Abstract
The fire blight pathogen Erwinia amylovora can be considered a psychrotrophic bacterial species since it can grow at temperatures ranging from 4 °C to 37 °C, with an optimum of 28 °C. In many plant pathogens the expression of virulence determinants is restricted to a certain range of temperatures. In the case of E. amylovora, temperatures above 18 °C are required for blossom blight epidemics under field conditions. Moreover, this bacterium is able to infect a variety of host tissues/organs apart from flowers, but it is still unknown how environmental temperatures, especially those below 18 °C, affect the pathogen ability to cause fire blight disease symptoms in such tissues/organs. There is also scarce information on how temperatures below 18 °C affect the E. amylovora starvation-survival responses, which might determine its persistence in the environment and probably contribute to the seasonal development of fire blight disease, as occurs in other pathogens. To characterize the virulence and survival of E. amylovora at temperate and low temperatures, we evaluated the effect of three temperatures (4 °C, 14 °C, 28 °C) on symptom development, and on different parameters linked to starvation and virulence. E. amylovora was pathogenic at the three assayed temperatures, with a slow-down of symptom development correlating with colder temperatures and slower growth rates. Siderophore secretion and motility also decreased in parallel to incubation temperatures. However, production of the exopolysaccharides amylovoran and levan was enhanced at 4 °C and 14 °C, respectively. Similarly, biofilm formation, and oxidative stress resistance were improved at 14 °C, with this temperature also favoring the maintenance of culturability, together with a reduction in cell size and the acquisition of rounded shapes in E. amylovora cells subjected to long-term starvation. However, starvation at 28 °C and 4 °C induced an enhanced viable but nonculturable (VBNC) response (to a lesser extent at 4 °C). This work reveals E. amylovora as a highly adaptable pathogen that retains its pathogenic potential even at the minimal growth temperatures, with an improved exopolysaccharide synthesis, biofilm formation or oxidative stress resistance at 14 °C, with respect to the optimal growth temperature (28 °C). Finally, our results also demonstrate the thermal modulation of starvation responses in E. amylovora, suggesting that the starvation-survival and the VBNC states are part of its life cycle. These results confirm the particular psychrotrophic adaptations of E. amylovora, revealing its pathogenic potential and survival at temperate and low environmental temperatures, which have probably contributed to its successful spread to countries with different climates. This knowledge might improve integrated control measures against fire blight.
Keywords: Fire blight, EPS, Biofilms, Oxidative stress, Siderophores, VBNC, Motility, Starvation, Virulence
Introduction
Erwinia amylovora is a Gram-negative bacterium that causes fire blight, a destructive plant disease affecting economically important fruit trees such as pear and apple, as well as ornamental plants of the family Rosaceae. Among the environmental factors affecting the development of fire blight, temperature is a critical one, directly affecting bacterial growth, the phenologic development of the host (and interactions among these factors), and determining the presence of vectors responsible for the spread of the pathogen (Thomson, 2000; Van der Zwet, Orolaza-Halbrendt & Zeller, 2012).
Temperature ranges allowing bacterial growth are usually linked to the ecology of each bacterial species, with the ability of bacteria to adapt and/or tolerate certain temperatures directly conditioning their pathogenicity, virulence and/or niches (Jay, 1998; Scherf, Milling & Allen, 2010; Burbank & Stenger, 2016; Du Raan, Coutinho & Van der Waals, 2016; Morris et al., 2016). Compared to other phytopathogens (Du Raan, Coutinho & Van der Waals, 2016; Burbank & Stenger, 2016), many of them mesophilic, E. amylovora possesses an unusually wide range of growth temperatures (4 °C–37 °C), the optimal temperature being 28 °C (Van der Zwet, Orolaza-Halbrendt & Zeller, 2012). It is for this reason that this pathogen can be considered a psychrotrophic microorganism (i.e., able to grow at low temperatures but with optimal and maximal growth temperatures above 15 °C and 20 °C, respectively) (Moyer & Morita, 2007). However, how this characteristic is connected to the E. amylovora life cycle is still non-fully understood.
The ability to cause disease of many pathogens is restricted to a certain range of temperatures, and suppressed or very reduced at different ones (Konkel & Tilly, 2000; Smirnova et al., 2001; Case et al., 2011; Kimes et al., 2012; Guijarro et al., 2015; Du Raan, Coutinho & Van der Waals, 2016). In plant pathogenic bacteria, the temperatures enhancing the deployment of virulence factors use to be lower than the optimal for growth (16–24 °C), coinciding with the ones favoring the formation of water aerosols or films near/on plant surfaces (which are usually required for efficient infection), or with the ones of water films (Smirnova et al., 2001). In the case of E. amylovora, a temperature of 18 °C seems required for blossom blight epidemics to occur under field conditions (Thomson, 2000; Van der Zwet, Orolaza-Halbrendt & Zeller, 2012), and rain and/or heavy dew at the end of warm periods promote infection events in the field (Johnson & Stockwell, 1998). Moreover, at 18 °C, the expression of pathogenicity and other function genes is enhanced with respect to higher temperatures (Wei, Sneath & Beer, 1992; Goyer & Ullrich, 2006). However, despite flowers are the main organ for the E. amylovora entry into the host, other infection routes (e.g., via stomata, wounds caused by hail, wind, insects, etc.) contribute to the phenomenology of fire blight epidemics, which difficult disease control (Thomson, 2000; Billing, 2011; Van der Zwet, Orolaza-Halbrendt & Zeller, 2012). In this regard, there is still scarce information on the E. amylovora ability to infect susceptible tissues/organs other than flowers at temperatures below 18 °C, or about how such temperatures may affect the secretion of virulence factors.
The effect of temperature on E. amylovora (and other pathogens) varies depending on the nutritional state of cells. When inside the host, or as an epiphyte on floral organs, nutrient availability together with appropriate environmental temperatures favor bacterial cell multiplication. However, when E. amylovora experiences nutrient limitation (e.g., on plant surfaces, rainwater, soil, vector insects, etc.) this stress induces, in a temperature dependent manner, growth arrest, morphological changes and the development of a starvation-survival response with subpopulations of cells entering the viable but nonculturable (VBNC) state (Santander et al., 2012; Santander, Oliver & Biosca, 2014; Ordax et al., 2015).
The VBNC state was firstly described in E. amylovora in response to copper stress (Ordax et al., 2006). However, regardless of the VBNC-inducing stress (e.g., copper, starvation, chlorine, etc.), VBNC cells are able to recover culturability under certain conditions (Ordax et al., 2009; Santander et al., 2012; Ordax et al., 2015), thus constituting potential and hard-to-detect inoculum sources of the pathogen in the environment.
The starvation-survival and the VBNC state are bacterial strategies to face harsh nutrient and temperature conditions, and may have an impact on the ecology of many pathogens and/or the phenomenology of the disease they cause (Biosca et al., 1996; Armada et al., 2003; Caruso et al., 2005; Vattakaven et al., 2006; Lutz et al., 2013; Ayrapetyan et al., 2015). However, the role of such strategies in the E. amylovora life cycle has not been well established, and little is known about the effects of environmental temperatures below 18 °C on the pathogen responses to starvation, or how such responses might be linked to its life cycle.
We hypothesize that the E. amylovora psychrotrophic adaptations may have repercussions on its ability to infect susceptible plant material and starvation responses at environmental temperatures below 18 °C. In this work, we investigated the effect of warm (28 °C), temperate (14 °C) and low (4 °C) temperatures on E. amylovora virulence and survival under natural starvation conditions, as well as on different factors related to virulence and/or environmental survival. Our results provide new knowledge on scarcely studied aspects of the E. amylovora life cycle, such as the temperature-dependent modulation of virulence and starvation responses which have probably contributed to the successful spread of this bacterial species to numerous countries worldwide with different climate areas (EPPO, 2017).
Material and Methods
Biosafety approval
The Universitat de València granted Biosafety approval to carry out the study within its facilities.
Bacterial strains and culture conditions
Five E. amylovora strains from different hosts and geographical origins frequently used in studies on fire blight were employed in this work: Ea 1/79 (Falkenstein et al., 1988), Ea 1189 (Burse, Weingart & Ullrich, 2004), CFBP 1430 (Paulin & Samson, 1973), NCPPB 2080 (Giorgi & Scortichini, 2005) and ATTC 49946 (Sebaihia et al., 2010). Unless otherwise specified, bacterial cultures were grown on LB agar (LBA) plates or in liquid LB at 28 °C with shaking (150 rpm). Strains were cryopreserved at −80 °C in 25% glycerol.
Virulence
The virulence of the five E. amylovora strains included in this study was tested using green loquats (cv Tanaka). One-off assays were additionally performed on green pears (cv Devoe) with the reference strain CFBP 1430, as well as on pear shoots (cv. Conference) with strains CFBP 1430 and ATCC 49946. To reduce fungal growth during prolonged incubation periods, particularly at low temperatures, fruits were first surface disinfected with 2% sodium hypochlorite as previously described (Santander et al., 2014) and then treated with 21.6 mg L−1 natamycin (Nataproq-G; Proquiga Biotech, Bergondo, Spain) (Pedersen, 1992) for 5 min. This compound is a broad-spectrum fungicide, with low toxicity, used in multiple applications (Pedersen, 1992; Pengfei, Junxing & Shaobo, 2013; Biosca et al., 2016). Based in our previous experience, applying natamycin to immature fruits prior to their inoculation efficiently delays fungal growth, allowing prolonged incubations without fungal contaminations. Natamycin treated fruits were let to dry in aseptic conditions under the hood, and then wound-inoculated with 103 CFU of E. amylovora per wound according to Santander et al. (2014), incubated in a wet chamber at 4 °C, 14 °C and 28 °C, and periodically monitored for fire blight symptoms development. One-off pear shoot assays were performed according to Ordax et al. (2009), at 28 °C and 14 °C, with four shoots per strain and temperature, incubated in a climatic chamber. E. amylovora was re-isolated from symptomatic fruits and shoots and identified by specific PCR as previously described (Santander et al., 2012). Ten fruits were inoculated per strain and temperature, and the experiment was performed in two independent repeats.
Growth curves
Growth curves were performed at 4 °C, 14 °C and 28 °C in LB broth under static conditions. For this purpose, overnight cultures in LB were adjusted to an OD600 nm of 1.0 (ca. 2 109 CFU mL−1), diluted 1:50 into fresh medium and incubated in parallel under static conditions at the above-mentioned temperatures. Aliquots of 0.5 mL were periodically taken from cultures and the OD600 nm measured. This experiment was performed in duplicate, per strain and temperature.
Siderophore detection
To induce the biosynthesis/secretion of siderophores, AB minimal medium (Chilton et al., 1974) containing 0.05 g L−1 nicotinic acid (ABN) to allow E. amylovora growth (Starr & Mandel, 1950) and lacking FeSO4 (ABN-Fe) was employed. E. amylovora overnight cultures in LB were pelleted, washed twice, resuspended in fresh ABN-Fe medium to an OD600 nm of 0.3, and incubated in parallel at 28°C, 14 °C and 4 °C. Siderophore levels were quantified after 0, 1, 3 and 7 days post-inoculation (dpi) by mixing 0.4 mL of supernatant with 0.3 mL of a fresh solution of 0.12 M FeCl3 in 5 mM HCl (Holzberg & Artis, 1983). The abundance of siderophores in culture supernatants was spectrophotometrically determined at A425 nm, and normalized to the OD600 nm of the analyzed cultures. This experiment was performed in duplicate, in two independent experiments.
Motility assays in soft agar
Swimming motility was evaluated using TG (1% tryptone, 0.5% glucose, pH 7.0) soft agar plates (0.3% agar). Briefly, a sterile toothpick previously dipped into an overnight culture of the E. amylovora strain to test was used to inoculate soft agar plates, which were sealed with parafilm and incubated at 4 °C, 14 °C and 28 °C. This assay was performed in two independent experiments, each with three technical repeats.
EPS relative quantification
To induce the production of the main E. amylovora EPSs amylovoran and levan, overnight cultures were washed and diluted to an OD600 nm of 0.4 in LB plus 1% sorbitol (LB Sor) or 5% sucrose (LB Suc), respectively (Geider, 2000). Cultures were incubated at 4 °C, 14 °C and 28 °C and EPS contents in supernatants were measured at times 1, 3 and 7 dpi.
Amylovoran was measured (OD600 nm) after a 10 min reaction of 0.8 mL LB Sor supernatants with 40 µL of 50 mg mL−1 cetylpyridinium chloride (CPC) (Ordax et al., 2010). Levan production was measured as an increase in turbidity (OD580 nm) in LB Suc culture supernatants (Ordax et al., 2010).
Amylovoran and levan levels were normalized to the OD600 nm of the analyzed culture in each case (Santander et al., 2014). This assay was performed in duplicate in two independent experiments.
Biofilm quantification by a microtiter assay
E. amylovora biofilms were quantitated using a microtiter assay based on Chelvam, Chai & Thong (2014). To this aim, E. amylovora overnight cultures were diluted to an OD600 nm of 0.5 into 0.5× LB broth, and transferred (160 µL per well) to polystyrene (surface-treated, hydrophylic) Nunc™ MicroWell™ 96-well microplates (Thermo Scientific, Waltham, MA, USA). Sterility controls consisting of non-inoculated 0.5× LB were included in each assay. Inoculated plates were incubated in parallel at 4 °C, 14 °C and 28 °C for 48 h. After this period, the plates were left to reach room temperature for 10 min, and planktonic cells and medium were removed by inversion, gentle tapping and incubation upside down for 5 min, inside a biosafety cabinet. Then, biofilms were heat-fixed in a Pasteur oven at 80 °C for 30 min and let to cool down to room temperature. Afterwards, 220 µL of 1% crystal violet (CV) were added to each well and incubated for 15 min at room temperature. The CV was removed by inversion, and the plates were thoroughly rinsed with distilled water and let to dry upside down inside the hood. Biofilm formation was determined spectrophotometrically (A600 nm) with a plate reader (FLUOstar OPTIMA; BMG Labtech, Ortenberg, Germany), after solubilizing the CV from biofilms with an 8:2 mixture of absolute ethanol and acetone (220 µL per well) for 20 min. This assay was carried out with, at least, seven replicates per strain and temperature, in two independent experiments.
Oxidative stress resistance
Oxidative stress sensitivity at 4 °C, 14 °C and 28 °C was assessed by an agar dilution assay, based on Wiegand, Hilpert & Hancock (2008). Briefly, plates of LBA (control) and LBA + 0.75 mM H2O2 were prepared and let to solidify under the hood for 30 min. The H2O2 concentration was selected based on preliminary assays. E. amylovora overnight cultures in LB were adjusted to an OD600 nm of 1.0 (ca. 109 CFU mL−1), serially tenfold diluted in sterile saline, and 5 µL drops of each dilution (from 10−1 to 10−6) were plated on the above-mentioned media. Plates were then sealed and incubated in parallel at 28 °C, 14 °C and 4 °C for 6, 18 and 41 days, respectively. The minimal dilution at which each strain grew on each medium at each temperature was recorded. This assay was performed in two independent repeats.
Microcosms preparation, monitoring of population dynamics and morphology analysis of starved cells
Oligotrophic water microcosms were prepared as previously described (Santander et al., 2012), inoculated with E. amylovora at a final density of ca. 2 107 CFU mL−1, and incubated in parallel at 4 °C, 14 °C and 28 °C. Culturable cell populations at different periods were determined by drop-plate on LBA, as described elsewhere (Santander et al., 2012). Viable and total cell population dynamics were monitored by flow cytometry, using a BD FACSVerse™ flow cytometer (BD Biosciences, San Jose, CA, USA), with 488 nm excitation. To this aim, microcosm aliquots were taken at different intervals throughout a 122-day period, and stained with the components of the Baclight Live/Dead viability kit (Life Technologies), according to the manufacturer’s instructions. Red and green fluorescence were measured with 700/54 and 527/32 filters, respectively, the trigger was set on FSC (forward scatter), and data were analyzed using the BD FACSuite™ software (BD Biosciences, San Jose, CA, USA). Each strain was inoculated in three independent microcosms per temperature.
Morphological changes occurring in E. amylovora cells starved in water microcosms were monitored by epifluorescence microscopy similar to Álvarez, López & Biosca (2008). Briefly, 20 µL microcosm aliquots were taken at times 0 and 122 dpi, fixed on a glass slide and stained for 10 min with 12 mM SYTO9 (Life Technologies, Carlsbad, CA, USA). Afterwards, the stained cells were covered with a cover slip and photographed at a magnification of ×1,000, using a digital camera adapted to a Nikon Eclipse E800 epifluorescence microscope, and the automatic camera tamer software ACT-1. Microscope images were thresholded to create binary images with IMAGEJ software (Schneider, Rasband & Eliceiri, 2012), and the parameters Feret diameter, area and circularity were measured to determine possible changes in cell size and shape due to starvation and/or temperature.
Statistical analysis
Prior to statistical analysis a log transformation of culturable, viable, and total cell counts was performed. In general, differences between means were determined by analysis of variance (ANOVA). The factors compared were temperature and strain, although the time factor was also included in certain analyses. In some experiments replicate means were compared using Bonferroni post-tests. P-values ≤0.05 were considered significant.
Results & Discussion
E. amylovora remains pathogenic even at 4 °C, with virulence, growth rates, siderophore secretion and motility decreasing in parallel to incubation temperature
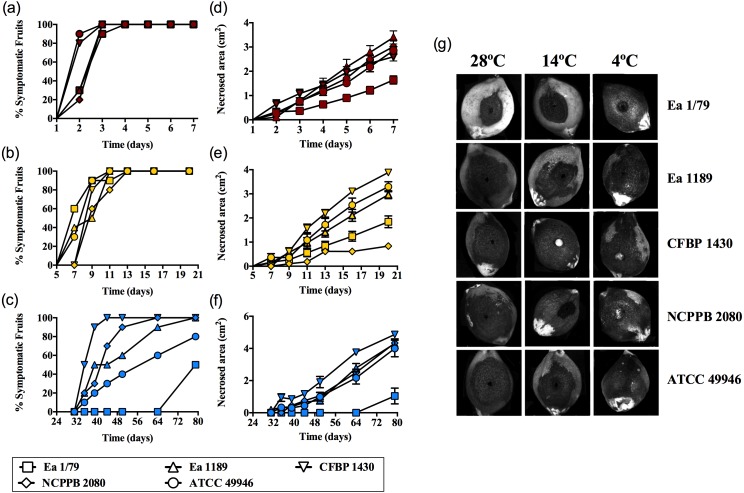
Results corresponding to virulence assays at 4 °C, 14 °C and 28 °C in green loquats are shown in Figs. 1A–1G. All the E. amylovora assayed strains were able to cause typical fruit blight symptoms (necrosis and exudates) at the three tested temperatures. However, the onset of symptoms and the progression of necrosis throughout time were strongly dependent on temperature (p < 0.001). Fruit blight symptoms developed earlier at 28 °C (2 dpi) (Fig. 1A), followed by 14 °C (7–9 dpi) (Fig. 1B) and 4 °C (35–79 dpi) (Fig. 1C). In the same way, the fastest necrosis expansion over time was observed at 28 °C, followed by 14 °C and 4 °C (Figs. 1D–1F). These results were similarly reproduced in virulence assays with green pears (cv. Devoe) inoculated with the E. amylovora strain CFBP 1430 (Fig. S1). In the case of one-off assays with pear shoots (P. communis var. Conference), symptoms also developed in 7–9 dpi at 14 °C, and at 2–3 dpi at 28°C.
Figure 1. E. amylovora virulence assays in immature loquats (cv. Tanaka) at 28 °C (A, D), 14 °C (B, E) and 4 °C (C, F).
Percentage of symptomatic fruits (total values) over time (A–C) and expansion of necrotic lesions over time (D–F). Representative pictures of fruits showing fire blight symptoms at the end of the experimental period (28 °C, 7 dpi; 14 °C, 20 dpi; 4 °C, 79 dpi) (G). These data show the results from a representative experiment performed with ten replicates and repeated in two independent experiments with equivalent results.
It is noteworthy that E. amylovora retained its pathogenicity at 14 °C and even at 4 °C. In this regard, despite flowers are the main E. amylovora entry site into the host, and a temperature of 18 °C is required for blossom blight epidemics development (Thomson, 2000; Van der Zwet, Orolaza-Halbrendt & Zeller, 2012), our results shed light on the potential ability of this pathogen to initiate infections and cause fire blight symptoms in other tissues, at lower environmental temperatures. This is in agreement with the reported progression of fire blight symptoms in perennial pear branches during winter in Israel, following autumn infections (Shtienberg et al., 2015). Moreover, the slow progression of symptom development at such temperatures might allow latent infections to keep hidden until higher environmental temperatures favor symptom emergence, although field studies are required to confirm this hypothesis.
The retention of pathogenicity at a wide range of environmental temperature is not a common behavior among pathogenic bacteria. As an example, the ability to cause disease and/or the production of some virulence factors in a variety of bacterial phytopathogens (e.g., species of Dickeya, Pectobacterium, Pseudomonas, Ralstonia, and Rhizobium) is restricted to a certain temperature range (Serfontein et al., 1991; Durand et al., 2000; Goyer & Ullrich, 2006; Bocsanczy et al., 2014; Du Raan, Coutinho & Van der Waals, 2016). This is due to the repression of virulence and/or pathogenicity genes by temperatures above or below this range (Konkel & Tilly, 2000; Smirnova et al., 2001).
Our results show E. amylovora as a highly adaptable pathogen to a range of environmental temperatures, more active at 28 °C, but retaining its pathogenic potential at lower temperatures (even at 14 °C and 4 °C). In this sense, the wide temperature range at which E. amylovora is potentially pathogenic is probably a crucial factor allowing the pathogen to successfully colonize new areas with diverse climate conditions.
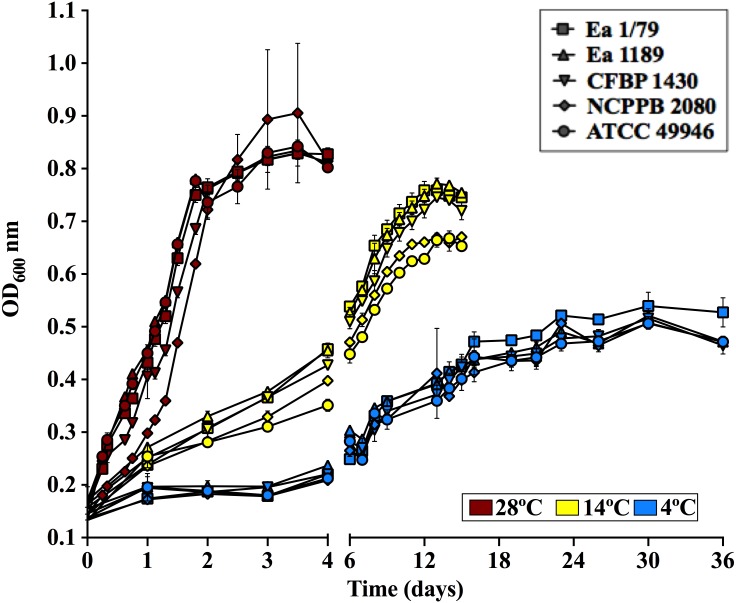
The effect of incubation temperature on symptom development was similar to that observed in growth rates (Fig. 2), siderophores secretion (Figs. 3A–3C) and motility (Fig. 4). Coinciding with fire blight symptom development, these virulence determinants reached lower values the lower the assayed incubation temperature was.
Figure 2. E. amylovora growth curves in LB at 28 °C, 14 °C and 4 °C under static conditions.
Vertical lines indicate the SD. These data show the results from a representative experiment performed in duplicate.
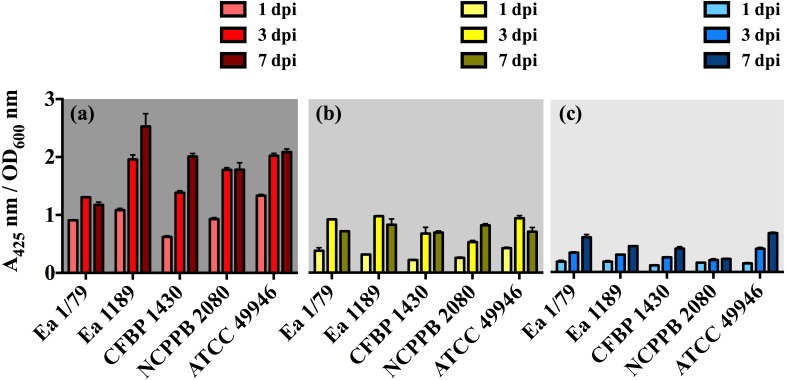
Figure 3. E. amylovora siderophore production at 28 °C (A), 14 °C (B) and 4 °C (C) in minimal medium ABN-Fe after 1, 3 and 7 dpi.
Vertical lines mark the SD. The two-way ANOVA analysis of these results showed that differences of siderophore production among the three temperatures were very significant (p < 0.0001). These data show the results from a representative experiment performed in duplicate and repeated twice, with similar results.
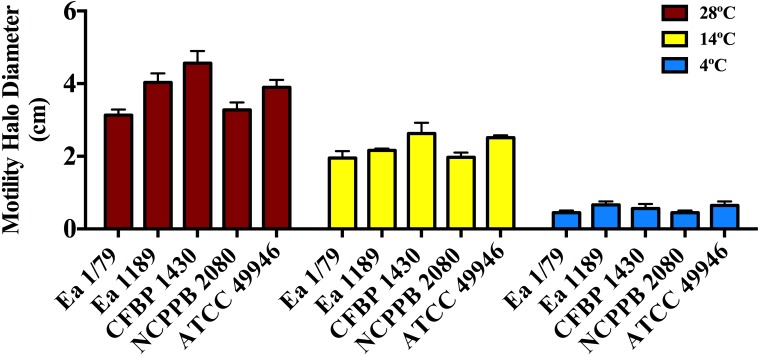
Figure 4. Motility of E. amylovora in soft agar plates at 28 °C, 14 °C and 4 °C after 2 days of incubation.
Vertical lines mark the SD. Swimming motility differences at the three temperatures assayed were very significant (p < 0.001), regardless of the tested strain. These data are from a representative experiment of two, performed in triplicate and with similar results.
Regarding growth rates, some virulence factors in E. amylovora are regulated in a cell population dependent manner via quorum sensing (Molina et al., 2005). Therefore, it is reasonable that fruit blight symptoms appear and develop faster at temperatures allowing E. amylovora to reach appropriate cell densities in shorter periods, which correlates with the higher growth rates observed at 28°C, followed by 14 °C and 4 °C (Fig. 2). Interestingly, Pusey & Curry (2004) demonstrated that the E. amylovora proliferation on stigma surfaces of crab apple flowers only occurs at temperatures above 12 °C, with temperatures ranging from 20 °C to 32 °C allowing the pathogen to reach cell densities greater than those of antagonistic bacteria. This fact, among others, might explain why blossom blight in field is often observed at temperatures above 18 °C (Van der Zwet, Orolaza-Halbrendt & Zeller, 2012). However, growth curves and virulence assays in immature fruits as well as in pear shoots indicate that E. amylovora not only grows at temperatures below 18 °C, but is also able to cause fire blight symptoms once it reaches susceptible host tissues.
With respect to siderophores, these iron chelating compounds have long been recognized as important virulence factors in E. amylovora, contributing to iron acquisition inside the host and survival under oxidative stress (Dellagi et al., 1998; Dellagi et al., 1999; Venisse, Gullner & Brisset, 2001; Venisse et al., 2003). In this study, we identified a decrease in siderophore production occurring in parallel with the diminution of incubation temperature, with the relative levels of these iron chelating compounds at 28 °C being 2.5 (p < 0.001) and 5.4 (p < 0.001) times greater than those determined at 14 °C and 4 °C, respectively (Figs. 3A–3C). This might be related to the diminished iron requirements as temperature decreases, as a consequence of a slowed down metabolism. The lower siderophore production, together with the reduced virulence as temperature decreased, might also support the important role of these iron-uptake molecules as E. amylovora virulence factors.
Motility in E. amylovora is necessary for full virulence, aiding bacterial cells to reach natural openings in apple blossoms (Bayot & Ries, 1986) or other plant organs (Cesbron et al., 2006). This phenotypic trait is modulated by temperature, pH and other environmental factors (Raymundo & Ries, 1980; Goyer & Ullrich, 2006). Once inside host tissues, E. amylovora cells lose flagella (Raymundo & Ries, 1981; Cesbron et al., 2006), avoiding the elicitation of plant defense responses by flagellins (Cesbron et al., 2006; Holtappels et al., 2015). However, non-motile cells from infected tissues can recover their motility if water films on plant surfaces are formed (Raymundo & Ries, 1981). Furthermore, cells expressing flagella can remain motile even under starvation conditions (Santander, Oliver & Biosca, 2014) and for variable periods, also depending on incubation temperatures (Raymundo & Ries, 1981). This probably makes it easier to reach new host entry sites and/or other nutrient sources.
As shown in Fig. 4, all the assayed E. amylovora strains were able to move throughout soft agar regardless of the tested temperature, with the greatest motility observed at 28 °C, followed by 14 °C and 4 °C. According to our results, E. amylovora has the potential to move towards new host entry sites even at suboptimal environmental growth temperatures, however chemotactic responses decrease above 28 °C and below 20 °C (Raymundo & Ries, 1980). This could suggest reduced chances for bacterial cells to locate nectaries at temperatures below 20 °C. Nevertheless, motility is repressed after entering into host tissues (Raymundo & Ries, 1981; Cesbron et al., 2006) and would not be necessary, for example when E. amylovora cells enter into the host through wounds, with these bacterial cells having the potential to invade host tissues even at minimal growth temperatures.
EPS production, biofilm formation and oxidative stress resistance are enhanced at temperatures below the optimal for growth
Two important virulence/pathogenicity factors in the fire blight pathogen and other phytopathogenic bacteria are the production of EPSs and the formation of biofilms (Koczan et al., 2009; Koczan et al., 2011; Van der Zwet, Orolaza-Halbrendt & Zeller, 2012; Piqué et al., 2015; Castiblanco & Sundin, 2016). EPSs contribute to pathogen spread and host colonization, by obstruction of the plant vascular system and masking the bacterial cell surface elicitors of plant defenses (Geider, 2000; Maes et al., 2001; Venisse, Gullner & Brisset, 2001; Ordax et al., 2015). Moreover, they might favor pathogen survival in the environment, acting as alternative carbon sources during starvation periods, and as a barrier against desiccation, bacteriophages and toxic compounds such as copper (Geider, 2000; Jock, Langlotz & Geider, 2005; Ordax et al., 2010; Van der Zwet, Orolaza-Halbrendt & Zeller, 2012; Born et al., 2014). With regard to biofilms, their role during the systemic invasion of plant hosts has been reported (Koczan et al., 2009; Koczan et al., 2011), as well as their relevance for the survival of the pathogen on plant surfaces and vectors (Ordax et al., 2009; Ordax et al., 2015).
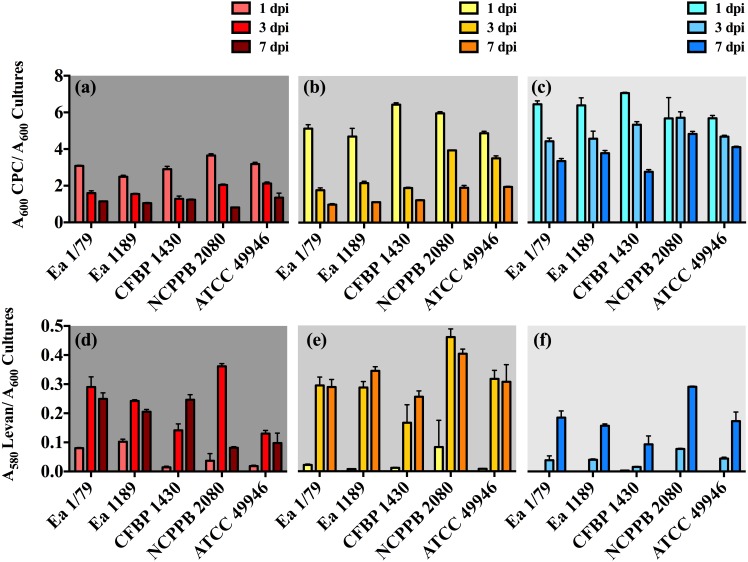
In our work, we provide for the first time data on the ability of E. amylovora to produce EPSs (Figs. 5A–5F) and biofilms (Fig. 6) at temperatures ranging from 4 °C to 28 °C, with these virulence factors being more intensely induced at temperatures below the optimal for growth (p < 0.0001).
Figure 5. Relative production of amylovoran (A–C) and levan (D–F) by E. amylovora at 28 °C (A, D), 14 °C (B, E) and 4 °C (C, F).
Vertical lines denote the SD. A two-way ANOVA analysis of data revealed that differences in EPS production at 28 °C, 14 °C and 4 °C were very significant (p < 0.0001), regardless of the assayed strain. These data are from a representative experiment performed in duplicate, being similar to those obtained in a second independent experiment.
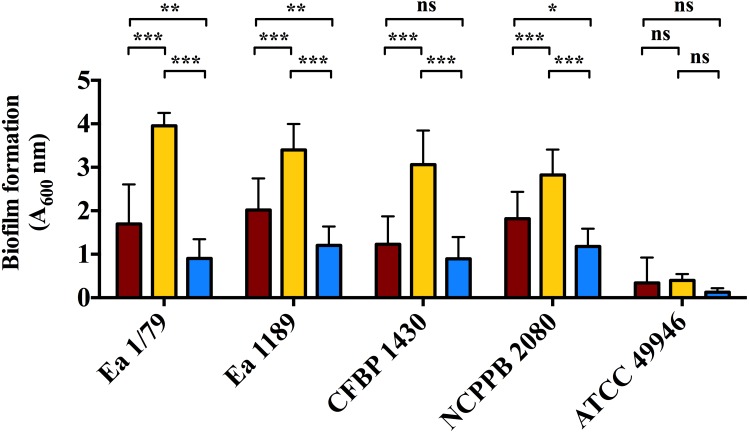
Figure 6. Quantification of biofilm formation by E. amylovora after 48 h of incubation at 28 °C (red), 14 °C (yellow) and 4 °C (blue).
Vertical lines indicate the SD. Asterisks above horizontal lines denote statistically significant differences between the two signaled columns. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, non-significant differences. These data are from a representative experiment carried out, at least, with seven replicates per strain and temperature. This experiment was repeated with the same number of replicates, showing similar results.
Our results also revealed an interesting differentiated temperature-dependent modulation of the two main EPSs in E. amylovora, amylovoran and levan (Figs. 5A–5F). Amylovoran was more intensively produced at 4 °C, followed by 14 °C and 28 °C, reaching maximum levels at 1 dpi (Figs. 5A–5C). Levan production, however, increased over time regardless of the assayed temperature, with levels of this EPS reaching maximum values at 3 and 7 dpi, and being greater at 14 °C, than at 28 °C or 4 °C (Figs. 5D–5F). These results might suggest a not yet described role for EPSs in E. amylovora facilitating growth at low temperatures and/or enhancing cold tolerance, as described in bacteria from cold environments (Nichols et al., 2005; Chrismas et al., 2016).
In some cases, a reduction in EPS levels (mainly amylovoran) over time was observed (Figs. 5A–5F). This phenomenon has been linked to their use as alternative carbon sources under nutrient limiting conditions (Ordax et al., 2010). In our case, transitory nutrient starvation of E. amylovora cells due to their incubation for a week under static conditions could explain the results obtained.
Analogous to levan production, biofilm formation in all the assayed E. amylovora strains was enhanced at 14 °C, followed by 28 °C and 4°C (Fig. 6). This demonstrates the potential of E. amylovora to form biofilms in plant tissues at 14 °C and even at 4 °C, suggesting that the systemic invasion of host plants is possible even at very low temperatures. Furthermore, the need of both amylovoran and levan for proper biofilm development in E. amylovora has been described (Koczan et al., 2009). Accordingly, the observed differences in the thermal modulation patterns of these two EPSs might indicate that their degree of contribution to biofilm formation might also vary depending on environmental temperatures.
Taken together the above-mentioned virulence related results, from assays with five different strains of the pathogen from different geographical areas, demonstrate the ability of E. amylovora to cause fire blight symptoms at 28 °C, 14 °C, and 4 °C. However, the apparent discrepancy between virulence results and the differential production of virulence determinants at each temperature, suggest the complex nature of E. amylovora interactions with its hosts, as well as the potential influence of other ecological abiotic and biotic conditions, such as temperature, surrounding environmental microbiota, etc.
Apart from producing EPSs and forming biofilms, the E. amylovora invasion of host tissues depends on other virulence/pathogenicity factors (Piqué et al., 2015) such as a type three secretion system (TTSS) and TTSS-related effector proteins, which are used to kill host plant cells by means of a strategy that distinguishes E. amylovora from other phytopathogens (Venisse, Gullner & Brisset, 2001; Venisse et al., 2003). While many plant pathogens avoid or reduce host responses via deployment of the TTSS (Grant et al., 2006), E. amylovora uses the same mechanism to provoke an oxidative burst, which is used to kill plant cells (Venisse, Gullner & Brisset, 2001; Venisse et al., 2003). Accordingly, antioxidant mechanisms are required to face the reactive oxygen species (ROS) released by host cells. In fact, catalases have been reported as virulence factors in E. amylovora (Santander, Figàs-Segura & Biosca, 2017). Our results indicate that E. amylovora resistance to oxidative stress is also temperature dependent, with resistance to H2O2 being enhanced at 14 °C (Fig. 7B), with respect to 28 °C (Fig. 7A) and 4 °C (Fig. 7C). Interestingly, the production of EPSs was also more efficiently induced at 14 °C than at 28 °C (Figs. 5A–5F). Although, the role of these macromolecules as protecting agents against H2O2 has been discussed (Venisse et al., 2003), they are induced by oxidative stress (Santander, Figàs-Segura & Biosca, 2017), suggesting their possible contribution to the protection against other ROS, or toxins secreted by plant cells, which indirectly might cause oxidative stress.
Figure 7. Quantification of the oxidative stress resistance of E. amylovora at 28 °C (A), 14 °C (B) and 4 °C (C) by an agar dilution assay using LBA plates containing 0.75 mM H2O2.
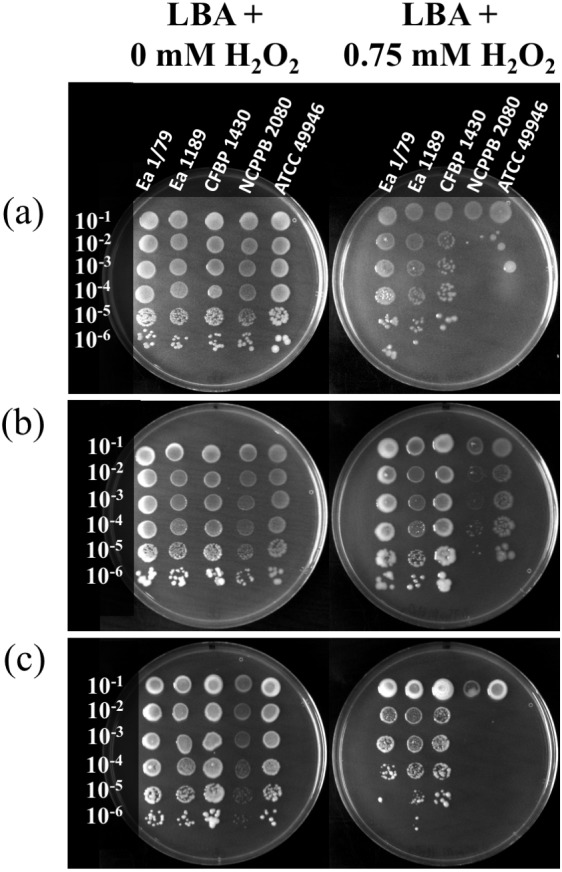
Plates inoculated with serial tenfold dilutions of E. amylovora overnight cultures were sealed, incubated at each temperature and photographed at 6, 18 and 41 dpi, respectively. Oxidative stress resistance was greater at 14 °C than at 28 °C or 4 °C. These pictures are representative of an experiment repeated twice with similar results.
It is remarkable that, despite the clear enhancement of H2O2 sensitivity at 4 °C and 28°C (Figs. 7A–7C), all the E. amylovora strains were able to cause regular fire blight symptoms in immature fruits (Figs. 1A–1G), with the strains which were more sensitive to oxidative stress (NCPPB 2080 and 49946) (Figs. 7A–7C) being similarly or even more virulent than some of the strains showing better resistance to H2O2. A strain-dependent production of factors reducing the effects of ROS on bacterial cells, such as the lipopolysaccharide (Berry et al., 2009) or cold shock proteins (Burbank & Stenger, 2016), probably also contributes to the phenotypes observed. Siderophore secretion has also been related to oxidative stress protection in E. amylovora (Dellagi et al., 1998; Dellagi et al., 1999; Venisse et al., 2003). However, although an enhanced siderophore production was observed at 28 °C, the higher resistance to H2O2 occurred at 14 °C, suggesting that other factors are involved in this stress resistance under the assayed conditions.
Starvation at 14 °C favors the persistence in a culturable state, while the VBNC response is enhanced at 28 °C and, to a lesser extent, at 4 °C
As part of its life cycle, E. amylovora has to deal with periods of starvation, either within host cankers, as an epiphyte, inside vectors, or when spread by rainwater, etc. (Thomson, 2000; Santander, Oliver & Biosca, 2014). In a previous work we showed the temperature-dependent nature of E. amylovora responses to nutrient limitation (Santander, Oliver & Biosca, 2014). However, the effect of temperatures below 18 °C on starvation responses had not yet been assessed. Results in this work demonstrated the modulation of E. amylovora long-term starvation responses by temperature (Figs. 8A–8D). A progressive drop of culturability over time was observed at 28 °C (Fig. 8A) and, to a lesser extent, at 4 °C (Fig. 8C). This phenotype, however, was not observed at 14 °C, a temperature at which E. amylovora cells developed a characteristic starvation-survival response, with culturability values being similar to those at initial time, with only a slight reduction of culturability at final time (Fig. 8B).
Figure 8. Analysis of E. amylovora culturable and VBNC cell populations during a long-term starvation period (122 days) at 28 °C (A), 14 °C (B) and 4 °C (C).
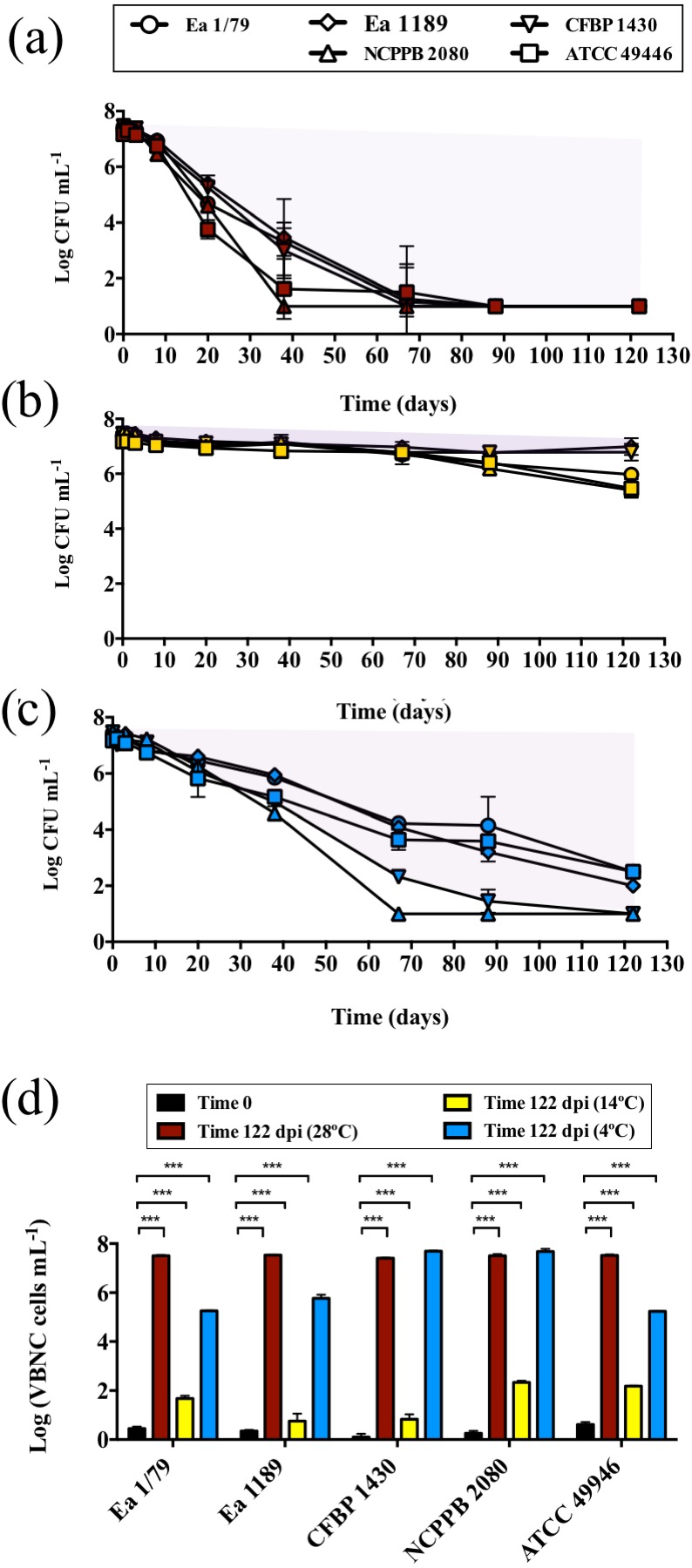
Culturable cell counts are represented with symbols (A–C). Purple areas indicating VBNC cell subpopulations are drawn (A–C). Given the similar trends in the VBNC induction of all the assayed E. amylovora strains, only results corresponding to strain CFBP 1430 are shown. VBNC cell populations at times 0 and 122 dpi are summarized in (D). Vertical lines indicate the SD, and asterisks denote statistically significant differences between temperatures (***, p < 0.001). These data are representative of an assay performed with three microcosms per strain and temperature, inoculated in independent experiments.
Flow cytometry analyses showed a temperature-dependent loss of viability (p < 0.05) between initial and final (122 days) times, which was greater at 28 °C, followed by 14 °C, but nonsignificant at 4 °C (Fig. S2). Nevertheless, compared to culturable cell counts, this decrease of viability was very reduced, indicating that the drop in culturability observed at 28 °C and 4 °C actually corresponded to a progressive entry into the VBNC state, with the number of VBNC cells at the end of the experiment at 14 °C being reduced compared to that observed at 28 °C or 4 °C (Fig. 8D). The flow cytometric analysis of E. amylovora total cell populations also revealed a certain cell lysis at final time, which was greater at 28 °C, followed by 14 °C and 4°C (p < 0.01) (Fig. S2).
As demonstrated in previous works, E. amylovora cells starved for long periods remain fully virulent while culturable (Santander et al., 2012; Santander, Oliver & Biosca, 2014). Starved cells entering the VBNC state, however, lose their ability to cause disease in immature fruits, although virulence can be recovered by passage through a susceptible host (Santander et al., 2012; Ordax et al., 2015). Interestingly, the loss of culturability characteristic of the VBNC state has been linked to a reduced resistance to oxidative stress (Oliver, 2010), and E. amylovora mutants defective in the sigma factor RpoS, which regulates a variety of antioxidant enzymes, or mutants lacking catalase activity, show an enhanced starvation-induced VBNC response (Santander et al., 2014; Santander, Figàs-Segura & Biosca, 2017), which agrees with results obtained in this work.
Temperature dependent alternating periods of VBNC and starvation-survival responses are characteristic stages of the life cycle of many non-obligated pathogens, and determine the seasonal incidence of the disease they cause (Armada et al., 2003; Caruso et al., 2005; Smith & Oliver, 2006; Vattakaven et al., 2006; Ayrapetyan et al., 2015). The knowledge of the roles that these survival responses play in the E. amylovora life cycle, or how environmental temperatures affect such responses may, indeed, allow for an improvement in control and/or prevention measures against fire blight.
Starvation induces morphological changes in a temperature-dependent manner
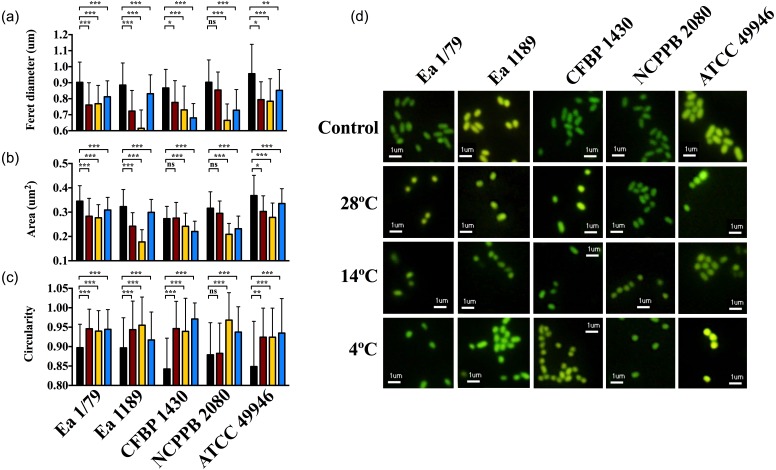
Similar to other bacteria that change their shape during their life cycle (Morita, 1997; Álvarez, López & Biosca, 2008; Chen et al., 2009; Oliver, 2010; Yang, Blair & Salama, 2016), E. amylovora responds to starvation with a reduction in cell size (dwarfing) and the acquisition of rounded shapes, probably via the degradation of endogenous material, cell walls and envelopes through the formation of surface vesicles (Santander, Oliver & Biosca, 2014). In many pathogens, morphological changes occurring under starvation conditions are dependent on temperature (Chen et al., 2009; Yang, Blair & Salama, 2016), however, the influence of this environmental factor on E. amylovora starved cells had not yet been explored.
At time 0, E. amylovora cells showed a typical rod shape (Figs. 9A–9D). However, in general, prolonged exposure to starvation provoked the acquisition of rounded shapes and dwarfing (Figs. 9A–9D). Except for strain NCPPB 2080, the acquisition of rounded shapes in response to oligotrophy was observed at all the temperatures assayed (p < 0.05). However, a strong effect of incubation temperature on the cell size of long-term starved E. amylovora cells was observed (p < 0.001). Most strains experienced enhanced dwarfing at 14 °C, while the effect of the other temperatures on size reduction varied depending on the assayed strain (Figs. 9A–9D).
Figure 9. E. amylovora morphological changes induced by starvation at different temperatures.
Variations in cell length (Feret diameter) (A), area (B) and circularity (C) (average values of more than 100 measurements per microcosm) in cells at time 0 (black columns) with respect to cells starved for 122 days at 28 °C (red columns), 14 °C (yellow columns) and 4 °C (blue columns). Representative pictures of the previous data (D). Asterisks above horizontal lines denote statistically significant differences between two conditions (*, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, non-significant differences). These data are representative of one of three assays (microcosms) per strain and temperature, inoculated in independent experiments and which showed similar results.
The reduction of bacterial cell size and the acquisition of coccoid shapes have been related to the optimization of energetic resources in starved cells, but also as a strategy to avoid predation by protists (Byrd, 2000; Nyström, 2004). Moreover, for some pathogens, morphological transitions occur in parallel to the colonization of different host tissues or cell types, transmission between hosts and/or shifts to environmental reservoirs (Yang, Blair & Salama, 2016). E. amylovora cells during infections are rod-shaped, similar to what occurs during growth in liquid medium. Our results indicate that prolonged starvation conditions such as in reservoirs outside the host, or also within the host in cankers, would induce size reduction and the adoption of circular shapes, with this response being favored at temperatures enhancing the persistence of the pathogen in a culturable and pathogenic state, such as 14 °C and also 20 °C (Santander, Oliver & Biosca, 2014).
Taken together the results in this work and a previous one (Santander, Oliver & Biosca, 2014), it can be deducted that temperatures ranging from 14 °C to 20 °C would favor the persistence of E. amylovora in the environment in a culturable and pathogenic state, reducing cell size and adopting coccoid shape in response to environmental starvation. Moreover, temperate temperatures would coincide with those ensuring a greater abundance of pollinating insects (or other biotic vectors), and contributing to the epiphytic growth on blossoms, motility, chemotactic responses, and fire blight symptom development when inside host tissues (Raymundo & Ries, 1980; Raymundo & Ries, 1981; Thomson, 2000; Pusey & Curry, 2004; Van der Zwet, Orolaza-Halbrendt & Zeller, 2012; Santander, Oliver & Biosca, 2014). However, lower and higher temperatures in other periods of the year would induce the development of the VBNC response (e.g., inside host cankers) in parallel to the loss of pathogenicity in immature fruits (Santander et al., 2012), but with an enhanced environmental stress resistance (Oliver, 2010; Nowakowska & Oliver, 2013). Nevertheless, such VBNC cells can recover their pathogenicity under appropriate conditions (e.g., by passage through pear plantlets) (Santander et al., 2012; Ordax et al., 2015).
Overall, this study provides, for the first time, new data on E. amylovora psychrotrophic adaptations, evidencing its pathogenic potential, production of different virulence factors, and survival at a wide range of environmental temperatures (4 °C–28 °C) shedding light on scarcely known aspects of the biology of this pathogen. This knowledge provides new and valuable information for the improvement of an integrated management of fire blight disease.
Supplemental Information
Graphs show the percentage of symptomatic fruits over time. Representative pictures of fruits showing fire blight symptoms at the end of the experimental period (28 °C, 10 dpi; 14 °C, 15 dpi; 4 °C, 51 dpi) are represented besides the corresponding graph. Vertical lines indicate the SD.
Strains Ea 1/79 (A), Ea 1189 (B), CFBP 1430 (C), NCPPB 2080 (D) and ATCC 49946. Vertical lanes indicate the SD. An ANOVA analysis of data revealed an effect of temperature on viability (p < 0.05) and total cell integrity (total cell counts) (p < 0.01).
Acknowledgments
We are grateful to A Flores and MJ Seguí (SCSIE, Universitat de València, Spain) for their valuable assistance in flow cytometry. We thank E Montesinos (Institute of Food and Agricultural Technology-CIDSAV-XaRTA, Universitat de Girona, Spain) for kindly providing immature pear fruits. We also thank À Figàs-Segura (Universitat de València) for her help, and MM López (Instituto Valenciano de Investigaciones Agrarias, Moncada, Spain) and GW Sundin (Michigan State University, Michigan, USA) for making some of the E. amylovora strains available to us.
Funding Statement
This work was supported by the project UV-INV-AE 112-66196 of the Universitat de València (UV), and also by the UV research support to the BACPLANT Laboratory. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Ricardo D. Santander conceived and designed the experiments, performed the experiments, analyzed the data, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Elena G. Biosca conceived and designed the experiments, contributed reagents/materials/analysis tools, wrote the paper, reviewed drafts of the paper.
Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
The Universitat de València granted ethical and biosafety approval to carry out the study within its facilities.
Data Availability
The following information was supplied regarding data availability:
The raw data from representative experiments used to create the figures in the paper have been provided as Supplemental Files.
References
- Álvarez, López & Biosca (2008).Álvarez B, López MM, Biosca EG. Survival strategies and pathogenicity of Ralstonia solanacearum phylotype II subjected to prolonged starvation in environmental water microcosms. Microbiology. 2008;154:3590–3598. doi: 10.1099/mic.0.2008/019448-0. [DOI] [PubMed] [Google Scholar]
- Armada et al. (2003).Armada SP, Farto R, Pérez MJ, Pérez-Nieto T. Long-term survival and the viable but nonculturable state as part of the life cycle of Listonella pelagia. Aquatic Microbial Ecology. 2003;33:207–216. doi: 10.3354/ame033207. [DOI] [Google Scholar]
- Ayrapetyan et al. (2015).Ayrapetyan M, Williams TC, Baxter R, Oliver JD. Viable but nonculturable and persister cells coexist stochastically and are induced by human serum. Infection and Immunity. 2015;83:4194–4203. doi: 10.1128/IAI.00404-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayot & Ries (1986).Bayot RG, Ries SM. Role of motility in apple blossom infection by Erwinia amylovora and studies of fire blight control with attractant and repellent compounds. Phytopathology. 1986;76:441–445. doi: 10.1094/Phyto-76-441. [DOI] [Google Scholar]
- Berry et al. (2009).Berry MC, McGhee GC, Zhao Y, Sundin GW. Effect of a waaL mutation on lipopolysaccharide composition, oxidative stress survival, and virulence in Erwinia amylovora. FEMS Microbiology Letters. 2009;291:80–87. doi: 10.1111/j.1574-6968.2008.01438.x. [DOI] [PubMed] [Google Scholar]
- Billing (2011).Billing E. Fire blight. Why do views on host invasion by Erwinia amylovora differ? Plant Pathology. 2011;60:178–189. doi: 10.1111/j.1365-3059.2010.02382.x. [DOI] [Google Scholar]
- Biosca et al. (1996).Biosca EG, Amaro C, Marco-Noales E, Oliver JD. Effect of low temperature on starvation-survival of the eel pathogen Vibrio vulnificus biotype 2. Applied and Environmetal Microbiology. 1996;62:450–455. doi: 10.1128/aem.62.2.450-455.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biosca et al. (2016).Biosca EG, Flores R, Santander RD, Díez-Gil JL, Barreno E. Innovative approaches using lichen enriched media to improve isolation and culturability of lichen associated bacteria. PLOS ONE. 2016;11:e0160328. doi: 10.1371/journal.pone.0160328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocsanczy et al. (2014).Bocsanczy AM, Achenbach UCM, Mangravita-Novo A, Chow M, Norman DJ. Proteomic comparison of Ralstonia solanacearum strains reveals temperature dependent virulence factors. BMC Genomics. 2014;15:280. doi: 10.1186/1471-2164-15-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born et al. (2014).Born Y, Fieseler L, Klumpp J, Eugster MR, Zurfluh K, Duffy B, Loessner MJ. The tail-associated depolymerase of Erwinia amylovora phage L1 mediates host cell adsorption and enzymatic capsule removal, which can enhance infection by other phage. Environmental Microbiology. 2014;16:2168–2180. doi: 10.1111/1462-2920.12212. [DOI] [PubMed] [Google Scholar]
- Burbank & Stenger (2016).Burbank LP, Stenger DC. A temperature-independent cold-shock protein homolog acts as a virulence factor in Xylella fastidiosa. Molecular Plant-Microbe Interactions. 2016;29:335–344. doi: 10.1094/MPMI-11-15-0260-R. [DOI] [PubMed] [Google Scholar]
- Burse, Weingart & Ullrich (2004).Burse A, Weingart H, Ullrich MS. NorM, an Erwinia amylovora multidrug efflux pump involved in in vitro competition with other epiphytic bacteria. Applied and Environmental Microbiology. 2004;70:693–703. doi: 10.1128/AEM.70.2.693-703.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd (2000).Byrd JJ. Morphological changes leading to the nonculturable state. In: Colwell RR, Grimes D, editors. Nonculturable microorganisms in the environment. Washington D.C.: ASM Press; 2000. pp. 7–18. [Google Scholar]
- Caruso et al. (2005).Caruso P, Palomo JL, Bertolini E, Álvarez B, López MM, Biosca EG. Seasonal variation of Ralstonia solanacearum biovar 2 populations in a Spanish river: recovery of stressed cells at low temperatures. Applied and Environmental Microbiology. 2005;71:140–148. doi: 10.1128/AEM.71.1.140-148.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case et al. (2011).Case RJ, Longford SR, Campbell AH, Low A, Tujula N, Steinberg PD, Kjelleberg S. Temperature induced bacterial virulence and bleaching disease in a chemically defended marine macroalga. Environmental Microbiology. 2011;13:529–537. doi: 10.1111/j.1462-2920.2010.02356.x. [DOI] [PubMed] [Google Scholar]
- Castiblanco & Sundin (2016).Castiblanco LF, Sundin GW. New insights on molecular regulation of biofilm formation in plant-associated bacteria. Journal of Integrative Plant Biology. 2016;58:362–372. doi: 10.1111/jipb.12428. [DOI] [PubMed] [Google Scholar]
- Cesbron et al. (2006).Cesbron S, Paulin JP, Tharaud M, Barny MA, Brisset MN. The alternative σ factor HrpL negatively modulates the flagellar system in the phytopathogenic bacterium Erwinia amylovora under hrp-inducing conditions. FEMS Microbiology Letters. 2006;257:221–227. doi: 10.1111/j.1574-6968.2006.00172.x. [DOI] [PubMed] [Google Scholar]
- Chelvam, Chai & Thong (2014).Chelvam KK, Chai LC, Thong KL. Variations in motility and biofilm formation of Salmonella enterica serovar typhi. Gut Pathogens. 2014;6:2. doi: 10.1186/1757-4749-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2009).Chen SY, Jane WN, Chen YS, Wong H. Morphological changes of Vibrio parahaemolyticus under cold and starvation stresses. International Journal of Food Microbiology. 2009;129:157–165. doi: 10.1016/j.ijfoodmicro.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Chilton et al. (1974).Chilton MD, Currier TC, Farrand SK, Bendich AJ, Gordon MP, Nester EW. Agrobacterium tumefaciens DNA and PS8 bacteriophage DNA not detected in crown gall tumors. Proceedings of the National Academy of Sciences of the United States of America. 1974;71:3672–3676. doi: 10.1073/pnas.71.9.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrismas et al. (2016).Chrismas NA, Barker G, Anesio AM, Sánchez-Baracaldo P. Genomic mechanisms for cold tolerance and production of exopolysaccharides in the Arctic cyanobacterium Phormidesmis priestleyi BC1401. BMC Genomics. 2016;2(17):533. doi: 10.1186/s12864-016-2846-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellagi et al. (1998).Dellagi A, Brisset MN, Paulin JP, Expert D. Dual role of desferrioxamine in Erwinia amylovora pathogenicity. Molecular Plant-Microbe Interactions. 1998;11:734–742. doi: 10.1094/MPMI.1998.11.8.734. [DOI] [PubMed] [Google Scholar]
- Dellagi et al. (1999).Dellagi A, Reis D, Vian B, Expert D. Expression of the ferrioxamine receptor gene of Erwinia amylovora CFBP 1430 during pathogenesis. Molecular Plant-Microbe Interactions. 1999;12:463–466. doi: 10.1094/MPMI.1999.12.5.463. [DOI] [PubMed] [Google Scholar]
- Du Raan, Coutinho & Van der Waals (2016).Du Raan S, Coutinho TA, Van der Waals JE. Cardinal temperature differences, determined in vitro, between closely related species and subspecies of pectinolytic bacteria responsible for blackleg and soft rot on potatoes. European Journal of Plant Pathology. 2016;144:361–369. doi: 10.1007/s10658-015-0773-x. [DOI] [Google Scholar]
- Durand et al. (2000).Durand JMB, Dagberg B, Uhlin BE, Björk GR. Transfer RNA modification, temperature and DNA superhelicity have a common target in the regulatory network of the virulence of Shigella flexneri: the expression of the virF gene. Molecular Microbiology. 2000;35:924–935. doi: 10.1046/j.1365-2958.2000.01767.x. [DOI] [PubMed] [Google Scholar]
- EPPO (2017).EPPO Global Database 2017. https://gd.eppo.int. [14 March 2017]. https://gd.eppo.int
- Falkenstein et al. (1988).Falkenstein H, Bellemann P, Walter S, Zeller W, Geider K. Identification of Erwinia amylovora, the fireblight pathogen, by colony hybridization with DNA from plasmid pEA29. Applied and Environmental Microbiology. 1988;54:2798–2802. doi: 10.1128/aem.54.11.2798-2802.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geider (2000).Geider K. Exopolysaccharides of Erwinia amylovora: structure, biosynthesis, regulation, role in pathogenicity of amylovoran and levan. In: Vanneste JL, editor. Fire blight: the disease and its causative agent, Erwinia amylovora. Wallingford: CAB International; 2000. pp. 117–140. [Google Scholar]
- Giorgi & Scortichini (2005).Giorgi S, Scortichini M. Molecular characterization of Erwinia amylovora strains from different host plants through RFLP analysis and sequencing of hrpN and dspA/E genes. Plant Pathology. 2005;54:789–798. doi: 10.1111/j.1365-3059.2005.01247.x. [DOI] [Google Scholar]
- Goyer & Ullrich (2006).Goyer C, Ullrich MS. Identification of low-temperature-regulated genes in the fire blight pathogen Erwinia amylovora. Canadian Journal of Microbiology. 2006;52:468–475. doi: 10.1139/w05-153. [DOI] [PubMed] [Google Scholar]
- Grant et al. (2006).Grant SR, Fisher EJ, Chang JH, Mole BM, Dangl JL. Subterfuge and manipulation: type III effector proteins of phytopathogenic bacteria. Annual Review of Microbiology. 2006;60:425–449. doi: 10.1146/annurev.micro.60.080805.142251. [DOI] [PubMed] [Google Scholar]
- Guijarro et al. (2015).Guijarro JA, Cascales D, García-Torrico AI, García-Domínguez M, Méndez J. Temperature-dependent expression of virulence genes in fish-pathogenic bacteria. Frontiers in Microbiology. 2015;6:700. doi: 10.3389/fmicb.2015.00700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtappels et al. (2015).Holtappels M, Vrancken K, Schoofs H, Deckers T, Remans T, Noben JP, Valcke R. A comparative proteome analysis reveals flagellin, chemotaxis regulated proteins and amylovoran to be involved in virulence differences between Erwinia amylovora strains. Journal of Proteomics. 2015;123:54–69. doi: 10.1016/j.jprot.2015.03.036. [DOI] [PubMed] [Google Scholar]
- Holzberg & Artis (1983).Holzberg M, Artis WM. Hydroxymate siderophore production by opportunistic and systemic fungal pathogens. Infection and Immunity. 1983;40:1134–1139. doi: 10.1128/iai.40.3.1134-1139.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay (1998).Jay JM. Intrinsic and extrinsic parameters of foods that affect microbial growth. In: Jay JM, editor. Modern food microbiology. 5th edition Boston: Springer; 1998. pp. 8–61. [Google Scholar]
- Jock, Langlotz & Geider (2005).Jock S, Langlotz C, Geider K. Survival and possible spread of Erwinia amylovora and related plant-pathogenic bacteria exposed to environmental stress conditions. Journal of Phytopathology. 2005;153:87–93. doi: 10.1111/j.1439-0434.2004.00934.x. [DOI] [Google Scholar]
- Johnson & Stockwell (1998).Johnson KB, Stockwell VO. Management of fire blight: a case study in microbial ecology. Annual Review of Phytopathology. 1998;36:227–248. doi: 10.1146/annurev.phyto.36.1.227. [DOI] [PubMed] [Google Scholar]
- Kimes et al. (2012).Kimes NE, Grim CJ, Johnson WR, Hasan NA, Tall BD, Kothary MH, Kiss H, Munk AC, Tapia R, Green L, Detter C, Bruce DC, Brettin TS, Collwell RR, Morris PJ. Temperature regulation of virulence factors in the pathogen Vibrio coralliilyticus. The ISME Journal. 2012;6:835–846. doi: 10.1038/ismej.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koczan et al. (2011).Koczan JM, Lenneman BR, McGrath MJ, Sundin GW. Cell surface attachment structures contribute to biofilm formation and xylem colonization by Erwinia amylovora. Applied and Environmental Microbiology. 2011;77:7031–7039. doi: 10.1128/AEM.05138-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koczan et al. (2009).Koczan JM, McGrath MJ, Zhao Y, Sundin GW. Contribution of Erwinia amylovora exopolysaccharides amylovoran and levan to biofilm formation: implications in pathogenicity. Phytopathology. 2009;99:1237–1244. doi: 10.1094/PHYTO-99-11-1237. [DOI] [PubMed] [Google Scholar]
- Konkel & Tilly (2000).Konkel ME, Tilly K. Temperature-regulated expression of bacterial virulence genes. Microbes and Infection. 2000;2:157–166. doi: 10.1016/S1286-4579(00)00272-0. [DOI] [PubMed] [Google Scholar]
- Lutz et al. (2013).Lutz C, Erken M, Noorian P, Sun S, McDougald D. Environmental reservoirs and mechanisms of persistence of Vibrio cholerae. Frontiers in Microbiology. 2013;4:375. doi: 10.3389/fmicb.2013.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes et al. (2001).Maes M, Orye K, Bobev S, Devreese B, Van Beeumen J, De Bruyn A, Busson R, Herdewijn P, Morreel K, Messens E. Influence of amylovoran production on virulence of Erwinia amylovora and a different amylovoran structure in E. amylovora isolates from Rubus. European Journal of Plant Pathology. 2001;107:839–844. doi: 10.1023/A:1012215201253. [DOI] [Google Scholar]
- Molina et al. (2005).Molina L, Rezzonico F, Défago G, Duffy B. Autoinduction in Erwinia amylovora: evidence of an acyl-homoserine lactone signal in the fire blight pathogen. Journal of Bacteriology. 2005;187:3206–3213. doi: 10.1128/JB.187.9.3206-3213.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita (1997).Morita RY. Bacteria in oligotrophic environments: starvation-survival lifestyle. New York: Chapman & Hall; 1997. [Google Scholar]
- Morris et al. (2016).Morris CE, Barny MA, Berge O, Kinkel LL, Lacroix C. Frontiers for research on the ecology of plant-pathogenic bacteria: fundamentals for sustainability. Molecular Plant Pathology. 2016;18:308–319. doi: 10.1111/mpp.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer & Morita (2007).Moyer CL, Morita RY. Encyclopedia of life sciences. John Wiley & Sons, Ltd; Chichester: 2007. Psychrophiles and psychrotrophs; pp. 1–6. [Google Scholar]
- Nichols et al. (2005).Nichols CM, Lardière SG, Bowman JP, Nichols PD, Gibson JAE, Guézennec J. Chemical characterization of exopolysaccharides from Antarctic marine bacteria. Microbial Ecology. 2005;49:578–589. doi: 10.1007/s00248-004-0093-8. [DOI] [PubMed] [Google Scholar]
- Nowakowska & Oliver (2013).Nowakowska J, Oliver JD. Resistance to environmental stresses by Vibrio vulnificus in the viable but nonculturable state. FEMS Microbiology Ecology. 2013;84:213–222. doi: 10.1111/1574-6941.12052. [DOI] [PubMed] [Google Scholar]
- Nyström (2004).Nyström T. Stationary-phase physiology. Annual Review of Microbiology. 2004;58:161–181. doi: 10.1146/annurev.micro.58.030603.123818. [DOI] [PubMed] [Google Scholar]
- Oliver (2010).Oliver JD. Recent findings on the viable but nonculturable state in pathogenic bacteria. FEMS Microbiology Reviews. 2010;34:415–425. doi: 10.1111/j.1574-6976.2009.00200.x. [DOI] [PubMed] [Google Scholar]
- Ordax et al. (2009).Ordax M, Biosca EG, Wimalajeewa SC, López MM, Marco-Noales E. Survival of Erwinia amylovora in mature apple fruit calyces through the viable but nonculturable (VBNC) state. Journal of Applied Microbiology. 2009;107:106–116. doi: 10.1111/j.1365-2672.2009.04187.x. [DOI] [PubMed] [Google Scholar]
- Ordax et al. (2006).Ordax M, Marco-Noales E, López MM, Biosca EG. Survival strategy of Erwinia amylovora against copper: induction of the viable-but-nonculturable state. Applied and Environmental Microbiology. 2006;72:3482–3488. doi: 10.1128/AEM.72.5.3482-3488.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordax et al. (2010).Ordax M, Marco-Noales E, López MM, Biosca EG. Exopolysaccharides favor the survival of Erwinia amylovora under copper stress through different strategies. Research in Microbiology. 2010;161:549–555. doi: 10.1016/j.resmic.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Ordax et al. (2015).Ordax M, Piquer-Salcedo JE, Santander RD, Sabater-Muñoz B, Biosca EG, López MM, Marco-Noales E. Medfly Ceratitis capitata as potential vector for fire blight pathogen Erwinia amylovora: survival and transmission. PLOS ONE. 2015;10:e0127560. doi: 10.1371/journal.pone.0127560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulin & Samson (1973).Paulin JP, Samson R. Le feu bacterien en France. II.—caracteres des souches d’ Erwinia amylovora (Burril) Winslow, others, 1920, isolees du foyer franco-belge. Annales de Phytopathologie. 1973;5:389–397. [Google Scholar]
- Pedersen (1992).Pedersen JC. Natamycin as a fungicide in agar media. Applied and Environmental Microbiology. 1992;58:1064–1066. doi: 10.1128/aem.58.3.1064-1066.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pengfei, Junxing & Shaobo (2013).Pengfei L, Junxing Z, Shaobo L. Effects of natamycin on the elimination of fungal contamination in rice and Arabidopsis thaliana tissue cultures for Agrobacterium-mediated transformation. Research Journal of Biotechnology. 2013;8:3–9. [Google Scholar]
- Piqué et al. (2015).Piqué N, Miñana Galbis D, Merino S, Tomás JM. Virulence factors of Erwinia amylovora: a review. International Journal of Molecular Sciences. 2015;16:12836–12854. doi: 10.3390/ijms160612836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusey & Curry (2004).Pusey PL, Curry EA. Temperature and pomaceous flower age related to colonization by Erwinia amylovora and antagonists. Phytopathology. 2004;94:901–911. doi: 10.1094/PHYTO.2004.94.8.901. [DOI] [PubMed] [Google Scholar]
- Raymundo & Ries (1980).Raymundo AK, Ries SM. Chemotaxis of Erwinia amylovora. Phytopathology. 1980;70:1066–1069. doi: 10.1094/Phyto-70-1066. [DOI] [Google Scholar]
- Raymundo & Ries (1981).Raymundo AK, Ries SM. Motility of Erwinia amylovora. Phytopathology. 1981;71:45–49. doi: 10.1094/Phyto-71-45. [DOI] [Google Scholar]
- Santander et al. (2012).Santander RD, Català-Senent JF, Marco-Noales E, Biosca EG. In planta recovery of Erwinia amylovora viable but nonculturable cells. Trees—Structure and Function. 2012;26:75–82. doi: 10.1007/s00468-011-0653-8. [DOI] [Google Scholar]
- Santander, Figàs-Segura & Biosca (2017).Santander RD, Figàs-Segura À, Biosca EG. Erwinia amylovora catalases KatA and KatG are virulence factors and delay the starvation-induced viable but nonculturable (VBNC) response. Molecular Plant Pathology. 2017 doi: 10.1111/mpp.12577. Epub ahead of print Sep 20 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santander et al. (2014).Santander RD, Monte-Serrano M, Rodríguez-Herva JJ, López-Solanilla E, Rodríguez-Palenzuela P, Biosca EG. Exploring new roles for the rpoS gene in the survival and virulence of the fire blight pathogen Erwinia amylovora. FEMS Microbiology Ecology. 2014;90:895–907. doi: 10.1111/1574-6941.12444. [DOI] [PubMed] [Google Scholar]
- Santander, Oliver & Biosca (2014).Santander RD, Oliver JD, Biosca EG. Cellular, physiological, and molecular adaptive responses of Erwinia amylovora to starvation. FEMS Microbiology Ecology. 2014;88:258–271. doi: 10.1111/1574-6941.12290. [DOI] [PubMed] [Google Scholar]
- Scherf, Milling & Allen (2010).Scherf JM, Milling A, Allen C. Moderate temperature fluctuations rapidly reduce the viability of Ralstonia solanacearum Race 3, Biovar 2, in infected geranium, tomato, and potato plants. Applied and Environmental Microbiology. 2010;76:7061–7067. doi: 10.1128/AEM.01580-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, Rasband & Eliceiri (2012).Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nature Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebaihia et al. (2010).Sebaihia M, Bocsanczy AM, Biehl BS, Quail MA, Perna NT, Glasner JD, DeClerck GA, Cartinhour S, Schneider DJ, Bentley SD, Parkhill J, Beer SV. Complete genome sequence of the plant pathogen Erwinia amylovora strain ATCC 49946. Journal of Bacteriology. 2010;192:2020–2021. doi: 10.1128/JB.00022-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serfontein et al. (1991).Serfontein S, Logan C, Swanepoel AE, Boelema BH, Theron DJ. A potato wilt disease in South Africa caused by Erwinia carotovora subspecies carotovora and E. chrysanthemi. Plant Pathology. 1991;40:382–386. doi: 10.1111/j.1365-3059.1991.tb02394.x. [DOI] [Google Scholar]
- Shtienberg et al. (2015).Shtienberg D, Manulis-Sasson S, Zilberstaine M, Oppenheim D, Shwartz H. The incessant battle against fire blight in pears: 30 years of challenges and successes in managing the disease in Israel. Plant Disease. 2015;99:1048–1058. doi: 10.1094/PDIS-01-15-0101-FE. [DOI] [PubMed] [Google Scholar]
- Smirnova et al. (2001).Smirnova A, Li H, Weingart H, Aufhammer S, Burse A, Finis K, Schenk A, Ullrich M. Thermoregulated expression of virulence factors in plant-associated bacteria. Archives of Microbiology. 2001;176:393–399. doi: 10.1007/s002030100344. [DOI] [PubMed] [Google Scholar]
- Smith & Oliver (2006).Smith B, Oliver JD. In Situ and in vitro gene expression by Vibrio vulnificus during entry into, persistence within, and resuscitation from the viable but nonculturable state. Applied and Environmental Mcrobiology. 2006;72:1445–1451. doi: 10.1128/AEM.72.2.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr & Mandel (1950).Starr MP, Mandel M. The nutrition of phytopathogenic bacteria. IV. Minimal nutritive requirements of the genus Erwinia. Journal of Bacteriology. 1950;60:669–677. doi: 10.1128/jb.60.5.669-672.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson (2000).Thomson SV. Epidemiology of fire blight. In: Vanneste JL, editor. Fire blight: the disease and its causative agent, Erwinia amylovora. Wallingford: CAB International; 2000. pp. 9–36. [Google Scholar]
- Van der Zwet, Orolaza-Halbrendt & Zeller (2012).Van der Zwet T, Orolaza-Halbrendt N, Zeller W. Fire blight: history, biology, and management. St. Paul: APS Press; 2012. [Google Scholar]
- Vattakaven et al. (2006).Vattakaven T, Bond P, Bradley G, Munn CB. Differential effects of temperature and starvation on induction of the viable-but-nonculturable state in the coral pathogens Vibrio shiloi and Vibrio tasmaniensis. Applied and Environmental Microbiology. 2006;72:6508–6513. doi: 10.1128/AEM.00798-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venisse et al. (2003).Venisse JS, Barny MA, Paulin JP, Brisset MN. Involvement of three pathogenicity factors of Erwinia amylovora in the oxidative stress associated with compatible interaction in pear. FEBS Letters. 2003;537:198–202. doi: 10.1016/S0014-5793(03)00123-6. [DOI] [PubMed] [Google Scholar]
- Venisse, Gullner & Brisset (2001).Venisse JS, Gullner G, Brisset MN. Evidence for the involvement of an oxidative stress in the initiation of infection of pear by Erwinia amylovora. Plant Physiology. 2001;125:2164–2172. doi: 10.1104/pp.125.4.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, Sneath & Beer (1992).Wei ZM, Sneath BJ, Beer SV. Expression of Erwinia amylovora hrp genes in response to environmental stimuli. Journal of Bacteriology. 1992;174:1875–1882. doi: 10.1128/jb.174.6.1875-1882.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand, Hilpert & Hancock (2008).Wiegand I, Hilpert K, Hancock REW. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nature Protocols. 2008;3:163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- Yang, Blair & Salama (2016).Yang DC, Blair KM, Salama NR. Staying in shape: the impact of cell shape on bacterial survival in diverse environments. Microbiology and Molecular Biology Reviews. 2016;80:187–203. doi: 10.1128/MMBR.00031-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Graphs show the percentage of symptomatic fruits over time. Representative pictures of fruits showing fire blight symptoms at the end of the experimental period (28 °C, 10 dpi; 14 °C, 15 dpi; 4 °C, 51 dpi) are represented besides the corresponding graph. Vertical lines indicate the SD.
Strains Ea 1/79 (A), Ea 1189 (B), CFBP 1430 (C), NCPPB 2080 (D) and ATCC 49946. Vertical lanes indicate the SD. An ANOVA analysis of data revealed an effect of temperature on viability (p < 0.05) and total cell integrity (total cell counts) (p < 0.01).
Data Availability Statement
The following information was supplied regarding data availability:
The raw data from representative experiments used to create the figures in the paper have been provided as Supplemental Files.