Abstract
Objectives
Obesity, typically defined as a body mass index (BMI) ≥ 30 kg/m2, is an established risk factor for renal cell carcinoma (RCC) but is paradoxically linked to less advanced disease at diagnosis and improved outcomes. However, BMI has inherent flaws, and alternate obesity-defining metrics that emphasize abdominal fat are available. We investigated three obesity-defining metrics, to better examine the associations of abdominal fat versus generalized obesity with renal tumor stage, grade, or R.E.N.A.L. nephrometry score.
Methods and Materials
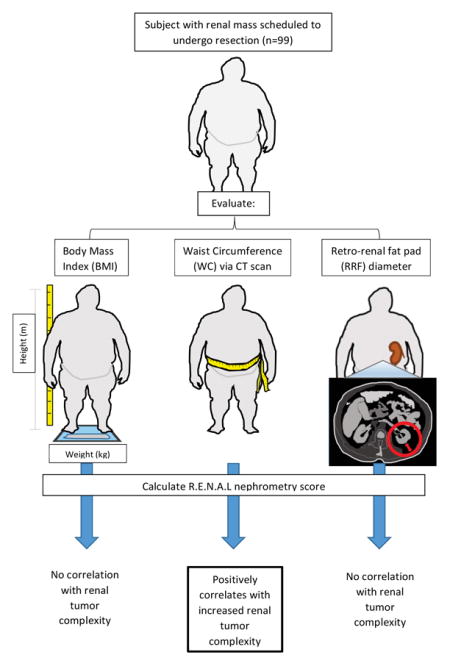
In a prospective cohort of 99 subjects with renal masses undergoing resection and no evidence of metastatic disease, obesity was assessed using three metrics: body mass index (BMI), radiographic waist circumference (WC), and retro-renal fat (RRF) pad distance. R.E.N.A.L. nephrometry scores were calculated based on preoperative CT or MRI. Univariate and multivariate analyses were performed to identify associations between obesity metrics and nephrometry score, tumor grade, and tumor stage.
Results
In the 99 subjects, surgery was partial nephrectomy in 51and radical nephrectomy in 48. Pathology showed benign masses in 11 and RCC in 88 (of which 20 had stage T3 disease). WC was positively correlated with nephrometry score, even after controlling for age, sex, race, and diabetes status (p = 0.02); whereas BMI and RRF were not (p = 0.13, and p = 0.57, respectively). WC in stage T2/T3 subjects was higher than in subjects with benign masses (p= 0.03). In contrast, subjects with Fuhrman grade 1&2 tumors had higher BMI (p < 0.01) and WC (p = 0.04) than subjects with grade 3&4 tumors.
Conclusions
Our data suggest that obesity measured by WC, but not BMI or RRF, is associated with increased renal mass complexity. Tumor Fuhrman grade exhibited a different trend, with both high WC and BMI associated with lower grade tumors. Our findings indicate that WC and BMI are not interchangeable obesity metrics. Further evaluation of RCC-specific outcomes using WC versus BMI is warranted to better understand the complex relationship between general versus abdominal obesity and RCC characteristics.
Keywords: renal cell carcinoma, obesity, waist circumference, body mass index, nephrometry
Graphical Abstract
1. Introduction
Obesity, defined as a body mass index (BMI) score of ≥ 30 kg/m2, is an established risk factor for renal cell carcinoma (RCC) with multiple studies showing a positive association between obesity as determined by BMI and increased RCC risk [1, 2]. Clear cell histology is the most common subtype of RCC and is most strongly associated with obesity [3]. Despite increasing RCC risk, prior studies have found that obesity as measured by BMI is associated with better pathologic features [4] and improved survival [5] in RCC patients with organ-confined tumors. These findings of increased survival in RCC patients with high BMI has been called an “obesity paradox” [1].
However, additional evidence suggests that associations between obesity and RCC are complex and not fully explained by the obesity paradox paradigm. One prior study found that increased visceral adipose tissue was an independent predictor of higher Fuhrman grade in T1a RCC [6]. This is notable as abdominal and subcutaneous adipose tissue may differentially contribute to obesity-related pathologies [7, 8], and abdominal obesity as measured by waist circumference (WC) has been associated with greater all-cause mortality in U.S. adults, independent of BMI [9]. Additionally, Gonzales et al. found that in breast and other cancer patients, the obesity paradox existed only when BMI was used to define obesity, but not the more definitive bioelectrical impedance analysis [10].
Given the growing discrepancies reported in biological and outcome data with respect to fat distribution and obesity characteristics, our goal was to examine potential associations between various metrics of obesity and indicators of renal tumor histology, stage, and complexity in one cohort of RCC subjects.
2. Materials and Methods
2.1 Study subjects
Between October 2012 and May 2014, adult patients with no evidence of metastatic disease undergoing resection of renal masses suspicious for RCC at the University of Iowa Hospitals and Clinics were offered enrollment in an Institutional Review Board-approved study. Informed consent was obtained for 99 subjects. Exclusion criteria included: active secondary malignancy, immune modulating medications, and metastatic disease. Demographic information, including age, race, BMI, and clinical data, including final pathology, were obtained from the electronic health record.
2.2 Evaluation of obesity
BMI was calculated from subject height/weight information as documented in patient medical records. As per World Health Organization guidelines, BMI ≥ 30 kg/m2 was used as the principal metric to define obesity. Subjects’ preoperative computed tomography (CT) and / or MRI scans were reviewed to determine two metrics of abdominal fat accumulation: retro-renal fat (RRF) pad distance (i.e. distance from the posterior aspect of the kidney to the posterior abdominal wall), and waist circumference (WC), which encompasses subcutaneous, visceral, and RRF depots. RRF pad distance was measured on the contra-lateral kidney at the level of the renal vein, as described [11]. Radiographic WC was utilized to measure WC at 1 cm above the umbilicus [12].
2.3 Evaluation of renal mass complexity
Preoperative imaging was available for 97 of 99 patients. R.E.N.A.L. nephrometry scores were calculated for each subject. R.E.N.A.L. nephrometry scoring includes tumor radius, exophytic versus endophytic characteristics, nearness to the collecting system, anterior/posterior location, and location to polar lines [13]. Maximum mass diameter was obtained from imaging (L.B.). Masses were categorized as hilar or non-hilar, as this has been shown to correlate with tumor grade [14]. Subjects who had undergone a previous radical or partial nephrectomy or those with polycystic kidney disease were excluded, given the effect their distorted anatomy might play in calculating both nephrometry score and RRF. A single investigator performed all RRF, WC, and nephrometry score calculations (L.B.) with randomly selected subjects’ measurements confirmed by a separate investigator (K.N.). Prior studies showed high inter-observer correlation and reproducibility when utilizing nephrometry scores [15].
2.3 Statistical analyses
Patients’ demographics and clinical characteristics were summarized as mean ± standard deviation (SD) for continuous variables and frequency (proportion) for categorical variables. The group comparison was conducted using a two-sample t test for continuous variables and Chi-square test or Fisher’s exact test for categorical variables. The obesity metrics among Fuhrman grade or tumor stage were evaluated using analysis of variance (ANOVA) and two-sample t test for group comparisons. Associations between obesity metrics and potential contributing factors were evaluated with univariate analyses. BMI and RRF were log-transformed to meet the normality assumption for hypothesis testing. The associations between R.E.N.A.L. score and obesity metrics were evaluated with univariate analyses, and multivariate analyses controlling for age, sex, race, and diabetes status, using general linear regression. The raw p values were presented and p <0.05 was considered as statistically significant. All analyses were conducted using SAS 9.4 (Cary, NC).
3. Results
3.1 Study subject characteristics
Tables 1 and 2 summarize the clinical characteristics of all consented study subjects (n = 99). The mean BMI of our overall cohort was 32.6 kg/m2. The majority of tumors were the clear cell histologic subtype (76.8%), and 12.1% were either papillary or chromophobe RCC. Another 11.1% of renal masses were benign. For obese versus non-obese subjects, age and biological sex were comparable. Tumor sizes were comparable in both cohorts (mean imaging mass diameter = 4.6 cm in non-obese and 5.2 cm in obese cohort; mean pathological mass diameter = 4.9 cm in non-obese and 5.2 cm in obese cohort) (p values = 0.57 and 0.94, respectively). Radical nephrectomy was performed in 31/56 (56.4%) of obese and 17/43 (39.5%) of non-obese subjects (p value = 0.16).
Table 1.
Subject demographics and clinical characteristics of subjects with a renal mass
| Variables |
Total Subjects Mean (SD) or proportion N=99 |
Obese Subjects (BMI ≥ 30kg/m2) Mean (SD) or proportion N= 56 |
Non-Obese Subjects (BMI < 30kg/m2) Mean (SD) or proportion N= 43 |
|---|---|---|---|
| Age (years) | 58.7 (11.5) | 57.9 (10.1) | 59.9 (13.07) |
| Sex | |||
| Male | 64 (64.6%) | 33 (75.7%) | 31 (72.1%) |
| Female | 35 (35.4%) | 23 (24.3%) | 12 (27.9%) |
| Race | |||
| Caucasian | 90 (90.9%) | 49 (87.5%) | 41 (95.3%) |
| AA | 7 (7.1%) | 6 (10.7%) | 1 (2.3%) |
| Other | 2 (2.0%) | 1 (1.8%) | 1 (2.3%) |
| Weight (kg) | 99.6 (32.3) | 117.2 (32.15) | 76.8 (11.6) |
| Height (m) | 1.7 (0.10) | 1.7 (0.11) | 1.8 (0.10) |
| BMI (kg/m2) | 32.6 (9.2) | 38.4 (8.04) | 25.0 (2.91) |
| WC | 96.9 (48.37) | 127.0 (18.1) | 97.8 (10.3) |
| RRF | 17.2 (12.01) | 21.5 (11.6) | 15.0 (11.58) |
Abbreviations: SD= standard deviation, BMI= body mass index, AA = African American, WC = waist circumference, RRF = retro-renal fat pad distance
Table 2.
Subject tumor characteristics
| Variables |
Total Subjects Mean (SD) or proportion N=99 |
Obese Subjects (BMI ≥ 30kg/m2) Mean (SD) or proportion N= 56 |
Non-Obese Subjects (BMI < 30kg/m2) Mean (SD) or proportion N= 43 |
|---|---|---|---|
| R.E.N.A.L. Score | 8.5 (2.3) | 8.6 (2.2) | 8.2 (2.5) |
| Mass size | |||
| Radiographic | 4.9 (2.8) | 5.2 (2.7) | 4.5 (2.9) |
| Pathological examination | 5.0 (2.9) | 5.2 (2.6) | 4.9 (3.3) |
| Fuhrman Grade | |||
| 1 | 2 (2.0%) | 2 (3.6%) | 0 (0%) |
| 2 | 41 (41.4%) | 29 (51.8%) | 12 (27.9%) |
| 3 | 28 (28.3%) | 12 (21.4%) | 16 (37.2%) |
| 4 | 11 (11.1%) | 5 (8.9%) | 6 (14.0%) |
| Not Reported | 17 (17.2%) | 8 (14.3%) | 9 (20.9%) |
| Stage | |||
| T1a | 39 (39.4%) | 20 (35.7%) | 19 (44.2%) |
| T1b | 23 (23.2%) | 18 (32.1%) | 5 (11.6%) |
| T2a | 3 (3.0%) | 2 (3.6%) | 1 (2.3%) |
| T2b | 2 (2.0%) | 1 (1.8%) | 1 (2.3%) |
| T3a | 19 (19.2%) | 10 (17.9%) | 9 (20.9%) |
| T3b | 1 (1.0%) | 0 (0%) | 1 (2.3%) |
| Not Reported | 12 (12.1%) | 5 (8.9%) | 7 (16.3%) |
| Pathology | |||
| Clear cell | 76 (76.8%) | 46 (82.1%) | 30 (69.8%) |
| Chromophobe | 4 (4.0%) | 2 (3.6%) | 2 (4.7%) |
| Papillary | 8 (8.1%) | 4 (7.1%) | 4 (9.3%) |
| AML (Benign) | 1 (1.0%) | 0 (0%) | 1 (2.3%) |
| Benign cyst | 1 (1.0%) | 1 (1.8%) | 0 (0%) |
| Cystic nephroma (Benign) | 1 (1.0%) | 0 (0%) | 1 (2.3%) |
| Mixed Epithelial (Benign) | 1 (1.0%) | 1 (1.8%) | 0 (0%) |
| Oncocytoma (Benign) | 6 (6.1%) | 2 (3.6%) | 4 (9.3%) |
| Not Reported (Benign) | 1 (1.0%) | 0 (0%) | 1 (2.3%) |
Abbreviations: SD= standard deviation, BMI= body mass index, AML = angiomyolipoma
We performed univariate analyses to examine potential associations between each obesity metric and age, sex, race, or diabetes status. No significant associations existed between BMI or WC with any these factors (Table 3). In contrast, higher RRF was significantly associated with increased age and male sex (both, p < 0.01); for every 1 year increase in age, the RRF distance increased 0.35 mm, and the mean RRF was 7.4 mm higher in males than females.
Table 3.
Univariate analysis for correlations among WC, BMI, RRF, and potential contributing factors
| BMI:
| |||||
|---|---|---|---|---|---|
| Parameter | Estimate | SE | 95%CI | P | |
| Age | −0.11 | 0.08 | −0.27 | 0.05 | 0.1836 |
| Sex (F) | 0.42 | 1.95 | −3.44 | 4.28 | 0.8304 |
| Race (C) | −1.30 | 3.23 | −7.72 | 5.12 | 0.6885 |
| Diabetes | 3.40 | 2.11 | −0.79 | 7.60 | 0.1107 |
| WC:
| |||||
|---|---|---|---|---|---|
| Parameter | Estimate | SE | 95%CI | P | |
| Age | −0.11 | 0.19 | −0.49 | 0.26 | 0.5553 |
| Sex (F) | −2.49 | 4.44 | −11.30 | 6.32 | 0.5760 |
| Race (C) | 2.96 | 7.25 | −11.44 | 17.36 | 0.6840 |
| Diabetes | 7.51 | 4.83 | −2.09 | 17.11 | 0.1235 |
| RRF
| |||||
|---|---|---|---|---|---|
| Parameter | Estimate | SE | 95%CI | P | |
| Age | 0.35 | 0.10 | 0.14 | 0.55 | 0.0011 |
| Sex (F) | −7.37 | 2.54 | −12.42 | −2.33 | 0.0046 |
| Race (C) | 4.62 | 4.52 | −4.36 | 13.61 | 0.3094 |
| Diabetes | 4.75 | 2.91 | −1.02 | 10.53 | 0.1057 |
Abbreviations: WC = waist circumference, BMI = body mass index, RRF = retro-renal fat pad distance, F = female, C = caucasian
3.2 Univariate analyses of individual obesity metrics with R.E.N.A.L. score, Fuhrman grade and tumor stage
Univariate analyses were used to examine potential associations between BMI, WC, and RRF, and R.E.N.A.L. nephrometry score. As shown in Table 4, nephrometry scores were positively associated with WC (p = 0.02), but not BMI (p = 0.13) or RRF (p = 0.57). Univariate analyses were also performed on potential contributing factors including age, sex, race, and diabetes status. Only biological sex was significantly associated with R.E.N.A.L. score (p = 0.02); males showed an average R.E.N.A.L score of 1.11 units > females.
Table 4.
Univariate analysis for correlations among BMI, WC, RRF and R.E.N.A.L. score
| Parameter | Estimate | SE | 95%CI | P | |
|---|---|---|---|---|---|
| BMI | 0.04 | 0.03 | −0.01 | 0.09 | 0.1253 |
| WC | 0.03 | 0.01 | 0.00 | 0.05 | 0.0215 |
| RRF | 0.01 | 0.02 | −0.03 | 0.05 | 0.5680 |
| Age | 0.01 | 0.02 | −0.03 | 0.05 | 0.5828 |
| Sex (F) | −1.11 | 0.48 | −2.08 | −0.15 | 0.0240 |
| Race (C) | 0.72 | 0.81 | −0.89 | 2.32 | 0.3778 |
| Diabetes | −0.01 | 0.55 | −1.10 | 1.07 | 0.9798 |
The univariate analyses suggest that WC and sex are significantly associated with the R.E.N.A.L. score (p=0.0215 and 0.0240, respectively). The R.E.N.A.L. score is positively correlated with WC (r=0.24, p=0.023) in the univariate analysis. The mean R.E.N.A.L. score in females is 1.11 units lower than in males.
Abbreviations: WC = waist circumference, BMI = body mass index, RRF = retro-renal fat pad distance, F = female, C = caucasian
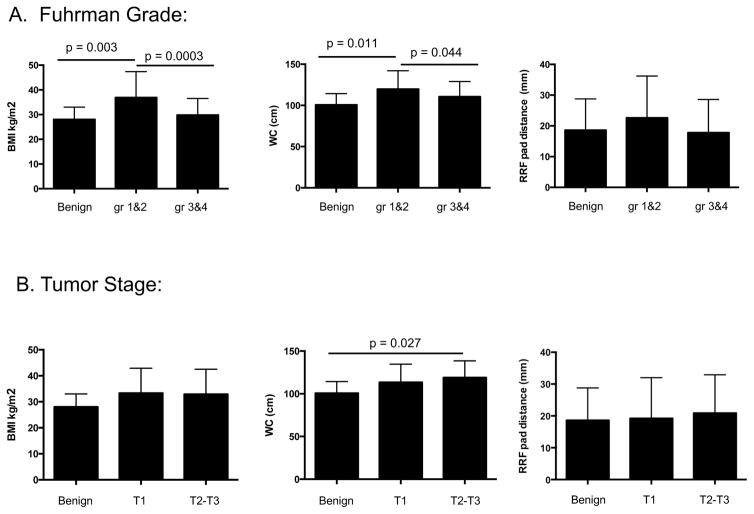
ANOVA was performed to compare BMI, WC, and RRF relative to renal tumor Fuhrman grade and stage (Figure 1). BMI was significantly different for benign versus Grade 1&2 tumors, and Grade 1&2 versus Grade 3&4 tumors (mean benign = 28.0 ± 5.0, grade 1&2, = 36.9 ± 10.5, or grade 3&4 = 29.8 ± 6.7). WC showed similar trends, with grade 1&2 tumors having the highest mean (mean benign = 100.7 cm ± 13.6, grade 1&2 = 119.8 cm ± 22.2, and grade 3&4 = 110.6 cm ± 18.5). No significant differences were observed when tumor grade was evaluated relative to RRF (p = 0.20) (Figure 1). For tumor T pathologic stage, no significant associations were found between BMI or RRF and tumor T stage (BMI, p = 0.21; or RRF, p = 0.81) (Figure 1). However, WC was higher in subjects with T2/3 versus benign tumors (p = 0.03).
Figure 1.
Increased BMI in subjects with low Fuhrman grade tumors. Subjects were categorized based upon tumor Fuhrman grade (A) or T stage (B), then mean BMI scores were calculated for each category. Category means showing statistically significant differences are indicated.
3.4 Multivariate analysis of WC and R.E.N.A.L. score
To more definitively evaluate associations between obesity as measured by WC and R.E.N.A.L. nephrometry score, multivariate analysis was performed to consider potential confounding effects of age, biological sex, race, and diabetes status. After controlling for all of these factors, higher WC remained significantly associated with increased R.E.N.A.L. nephrometry score (p = 0.02), such that for every 1 cm increase in WC the R.E.N.A.L. score increased 0.03 units (Table 5). Males had higher R.E.N.A.L. scores than females (average of 1.1 units higher) when WC, age, rage, and diabetes status were controlled.
Table 5.
Multivariate analyses for WC, BMI, RRF and R.E.N.A.L. score associations
| WC: | |||||
|---|---|---|---|---|---|
| Parameter | Estimate | SE | 95%CI | p | |
| Intercept | 4.82 | 1.88 | 1.08 | 8.56 | 0.0122 |
| WC | 0.03 | 0.01 | 0.00 | 0.05 | 0.0219 |
| Age | 0.02 | 0.02 | −0.02 | 0.06 | 0.4630 |
| Sex (F vs M) | −1.09 | 0.51 | −2.10 | −0.09 | 0.0338 |
| Race (C vs Non-C) | 0.05 | 0.86 | −1.67 | 1.76 | 0.9550 |
| Diabetes | −0.39 | 0.56 | −1.50 | 0.72 | 0.4853 |
After controlling for age, sex, race and diabetes status, WC is significantly associated with the renal score (p=0.0219). For every 1 unit increase of WC, the renal score will increase 0.03 units. After controlling for WC, age, Race and diabetes status, females have average of 1.09 unit lower renal score than males (p=0.0338). However, there is no significant association between renal score and diabetes status.
Abbreviations: WC = waist circumference, BMI = body mass index, RRF = retro-renal fat pad distance, F = female, M = male, C = Caucasian, Non-C = non-caucasian
4. Discussion
We examined associations between multiple obesity metrics and renal tumor characteristics, to determine if evaluation of abdominal obesity via WC measurement would reveal associations not indicated when BMI was used to define obesity. R.E.N.A.L. nephrometry scoring was used, as higher R.E.N.A.L. scores have been linked to post-surgical complications, tumor recurrence, and decreased survival [16–19]. Our study identified a significant positive association between WC and R.E.N.A.L. nephrometry score, and subjects with stage T2/T3 tumors had higher WC than subjects with benign renal masses. In contrast, both high WC and high BMI were seen in individuals with low Fuhrman grade RCC. Thus, our study highlights the complexity of the relationship between obesity and RCC, as no unifying trend existed when different obesity metrics or tumor characterization standards were used.
A few prior reports have examined abdominal obesity, rather than BMI, as a contributor to RCC. When visceral fat accumulation was examined by CT/MRI, Wang et al. found a positive association with clear cell RCC occurrence [20], and Zhu et al. determined that increased visceral adiposity was an independent predictor of higher Fuhrman grade in localized T1a clear cell RCC [6]. Our report finds that increased abdominal adiposity as defined by WC, which includes subcutaneous, visceral, and RRF depots, is associated with greater renal tumor complexity, but, in contrast to Zhu et al., lower tumor grade. RRF was not associated with any tumor metric, which suggests that other fat depots are driving the observed association between WC and R.E.N.A.L. score. Collectively, these findings suggest that continued evaluation of associations between obesity and RCC are needed, and obesity metrics other than BMI should be included, as WC may be identifying some element of renal mass development that is not evident when BMI is used.
Obesity defined by BMI has been associated with improved survival [5] and treatment outcomes [4] in RCC patients. Hakimi et al. reported that high BMI was associated with reduced RCC stage at diagnosis and improved survival on univariate analysis; however, high BMI had no relationship with cancer-specific mortality after stage and grade were controlled [21]. Albiges et al. compared survival in over 5,000 subjects with metastatic RCC, and found that high BMI (BMI > 25 kg/m2, including both overweight and obese subjects) was associated with increased OS following targeted therapy [22]. These and other findings have led to the conclusion that an obesity paradox exists for RCC, wherein obesity defined by high BMI is associated with less advanced disease and better outcomes.
The obesity paradox phenomenon has been refuted by a growing number of publications. The 2016 Aune et al. meta-analysis of >30 million participants in 230 independent studies reported that overweight and obesity are indeed associated with increased all-cause mortality [23]. The authors posited that prior obesity paradox reports had skewed outcome data by failing to exclude from the lean category prior or current smokers, or individuals with disease-related weight loss. A 2016 review suggested that the RCC obesity paradox may also arise from increased incidental diagnoses in individuals with obesity-associated comorbidities [2]. Additionally, Bagheri et al. found that BMI-defined obesity was associated with improved RCC-specific survival, but decreased OS in these same individuals, suggesting different effects of obesity on outcomes from cancer and other cause mortality [24]. Given links between R.E.N.A.L. scores and RCC recurrence and worse OS [16–19], our data suggest that further evaluation of outcome data in larger RCC cohorts may reveal important new associations between obesity and RCC outcomes if abdominal versus general obesity are compared.
Our study does have limitations. Key among these is the small sample size (n=99) in our cohort. Our findings should be confirmed by further evaluation of larger RCC subject cohorts. Also, the relationship between a complex disease such as cancer and obesity is unlikely to be straightforward. Other factors that could be examined in future studies include physical activity, socioeconomic status, and alcohol and tobacco use, the latter two of which may correlate with lower body weights but would be associated with poorer overall health status. Of note, our study subjects were overwhelmingly Caucasian due to Iowa’s state demographics. A prior study found stronger associations between obesity and RCC incidence in Caucasians than African Americans [25]. Additionally, prior studies have shown links between specific genes, namely estrogen receptor-α (ESR1), opioid receptor (OPRM1), and neuromedin B receptor (NMBR), and abdominal adiposity [26, 27]. However, a full genetic investigation of such markers was beyond the scope of this study. Finally, as our study cohort is relatively new, we focused on data available at surgery and do not yet have five-year survival data, which will be needed to fully interpret identified associations and subsequent effects on outcome and prognosis.
5. Conclusions
Our findings highlight the need for continued examination into associations between obesity and RCC biology and outcomes. Our study illustrates a significant positive association between WC and R.E.N.A.L. nephrometry score, but an opposing trend for WC or BMI versus Fuhrman grade. We propose that both WC and BMI should be used in future studies to gain a deeper understanding of the complex interplay between general and abdominal obesity in RCC.
Highlights.
The obesity paradox in RCC is based upon BMI to define obesity
Abdominal fat is a key contributor to general obesity-related pathologies
Increasing waist circumference correlated positively with R.E.N.A.L. score
High BMI was associated with low Fuhrman grade but not R.E.N.A.L. score
Waist circumference and BMI are not interchangeable obesity metrics in RCC
Acknowledgments
Funding
This study was supported by NIH grant R01CA181088 to LAN, UL1TR001417 to PL, NIH/NCI Cancer Prevention and Control Training Pre-Doctoral Program Grant #5-R25-CA47888 to SKB, and UAB NIH Nutrition Obesity Research Center (NORC) Pre-Doctoral Training Grant #T32HL105349 to RMO.
8. References
- 1.Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010;7:245–57. doi: 10.1038/nrurol.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson KM, Cho E. Obesity and Kidney Cancer. Recent Results Cancer Res. 2016;208:81–93. doi: 10.1007/978-3-319-42542-9_5. [DOI] [PubMed] [Google Scholar]
- 3.Lowrance WT, Thompson RH, Yee DS, Kaag M, Donat SM, Russo P. Obesity is associated with a higher risk of clear-cell renal cell carcinoma than with other histologies. BJU Int. 2010;105:16–20. doi: 10.1111/j.1464-410X.2009.08706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi Y, Park B, Jeong BC, Seo SI, Jeon SS, Choi HY, et al. Body mass index and survival in patients with renal cell carcinoma: a clinical-based cohort and meta-analysis. Int J Cancer. 2013;132:625–34. doi: 10.1002/ijc.27639. [DOI] [PubMed] [Google Scholar]
- 5.Waalkes S, Merseburger AS, Kramer MW, Herrmann TR, Wegener G, Rustemeier J, et al. Obesity is associated with improved survival in patients with organ-confined clear-cell kidney cancer. Cancer Causes Control. 2010;21:1905–10. doi: 10.1007/s10552-010-9618-2. [DOI] [PubMed] [Google Scholar]
- 6.Zhu Y, Wang HK, Zhang HL, Yao XD, Zhang SL, Dai B, et al. Visceral obesity and risk of high grade disease in clinical t1a renal cell carcinoma. J Urol. 2013;189:447–53. doi: 10.1016/j.juro.2012.09.030. [DOI] [PubMed] [Google Scholar]
- 7.Grignol VP, Smith AD, Shlapak D, Zhang X, Del Campo SM, Carson WE. Increased visceral to subcutaneous fat ratio is associated with decreased overall survival in patients with metastatic melanoma receiving anti-angiogenic therapy. Surg Oncol. 2015;24:353–8. doi: 10.1016/j.suronc.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahima RS, Lazar MA. Physiology. The health risk of obesity--better metrics imperative. Science. 2013;341:856–8. doi: 10.1126/science.1241244. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs EJ, Newton CC, Wang Y, Patel AV, McCullough ML, Campbell PT, et al. Waist circumference and all-cause mortality in a large US cohort. Arch Intern Med. 2010;170:1293–301. doi: 10.1001/archinternmed.2010.201. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez MC, Pastore CA, Orlandi SP, Heymsfield SB. Obesity paradox in cancer: new insights provided by body composition. Am J Clin Nutr. 2014;99:999–1005. doi: 10.3945/ajcn.113.071399. [DOI] [PubMed] [Google Scholar]
- 11.Clark W, Siegel EM, Chen YA, Zhao X, Parsons CM, Hernandez JM, et al. Quantitative measures of visceral adiposity and body mass index in predicting rectal cancer outcomes after neoadjuvant chemoradiation. J Am Coll Surg. 2013;216:1070–81. doi: 10.1016/j.jamcollsurg.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciudin A, Salvador R, Budoy A, Ciudin A, Spinu C, Diaconu MG, et al. Measurement of waist circumference for retrospective studies - prospective validation of use of CT images to assess abdominal circumference. Endocrinol Nutr. 2014;61:147–52. doi: 10.1016/j.endonu.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009;182:844–53. doi: 10.1016/j.juro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 14.Kutikov A, Smaldone MC, Egleston BL, Manley BJ, Canter DJ, Simhan J, et al. Anatomic features of enhancing renal masses predict malignant and high-grade pathology: a preoperative nomogram using the RENAL Nephrometry score. Eur Urol. 2011;60:241–8. doi: 10.1016/j.eururo.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montag S, Waingankar N, Sadek MA, Rais-Bahrami S, Kavoussi LR, Vira MA. Reproducibility and fidelity of the R.E.N.A.L. nephrometry score. J Endourol. 2011;25:1925–8. doi: 10.1089/end.2011.0217. [DOI] [PubMed] [Google Scholar]
- 16.Kriegmair MC, Mandel P, Moses A, Lenk J, Rothamel M, Budjan J, et al. Defining Renal Masses: Comprehensive Comparison of RENAL, PADUA, NePhRO, and C-Index Score. Clin Genitourin Cancer. 2016 doi: 10.1016/j.clgc.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 17.Kopp RP, Mehrazin R, Palazzi KL, Liss MA, Jabaji R, Mirheydar HS, et al. Survival outcomes after radical and partial nephrectomy for clinical T2 renal tumours categorised by R.E.N.A.L. nephrometry score. BJU Int. 2014;114:708–18. doi: 10.1111/bju.12580. [DOI] [PubMed] [Google Scholar]
- 18.Camacho JC, Kokabi N, Xing M, Master VA, Pattaras JG, Mittal PK, et al. R.E.N.A.L. (Radius, exophytic/endophytic, nearness to collecting system or sinus, anterior/posterior, and location relative to polar lines) nephrometry score predicts early tumor recurrence and complications after percutaneous ablative therapies for renal cell carcinoma: a 5-year experience. J Vasc Interv Radiol. 2015;26:686–93. doi: 10.1016/j.jvir.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Nagahara A, Uemura M, Kawashima A, Ujike T, Fujita K, Miyagawa Y, et al. R.E.N.A.L. nephrometry score predicts postoperative recurrence of localized renal cell carcinoma treated by radical nephrectomy. Int J Clin Oncol. 2016;21:367–72. doi: 10.1007/s10147-015-0879-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang HK, Song XS, Cheng Y, Qu YY, Zhang SL, Dai B, et al. Visceral fat accumulation is associated with different pathological subtypes of renal cell carcinoma (RCC): a multicentre study in China. BJU Int. 2014;114:496–502. doi: 10.1111/bju.12592. [DOI] [PubMed] [Google Scholar]
- 21.Hakimi AA, Furberg H, Zabor EC, Jacobsen A, Schultz N, Ciriello G, et al. An epidemiologic and genomic investigation into the obesity paradox in renal cell carcinoma. J Natl Cancer Inst. 2013;105:1862–70. doi: 10.1093/jnci/djt310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albiges L, Hakimi AA, Xie W, McKay RR, Simantov R, Lin X, et al. Body Mass Index and Metastatic Renal Cell Carcinoma: Clinical and Biological Correlations. J Clin Oncol. 2016 doi: 10.1200/JCO.2016.66.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aune D, Sen A, Prasad M, Norat T, Janszky I, Tonstad S, et al. BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ. 2016;353:i2156. doi: 10.1136/bmj.i2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bagheri M, Speakman JR, Shemirani F, Djafarian K. Renal cell carcinoma survival and body mass index: a dose-response meta-analysis reveals another potential paradox within a paradox. Int J Obes (Lond) 2016;40:1817–22. doi: 10.1038/ijo.2016.171. [DOI] [PubMed] [Google Scholar]
- 25.Beebe-Dimmer JL, Colt JS, Ruterbusch JJ, Keele GR, Purdue MP, Wacholder S, et al. Body mass index and renal cell cancer: the influence of race and sex. Epidemiology. 2012;23:821–8. doi: 10.1097/EDE.0b013e31826b7fe9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox CS, Heard-Costa NL, Wilson PW, Levy D, D’Agostino RB, Sr, Atwood LD. Genome-wide linkage to chromosome 6 for waist circumference in the Framingham Heart Study. Diabetes. 2004;53:1399–402. doi: 10.2337/diabetes.53.5.1399. [DOI] [PubMed] [Google Scholar]
- 27.Fox CS, Heard-Costa N, Cupples LA, Dupuis J, Vasan RS, Atwood LD. Genome-wide association to body mass index and waist circumference: the Framingham Heart Study 100K project. BMC Med Genet. 2007;8(Suppl 1):S18. doi: 10.1186/1471-2350-8-S1-S18. [DOI] [PMC free article] [PubMed] [Google Scholar]