Abstract
Recent metabonomic studies have identified an important role of bile acids in patients with liver cirrhosis. Serum bile acids, such as glycocholate (GCA), glycochenodeoxycholate (GCDCA), taurocholate (TCA) and taurochenodeoxycholate (TCDCA), increased significantly in liver cirrhosis patients. Our recently published urinary metabonomic study showed that glycocholate 3-glucuronide, taurohyocholate, TCA, glycolithocholate 3-sulfate and glycoursodeoxycholate (GUDCA) were markedly increased in hepatitis B-induced cirrhotic patients (n = 63) compared with healthy controls (n = 31). The urinary levels of GUDCA were able to differentiate among three stages of cirrhotic patients with Child Pugh (CP) score A, B and C. In this study, we recruited two new cohorts of patients with hepatitis B-induced cirrhosis and healthy control subjects and quantitatively profiled their serum bile acids using ultra performance liquid chromatography triple quadrupole mass spectrometry. Serum bile acid profile and corresponding differential bile acids were characterized, in addition to the blood routine, liver and renal function tests. The alterations of bile acids contributing to the inter-group variation between healthy controls and cirrhotic patients, and among pathological stages of CP grade A, B and C were also investigated. Five bile acids, GCA, GCDCA, TCA, TCDCA and GUDCA were significantly altered among different stages of liver cirrhosis (n = 85), which was validated with an independent cohort of cirrhotic patients (n = 53). Our results show that dynamic alteration of serum bile acids is indicative of an exacerbated liver function, highlighting their potential as biomarkers for staging the liver cirrhosis and monitoring its progression.
Keywords: cirrhosis, hepatitis B, serum, bile acid, Child-Pugh classification
Graphical abstract
INTRODUCTION
Cirrhosis is the final stage of chronic liver damage characterized by a substitution of the lobular liver architecture with regenerative nodules surrounded by fibrous septa, leading to portal hypertension and end-stage liver disease.1 Cirrhosis is the twelfth leading cause of death in the United States, accounting for nearly 32,000 deaths each year.2 The most common causes of cirrhosis include hepatitis B virus (HBV)- and hepatitis C virus (HCV)-related viral hepatitis, alcohol-related liver diseases, autoimmune and metabolic disorders, representing a major health problem. In cirrhosis due to HBV, which is the major cause of deaths related to HCC worldwide,3, 4 the 5-year cumulative occurrence of HCC is 15% in highly endemic areas and 10% in the USA and Europe.1
Cirrhosis is irreversible, and treatment usually focuses on preventing progression and complications. In advanced stages of cirrhosis the only option is a liver transplant. Understanding the severity of cirrhosis as well as the range of potential outcomes is essential to predict treatment outcomes and individualize therapy. Many studies have attempted to develop a classification system that can both characterize the degree of liver injury and predict the prognosis of patients with cirrhosis on the basis of clinical and laboratory parameters, including the most widely used Child–Pugh (CP) classification5, 6 and the model for end-stage liver disease (MELD).7, 8 Liver cirrhosis patients can be classified into three groups, A (least), B (moderate), and C (worst) according to the CP score,5, 6 with one-year survival rates of 100%, 81% and 45%, respectively. To date, the CP classification is still considered the cornerstone in prognostic evaluation of liver cirrhosis,9, 10 however, the CP score does not provide direct evidence of the stage of a patient’s cirrhosis and it has some disadvantage that clinical evaluation might be imperfect and biochemical parameters could have similar physiopathological meanings.11–14 Doctors must make a diagnosis using past clinical experience or an invasive liver biopsy.
With the increasing use of effective anti-viral treatments and the emergence of effective anti-fibrotic agents, there is a pressing necessity for establishing a more refined cirrhosis staging method for the management of liver cirrhosis. Moreover, there is a strong need to redefine cirrhosis in a manner that better recognizes its underlying relationship to portal hypertension and related circulatory changes, and more accurately reflects its progression, reversibility and prognosis, ultimately linking these parameters to clinically relevant outcomes and therapeutic strategies.13 Correct disease staging is also the main step towards improving the timing of listing for liver transplantation so as to avoid premature or late entry. Novel biomarkers that are non-invasive, reliable, and cost-effective are urgently needed for the clinical classification of cirrhosis, as well as assessing the disease risk and monitoring disease progression in a timely manner.
Emerging evidence showed that the gut milieu plays an important role in the progression of complications of cirrhosis.15–17 Studies have found dysbiosis in the gut microbiota, e.g. Bacteroides and Firmicutes in patients with cirrhosis.23, 24 One critical component of the intestinal milieu is bile acids,18 alteration of which has been closely associated with liver cirrhosis.19–21 Cirrhotic patients have been shown to have a lower proportion of secondary bile acids in their bile22 and serum primary bile acids were higher in advanced cirrhosis compared to the rest.23 We also observed that HCC and liver cirrhosis is in direct association with significantly altered bile acid profiles in blood and urine.19, 20 Particularly, urinary glycoursodeoxycholate (GUDCA) was significantly altered among the three CP grades, A, B, and C, suggesting that bile acids could be potential biomarkers for patient’s stratification at different pathological stages of cirrhosis.
In this study, we used a targeted metabonomics approach with ultra-performance liquid chromatography triple quadrupole mass spectrometry (UPLC-TQMS) to characterize the bile acid profiles in serum of patients with liver cirrhosis and healthy subjects. The bile acids significantly altered among the three CP grades in the discovery set were validated using an independent group of liver cirrhotic patients.
Materials and Methods
Participants
Two independent groups of participants were enrolled in this study. The demographic information and clinical characteristics of all subjects are shown in Table 1. In the first group (discovery set), a total of 85 patients diagnosed with liver cirrhosis were recruited at Shuguang Hospital affiliated with Shanghai University of Traditional Chinese Medicine (Shanghai, China), and Xiamen Hospital of Traditional Chinese Medicine (Xiamen, China) from April 2013 to December 2013. Patients were clinically diagnosed with post-hepatitis B liver cirrhosis according to the “Guideline on prevention and treatment of chronic hepatitis B in China (2005)”.24 Patients with human immunodeficiency virus (HIV), hepatitis C virus (HCV), hepatitis D virus (HDV), or hepatitis E virus (HEV) infection, alcohol consumption, neoplastic liver diseases and hepatotoxic medication in the past 6 months were ruled out before entering the study. Patients were divided into three subgroups, class A (n=39), class B (n=30) and class C (n=16) according to CP scores. A cohort of 88 participants was recruited as healthy controls from the Physical Examination Center of Shuguang Hospital. There was no significant difference in age and BMI between healthy controls and liver cirrhosis patients (Table 1 and 2). In the second group (validation set), serum samples were obtained from 53 liver cirrhosis patients and 50 healthy controls at First Hospital Affiliated to Guangxi University of Traditional Chinese Medicine, Guangxi, China. Inclusion and exclusion criteria were the same as for the discovery set samples. There is a difference in the information summarized in Table 1 for the discovery vs. validation set due to the difference between the biochemical testing performed at the two different collection sites.
Table 1.
Clinical information of human subjects.
| Discovery Set | Validation Set | |||
|---|---|---|---|---|
|
| ||||
| Group No. |
Control n=88 |
liver cirrhosis n=85 |
Control n=50 |
liver cirrhosis n=53 |
| CP A/B/C | 39/30/16 | 16/26/11 | ||
| BMI | 23.6±0.32 | 22.87±0.32 | 23.42±0.33 | 22.56±0.45 |
| Sex (M/F) | 55/23 | 53/32 | 31/19 | 37/16 |
| age | 48.26±1.22 | 48.65±1.25 | 50.68±7.46 | 52.85±1.36 |
| ALB | 48.26±0.27 | 32.35±0.93** | 47.86±3.10 | 32.59±1.11** |
| ALP | 87.52±2.07 | 125.07±12.79** | ||
| ALT | 30.43±1.48 | 91.8±12.97** | 24.26±9.48 | 48.91±9.85* |
| AST | 21.65±0.49 | 76.14±9.17** | 23.82±5.63 | 82.89±23.56** |
| BUN | 5.05±0.13 | 4.99±0.29* | ||
| CREA | 75.54±0.79 | 78.58±3.62* | 74.84±14.41 | 78.25±4.56* |
| CHOL | 5.46±0.11 | 4.17±0.21** | 5.01±0.73 | 4.11±0.17** |
| TG | 1.6±0.06 | 1.16±0.12* | 1.44±0.55 | 0.96±0.06* |
| HDL-C | 1.25±0.23 | 0.97±0.05* | 1.38±0.35 | 1.10±0.06* |
| LDL-C | 3.44±0.56 | 2.73±0.15* | 3.04±0.62 | 2.45±1.05* |
| DBIL | 2.18±0.08 | 15.33±2.89** | 2.19±0.70 | 29.12±7.67** |
| GGT | 15.74±0.89 | 79.49±17.97** | ||
| GLB | 29.96±0.28 | 35.28±0.77** | ||
| GLU | 5.51±0.11 | 5.65±0.23 | ||
| HCT | 43.67±0.44 | 37.02±0.7** | ||
| HGB | 138.12±2.27 | 110.19±3.47** | ||
| IBIL | 10.09±0.45 | 32.76±3.64** | ||
| MCH | 28.16±0.2 | 30.19±0.35** | ||
| MCHC | 352.5±0.92 | 338.69±2.12** | ||
| MPV | 8.17±0.06 | 9.07±0.13** | ||
| PALB | 339.03±3.9 | 165.42±7.21* | ||
| PCT | 0.14±0.01 | 0.08±0** | ||
| PDW | 15.35±0.05 | 16.42±0.13** | ||
| PLT | 267.35±8.21 | 92.56±6.94** | ||
| RBC | 4.64±0.05 | 3.79±0.09** | ||
| TBA | 4.93±0.29 | 63.07±6.73** | 3.30±2.42 | 74.17±12.09** |
| TBIL | 14.88±0.49 | 50.09±6.53** | 11.30±3.23 | 54.27±11.34** |
| TP | 74.05±0.51 | 63.67±1.47** | ||
| WBC | 6.53±0.17 | 4.57±0.24** | ||
Note: Values are expressed as mean ± SEM.
, p <0.05;
, p<0.01 when compared to healthy controls.
ALT, Alanine transaminase; AST, Aspartate transaminase; TBIL, Total bilirubin; DBIL, direct bilirubin; IBIL, indirect bilirubin; ALP, alkaline phosphatase; GGT, gamma-glutamyl transferase; GLB, globin; TP, Total Protein; ALB, Albumin; PALB, prealbumin; TBA, Total bile acid; CREA, creatinine; BUN, Blood urea nitrogen; CHOL, cholesterol; TG, Triglyceride; HDLC, High-Density Lipoprotein Cholesterol; LDLC, low-density lipoprotein cholesterol; GLU, Glucose; RBC, red blood cell; WBC, white blood cell; HCT, hematocrit; HGB, hemoglobin; MCH, mean corpuscular hemoglobin; MCHC, Mean corpuscular hemoglobin concentration; MPV, Mean platelet volume; PLT, platelet; PCT, plateletocrit; PDW, platelet distribution width
Table 2.
Clinical information and characteristics of liver cirrhotic patients in the discovery set.
| Child-Pugh grade | p | |||
|---|---|---|---|---|
|
| ||||
| Variable | Class A (score 5–6) | Class B (score 7–9) | Class C (score 10–13) |
|
| Patients (n) | 39 | 30 | 16 | |
| Age (mean± SD) | 44.1±1.7 | 54.33±1.65 | 49.06±3.35 | |
| BMI (kg/m2) | 23.63±0.53 | 21.86±0.42 | 22.89±0.69 | ns |
| ALB | 39.58±0.76 | 27.97±1.05 | 22.92±1.12 | * † ‡ |
| ALP | 116.38±26.32 | 113.55±7.33 | 167.85±15.5 | † ‡ |
| ALT | 93.02±13.19 | 99.91±28.28 | 73.62±31.59 | ns |
| AST | 64.22±10.36 | 81.67±18.04 | 94.84±24.69 | ns |
| BUN | 4±0.18 | 5.42±0.47 | 6.58±1.08 | * ‡ |
| CHOL | 4.61±0.22 | 4.13±0.34 | 3.17±0.71 | ‡ |
| DBIL | 6.7±1.66 | 10.39±1.19 | 45.64±12.24 | † ‡ |
| GGT | 102.86±36.44 | 61.41±16.86 | 56.44±14.44 | ns |
| GLB | 35.41±1.44 | 34.74±0.89 | 35.98±1.31 | ns |
| GLU | 5.33±0.15 | 5.76±0.33 | 6.22±1.01 | ns |
| HCT | 40.85±0.83 | 35.43±0.96 | 30.69±1.32 | * † ‡ |
| HGB | 130.41±3.74 | 101.21±5.1 | 77.73±5.51 | * † ‡ |
| IBIL | 16.28±1.17 | 30.2±2.42 | 77.73±13.58 | * † ‡ |
| MCH | 30.14±0.36 | 29.51±0.65 | 31.62±1.01 | ns |
| MCHC | 337.11±2.51 | 338.26±3.9 | 343.34±6.14 | ns |
| MPV | 9.39±0.17 | 8.85±0.26 | 8.7±0.24 | * † |
| PALB | 179.82±9.75 | 158.62±13.32 | 143.06±15.63 | ns |
| PCT | 0.1±0.01 | 0.06±0 | 0.06±0 | * ‡ |
| PDW | 16.59±0.13 | 16.34±0.27 | 16.18±0.35 | ns |
| PLT | 124.58±10.54 | 64.27±8.96 | 67.55±12.15 | * ‡ |
| RBC | 4.32±0.08 | 3.61±0.12 | 2.81±0.13 | * † ‡ |
| TBA | 35.34±6.36 | 67.92±8.98 | 121.59±21.21 | * † ‡ |
| TBIL | 22.28±1.87 | 42.63±3.2 | 131.86±25.1 | * † ‡ |
| TP | 73.18±1.62 | 57±2.03 | 53.01±2.32 | * ‡ |
| WBC | 5.14±0.28 | 3.74±0.4 | 4.75±0.68 | * |
Note: Values are expressed as mean ± SEM.
P<0.05, Class A vs. Class B;
P<0.05, Class B vs. Class C;
P<0.05, Class A vs. Class C, ns, non-significant
ALT, Alanine transaminase; AST, Aspartate transaminase; TBIL, Total bilirubin; DBIL, direct bilirubin; IBIL, indirect bilirubin; ALP, alkaline phosphatase; GGT, gamma-glutamyl transferase; GLB, globin; TP, Total Protein; ALB, Albumin; PALB, prealbumin; TBA, Total bile acid; CREA, creatinine; BUN, Blood urea nitrogen; CHOL, cholesterol; TG, Triglyceride; HDLC, High-Density Lipoprotein Cholesterol; LDLC, low-density lipoprotein cholesterol; GLU, Glucose; RBC, red blood cell; WBC, white blood cell; HCT, hematocrit; HGB, hemoglobin; MCH, mean corpuscular hemoglobin; MCHC, Mean corpuscular hemoglobin concentration; MPV, Mean platelet volume; PLT, platelet; PCT, plateletocrit; PDW, platelet distribution width
Ethical approval for these studies was obtained from the ethics committee of the above three hospitals and all participants signed the informed consent prior to the study.
Biochemistry Tests
Serum biochemical assay was performed with an automatic biochemistry analyzer (Hitachi Ltd, Tokyo, Japan) for the analysis of blood routine, liver, and renal function markers. Ascites was examined by ultrasonography. The biochemical determinations were performed on the same day as blood was taken.
Serum Sample Collection
Serum samples were obtained in the morning from cirrhotic patients and control subjects before breakfast and stored at −80 °C until UPLC-TQMS analysis.
Serum Sample Preparation and Bile Acid Detection
Serum bile acids were prepared and measured according to our previously published method.25 Briefly, the serum samples were extracted with methanol and the supernatant were transferred and vacuum-dried. After reconstituted with mobile phase, the serum extract as well as the bile acid reference standards were analyzed with a Waters ACQUITY ultra performance liquid chromatography coupled with a Waters XEVO TQ-S mass spectrometer with an ESI source (Waters, Milford, MA). The entire UPLC–MS/MS system was controlled by MassLynx 4.1 software. All chromatographic separations were performed with an ACQUITY BEH C18 column (1.7 µm, 100 mm × 2.1 mm internal dimensions) (Waters, Milford, MA) and the injection volume was 5 µL. UPLC-MS raw data obtained with negative mode were analyzed using TargetLynx applications manager version 4.1 (Waters Corp., Milford, MA) to obtain calibration equations and the quantitative concentration of each bile acid in the samples.
Statistical Analysis
All the demographic data were analyzed at a univariate level using SPSS 22 (SPSS Inc). The continuous variables were analyzed using the Mann-Whitney U test while the categorical variables were analyzed with chi-square test. The bile acid data generated from the UPLC-TQMS analysis were imported to SIMCA P+ 13.0 (Umetrics, Umeå, Sweden) for multivariate statistical analysis. Orthogonal partial least squares-discriminant analysis (OPLS-DA)26 was performed to discriminate between the cirrhosis patients and healthy controls. The differences between the groups in bile acids measurements were analyzed by t tests with Holm-Sidak method for multiple comparisons correction using the Graphpad Prism 6.0 (GraphPad software, CA, USA). We regarded p values of < 0.05 as significant.
Results
Demographic Information and Clinical Characteristics of Liver Cirrhosis Patients
Demographic and clinical parameters including age, gender, body mass index (BMI), liver function, kidney function, and serum biochemical markers were listed in Table 1. Age, gender and BMI showed no significant differences among all the groups. Cirrhotic patients have significantly elevated levels of alanine transaminase (ALT), aspartate transaminase (AST), creatinine (CREA), direct bilirubin (DBIL), total bilirubin (TBIL) and total bile acids (TBA) while have significantly decreased levels of albumin (ALB), cholesterol (CHOL), triglyceride (TG), high density lipoprotein-cholesterol (HDL-C), and low density lipoprotein-cholesterol (LDL-C) as compared to healthy controls in both discovery set and validation set. The levels of indirect bilirubin (IBIL), gamma-glutamyl transferase (GGT), globin (GLB), alkaline phosphatase (ALP), and mean corpuscular hemoglobin (MCH), mean platelet volume (MPV), platelet distribution width (PDW) were increased while the levels of blood urea nitrogen (BUN), hematocrit (HCT), hemoglobin (HGB), prealbumin (PALB), mean corpuscular hemoglobin concentration (MCHC), plateletocrit (PCT), platelet (PLT), total protein (TP), red blood cell (RBC) and white blood cell (WBC) were decreased in cirrhotic patients as compared to healthy controls in the discovery set. However, these biochemical markers cannot be compared in the validation set due to the lack of information.
Patients’ clinical characteristics of the three stage subgroups in the discovery set were summarized in Table 2. As liver function gradually aggravated with increased CP scores, serum levels of ALB, HGB, PALB, GGT, RBC, TP, and CHOL were decreased progressively, while serum levels of AST, TBA, BUN, TBIL, DBIL, and IBIL were significantly increased.
Bile Acid Profiles of Cirrhosis Patients in the Discovery Set
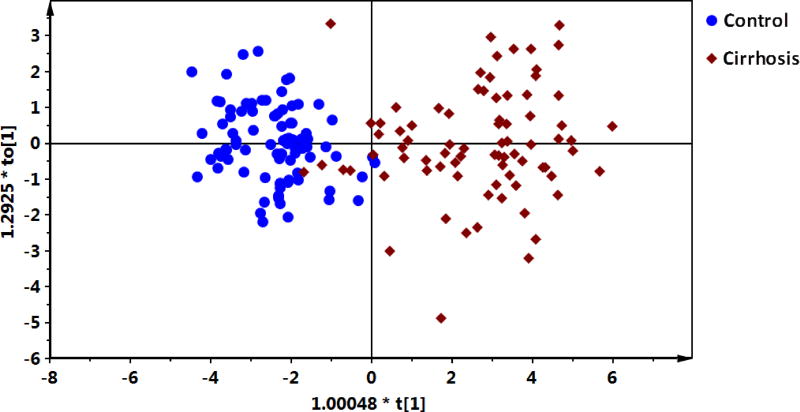
A number of 16 bile acids were significantly increased in cirrhotic patients compared to healthy controls. The comparison of the differentially expressed bile acids between control and CP A, B, C liver cirrhotic patients were summarized in Table 3. OPLS-DA revealed a separation between liver cirrhotic patients and healthy controls (Figure 1, R2Xcum=0.719, R2Ycum = 0.778, Q2Ycum = 0.744) using the 16 bile acids.
Table 3.
List of bile acids in cirrhotic patients and among Child-Pugh A, B and C classes relative to controls.
| Bile acids | Liver cirrhosis vs. control | CP A vs. control | CP B vs. control | CP C vs. control | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| FCa | Pe | FCb | Pe | FCc | Pe | FCd | Pe | |
| Glycochenodeoxycholate (GCDCA) | 14.97 | 7.67E-14 | 8.99 | 3.18E-09 | 12.09 | 2.39E-26 | 34.93 | 2.10E-27 |
| Glycocholate(GCA) | 44.39 | 1.35E-19 | 24.64 | 4.48E-10 | 51.66 | 4.85E-27 | 78.90 | 2.12E-36 |
| Taurocholate(TCA) | 225.79 | 5.06E-12 | 117.97 | 2.16E-05 | 251.81 | 1.32E-17 | 439.90 | 3.77E-23 |
| Taurochenodeoxycholate (TCDCA) | 88.36 | 5.96E-12 | 42.52 | 7.18E-10 | 97.69 | 4.99E-15 | 182.48 | 4.74E-19 |
| Glycodeoxycholate (GDCA) | 5.02 | 2.53E-04 | 6.20 | 5.49E-05 | 3.02 | 6.52E-05 | 5.93 | 6.20E-04 |
| Glycoursodeoxycholate (GUDCA) | 20.62 | 3.44E-03 | 7.77 | 3.05E-05 | 22.97 | 7.39E-05 | 47.53 | 4.00E-04 |
| Glyco-λ-muricholate (G λ MCA) | 12.53 | 1.24E-08 | 9.02 | 6.10E-08 | 13.73 | 1.06E-09 | 18.67 | 4.57E-09 |
| Glycolithocholate (GLCA) | 2.43 | 4.87E-05 | 2.54 | 2.31E-05 | 2.26 | 5.70E-03 | 2.49 | 2.64E-03 |
| Chenodeoxycholate (CDCA) | 4.15 | 1.68E-05 | 3.34 | 2.24E-06 | 4.14 | 1.01E-03 | 6.16 | 4.34E-09 |
| Cholate (CA) | 7.67 | 1.48E-05 | 5.81 | 5.72E-06 | 7.94 | 1.13E-04 | 11.72 | 3.19E-07 |
| Ursodeoxycholate (UDCA) | 5.98 | 9.95E-03 | 2.25 | 6.53E-03 | 11.20 | 6.20E-04 | 5.27 | 7.76E-03 |
| Glyco-λ-muricholate (λ MCA) | 7.25 | 1.01E-04 | 6.45 | 2.69E-04 | 7.68 | 1.74E-05 | 8.42 | 3.01E-04 |
| Tauroursodeoxycholate (TUDCA) | 56.10 | 1.91E-03 | 26.34 | 9.44E-03 | 96.35 | 4.11E-04 | 52.72 | 2.36E-10 |
| Taurodeoxycholate (TDCA) | 17.12 | 1.60E-04 | 23.43 | 4.30E-05 | 8.48 | 8.78E-08 | 18.03 | 1.90E-04 |
| Tauro-λ-muricholate (T λ MCA) | 30.79 | 2.41E-03 | 20.02 | 7.54E-02 | 29.91 | 3.45E-13 | 58.48 | 6.91E-05 |
| Taurolitholate (TLCA) | 2.06 | 1.63E-03 | 2.21 | 1.19E-03 | 2.14 | 3.69E-03 | 1.52 | 2.14E-03 |
Note:
fold change (FC) was obtained by comparing those bile acids in liver cirrhotic patients to controls;
FC was obtained by comparing those bile acids in CP A liver cirrhotic patients to controls;
FC was obtained by comparing those bile acids CP B liver cirrhotic patients to controls;
FC was obtained by comparing those bile acids CP C liver cirrhotic patients to controls;
p values were calculated from t tests with Holm-Sidak method for multiple comparisons correction. FC with a value >1 indicates a relatively higher concentration present in liver cirrhotic patients or CP A, B, C liver cirrhotic patients while a value <1 means a relatively lower concentration as compared to the controls.
Figure 1.
OPLS-DA scores plot of the serum bile acids from liver cirrhotic patients and healthy controls.
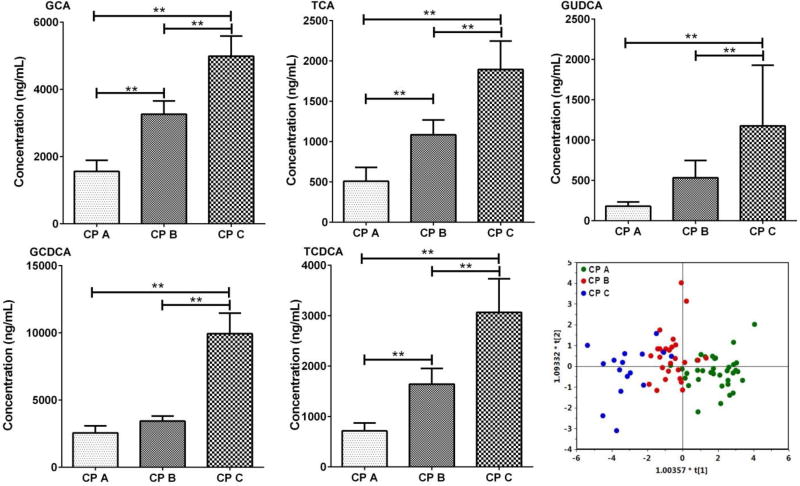
Additionally, as illustrated in Figure 2, five bile acids, including GCA, GCDCA, TCA, TCDCA and GUDCA were significantly altered among the three CP grades, A, B, and C, suggesting that these metabolites could be potential biomarkers for patients stratification at different pathological stages of liver cirrhosis. We also performed the OPLS-DA (R2Xcum=0.802, R2Ycum = 0.448, Q2Ycum = 0.379) based on the five representative bile acids among the three groups to visualize the relationship between the differential bile acids and the cirrhosis progression from CP A to B and C. As a result, distinct separation was seen among the bile acid profiles of the 3-staged cirrhotic patients (A, B and C), indicative of the progressive aggravation of liver function (Figure 2).
Figure 2.
Bar charts of five bile acids (ng/mL, mean ± SEM) that are differentially expressed among Child-Pugh A, B and C cirrhotic patients in the discovery set, ** p<0.01 from a Student’s t test. OPLS-DA scores plot of bile acids in Child-Pugh class A, B and C of liver cirrhotic patients in the discovery set.
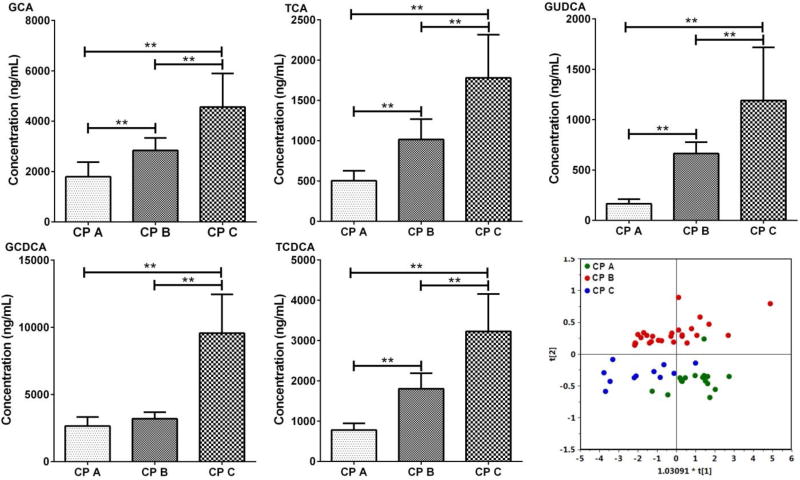
GCA, GCDCA, TCA, TCDCA and GUDCA in the Validation Set
The results obtained with the samples in the discovery set revealed that five bile acids, GCA, GCDCA, TCA, TCDCA and GUDCA were significantly altered among the three CP grades, A, B, and C. In the validation set, we focused on the levels of GCA, GCDCA, TCA, TCDCA and GUDCA in serum (Figure 3). The results were similar to those obtained in the discovery set. The levels of the five bile acids were higher in cirrhotic patients and were significantly altered among CP A, B and C. OPLS-DA (R2Xcum=1.000, R2Ycum = 0.571, Q2Ycum = 0.437) scores plot constructed with the five bile acids demonstrated a clear separation among the three groups (Figure 3).
Figure 3.
Bar charts of five bile acids (ng/mL, mean ± SEM) that are differentially expressed among Child-Pugh A, B and C cirrhotic patients in the validation set, ** p<0.01 from a Student’s t test. OPLS-DA scores plot of bile acids in Child-Pugh class A, B and C of hepatic cirrhotic patients in the validation set.
Discussion
Increasing evidence demonstrated that cirrhosis is closely associated with the significantly altered bile acid levels in serum.19, 21 Elevated serum bile acid concentrations have been shown to be a more sensitive test for the detection of liver cirrhosis than conventional liver function tests.27, 28 However, the level of TBA in serum does not seem to offer any help in diagnosis or prognosis.29, 30 The present study is designed to characterize the alteration of serum bile acid profiles associated with the pathophysiology of cirrhosis complementary to the conventional biochemical indices. Sixteen bile acids including GCDCA, GCA, TCA, TCDCA, GDCA, GUDCA, G λ-MCA, GLCA, CDCA, CA, UDCA, λ-MCA, TUDCA, TDCA, T λ-MCA, and TLCA were significantly increased in cirrhotic patients (Table 3). The increased bile acids in cirrhotic patients compared to healthy controls were consistent with the clinical biochemical measurement that the concentrations of TBA were increased in liver cirrhosis (Table 1). The OPLS-DA models derived from our current bile acid analysis showed good separations between cirrhotic patients and healthy controls, highlighting the diagnostic potential of this noninvasive analytical approach. Additionally, as shown in Figure 2 and 3, five serum bile acids, GCA, TCA, GCDCA, TCDCA and GUDCA were significantly altered among the three CP grades, A, B, and C, suggesting that these metabolites could be potential biomarkers for patients stratification at different pathological stages of cirrhosis.
Cirrhosis is associated with significant alterations in the gut microbiome compared with healthy subjects,15, 31 which in turn significantly influence the serum bile acid profile due to the altered gut microbiome.32–34 Among the 16 significantly altered bile acids, GCA, GCDCA, TCA and TCDCA, the conjugated BAs, were found significantly increased in the serum of cirrhotic patients, which have been demonstrated by other research groups that have found significant increase in bile acids similar to ours.21 It was believed that the conjugated bile acids could be the indicators of liver dysfunction in cirrhosis or chronic hepatitis.21, 35, 36 When hepatic trauma occurred, the hepatic clearance of bile acids decreased, leading to an increased serum bile acids concentration.37 Increased serum levels of bile acids in cirrhotic patients probably reflect a modification in the proportions of the bile acids present in the enterohepatic and systemic circulations. It is also possible that the increase in serum bile acids in cirrhosis is their reduced clearance that is manifested in various hepatopathies,38 which is different from that in normal populations, the efficiency of the liver in removing bile acids from portal blood is notable and only very small quantities of bile acids pass through the liver daily. The presence of serum GLCA, TLCA, GDCA, and TDCA suggest an enterohepatic circulation in cirrhotic patients and healthy controls, since DCA and LCA are formed by bacterial biotransformation through 7α-dehydroxylation from CA and CDCA, respectively, in the large intestine and returned to liver via portal circulation.39 DCA and LCA are reconjugated with glycine and taurine, accumulated in hepatocytes, and released to the blood pool through BA efflux transporters, leading to significantly increased GLCA, TLCA, GDCA, and TDCA levels in serum. It is well known that the LCA and its conjugates are cirrhogenics40 and may induce intrahepatic cholestasis in a variety of experimental animals.41 This could set up a vicious circle in which the increase in serum LCA conjugates, TLCA and GLCA, determines further hepatic damage.28
Conclusion
Bile acid analysis identifies a panel of bile acid markers that are of clinical potential for disease diagnosis and patient stratification for liver cirrhosis. The significantly increased serum bile acids warrant further validation as biomarkers for diagnosis and prognosis of post-hepatitis B cirrhosis. Five bile acids, GCA, TCA, GCDCA, TCDCA and GUDCA, were significantly altered among cirrhotic patients with CP A, B and C, reflecting abnormal metabolism of bile acids and intestinal microbial metabolism and showing a mechanistic association between serum bile acid alteration and pathological progression of the post-hepatitis B cirrhotic patients.
Acknowledgments
This study was financially supported by the National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism Grant R01 AA020212, in part by Program for Budgeted Scientific Research Project of Shanghai Municipal Education Commission, China (I3YZ044, 14ZZ119), Natural Science Foundation of Shanghai, China (14ZR1441400, 12ZR1432300), and E-institutes of Shanghai Municipal Education Commission, China (E03008, 085ZY1205).
References
- 1.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371(9615):838–51. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy S, Xu J, Kochanek K. Deaths: Final Data for 2010. National Vital Statistics Reports (Centers for Disease Control and Prevention) 2013;61(4):1–118. [PubMed] [Google Scholar]
- 3.Lok AS, McMahon BJ. Chronic hepatitis B: update of recommendations. Hepatology. 2004;39(3):857–61. doi: 10.1002/hep.20110. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362(9399):1907–17. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 5.Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1–85. [PubMed] [Google Scholar]
- 6.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–9. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 7.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31(4):864–71. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 8.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464–70. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 9.Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL. Harrison's Principles of Internal Medicine. 16. McGRaw-Hill Co; New York: 2005. [Google Scholar]
- 10.Benedeto-Stojanov D, Nagorni A, Bjelakovic G, Stojanov D, Mladenovic B, Djenic N. The model for the end-stage liver disease and Child-Pugh score in predicting prognosis in patients with liver cirrhosis and esophageal variceal bleeding. Vojnosanit Pregl. 2009;66(9):724–8. doi: 10.2298/vsp0909724b. [DOI] [PubMed] [Google Scholar]
- 11.Testa R, Valente U, Risso D, Caglieris S, Giannini E, Fasoli A, Botta F, Dardano G, Lantieri PB, Celle G. Can the MEGX test and serum bile acids improve the prognostic ability of Child-Pugh's score in liver cirrhosis? Eur J Gastroenterol Hepatol. 1999;11(5):559–63. doi: 10.1097/00042737-199905000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Oellerich M, Burdelski M, Lautz HU, Rodeck B, Duewel J, Schulz M, Schmidt FW, Brodehl J, Pichlmayr R. Assessment of pretransplant prognosis in patients with cirrhosis. Transplantation. 1991;51(4):801–6. doi: 10.1097/00007890-199104000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Tsao G, Friedman S, Iredale J, Pinzani M. Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology. 2010;51(4):1445–9. doi: 10.1002/hep.23478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christensen E. Prognostic models in chronic liver disease: validity, usefulness and future role. J Hepatol. 1997;26(6):1414–24. doi: 10.1016/s0168-8278(97)80481-3. [DOI] [PubMed] [Google Scholar]
- 15.Bajaj JS, Ridlon JM, Hylemon PB, Thacker LR, Heuman DM, Smith S, Sikaroodi M, Gillevet PM. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol. 2012;302(1):G168–75. doi: 10.1152/ajpgi.00190.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bajaj JS, Hylemon PB, Ridlon JM, Heuman DM, Daita K, White MB, Monteith P, Noble NA, Sikaroodi M, Gillevet PM. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrointest Liver Physiol. 2012;303(6):G675–85. doi: 10.1152/ajpgi.00152.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Yang F, Lu H, Wang B, Lei D, Wang Y, Zhu B, Li L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54(2):562–72. doi: 10.1002/hep.24423. [DOI] [PubMed] [Google Scholar]
- 18.Islam KB, Fukiya S, Hagio M, Fujii N, Ishizuka S, Ooka T, Ogura Y, Hayashi T, Yokota A. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology. 2011;141(5):1773–81. doi: 10.1053/j.gastro.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 19.Chen T, Xie G, Wang X, Fan J, Qiu Y, Zheng X, Qi X, Cao Y, Su M, Xu LX, Yen Y, Liu P, Jia W. Serum and urine metabolite profiling reveals potential biomarkers of human hepatocellular carcinoma. Molecular & cellular proteomics : MCP. 2011;10(7):M110 004945. doi: 10.1074/mcp.M110.004945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Xie G, Zhou M, Yu H, Lin Y, Du G, Luo G, Jia W, Liu P. Urinary metabolite variation is associated with pathological progression of the post-hepatitis B cirrhosis patients. J Proteome Res. 2012;11(7):3838–47. doi: 10.1021/pr300337s. [DOI] [PubMed] [Google Scholar]
- 21.Yin P, Wan D, Zhao C, Chen J, Zhao X, Wang W, Lu X, Yang S, Gu J, Xu G. A metabonomic study of hepatitis B-induced liver cirrhosis and hepatocellular carcinoma by using RP-LC and HILIC coupled with mass spectrometry. Mol Biosyst. 2009;5(8):868–76. doi: 10.1039/b820224a. [DOI] [PubMed] [Google Scholar]
- 22.Vlahcevic ZR, Buhac I, Bell CC, Jr, Swell L. Abnormal metabolism of secondary bile acids in patients with cirrhosis. Gut. 1970;11(5):420–2. doi: 10.1136/gut.11.5.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kakiyama G, Pandak WM, Gillevet PM, Hylemon PB, Heuman DM, Daita K, Takei H, Muto A, Nittono H, Ridlon JM, White MB, Noble NA, Monteith P, Fuchs M, Thacker LR, Sikaroodi M, Bajaj JS. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol. 2013;58(5):949–55. doi: 10.1016/j.jhep.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chinese Society of Hepatology, Chinese Medical Association and Chinese Society of Infectious Diseases, Chinese Medical Association. Guideline on prevention and treatment of chronic hepatitis B in China(2005) Chin Med J (Engl) 2007;120(24):2159–73. [PubMed] [Google Scholar]
- 25.Xie G, Wang Y, Wang X, Zhao A, Chen T, Ni Y, Wong L, Zhang H, Zhang J, Liu C, Liu P, Jia W. Profiling of Serum Bile Acids in a Healthy Chinese Population Using UPLC-MS/MS. J Proteome Res. 2015;14(2):850–859. doi: 10.1021/pr500920q. [DOI] [PubMed] [Google Scholar]
- 26.Trygg J, Wold S. Orthogonal projections to latent structures (O-PLS) J Chemometr. 2002;16(3):119–128. [Google Scholar]
- 27.Mannes GA, Thieme C, Stellaard F, Wang T, Sauerbruch T, Paumgartner G. Prognostic significance of serum bile acids in cirrhosis. Hepatology. 1986;6(1):50–3. doi: 10.1002/hep.1840060110. [DOI] [PubMed] [Google Scholar]
- 28.Greco AV, Mingrone G. Serum bile acid concentrations in mild liver cirrhosis. Clin Chim Acta. 1993;221(1–2):183–9. doi: 10.1016/0009-8981(93)90032-y. [DOI] [PubMed] [Google Scholar]
- 29.Jonsson G, Hedenborg G, Wisen O, Norman A. Serum concentrations and excretion of bile acids in cirrhosis. Scand J Clin Lab Invest. 1992;52(7):599–605. doi: 10.1080/00365519209115502. [DOI] [PubMed] [Google Scholar]
- 30.Cravetto C, Molino G, Biondi AM, Cavanna A, Avagnina P, Frediani S. Evaluation of the diagnostic value of serum bile acid in the detection and functional assessment of liver diseases. Ann Clin Biochem. 1985;22(Pt 6):596–605. doi: 10.1177/000456328502200608. [DOI] [PubMed] [Google Scholar]
- 31.Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, Guo J, Le Chatelier E, Yao J, Wu L, Zhou J, Ni S, Liu L, Pons N, Batto JM, Kennedy SP, Leonard P, Yuan C, Ding W, Hu X, Zheng B, Qian G, Xu W, Ehrlich SD, Zheng S, Li L. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513(7516):59–64. doi: 10.1038/nature13568. [DOI] [PubMed] [Google Scholar]
- 32.Swann JR, Want EJ, Geier FM, Spagou K, Wilson ID, Sidaway JE, Nicholson JK, Holmes E. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc Natl Acad Sci U S A. 2011;1:4523–30. doi: 10.1073/pnas.1006734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sayin SI, Wahlstrom A, Felin J, Jantti S, Marschall HU, Bamberg K, Angelin B, Hyotylainen T, Oresic M, Backhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell metabolism. 2013;17(2):225–35. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Claus SP, Tsang TM, Wang Y, Cloarec O, Skordi E, Martin FP, Rezzi S, Ross A, Kochhar S, Holmes E, Nicholson JK. Systemic multicompartmental effects of the gut microbiome on mouse metabolic phenotypes. Molecular systems biology. 2008;4:219. doi: 10.1038/msb.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paumgartner G. Serum bile acids. Physiological determinants and results in liver disease. J Hepatol. 1986;2(2):291–8. doi: 10.1016/s0168-8278(86)80088-5. [DOI] [PubMed] [Google Scholar]
- 36.Yang J, Zhao X, Liu X, Wang C, Gao P, Wang J, Li L, Gu J, Yang S, Xu G. High performance liquid chromatography-mass spectrometry for metabonomics: potential biomarkers for acute deterioration of liver function in chronic hepatitis B. J Proteome Res. 2006;5(3):554–61. doi: 10.1021/pr050364w. [DOI] [PubMed] [Google Scholar]
- 37.Yin P, Zhao X, Li Q, Wang J, Li J, Xu G. Metabonomics study of intestinal fistulas based on ultraperformance liquid chromatography coupled with Q-TOF mass spectrometry (UPLC/Q-TOF MS) J Proteome Res. 2006;5(9):2135–43. doi: 10.1021/pr060256p. [DOI] [PubMed] [Google Scholar]
- 38.Thjodleifsson B, Barnes S, Chitranukroh A, Billing BH, Sherlock S. Assessment of the plasma disappearance of cholyl'l14C-glycine as a test of hepatocellular disease. Gut. 1977;18(9):697–702. doi: 10.1136/gut.18.9.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hylemon PB, Zhou HP, Pandak WM, Ren SL, Gil G, Dent P. Bile acids as regulatory molecules. J Lipid Res. 2009;50(8):1509–1520. doi: 10.1194/jlr.R900007-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holsti P. Cirrhosis of the Liver induced in Rabbits by Gastric Instillation of 3-Monohydroxycholanic Acid. Nature. 1960;186(4720):250–250. [Google Scholar]
- 41.Yousef IM, Tuchweber B, Vonk RJ, Massé D, Audet M, Roy CC. Lithocholate cholestasis—Sulfated glycolithocholate-induced intrahepatic cholestasis in rats. Gastroenterology. 80(2):233–241. [PubMed] [Google Scholar]