Abstract
Cell size control and homeostasis is a long-standing subject in biology. Recent experimental work provides extensive evidence for a simple, quantitative size homeostasis principle coined adder (as opposed to sizer or timer). The adder principle provides unexpected insights into how bacteria maintain their size without employing a feedback mechanism. We review the genesis of adder and recent cell size homeostasis study on evolutionarily divergent bacterial organisms and beyond. We propose new coarse-grained approaches to understand the underlying mechanisms of cell size control at the whole cell level.
Introduction
Size homeostasis is a basic feature of life. A group of newly born or dividing cells will exhibit a distinct distribution of sizes (Figure 1a). Without underlying coordination of growth and the cell cycle, this distribution would diverge over generations, leading to cell sizes that span many orders of magnitude. However, most microorganisms change their size by only twofold between birth and division, with the width of the size distributions being much smaller than their mean. This fidelity in the cell size maintenance points to the existence of size control mechanisms.
Figure 1.
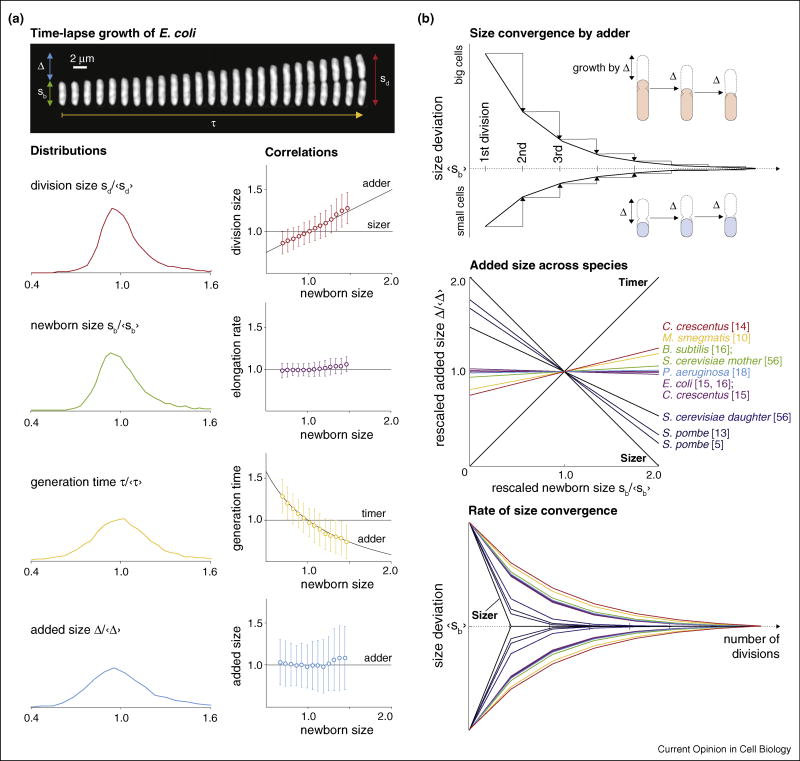
Single cell growth parameters and size convergence by adder. (a) Top panel: timelapse images of an E. coli cell from birth to division in rich soytone medium at 37°C. Interval between images is 45 s. Bottom panel: distributions of four growth parameters in increasing order of coefficient of variance (CV). The x-axes of the distributions are normalized by their respective means. The single cell correlations between the growth parameters and newborn size can be used to test sizer, timer, and adder. (b) Top panel: adder corrects size deviations passively by adding constant Δ between birth and division and dividing in the middle, irrespective of the birth size. Every generation cuts the size difference between the mother’s newborn size and the average newborn size by half, following a geometric series. Newborn size converges to the average, which is equivalent to Δ. Middle panel: most published single-cell data show bacteria in general behave as perfect adder or near-perfect adder, whereas yeast behave closer to sizer. Bottom panel: using the Δ vs. sb correlations, we can predict size convergence (colors correspond to those in the middle panel). Perfect sizer would reach the target size just after one generation, whereas timers would never converge their size.
Cell size control research requires quantitative information from single-cell data. Indeed, the field has benefited from single-cell measurement of cellular growth from time-lapse images since it began a century ago [1–3]. However, only recently has experimental technology become sophisticated enough to generate data with the throughput and spatiotemporal resolution required to rigorously test previously proposed hypotheses [4–8,9•]. With the new single-cell data, notions that microbes measure a critical size (‘sizer’) or a specific time elapsed during the cell cycle (‘timer’) to trigger division are no longer supported [10•,11–14,15••,16••,17,18].
Two recent papers independently provided extensive experimental evidence for the ‘adder’ principle of size homeostasis [15••,16••].2 This principle states that all cells under a steady-state growth condition add a nearly constant size between birth and division, regardless of their birth size (Figure 1b). The model has been used to explain size convergence in evolutionarily divergent bacterial organisms [17,19]. Size homeostasis by adder represents a fundamental departure from a feedback-based view.
This article is organized as follows. In the first half, we describe the origin of several cornerstone models in cell size control and homeostasis in bacteria. This is continued with a more in-depth discussion of the adder principle and its implications. In the second half, we discuss the challenges involved in studying the underlying molecular mechanism. We explain the power of a coarse-grained approach using recent examples in bacterial physiology and cell cycle research.
From sizer and timer to adder in cell size homeostasis
Consider the life cycle of Escherichia coli from birth to division (Figure 1a). All growth parameters show distributions with a well-defined peak. One naturally associates the width of the distributions with the ‘tightness’ of regulation of these parameters. Early on, researchers measured the coefficient of variation (CV) of the distributions of the division size and the generation time [21,22]. This led to the first quantitative models on size control. For example, Koch and Schaechter concluded that “a cell grows until it has reached a critical size” [4], because the division size distribution was narrower than that of the generation time, thus sizer. Had the CV of the generation time been smaller than that of the division size, they may instead have concluded timer.
While both sizer and timer are obvious models for size control, they fail to explain single-cell growth data [10•,11–14,15••,16••,17,18]. The failure of these models is not apparent from the distributions, but requires correlations between the growth parameters. That is, if a cell divided at a critical size (sizer), then its size at division would be constant despite its birth size (Figure 1a). If the cell instead divided after a constant time has elapsed since birth (timer), then generation time would be independent of the birth size. Neither the division size nor the generation time is constant relative to the birth size, inconsistent with the data.
In contrast to sizer or timer, cells following adder simply add on average a constant size, Δ, between birth and division regardless of their birth size (Figure 1a, bottom right panel). This alone ensures size homeostasis (Figure 1b, top panel). To see this, consider a cell born exactly at Δ. This cell grows by Δ, doubling its size. When it divides in the middle its two daughters are born at the same size as the previous generation. However, a cell born smaller than Δ will more than double its size, and its daughters are born slightly bigger than it was. Over successive generations, the difference between the initial newborn cell size and Δ progressively diminishes. Analogously, cells born larger than Δ also dilute out their size deviations with every division. The size of all newborn cells will converge to Δ after several consecutive divisions irrespective of their initial size. Consequently, the average newborn size of a steady-state population is identical to Δ, which is determined by the growth condition.
So far, this has been shown for several evolutionarily divergent bacterial species such as Caulobacter crescentus, E. coli, Bacillus subtilis, and Pseudomonas aeruginosa [12,15••,16••,17,18]. For the summary and comparison of these studies, see Table 1.
Table 1.
Summary of select single-cell experiments for microbial cell size study.
| Organism | Methods | Experimental conditions |
Cell number | Definition of cell size | Slope of Δ vs. sb |
|---|---|---|---|---|---|
| S. pombe [5] | Agarose pad | 1 | <100 | Length | −0.76 |
| S. pombe [13] | Microfluidics | 5 | 347 926 | Length | −0.67 ± 0.053 |
| S. cerevisiae [56] | Agarose pad | 6 | 50–200 | Volumea | 0.06 ± 0.08 (mother) |
| −0.47 ± 0.08 (daughter) | |||||
| M. smegmatis [10•] | Microfluidics | 1 | 520 | Length | 0.19 ± 0.065 |
| C. crescentus [14] | Microfluidics | 5 | ~104–105 | Area | 0.25 ± 0.06 |
| C. crescentus [15••] | Agarose pad | 1 | 565 (stalked) | Length | 0.0023 (stalked) |
| 141 (swarmer) | −0.006 (swarmer) | ||||
| E. coli [15••] | Microfluidics | 2 (wildtype) | ~103 | Length | 0.0147; 0.0189 |
| 1 (mutants) | (LB media; M9 media) | ||||
| E. coli [16••] | Microfluidics | 7 (wildtype) | 84 699 | Length, volume | −0.01 ± 0.012 |
| 2 (mutants) | 8105 | 0.01 ± 0.055 | |||
| B. subtilis [16••] | Microfluidics | 4 | 24 758 | Length, volume | 0.06 ± 0.021 |
| P. aeruginosa [18] | Agarose pad | 2 | 117 | Length | 0.03 ± 0.023 (wildtype) |
Volume was measured by the total fluorescence level per cell.
The adder principle has several profound implications. First, adder describes cell size homeostasis without feedback. Cells employing adder passively converge their size within a few generations without the need of active measurement of their absolute size (Figure 1b). This is in stark contrast with the checkpoint-based models in eukaryotes for coordinating growth and the cell cycle, where each cell is described to actively control its own size.
Second, adder provides a general quantitative framework to help us understand size homeostasis for a wide range of organisms, including eukaryotes. Reanalysis of published data predicts that cell size converges like adder even when the added size is linearly correlated with birth size (Figure 1b).3 Surprisingly, the flatworm Dugesia tigrina behaves according to adder by growing a constant bodily area from the head piece after transverse splitting [19]!
Third, adder reveals a hierarchy of physiological control of an exponentially elongating cell. The added size and the elongation rate are the primary spatial and temporal controls of cellular reproduction, respectively. Other growth parameters such as birth size, division size, or generation time are completely characterized by and slaved to these two basic control parameters [16••]. Septum position exhibits a tight distribution independent of the other growth parameters.
The discovery of adder was made possible by modern single-cell data with throughput and precision that was not previously available. We anticipate more basic principles will be discovered by exploring other phenomenological parameters such as cell shape [23•], which will be aided by new and precise measurements [24,25]. Inevitably, we will also need to address the mechanisms underlying these phenomenological principles. This requires a new approach discussed below.
Mechanism of size control
Adder presents a unifying phenomenological principle for size homeostasis, but its mechanism is challenging to study for two reasons. First, it is the generality of the adder principle, which describes size homeostasis independent of the major molecular differences among organisms. As illustrated below, two evolutionarily distant organisms use different sets of genes for their cell cycle regulation, yet they can follow the same phenomenological principles at the cellular level. Second, it is the holistic nature of cell size control, which makes it difficult to rely on genetic approaches aimed at identifying genes involved in cell size control [26,27]. Mutations in any genes that affect cellular physiology will cause changes in the cell size.
For molecular mechanisms of size control, consider E. coli and B. subtilis. They are a textbook example of how Gram-negative and Gram-positive organisms are fundamentally different in their cell cycle at the molecular level. In E. coli, premature replication initiation is prevented by SeqA, which sequesters hemi-methylated DNA after initiation, but no seqA homologs exist in B. subtilis [28,29]. In E. coli, Min proteins oscillate from pole to pole to prevent formation of DNA-less minicells, whereas Min proteins show no oscillations in B. subtilis [30]. Despite these molecular differences, both E. coli and B. subtilis share common phenomenological characteristics at the cellular level such as multifork replication for cell cycle progression and the adder principle for cell size homeostasis.
Genetic screening in cell size study can be further limited because mutations in many genes can have secondary effects on cell size. Imagine, for instance, E. coli strains with mutations in topo IV (a type II topo), which would resolve topological catenations of replicating circular chromosome less efficiently than the wildtype. As a consequence, chromosome segregation and cell division would be delayed, thus causing an increase in cell size. However, it would be absurd to consider topo IV as a cell size control gene.
In our view, cell size control research will greatly benefit from a coarse-grained approach from physics or engineering as proposed before [31,32]. Without referring to any molecular details, cellular reproduction can be understood in terms of discrete ‘modules’ that carry out distinct functions [31]. These modules can be grouped into ‘kernels’, namely, major processes for cellular reproduction such as the cell cycle, cell wall synthesis, protein production, and metabolism. Each of these kernels is governed by an organism-specific set of genes, but their overall function is conserved from organism to organism. The cell must coordinate different kernels in space and time to maintain physiological homeostasis during cellular growth. The coordination in steady-state growth requires balance of flux, or flow of information, metabolites, or substrates, from one kernel to another.
Below are two examples of how coarse-grained models allow for a quantitative understanding of cellular systems. Both use distinct methods which are devoid of molecular details, yet give insight into how the cell coordinates major processes. A third example illustrates how molecular mechanisms can be understood in the context of a coarse-grained view of cellular processes in the first two examples.
Coarse-grained approach to protein composition
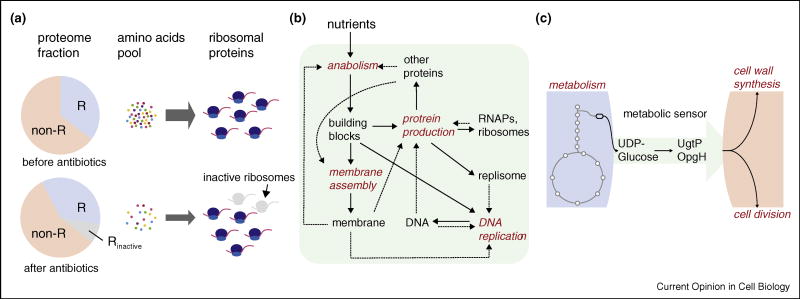
The power of the coarse-grained approach is that it allows for experimentally testable, quantitative predictions without the knowledge of individual molecules. A recent body of work successfully describes the proteome composition by partitioning it into ‘sectors’ which comprise functional sets of proteins [33,34]. The fraction of the ribosomal sector with respect to the rest can be understood as a result of balancing the amino acid flux between the protein production kernel and the metabolism kernel. If translation is partially inhibited by antibiotics which target ribosomes, the demand for amino acids reduces. As a result, the fraction of metabolic enzymes in the total proteome reduces, and cells re-establish the relative fraction of the ribosomal and non-ribosomal proteome sectors as they reach new steady-state growth (Figure 2a, from top to bottom panels). The total fraction of ribosomal proteins (both active and inactive) was shown to increase linearly with the growth rate [33]. The measured changes in proteome composition and the growth rate are in quantitative agreement with predictions based on coarse-grained flux balance analysis.4 A next step would be to apply a similar approach to cell size control [16••,35], by addressing the total number of proteins per cell, which is a good proxy of cell size.
Figure 2.
Examples of coarse-grained approach to physiology and cell cycle study. (a) Proteome partitioning: in this study of coordination between cell growth and protein composition, the proteome is divided into two ‘sectors’ depending on whether the protein is ribosomal (R) or non-ribosomal (non-R). In steady-state growth, the ratio between R and non-R sectors is determined by balance between the supply and demand of amino acids. If some ribosomes become inactive by sub-lethal concentration of antibiotics that inhibits translation, demand for amino acid reduces. This eventually also reduces supply of amino acids because less ribosomes are available to make the proteins for catabolism, causing reduced amino acid flux. (b) Coarse-grained view of intracellular processes underlying cellular reproduction presented in recent study [38•]. (c) A ‘metabolic sensor’ that coordinates metabolism and cell division. The common substrate UDP-glucose is converted from Glucose-6-P (shown as a hexagon) in the glycolysis pathway after two consecutive steps. These enzymes together with UDP-glucose serve as the metabolic sensor that passes the information about nutrient quality from the metabolism kernel to the cell division kernel.
The cell as a self-replicating factory
Another example of the coarse-grained approach is to view the cell as a ‘self-replicating factory’, in the vein of von Neumann’s self-reproducing automaton [37,38•]. Recent work presents cellular growth as six ‘processing units’ of cellular production machinery working in concert to produce more machinery in the presence of unlimited supply of raw materials [38•]. In this case, the growth rate during balanced growth is the production rate of a self-replicating factory consisting of optimally scheduled processing units. The predicted distribution of doubling time is a universal Log-Frechet form, in remarkable quantitative agreement with the recent single-cell data [38•,39,40]. We believe that, in future study, the language of proteome ‘sectors’ and ‘processing units’ can be brought together to formulate a quantitative framework to address the relationship between cell size and growth kinetics.
The role of molecules in the coarse-grained approach
Coarse-grained approaches can be powerful in that they can make predictions without the knowledge of molecular details. However, they are often based on correlations and can be limited in providing mechanistic insights. For coarse-grained models to be biologically meaningful, they should be able to provide context for molecules, for example, in coordinating growth, the cell cycle, and cell size control [41]. One such example is a ‘metabolic sensor’, which has been described to transmit information about nutrient conditions to cell division machinery [42•,43]. Experimental data suggest that glucosyltransferases (UgtP in B. subtilis and OpgH in E. coli) and their substrates UDP-glucose can cause delay in cell division by preventing FtsZ ring formation in a nutrient-dependent manner. In nutrient rich conditions, UgtP/OpgH is abundant and accumulates in the midcell to prevent premature cell division, whereas in nutrient poor conditions the enzyme is too sparse to inhibit FtsZ ring formation. Thus, a metabolic sensor is a mediator of flux between the metabolism kernel and the cell cycle (division) kernel, which manifests in cell size. Many additional papers have been aimed at discovering these coordinating components [44–51]. Such molecular and biochemical information is useful, since it can help quantify the flux and the dynamic properties of the metabolic sensor, revealing how information flows in the system.
Concluding remarks
Cell size and growth rate are the ultimate outcomes of all intracellular processes. This holistic nature of cell size control is the source of both fascination and frustration in studying its underlying mechanism [52–55]. The adder principle of size homeostasis in bacteria is a telling example of how we can find new ways to think about this long-standing problem with the help of quantitative and phenomenological approaches. Future challenges will involve bridging the gaps between phenomenological principles and molecular mechanisms. To this end, and inspired by the generality of the adder principle discussed in this article, we propose a coarse-grained view of cellular growth consisting of kernels, namely, intracellular processes grouped by their functions. Predictability of the cell’s response to perturbations to the coordinations between kernels will be an important step forward in understanding the mechanism of cell size control and homeostasis.
Acknowledgments
This work was supported by the Paul G Allen Foundation, the Pew Charitable Trusts, and the National Science Foundation CAREER Award (to S.J.). We thank all members of the Jun lab for numerous helpful discussions.
Footnotes
C. crescentus has been shown to add a constant length [15••], but a cellular area which is positively correlated with birth size [14,17]. Nevertheless, size homeostasis can be understood using the same convergence principle.
This coarse-grained approach to flux balance is complemented by network methods popular in metabolic engineering [36].
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Orskov J. Method for the isolation of bacteria in pure culture from single cells and procedure for the direct tracing of bacterial growth on a solid medium. J Bact. 1922;7:537–549. doi: 10.1128/jb.7.6.537-549.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayne-Jones S, Adolph EF. Growth in size of micro-organisms measured from motion pictures. III. Bacterium coli. J Cell Comp Physiol. 1932;2:329–348. http://dx.doi.org/10.1002/jcp.1030020308. [Google Scholar]
- 3.Kelly CD, Rahn O. The growth rate of individual bacterial cells. J Bact. 1932;23:147–153. doi: 10.1128/jb.23.2.147-153.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koch AL, Schaechter M. A model for statistics of the cell division process. J Gen Microbiol. 1962;29:435–454. doi: 10.1099/00221287-29-3-435. http://dx.doi.org/10.1099/00221287-29-3-435. [DOI] [PubMed] [Google Scholar]
- 5.Fantes PA, Grant WD, Pritchard RH, Sudbery PE, Wheals AE. The regulation of cell size and the control of mitosis. J Theor Biol. 1975;50:213–244. doi: 10.1016/0022-5193(75)90034-x. http://dx.doi.org/10.1016/0022-5193(75)90034-X. [DOI] [PubMed] [Google Scholar]
- 6.Tyson JJ, Diekmann O. Sloppy size control of the cell division cycle. J Theor Biol. 1986;118:405–426. doi: 10.1016/s0022-5193(86)80162-x. http://dx.doi.org/10.1016/S0022-5193(86)80162-X. [DOI] [PubMed] [Google Scholar]
- 7.Voorn WJ, Koppes LJ, Grover N. Mathematics of cell division in Escherichia coli. Curr Top Mol Genet. 1993;1:187–194. [Google Scholar]
- 8.Taheri-Araghi S, Brown SD, Sauls JT, McIntosh DB, Jun S. Single-cell physiology. Annu Rev Biophys. 2015;44:123–142. doi: 10.1146/annurev-biophys-060414-034236. http://dx.doi.org/10.1146/annurev-biophys-060414-034236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9•.Taheri-Araghi S. Self-consistent examination of Donachie’s constant initiation size at the single-cell level. Front Microbiol. 2015;6:1–11. doi: 10.3389/fmicb.2015.01349. http://dx.doi.org/10.3389/fmicb.2015.01349. Reassess a classic model which connects cell size to cell cycle, raising question to the one-to-one correspondence between replication initiation and division. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10•.Santi I, Dhar N, Bousbaine D, Wakamoto Y, McKinney JD. Single-cell dynamics of the chromosome replication and cell division cycles in mycobacteria. Nat Commun. 2013;4:2470. doi: 10.1038/ncomms3470. http://dx.doi.org/10.1038/ncomms3470. Thoroughly measures single cell growth, including size and cell cycle parameters, in the less well characterized tip-grower M. smegmatis. Disproved both sizer and timer. [DOI] [PubMed] [Google Scholar]
- 11.Robert L, Hoffmann M, Krell N, Aymerich S, Robert J, Doumic M. Division in Escherichia coli is triggered by a size-sensing rather than a timing mechanism. BMC Biol. 2014;12:17. doi: 10.1186/1741-7007-12-17. http://dx.doi.org/10.1186/1741-7007-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osella M, Nugent E, Cosentino Lagomarsino M. Concerted control of Escherichia coli cell division. Proc Natl Acad Sci U S A. 2014;111:3431–3435. doi: 10.1073/pnas.1313715111. http://dx.doi.org/10.1073/pnas.1313715111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nobs JB, Maerkl SJ. Long-term single cell analysis of S. pombe on a microfluidic microchemostat array. PLOS ONE. 2014;9:e93466. doi: 10.1371/journal.pone.0093466. http://dx.doi.org/10.1371/journal.pone.0093466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iyer-Biswas S, Wright CS, Henry JT, Lo K, Burov S, Lin Y, Crooks GE, Crosson S, Dinner AR, Scherer NF. Scaling laws governing stochastic growth and division of single bacterial cells. Proc Natl Acad Sci U S A. 2014;111:15912–15917. doi: 10.1073/pnas.1403232111. http://dx.doi.org/10.1073/pnas.1403232111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15••.Campos M, Surovtsev IV, Kato S, Paintdakhi A, Beltran B, Ebmeier SE, Jacobs-Wagner C. A constant size extension drives bacterial cell size homeostasis. Cell. 2014;159:1433–1446. doi: 10.1016/j.cell.2014.11.022. http://dx.doi.org/10.1016/j.cell.2014.11.022. Shows that C. crescentus and E. coli follow the adder principle to maintain size homeostasis and presents models to test during what cell cycle interval the cell measures added size. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16••.Taheri-Araghi S, Bradde S, Sauls JT, Hill NS, Levin PA, Paulsson J, Vergassola M, Jun S. Cell-size control and homeostasis in bacteria. Curr Biol. 2015;25:385–391. doi: 10.1016/j.cub.2014.12.009. http://dx.doi.org/10.1016/j.cub.2014.12.009. Examines cell size homeostasis in E. coli and B. subtilis in numerous growth conditions, strongly supporting the adder principle. Added size and elongation rate are shown to independently determine all other growth parameters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jun S, Taheri-Araghi S. Cell-size maintenance: universal strategy revealed. Trends Microbiol. 2015;23:4–6. doi: 10.1016/j.tim.2014.12.001. http://dx.doi.org/10.1016/j.tim.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Deforet M, van Ditmarsch D, Xavier JB. Cell-size homeostasis and the incremental rule in a bacterial pathogen. Biophys J. 2015;109:521–528. doi: 10.1016/j.bpj.2015.07.002. http://dx.doi.org/10.1016/j.bpj.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carter JA, Lind CH, Truong MP, Collins EMS. To each his own. J Stat Phys. 2015;161:250–272. http://dx.doi.org/10.1007/s10955-015-1310-1. [Google Scholar]
- 20.Voorn WJ, Koppes LJ. Skew or third moment of bacterial generation times. Arch Microbiol. 1998;169:43–51. doi: 10.1007/s002030050539. http://dx.doi.org/10.1007/s002030050539. [DOI] [PubMed] [Google Scholar]
- 21.Collins JF, Richmond MH. Rate of growth of Bacillus cereus between divisions. J Gen Microbiol. 1962;28:15–33. doi: 10.1099/00221287-28-1-15. http://dx.doi.org/10.1099/00221287-28-1-15. [DOI] [PubMed] [Google Scholar]
- 22.Schaechter M, Williamson JP, Hood JR, Koch AL. Growth, cell and nuclear divisions in some bacteria. J Gen Microbiol. 1962;29:421–434. doi: 10.1099/00221287-29-3-421. http://dx.doi.org/10.1099/00221287-29-3-421. [DOI] [PubMed] [Google Scholar]
- 23•.Zaritsky A. Cell-shape homeostasis in Escherichia coli is driven by growth, division, and nucleoid complexity. Biophys J. 2015;109:178–181. doi: 10.1016/j.bpj.2015.06.026. http://dx.doi.org/10.1016/j.bpj.2015.06.026. The relationship between the aspect ratio of the cell and the number of overlapping cell cycles is explored in search of the principles governing cell shape in bacteria. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Godin M, Delgado FF, Son S, Grover WH, Bryan AK, Tzur A, Jorgensen P, Payer K, Grossman AD, Kirschner MW, Manalis SR. Using buoyant mass to measure the growth of single cells. Nat Methods. 2010;7:387–390. doi: 10.1038/nmeth.1452. http://dx.doi.org/10.1038/nmeth.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Durmus NG, Tekin HC, Guven S, Sridhar K, Arslan Yildiz A, Calibasi G, Ghiran I, Davis RW, Steinmetz LM, Demirci U. Magnetic levitation of single cells. Proc Natl Acad Sci U S A. 2015;112:E3661–8. doi: 10.1073/pnas.1509250112. http://dx.doi.org/10.1073/pnas.1509250112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ginzberg MB, Kafri R, Kirschner M. On being the right (cell) size. Science (New York, NY) 2015;348:1245075. doi: 10.1126/science.1245075. http://dx.doi.org/10.1126/science.1245075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jorgensen P, Nishikawa JL, Breitkreutz BJ, Tyers M. Systematic identification of pathways that couple cell growth and division in yeast. Science. 2002;297:395–400. doi: 10.1126/science.1070850. http://dx.doi.org/10.1126/science.1070850. [DOI] [PubMed] [Google Scholar]
- 28.Skarstad K, Katayama T. Regulating DNA replication in bacteria. Cold Spring Harb Perspect Biol. 2013;5:a012922. doi: 10.1101/cshperspect.a012922. http://dx.doi.org/10.1101/cshperspect.a012922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katayama T, Ozaki S, Keyamura K, Fujimitsu K. Regulation of the replication cycle: conserved and diverse regulatory systems for DnaA and oriC. Nat Rev Microbiol. 2010;8:163–170. doi: 10.1038/nrmicro2314. http://dx.doi.org/10.1038/nrmicro2314. [DOI] [PubMed] [Google Scholar]
- 30.Lutkenhaus J. Assembly dynamics of the bacterial MinCDE system and spatial regulation of the Z ring. Annu Rev Biochem. 2007;76:539–562. doi: 10.1146/annurev.biochem.75.103004.142652. http://dx.doi.org/10.1146/annurev.biochem.75.103004.142652. [DOI] [PubMed] [Google Scholar]
- 31.Hartwell LH, Hopfield JJ, Leibler S, Murray aW. From molecular to modular cell biology. Nature. 1999;402(Suppl):C47–52. doi: 10.1038/35011540. http://dx.doi.org/10.1038/35011540. [DOI] [PubMed] [Google Scholar]
- 32.Ingolia NT, Murray AW. The ups and downs of modeling the cell cycle. Curr Biol. 2004;14:R771–7. doi: 10.1016/j.cub.2004.09.018. http://dx.doi.org/10.1016/j.cub.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 33.Scott M, Gunderson CW, Mateescu EM, Zhang Z, Hwa T. Interdependence of cell growth and gene expression: origins and consequences. Science. 2010;330:1099–1102. doi: 10.1126/science.1192588. http://dx.doi.org/10.1126/science.1192588. [DOI] [PubMed] [Google Scholar]
- 34.Scott M, Klumpp S, Mateescu EM, Hwa T. Emergence of robust growth laws from optimal regulation of ribosome synthesis. Mol Syst Biol. 2014;10:747. doi: 10.15252/msb.20145379. http://dx.doi.org/10.15252/msb.20145379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Basan M, Zhu M, Dai X, Warren M, Sévin D, Wang Yp, Hwa T. Inflating bacterial cells by increased protein synthesis. Mol Syst Biol. 2015;11:836. doi: 10.15252/msb.20156178. http://dx.doi.org/10.15252/msb.20156178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schellenberger J, Que R, Fleming RMT, Thiele I, Orth JD, Feist AM, Zielinski DC, Bordbar A, Lewis NE, Rahmanian S, Kang J, Hyduke DR, Palsson Bø. Quantitative prediction of cellular metabolism with constraint-based models: the COBRA Toolbox v2.0. Nat Prot. 2011:1290–1307. doi: 10.1038/nprot.2011.308. http://dx.doi.org/10.1038/nprot.2011.308. [DOI] [PMC free article] [PubMed]
- 37.von Neumann JBA. Theory of Self-Reproducing Automata. :19660598377980. http://dx.doi.org/10.1016/0020-0271(69)90026-6.
- 38•.Pugatch R. Greedy scheduling of cellular self-replication leads to optimal doubling times with a log-Frechet distribution. Proc Natl Acad Sci U S A. 2015;112:2611–2616. doi: 10.1073/pnas.1418738112. http://dx.doi.org/10.1073/pnas.1418738112. An outstanding example of how cell physiology study can benefit from a coarse-grained model. Despite the lack of any molecular detail, the match between the predicted and experimental doubling time distribution proved its utility. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang P, Robert L, Pelletier J, Dang WL, Taddei F, Wright A, Jun S. Robust growth of Escherichia coli. Curr Biol. 2010;20:1099–1103. doi: 10.1016/j.cub.2010.04.045. http://dx.doi.org/10.1016/j.cub.2010.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ullman G, Wallden M, Marklund EG, Mahmutovic A, Razinkov I, Elf J. High-throughput gene expression analysis at the level of single proteins using a microfluidic turbidostat and automated cell tracking. Philos Trans R Soc Lond B. 2013;368:20120025. doi: 10.1098/rstb.2012.0025. http://dx.doi.org/10.1098/rstb.2012.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jonas K. To divide or not to divide: control of the bacterial cell cycle by environmental cues. Curr Opin Microbiol. 2014;18:54–60. doi: 10.1016/j.mib.2014.02.006. http://dx.doi.org/10.1016/j.mib.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 42•.Hill NS, Buske PJ, Shi Y, Levin PA. A moonlighting enzyme links Escherichia coli cell size with central metabolism. PLoS Gen. 2013;9:e1003663. doi: 10.1371/journal.pgen.1003663. http://dx.doi.org/10.1371/journal.pgen.1003663. This study discovers a ‘metabolic sensor’ in E. coli and compares it to the functional homologue in B. subtilis. It sheds light to a shared strategy of coordinating metabolism and cell size control in bacteria. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weart RB, Lee AH, Chien AC, Haeusser DP, Hill NS, Levin PA. A metabolic sensor governing cell size in bacteria. Cell. 2007;130:335–347. doi: 10.1016/j.cell.2007.05.043. http://dx.doi.org/10.1016/j.cell.2007.05.043 arXiv:NIHMS150003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao Z, Davis RM, Kishony R, Kahne D, Ruiz N. Regulation of cell size in response to nutrient availability by fatty acid biosynthesis in Escherichia coli. Proc Natl Acad Sci U S A. 2012;109:E2561–8. doi: 10.1073/pnas.1209742109. http://dx.doi.org/10.1073/pnas.1209742109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pan KZ, Saunders TE, Flor-Parra I, Howard M, Chang F. Cortical regulation of cell size by a sizer cdr2p. eLife. 2014;2014:1–24. doi: 10.7554/eLife.02040. http://dx.doi.org/10.7554/eLife.02040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Monahan LG, Hajduk IV, Blaber SP, Charles IG, Harry EJ. Coordinating bacterial cell division with nutrient availability: a role for glycolysis. mBio. 2014;5:e00935–14. doi: 10.1128/mBio.00935-14. http://dx.doi.org/10.1128/mBio.00935-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tropini C, Lee TK, Hsin J, Desmarais SM, Ursell T, Monds RD, Huang KC. Principles of bacterial cell-size determination revealed by cell-wall synthesis perturbations. Cell Rep. 2014;9:1520–1527. doi: 10.1016/j.celrep.2014.10.027. http://dx.doi.org/10.1016/j.celrep.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lori C, Ozaki S, Steiner S, Böhm R, Abel S, Dubey BN, Schirmer T, Hiller S, Jenal U. Cyclic di-GMP acts as a cell cycle oscillator to drive chromosome replication. Nature. 2015;523:236–239. doi: 10.1038/nature14473. http://dx.doi.org/10.1038/nature14473. [DOI] [PubMed] [Google Scholar]
- 49.Robert L. Size sensors in bacteria, cell cycle control, and size control. Front Microbiol. 2015;6:515. doi: 10.3389/fmicb.2015.00515. http://dx.doi.org/10.3389/fmicb.2015.00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stott KV, Wood SM, Blair Ja, Nguyen BT, Herrera A, Mora YGP, Cuajungco MP, Murray SR. (p)ppGpp modulates cell size and the initiation of DNA replication in Caulobacter crescentus in response to a block in lipid biosynthesis. Microbiology. 2015;161(Pt 3):553–564. doi: 10.1099/mic.0.000032. http://dx.doi.org/10.1099/mic.0.000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wheeler R, Turner RD, Bailey RG, Salamaga B, Mesnage S, Mohamad SaS, Hayhurst EJ, Horsburgh M, Hobbs JK, Foster SJ. Bacterial cell enlargement requires control of cell wall stiffness mediated by peptidoglycan hydrolases. mBio. 2015;6:e00660. doi: 10.1128/mBio.00660-15. http://dx.doi.org/10.1128/mBio.00660-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vadia S, Levin P. Growth rate and cell size: a re-examination of the growth law. Curr Opin Microbiol. 2015;24:96–103. doi: 10.1016/j.mib.2015.01.011. http://dx.doi.org/10.1016/j.mib.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zaritsky A, Woldringh CL. Chromosome replication, cell growth, division and shape: a personal perspective. Front Microbiol. 2015;6:756. doi: 10.3389/fmicb.2015.00756. http://dx.doi.org/10.3389/fmicb.2015.00756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Norris V. Why do bacteria divide? Front Microbiol. 2015;6:322. doi: 10.3389/fmicb.2015.00322. http://dx.doi.org/10.3389/fmicb.2015.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levin PA, Angert ER. Small but mighty: cell size and bacteria. Cold Spring Harb Perspect Biol. 2015;7:a019216. doi: 10.1101/cshperspect.a019216. http://dx.doi.org/10.1101/cshperspect.a019216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Di Talia S, Skotheim JM, Bean JM, Siggia ED, Cross FR. The effects of molecular noise and size control on variability in the budding yeast cell cycle. Nature. 2007;448:947–951. doi: 10.1038/nature06072. http://dx.doi.org/10.1038/nature06072. [DOI] [PubMed] [Google Scholar]