
- 43.Samy K. P., Anderson D. J., Lo D. J., et al. Selective targeting of high-affinity LFA-1 does not augment costimulation blockade in a nonhuman primate renal transplantation model. American Journal of Transplantation. 2017;17(5):1193–1203. doi: 10.1111/ajt.14141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirk A. D., Harlan D. M., Armstrong N. N., et al. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(16):8789–8794. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weaver T. A., Charafeddine A. H., Agarwal A., et al. Alefacept promotes co-stimulation blockade based allograft survival in nonhuman primates. Nature Medicine. 2009;15(7):746–749. doi: 10.1038/nm.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Badell I. R., Russell M. C., Cardona K., et al. CTLA4Ig prevents alloantibody formation following nonhuman primate islet transplantation using the CD40-specific antibody 3A8. American Journal of Transplantation. 2012;12(7):1918–1923. doi: 10.1111/j.1600-6143.2012.04029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lo D. J., Anderson D. J., Weaver T. A., et al. Belatacept and sirolimus prolong nonhuman primate renal allograft survival without a requirement for memory T cell depletion. American Journal of Transplantation. 2013;13(2):320–328. doi: 10.1111/j.1600-6143.2012.04342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lowe M. C., Badell I. R., Turner A. P., et al. Belatacept and sirolimus prolong nonhuman primate islet allograft survival: adverse consequences of concomitant alefacept therapy. American Journal of Transplantation. 2013;13(2):312–319. doi: 10.1111/j.1600-6143.2012.04341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freitas A. M., Samy K. P., Farris A. B., et al. Studies introducing costimulation blockade for vascularized composite allografts in nonhuman primates. American Journal of Transplantation. 2015;15(8):2240–2249. doi: 10.1111/ajt.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lo D. J., Anderson D. J., Song M., et al. A pilot trial targeting the ICOS-ICOS-L pathway in nonhuman primate kidney transplantation. American Journal of Transplantation. 2015;15(4):984–992. doi: 10.1111/ajt.13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kirk A. D., Burkly L. C., Batty D. S., et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nature Medicine. 1999;5(6):686–693. doi: 10.1038/9536. [DOI] [PubMed] [Google Scholar]
- 52.Espinosa J., Herr F., Tharp G., et al. CD57+ CD4 T cells underlie belatacept-resistant allograft rejection. American Journal of Transplantation. 2016;16(4):1102–1112. doi: 10.1111/ajt.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vivot K., Langlois A., Jeandidier N., et al. Instant blood-mediated inflammatory reaction during islet transplantation: the role of Toll-like receptors signaling pathways. Transplantation Proceedings. 2011;43(9):3192–3194. doi: 10.1016/j.transproceed.2011.09.056. [DOI] [PubMed] [Google Scholar]
- 54.Badell I. R., Russell M. C., Thompson P. W., et al. LFA-1-specific therapy prolongs allograft survival in rhesus macaques. The Journal of Clinical Investigation. 2010;120(12):4520–4531. doi: 10.1172/jci43895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dobbels F., Wong S., Min Y., Sam J., Kalsekar A. Beneficial effect of belatacept on health-related quality of life and perceived side effects: results from the BENEFIT and BENEFIT-EXT trials. Transplantation. 2014;98(9):960–968. doi: 10.1097/tp.0000000000000159. [DOI] [PubMed] [Google Scholar]
- 56.Durrbach A., Pestana J. M., Pearson T., et al. A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT study) American Journal of Transplantation. 2010;10(3):547–557. doi: 10.1111/j.1600-6143.2010.03016.x. [DOI] [PubMed] [Google Scholar]
- 57.Larsen C. P., Grinyo J., Medina-Pestana J., et al. Belatacept-based regimens versus a cyclosporine A-based regimen in kidney transplant recipients: 2-year results from the BENEFIT and BENEFIT-EXT studies. Transplantation. 2010;90(12):1528–1535. doi: 10.1097/tp.0b013e3181ff87cd. [DOI] [PubMed] [Google Scholar]
- 58.Vincenti F., Charpentier B., Vanrenterghem Y., et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study) American Journal of Transplantation. 2010;10(3):535–546. doi: 10.1111/j.1600-6143.2009.03005.x. [DOI] [PubMed] [Google Scholar]
- 59.Rostaing L., Vincenti F., Grinyo J., et al. Long-term belatacept exposure maintains efficacy and safety at 5 years: results from the long-term extension of the BENEFIT study. American Journal of Transplantation. 2013;13(11):2875–2883. doi: 10.1111/ajt.12460. [DOI] [PubMed] [Google Scholar]
- 60.Krummey S. M., Cheeseman J. A., Conger J. A., et al. High CTLA-4 expression on Th17 cells results in increased sensitivity to CTLA-4 coinhibition and resistance to belatacept. American Journal of Transplantation. 2014;14(3):607–614. doi: 10.1111/ajt.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mathews D. V., Wakwe W. C., Kim S. C., et al. Belatacept resistant rejection is associated with CD28+ memory CD8 T cells. American Journal of Transplantation. 2017;17(9):2285–2299. doi: 10.1111/ajt.14349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cortes-Cerisuelo M., Laurie S. J., Mathews D. V., et al. Increased pre-transplant frequency of CD28+ CD4+ TEM predicts belatacept-resistant rejection in human renal transplant recipients. American Journal of Transplantation. 2017;17(9):2350–2362. doi: 10.1111/ajt.14350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kirk A. D., Guasch A., Xu H., et al. Renal transplantation using belatacept without maintenance steroids or calcineurin inhibitors. American Journal of Transplantation. 2014;14(5):1142–1151. doi: 10.1111/ajt.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ferguson R., Grinyo J., Vincenti F., et al. Immunosuppression with belatacept-based, corticosteroid-avoiding regimens in de novo kidney transplant recipients. American Journal of Transplantation. 2011;11(1):66–76. doi: 10.1111/j.1600-6143.2010.03338.x. [DOI] [PubMed] [Google Scholar]
- 65.Gupta G., Regmi A., Kumar D., et al. Safe conversion from tacrolimus to belatacept in high immunologic risk kidney transplant recipients with allograft dysfunction. American Journal of Transplantation. 2015;15(10):2726–2731. doi: 10.1111/ajt.13322. [DOI] [PubMed] [Google Scholar]
- 66.Law C. L., Grewal I. S. Therapeutic interventions targeting CD40L (CD154) and CD40: the opportunities and challenges. Advances in Experimental Medicine and Biology. 2009;647:8–36. doi: 10.1007/978-0-387-89520-8_2. [DOI] [PubMed] [Google Scholar]
- 67.Inwald D. P., McDowall A., Peters M. J., Callard R. E., Klein N. J. CD40 is constitutively expressed on platelets and provides a novel mechanism for platelet activation. Circulation Research. 2003;92(9):1041–1048. doi: 10.1161/01.res.0000070111.98158.6c. [DOI] [PubMed] [Google Scholar]
- 68.Knosalla C., Gollackner B., Cooper D. K. Anti-CD154 monoclonal antibody and thromboembolism revisted. Transplantation. 2002;74(3):416–417. doi: 10.1097/00007890-200208150-00024. [DOI] [PubMed] [Google Scholar]
- 69.Kim S. C., Wakwe W., Higginbotham L. B., et al. Fc-silent anti-CD154 domain antibody effectively prevents nonhuman primate renal allograft rejection. American Journal of Transplantation. 2017;17(5):1182–1192. doi: 10.1111/ajt.14197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oshima S., Karrer E. E., Kawato Y., et al. The effect of ASP2409, a novel CD86-selective variant of CTLA4-Ig, on renal allograft rejection in nonhuman primates. Transplantation. 2016;100(12):2611–2620. doi: 10.1097/tp.0000000000001397. [DOI] [PubMed] [Google Scholar]
- 71.Poirier N., Blancho G., Hiance M., et al. First-in-human study in healthy subjects with FR104, a pegylated monoclonal antibody fragment antagonist of CD28. Journal of Immunology. 2016;197(12):4593–4602. doi: 10.4049/jimmunol.1601538. [DOI] [PubMed] [Google Scholar]
- 72.Song L., Ma A., Dun H., et al. ASP2409, a next-generation CTLA4-Ig, versus belatacept in renal allograft survival in cynomolgus monkeys. American Journal of Transplantation. 2017;17(3):635–645. doi: 10.1111/ajt.14039. [DOI] [PubMed] [Google Scholar]
- 73.Cozzi E., Bhatti F., Schmoeckel M., et al. Long-term survival of nonhuman primates receiving life-supporting transgenic porcine kidney xenografts. Transplantation. 2000;70(1):15–21. [PubMed] [Google Scholar]
- 74.Buhler L., Awwad M., Basker M., et al. High-dose porcine hematopoietic cell transplantation combined with CD40 ligand blockade in baboons prevents an induced anti-pig humoral response. Transplantation. 2000;69(11):2296–2304. doi: 10.1097/00007890-200006150-00013. [DOI] [PubMed] [Google Scholar]
- 75.Yamada K., Yazawa K., Shimizu A., et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of α1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nature Medicine. 2005;11(1):32–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 76.Kim S., Higginbotham L., Mathews D., et al. CD4 depletion is necessary and sufficient for long-term nonhuman primate xenotransplant survival. American Journal of Transplantation. 2017;17(Supplement 3):374–374. [Google Scholar]
- 77.Iwase H., Ekser B., Satyananda V., et al. Initial in vivo experience of pig artery patch transplantation in baboons using mutant MHC (CIITA-DN) pigs. Transplant Immunology. 2015;32(2):99–108. doi: 10.1016/j.trim.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mohiuddin M. M., Singh A. K., Corcoran P. C., et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nature Communications. 2016;7, article 11138 doi: 10.1038/ncomms11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thompson P., Badell I. R., Lowe M., et al. Alternative immunomodulatory strategies for xenotransplantation: CD40/154 pathway-sparing regimens promote xenograft survival. American Journal of Transplantation. 2012;12(7):1765–1775. doi: 10.1111/j.1600-6143.2012.04031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Phelps C. J., Ball S. F., Vaught T. D., et al. Production and characterization of transgenic pigs expressing porcine CTLA4-Ig. Xenotransplantation. 2009;16(6):477–485. doi: 10.1111/j.1399-3089.2009.00533.x. [DOI] [PubMed] [Google Scholar]
- 81.Klymiuk N., van Buerck L., Bahr A., et al. Xenografted islet cell clusters from INSLEA29Y transgenic pigs rescue diabetes and prevent immune rejection in humanized mice. Diabetes. 2012;61(6):1527–1532. doi: 10.2337/db11-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wheeler D. G., Joseph M. E., Mahamud S. D., et al. Transgenic swine: expression of human CD39 protects against myocardial injury. Journal of Molecular and Cellular Cardiology. 2012;52(5):958–961. doi: 10.1016/j.yjmcc.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hara H., Witt W., Crossley T., et al. Human dominant-negative class II transactivator transgenic pigs - effect on the human anti-pig T-cell immune response and immune status. Immunology. 2013;140(1):39–46. doi: 10.1111/imm.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu Z., Hu W., He T., et al. Pig-to-primate islet xenotransplantation: past, present, and future. Cell Transplantation. 2017;26(6):925–947. doi: 10.3727/096368917x694859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ekser B., Bottino R., Cooper D. K. Clinical islet xenotransplantation: a step forward. eBioMedicine. 2016;12:22–23. doi: 10.1016/j.ebiom.2016.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Buhler L., Basker M., Alwayn I. P., et al. Coagulation and thrombotic disorders associated with pig organ and hematopoietic cell transplantation in nonhuman primates. Transplantation. 2000;70(9):1323–1331. doi: 10.1097/00007890-200011150-00010. [DOI] [PubMed] [Google Scholar]
- 87.Houser S. L., Kuwaki K., Knosalla C., et al. Thrombotic microangiopathy and graft arteriopathy in pig hearts following transplantation into baboons. Xenotransplantation. 2004;11(5):416–425. doi: 10.1111/j.1399-3089.2004.00155.x. [DOI] [PubMed] [Google Scholar]
- 88.Dor F. J., Kuwaki K., Tseng Y. L., et al. Potential of aspirin to inhibit thrombotic microangiopathy in α1,3-galactosyltransferase gene-knockout pig hearts after transplantation in baboons. Transplantation Proceedings. 2005;37(1):489–490. doi: 10.1016/j.transproceed.2004.12.235. [DOI] [PubMed] [Google Scholar]
- 89.Kuwaki K., Tseng Y. L., Dor F. J., et al. Heart transplantation in baboons using α1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nature Medicine. 2005;11(1):29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 90.Wu G., Pfeiffer S., Schroder C., et al. Co-stimulation blockade targeting CD154 and CD28/B7 modulates the induced antibody response after a pig-to-baboon cardiac xenograft. Xenotransplantation. 2005;12(3):197–208. doi: 10.1111/j.1399-3089.2005.00221.x. [DOI] [PubMed] [Google Scholar]
- 91.Wu G., Pfeiffer S., Schroder C., et al. Coagulation cascade activation triggers early failure of pig hearts expressing human complement regulatory genes. Xenotransplantation. 2007;14(1):34–47. doi: 10.1111/j.1399-3089.2006.00362.x. [DOI] [PubMed] [Google Scholar]
- 92.Ezzelarab M., Garcia B., Azimzadeh A., et al. The innate immune response and activation of coagulation in α1,3-galactosyltransferase gene-knockout xenograft recipients. Transplantation. 2009;87(6):805–812. doi: 10.1097/tp.0b013e318199c34f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mohiuddin M. M., Corcoran P. C., Singh A. K., et al. B-cell depletion extends the survival of GTKO.hCD46Tg pig heart xenografts in baboons for up to 8 months. American Journal of Transplantation. 2012;12(3):763–771. doi: 10.1111/j.1600-6143.2011.03846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim H., Chee H. K., Yang J., et al. Outcomes of alpha 1,3-GT-knockout porcine heart transplants into a preclinical nonhuman primate model. Transplantation Proceedings. 2013;45(8):3085–3091. doi: 10.1016/j.transproceed.2013.08.049. [DOI] [PubMed] [Google Scholar]
- 95.Ezzelarab M. B., Ekser B., Azimzadeh A., et al. Systemic inflammation in xenograft recipients precedes activation of coagulation. Xenotransplantation. 2015;22(1):32–47. doi: 10.1111/xen.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Iwase H., Ekser B., Satyananda V., et al. Pig-to-baboon heterotopic heart transplantation--exploratory preliminary experience with pigs transgenic for human thrombomodulin and comparison of three costimulation blockade-based regimens. Xenotransplantation. 2015;22(3):211–220. doi: 10.1111/xen.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Buhler L., Yamada K., Kitamura H., et al. Pig kidney transplantation in baboons: anti-Gal(alpha)1-3Gal IgM alone is associated with acute humoral xenograft rejection and disseminated intravascular coagulation. Transplantation. 2001;72(11):1743–1752. doi: 10.1097/00007890-200112150-00007. [DOI] [PubMed] [Google Scholar]
- 98.Barth R. N., Yamamoto S., LaMattina J. C., et al. Xenogeneic thymokidney and thymic tissue transplantation in a pig-to-baboon model: I. evidence for pig-specific T-cell unresponsiveness. Transplantation. 2003;75(10):1615–1624. doi: 10.1097/01.tp.0000064335.50622.20. [DOI] [PubMed] [Google Scholar]
- 99.Gollackner B., Mueller N. J., Houser S., et al. Porcine cytomegalovirus and coagulopathy in pig-to-primate xenotransplantation. Transplantation. 2003;75(11):1841–1847. doi: 10.1097/01.tp.0000065806.90840.c1. [DOI] [PubMed] [Google Scholar]
- 100.Knosalla C., Gollackner B., Bühler L., et al. Correlation of biochemical and hematological changes with graft failure following pig heart and kidney transplantation in baboons. American Journal of Transplantation. 2003;3(12):1510–1519. doi: 10.1046/j.1600-6135.2003.00258.x. [DOI] [PubMed] [Google Scholar]
- 101.Shimizu A., Yamada K., Yamamoto S., et al. Thrombotic microangiopathic glomerulopathy in human decay accelerating factor-transgenic swine-to-baboon kidney xenografts. Journal of the American Society of Nephrology. 2005;16(9):2732–2745. doi: 10.1681/asn.2004121148. [DOI] [PubMed] [Google Scholar]
- 102.Griesemer A. D., Hirakata A., Shimizu A., et al. Results of gal-knockout porcine thymokidney xenografts. American Journal of Transplantation. 2009;9(12):2669–2678. doi: 10.1111/j.1600-6143.2009.02849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lin C. C., Ezzelarab M., Shapiro R., et al. Recipient tissue factor expression is associated with consumptive coagulopathy in pig-to-primate kidney xenotransplantation. American Journal of Transplantation. 2010;10(7):1556–1568. doi: 10.1111/j.1600-6143.2010.03147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nishimura H., Scalea J., Wang Z., et al. First experience with the use of a recombinant CD3 immunotoxin as induction therapy in pig-to-primate xenotransplantation: the effect of T-cell depletion on outcome. Transplantation. 2011;92(6):641–647. doi: 10.1097/tp.0b013e31822b92a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim K., Schuetz C., Elias N., et al. Up to 9-day survival and control of thrombocytopenia following alpha1,3-galactosyl transferase knockout swine liver xenotransplantation in baboons. Xenotransplantation. 2012;19(4):256–264. doi: 10.1111/j.1399-3089.2012.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Navarro-Alvarez N., Shah J. A., Zhu A., et al. The effects of exogenous administration of human coagulation factors following pig-to-baboon liver xenotransplantation. American Journal of Transplantation. 2016;16(6):1715–1725. doi: 10.1111/ajt.13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hering B. J., Wijkstrom M., Graham M. L., et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nature Medicine. 2006;12(3):301–303. doi: 10.1038/nm1369. [DOI] [PubMed] [Google Scholar]
- 108.Cardona K., Korbutt G. S., Milas Z., et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nature Medicine. 2006;12(3):304–306. doi: 10.1038/nm1375. [DOI] [PubMed] [Google Scholar]
- 109.Rood P. P., Bottino R., Balamurugan A. N., et al. Reduction of early graft loss after intraportal porcine islet transplantation in monkeys. Transplantation. 2007;83(2):202–210. doi: 10.1097/01.tp.0000250680.36942.c6. [DOI] [PubMed] [Google Scholar]
- 110.Casu A., Bottino R., Balamurugan A. N., et al. Metabolic aspects of pig-to-monkey (Macaca fascicularis) islet transplantation: implications for translation into clinical practice. Diabetologia. 2008;51(1):120–129. doi: 10.1111/j.1748-5827.1975.tb05802.x. [DOI] [PubMed] [Google Scholar]
- 111.Bottino R., Wijkstrom M., van der Windt D. J., et al. Pig-to-monkey islet xenotransplantation using multi-transgenic pigs. American Journal of Transplantation. 2014;14(10):2275–2287. doi: 10.1111/ajt.12868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shin J. S., Kim J. M., Kim J. S., et al. Long-term control of diabetes in immunosuppressed nonhuman primates (NHP) by the transplantation of adult porcine islets. American Journal of Transplantation. 2015;15(11):2837–2850. doi: 10.1111/ajt.13345. [DOI] [PubMed] [Google Scholar]
- 113.Hecht G., Eventov-Friedman S., Rosen C., et al. Embryonic pig pancreatic tissue for the treatment of diabetes in a nonhuman primate model. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(21):8659–8664. doi: 10.1073/pnas.0812253106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Graham M. L., Schuurman H. J. The usefulness and limitations of the diabetic macaque model in evaluating long-term porcine islet xenograft survival. Xenotransplantation. 2013;20(1):5–17. doi: 10.1111/xen.12012. [DOI] [PubMed] [Google Scholar]