Abstract
Antipsychotic Induced Weight Gain (AIWG) is a common and severe side effect of many antipsychotic medications. Mitochondria play a vital role for whole-body energy homeostasis and there is increasing evidence that antipsychotics modulate mitochondrial function. This study aimed to examine the role of variants in nuclear-encoded mitochondrial genes and the mitochondrial DNA (mtDNA) in conferring risk for AIWG. We selected 168 European-Caucasian individuals from the CATIE sample based upon meeting criteria of multiple weight measures while taking selected antipsychotics (risperidone, quetiapine or olanzapine). We tested the association of 670 nuclear-encoded mitochondrial genes with weight change (%) using MAGMA software. Thirty of these genes showed nominally significant P-values (<0.05). We were able to replicate the association of three genes, CLPB, PARL, and ACAD10, with weight change (%) in an independent prospectively assessed AIWG sample. We analyzed mtDNA variants in a subset of 74 of these individuals using next-generation sequencing. No common or rare mtDNA variants were found to be significantly associated with weight change (%) in our sample. Additionally, analysis of mitochondrial haplogroups showed no association with weight change (%). In conclusion, our findings suggest nuclear-encoded mitochondrial genes play a role in AIWG. Replication in larger sample is required to validate our initial report of mtDNA variants in AIWG.
Keywords: Mitochondria, Mitochondrial DNA variants, Nuclear-encoded mitochondrial genes, Schizophrenia, Antipsychotic-induced weight gain, Next generation sequencing
1. Introduction
Schizophrenia (SCZ) is a complex disorder characterized by psychosis and disturbed behavior. It is estimated to affect approximately 1% of the adult population worldwide (McGuffin et al., 1995). SCZ is ranked among the top 10 causes of disability among people in developed countries (Kahn et al., 2015). Antipsychotic medications are an important, effective therapeutic intervention for controlling the major symptoms of SCZ (Allison et al., 1999). However, weight gain is a common side effect of treatment with antipsychotics, and is particularly pronounced with clozapine and olanzapine, and is becoming a major health concern (Gebhardt et al., 2010). Antipsychotic-induced weight gain (AIWG) is a severe side effect observed in up to 40% of patients taking medications referred to as second-generation or atypical antipsychotics (Lett et al., 2012; Ucok and Gaebel, 2008). Weight gain leads to increased risk for cardiovascular morbidity and mortality. In addition, excessive weight and obesity can have important effects on an individual's' adjustment in the community, ability to participate in rehabilitation efforts and self-image, contributing to a main reason for non-adherence (Ucok and Gaebel, 2008). The heritability of AIWG has been suggested to be between 60 and 80% based on twin and family studies, indicating the role of genetic factors in the pathophysiology of AIWG (Gebhardt et al., 2010). Several studies have addressed the genetic basis of AIWG in terms of the nuclear genome, and it is a well-established area of research (MacNeil and Muller, 2016; Muller et al., 2013; Muller and Kennedy, 2006).
The mitochondrial system is an interesting target to be examined in AIWG studies. First, mitochondria are the main source of aerobic energy for brain cellular functioning. Second, these organelles have been shown to be involved in appetite and satiety regulation through hypothalamic signaling mechanisms. Briefly, the arcuate nucleus in the hypothalamus is the main central regulator for energy metabolism. In this region, there are two specific types of neurons: anorectic (POMC) and orexigenic (NPY/AgRP). When glucose levels are high in the cell, POMC neurons are active and promote satiety in association with elevated production of the mitochondrial reactive oxygen species (mtROS), and increased mitochondrial fusion. When glucose levels are low, NPY-AgRP neurons are active and promote hunger associated with fatty acid metabolism, low levels of mtROS, and increased mitochondria fission (Jordan et al., 2010; Nasrallah and Horvath, 2014). Finally, antipsychotic medications are reported to alter mitochondrial function although the molecular mechanisms are incompletely understood (see (Goncalves et al., 2014) for a review). Despite the rationale for mitochondrial involvement, there are not many studies examining mitochondrial genetic variance in AIWG. In one such study, our group reported the association between the NDUFS1 mitochondrial gene with AIWG (Goncalves et al., 2014). This study was the first to highlight the role of mitochondrial variation in AIWG and it prompted further, more thorough investigation of our hypothesis.
The mtDNA is a circular molecule containing 16,569 base pairs that encodes 37 genes: 13 subunits of the mitochondrial electron transport chain and a distinct set of rRNAs and tRNAs, all of which are critical for life-sustaining oxidative phosphorylation and energy generation (Wallace, 1994). Additionally, more than 1000 nuclear-encoded mitochondrial genes are necessary for mitochondrial functioning and biogenesis (Wallace, 2013). Here we hypothesize that nuclear-encoded mitochondrial genes are enriched in variants associated with AIWG. We also hypothesize that variants in the mtDNA are associated with AIWG.
2. Methods
2.1. Methods for nuclear-encoded mitochondrial genes
2.1.1. CATIE GWAS imputation
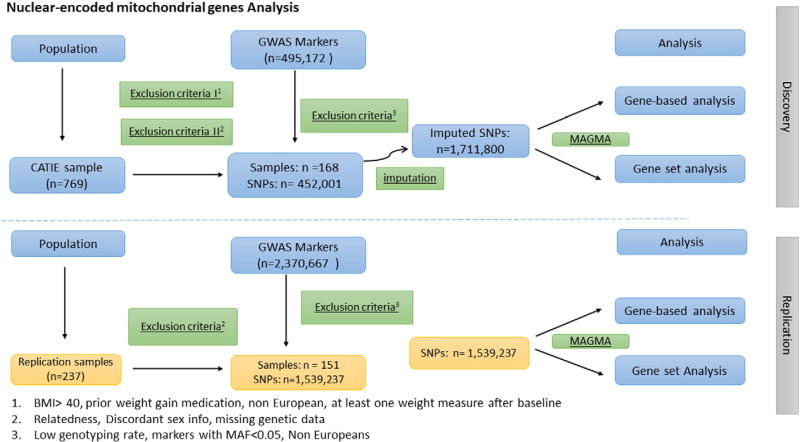
The genome-wide genotyping in the CATIE samples was performed using Affymetrix 500 k/Perlegen's custom 164 K chips. A total of 495,172 SNPs were available. We performed standard quality control (QC) measures in CATIE sample before imputation (Anderson et al., 2010; Clarke et al., 2011). Briefly, we removed individuals with <95% of the markers genotyped, and markers that were <95% genotyped. We checked cryptic relatedness, and one individual of each pair of related individuals was removed (PI^ HAT > 0.05), choosing preferably that individual with the missing phenotype or more missing genotypes. Mean heterozygosity was calculated and outliers (±4 SD) were removed. In a subsequent step, some SNPs were filtered out if the χ2-test for Hardy-Weinberg equilibrium was <0.001. Multi-dimensional scaling (MDS) analysis of the genotypes was used to check for population stratification, and outliers were removed through visual inspection of scatter plots. During QC, we excluded: (1) 0 subjects for relatedness; (2) 3 subjects for discordant sex info; (3) 0 subjects for missing genetic data; (4) 382 markers for low genotyping rate; and (5) 42,789 markers for minor allele frequency of <0.05. After QC, we had total sample of 168 individuals which also met the inclusion criteria for AIWG study as described below.
Whole-genome imputation was conducted using IMPUTE2 (Howie et al., 2012) in 5-Mb segments after pre-phasing the data in SHAPEIT2 (Delaneau et al., 2013) using the 1000 Genomes Project Phase 3 dataset (Genomes Project et al., 2015) as reference panel. The imputation output (.gen) was then converted to bed/bim/fam format using PLINK2 with an imputation score threshold of 0.8. Post-imputation QC was performed, removing individuals with <95% of the markers genotyped (N = 0), and markers that were <95% genotyped (N = 594,431). The χ2-test for Hardy-Weinberg equilibrium was <0.000001 (N = 0). A total of 1,711,800 biallelic SNPs were available for analyses after imputation.
2.1.2. Analysis of nuclear-encoded mitochondrial gene set
The mitochondrial set of genes included 670 mitochondrial genes from MitoCarta v.2.0 (release 2015). For the gene-set competitive analysis, we also included the remaining “protein-coding genes” (i.e., all other genes present in our data that were not listed above). MAGMA software was used (default settings) for gene-based and gene-set analyses (de Leeuw et al., 2015) for our imputed genotype data in the CATIE sample. MAGMA uses three basic steps to calculate P-values for genes and pathways/gene-sets. In step #1, the PLINK binary map file is used as input to annotate SNPs to genes (present in human genome build 37). In step #2, P-values are calculated for all genes present in the data set, excluding SNPs in linkage disequilibrium and taking into account gene sizes. The P-values are corrected for the number of tests used (i.e. total number of genes (N = 670 in the current study)). Step #3 calculates P-values for the pathways/gene-sets of interest. A competitive analysis model was used as it corrects for the baseline association, and verifies whether gene sets of interest are more strongly associated with the phenotype than other sets of genes in the data set.
We used an independent sample (N =151) to replicate our findings from gene-based analysis. This sample was described elsewhere (Tiwari et al., 2016). Briefly, we conducted genome-wide genotyping using the Illumina Omni 2.5 M Array. In total, 2,370,667 SNPs were genotyped for 237 participants at The Centre for Applied Genomics (TCAG) at The Hospital for Sick Children in Toronto, Canada. QC methods were selected according to Anderson et al. (Anderson et al., 2010) and Clarke et al. (Clarke et al., 2011) and which we have applied in previous studies (Maciukiewicz et al., in press). Across individuals within our sample, we investigated sex discordance, individual genotype missingness rates (<5%), heterozygosity rate, relatedness (i.e. identity-by-descent, IBD) and genetic ancestry to confirm self-reported ancestry, as well as to control for fine population structure. To avoid issues of sample contamination or the possibility of inbreeding, we ensured a heterozygosity rate of ±3 standard deviations from the mean. Furthermore, we restricted individual genotype missingness to <5%. To avoid spurious associations due to population stratification, we confirmed self-reported ancestry with genetic ancestry using MDS in PLINK. We plotted our sample versus the HapMap reference populations for Europeans and African Americans using R. We defined ethnic outliers as those individuals located ±6 standard deviations from the mean. After individual QC, we excluded: (1) 5 subjects with excessive relatedness; (2) 8 subjects with discordant sex info; (3) 2 subjects with low genotyping rate; (4) 10 subjects with missing genetic data and ancestry different than African American or European. Some of individuals failed more than one QC step. As a result, we had a total sample of 202 individuals (51 and 151 of African-American and European ancestry respectively). An analytical workflow for nuclear-encoded mitochondrial genes is shown in the Fig. 1.
Fig. 1.
Overall analytical workflow for nuclear-encoded mitochondrial genes.
2.2. Methods for mtDNA
2.2.1. CATIE sample
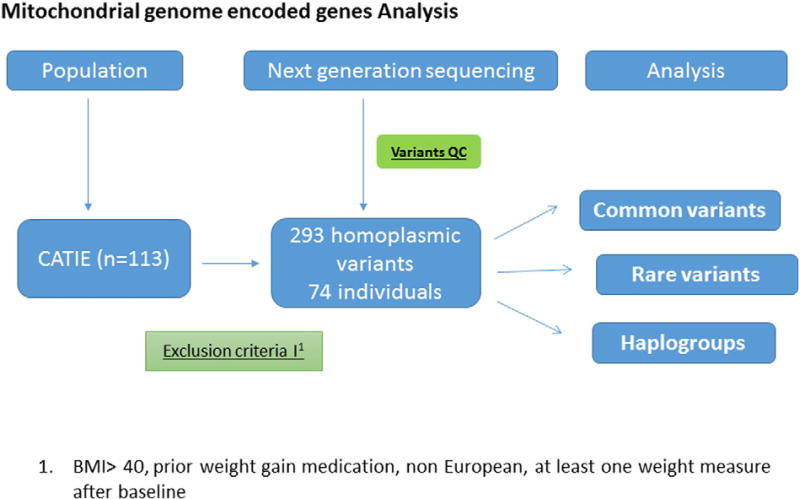
The description of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) sample has been previously published (Lieberman et al., 2005). We sequenced the mtDNA of 113 individuals from the CATIE sample. However in the association analysis, we included individuals who fulfilled the following criteria: study medication with at least medium or high risk for weight gain (risperidone, quetiapine or olanzapine), no marked obesity (BMI < 40 kgm−2) at study baseline, no medication with high risk for weight gain before baseline (start of the study) for >14 days, European ancestry and more than one weight measure available after baseline. This excluded patients who had received antipsychotics (ziprasidone or perphenazine) that are not associated with significant weight gain. Similarly, patients with baseline BMI ≥ 40 kgm−2, and with prior exposure to medication with high weight gain risk (Olanzapine) are less likely to gain weight during the course of the study (Brandl et al., 2016). After the exclusion of confounding factors, our refined sample size was 74. Demographic and clinical characteristics of the samples are provided in Table 1. Weight gainers were defined as those individuals who showed weight gain greater than or equal to 7% from baseline (N= 24).
Table 1.
Demographic and clinical characteristic of the sample.
| Total Number | Mean | SD | ||
|---|---|---|---|---|
| Sex | Male | 61 | ||
| Female | 13 | |||
| Age | 40 | 11.98 | ||
| Treatment duration | 143 | 43.52 | ||
| Percentage of weight gain | 4% | 8.62 | ||
| Olanzapine | 42 | |||
| Risperidone | 32 | |||
| Individuals with >7% weight gain | 24 |
SD: standard deviation.
2.2.2. Next-generation sequencing (NGS)
We sequenced the mtDNA genome in 113 schizophrenia subjects using methods described previously (Sequeira et al., 2012). Briefly, the mitochondrial genome was initially enriched by long-range PCR amplification of two overlapping amplicons, which were then purified and sequenced on the Illumina HiSeq 2500 according to manufacturer's protocols. Reads from the Illumina HiSeq 2500 (single end fastaQ) were analyzed using mtDNA-Server v.1.0.6 (https://mtdnaserver.uibk.ac.at/index.html) with default parameters. mtDNA-Server is a free web tool for mtDNA NGS analysis (for details see (Kloss-Brandstatter et al., 2015), (Weissensteiner et al., 2016)). Briefly, the server validates the input format (fastaQ/BAM) and aligns the reads to revised Cambridge Reference Sequence (rCRS, GenBank accession number NC_012920) using Burrows-Wheeler Aligner Maximum Exact Match algorithm (BWA MEM). Then, the bases (only with PHRED score ≥ 30) for each position relative to the rCRS are extracted. Details regarding the parameters used by the server can be found on the website (https://mtdna-server.uibk.ac.at/index.html#!pages/help). Homoplasmic variants were defined as those with the major allele found to differ from the rCRS and constituted 95–100% of the reads.
2.2.3. Statistical analysis
Linear regression was used to test the association of genotypes with weight change (%) using an additive genetic model (Purcell et al., 2007). Weight change (%) from baseline was used as the dependent variable, and genotypes were entered as predictors. To account for multiple tested, the number of independent SNPs (N = 167) was estimated using SNP Spectral Decomposition Lite (Nyholt, 2004), and a Bonferroni-corrected significance threshold of 0.0003 was applied. Haplogroups were identified using HaploGrep through the mtDNA-Server. All of them were assigned with a quality score of 0.8 or higher by HaploGrep. Due to the small sample size, phylogenetically related haplogroups were combined and analyzed together. Thus, three European groups were tested for association with weight change (%): (“H-HV-V”, “J-T”, and “K-U”). Individuals assigned as non-European based on their haplogroups were filtered out (N = 14). The association between haplogroups and weight change (%) was tested using linear regression in the Statistical Package for the Social Sciences (SPSS) v24 (IBM Corporation, Armonk, N.Y., USA). Rare variant analyses on the genotyped markers was performed using Sequence Kernel Association Test (SKAT) (Ionita-Laza et al., 2013), as described previously (Gonçalves et al., under review). We did not observe differences in allele calls when comparing cell lines with blood-derived mtDNA. However, in cell lines, there is a trend for increasing heteroplasmy compared to blood-derived mtDNA (Vawter, M., unpublished observation). Since heteroplasmy was not analyzed in this study, this observation does not affect the present results. Power was calculated using Quanto (Morrison, 2006) assuming an additive model and using the following parameters: mean weight change of 4% (±8.62), sample size of N = 74, the observed beta and minor allele frequency of the SNP. An analytical workflow for mtDNA variants is shown in the Fig. 2.
Fig. 2.
Overall analytical workflow for mtDNA variants.
3. Results
3.1. Analysis of nuclear-encoded mitochondria gene set
Nuclear-encoded mitochondrial genes were investigated for enrichment across variants associated with weight change (%). MAGMA annotated SNPs in 11,980 genes present in our CATIE imputed data (this included 670 mitochondrial genes). In the gene-based analysis (which tests the association of each gene with the phenotype), we identified 30 mitochondrial genes with P-value < 0.05, although these observations did not survive multiple-testing correction (Table 2). We performed the gene-based analysis in our second independent data set. While the gene-set analysis showed no enrichment of variants associated with weight change (%) for the nuclear-encoded mitochondrial gene set (N=670 genes; P-value=0.88), we were able to replicate three of the 30 mitochondrial genes (P-value <0.05) from the CATIE data set: (1) ClpB Homolog, Mitochondrial AAA ATPase Chaperonin (CLPB), (2) Presenilin Associated Rhomboid Like (PARL), and (3) Acyl-coenzyme A dehydrogenase 10 (ACAD10) (Table 3).
Table 2.
Nominally significant hits from gene-based analysis in MAGMA.
| Gene name | CHR | Start | Stop | NSNPS | NPARAM | N | ZSTAT | P |
|---|---|---|---|---|---|---|---|---|
| GRSF1 | 4 | 71,681,499 | 71,705,627 | 46 | 2 | 168 | 3.429 | 0.0003 |
| CPS1 | 2 | 211,342,406 | 211,543,831 | 154 | 8 | 168 | 3.259 | 0.0006 |
| NDUFB7 | 19 | 14,676,890 | 14,682,889 | 1 | 1 | 168 | 3.223 | 0.0006 |
| CRLS1 | 20 | 5,986,739 | 6,020,699 | 1 | 1 | 168 | 3.187 | 0.0007 |
| MAVS | 20 | 3,827,446 | 3,856,770 | 1 | 1 | 168 | 2.839 | 0.0023 |
| C6orf201 | 6 | 4,079,440 | 4,131,000 | 3 | 2 | 168 | 2.585 | 0.0049 |
| HMGCS2 | 1 | 120,290,619 | 120,311,555 | 11 | 2 | 168 | 2.379 | 0.0087 |
| IMMP2L | 7 | 110,303,106 | 111,202,588 | 521 | 27 | 168 | 2.377 | 0.0087 |
| BCL2 | 18 | 60,790,579 | 60,987,011 | 32 | 5 | 168 | 2.133 | 0.0164 |
| ARMC4 | 10 | 28,101,095 | 28,287,977 | 152 | 5 | 168 | 2.010 | 0.0222 |
| FH | 1 | 241,660,857 | 241,683,085 | 19 | 2 | 168 | 1.966 | 0.0247 |
| PANK2 | 20 | 3,869,486 | 3,904,538 | 2 | 1 | 168 | 1.961 | 0.0249 |
| MRPS18C | 4 | 84,377,085 | 84,382,929 | 1 | 1 | 168 | 1.936 | 0.0264 |
| CLPP | 19 | 6,361,463 | 6,368,915 | 13 | 2 | 168 | 1.935 | 0.0265 |
| MRPS27 | 5 | 71,515,236 | 71,616,084 | 41 | 4 | 168 | 1.932 | 0.0267 |
| MTIF3 | 13 | 28,009,776 | 28,024,739 | 13 | 2 | 168 | 1.924 | 0.0272 |
| PARL | 3 | 183,543,522 | 183,602,693 | 72 | 3 | 168 | 1.900 | 0.0287 |
| CLPB | 11 | 72,003,469 | 72,145,724 | 9 | 3 | 168 | 1.890 | 0.0294 |
| MRPL35 | 2 | 86,426,478 | 86,440,913 | 18 | 1 | 168 | 1.865 | 0.0311 |
| TRAP1 | 16 | 3,708,038 | 3,767,598 | 7 | 2 | 168 | 1.852 | 0.0320 |
| FAM210A | 18 | 13,663,346 | 13,726,591 | 3 | 2 | 168 | 1.851 | 0.0321 |
| SFXN1 | 5 | 174,905,514 | 174,956,745 | 1 | 1 | 168 | 1.804 | 0.0356 |
| COX5A | 15 | 75,212,616 | 75,230,495 | 27 | 2 | 168 | 1.781 | 0.0374 |
| IMMT | 2 | 86,371,055 | 86,423,264 | 114 | 2 | 168 | 1.760 | 0.0392 |
| ECI2 | 6 | 4,115,927 | 4,135,831 | 7 | 3 | 168 | 1.760 | 0.0392 |
| SQRDL | 15 | 45,923,346 | 45,983,492 | 81 | 7 | 168 | 1.752 | 0.0399 |
| PABPC1L | 20 | 43,538,701 | 43,587,585 | 30 | 3 | 168 | 1.720 | 0.0427 |
| ACAD10 | 12 | 112,123,857 | 112,194,911 | 51 | 2 | 168 | 1.704 | 0.0442 |
| NUDT19 | 19 | 33,157,186 | 33,204,702 | 12 | 2 | 168 | 1.682 | 0.0462 |
| L2HGDH | 14 | 50,704,285 | 50,778,947 | 108 | 6 | 168 | 1.660 | 0.0485 |
Table 3.
Results for gene-based analysis in MAGMA for discovery and replication sample.
| Discovery sample | Replication sample | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||
| Gene | CHR | Start | Stop | NSNPS | NPARAM | N | ZSTAT | P | NSNPS | NPARAM | N | ZSTAT | P |
| CLPB | 11 | 72,003,469 | 72,145,724 | 9 | 3 | 168 | 1.89 | 0.03 | 37 | 11 | 151 | 2.34 | 0.01 |
| PARL | 3 | 183,543,522 | 183,602,693 | 72 | 3 | 168 | 1.90 | 0.03 | 34 | 5 | 151 | 1.89 | 0.03 |
| ACAD10 | 12 | 112,123,857 | 112,194,911 | 51 | 2 | 168 | 1.70 | 0.04 | 14 | 4 | 151 | 1.77 | 0.04 |
3.2. Analysis of variants in mtDNA
We analyzed 74 complete mtDNA sequences. The average coverage for the variants was 12,000× (min = 1,895×, max = 38,894×).
3.2.1. Homoplasmic variants in mtDNA
A total of 293 homoplasmic variants were identified in our sample. Of these, 282 were transitions (95.3%) and 11 were transversions (4.7%) (Ti/Tv ratio is 25:1). This ratio is within the expected range of between 20:1 and 38:1 for humans (Guo et al., 2012; Pereira et al., 2009). A total of 56 were non-synonymous based on MutPred (Li et al., 2009) and Selection Score (Pereira et al., 2011), and 19 were classified as harmful in both tools (Supplemental Table 1).
Eighty-seven of 293 variants were uniquely found in weight gainers, and 18 of the 87 were non-synonymous substitutions (Table 4). There was no statistically significant difference for the proportion of synonymous versus non-synonymous SNPs between weight gainers and non-weight gainers in our sample (0.19 versus 0.18, P-value= 1).
Table 4.
List of 18 non-synonymous homoplasmic variants identified in individuals showing >7% weight gain in our study.
| Functional prediction | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| POS | MUT | Locus | AAC | MutPred | Selection_Score | HaploGrep_Weight |
| 3505 | A > G | MT-ND1 | T67A | 0.455 | 0.4 | 7.2 |
| 3992 | C > T | MT-ND1 | T229 M | 0.664 | 0.893 | 8.1 |
| 4024 | A > G | MT-ND1 | T240A | 0.326 | 0.243 | 8.8 |
| 4216 | T > C | MT-ND1 | Y304H | 0.611 | 0.728 | 5.6 |
| 4917 | A > G | MT-ND2 | N150D | 0.628 | 0.777 | 7.6 |
| 5046 | G > A | MT-ND2 | V193I | 0.483 | 0.445 | 7.2 |
| 5277 | T > C | MT-ND2 | F270 L | 0.554 | 0.585 | 8.8 |
| 5460 | G > A | MT-ND2 | A331T | 0.505 | 0.484 | 3.9 |
| 6489 | C > A | MT-CO1 | L196I | 0.641 | 0.817 | 10 |
| 7080 | T > C | MT-CO1 | F393 L | 0.561 | 0.601 | 10 |
| 9468 | A > G | MT-CO3 | T88A | 0.343 | 0.26 | 8.1 |
| 10,192 | C > T | MT-ND3 | S45F | 0.451 | 0.394 | 10 |
| 11,984 | T > C | MT-ND4 | Y409H | 0.738 | 1.187 | 8.1 |
| 13,285 | A > G | MT-ND5 | I317V | 0.507 | 0.488 | 10 |
| 14,582 | A > G | MT-ND6 | V31A | 0.452 | 0.395 | 10 |
| 14,766 | C > T | MT-CYB | T7I | 0.165 | 0.131 | 10 |
| 15,452 | C > A | MT-CYB | L236I | 0.098 | 0.101 | 10 |
| 15,884 | G > C | MT-CYB | A380P | 0.29 | 0.212 | 8.8 |
AAC: amino acid change.
We next performed linear regression with the weight change (%) as the dependent variable and the mtDNA genotypes as predictors. The raw sequence data was converted to VCF files and then to PLINK format to perform single SNP analysis. There were 75 bi-allelic variants identified by mtDNA NGS with MAF of at least 5%. From the association analysis, no SNPs were found to be significant in association with weight change (P < 0.05) (see Table 5 for top hits). Rare variant analysis conducted (N = 308 SNPs, MAF ≤ 5%) using SKAT (Ionita-Laza et al., 2013) yielded a P-value of 0.8. Considering that age can be a confounder for analysis of mtDNA variants, a Pearson correlation was performed to assess the relationship between age and weight change (%). There was no significant correlation between the two variables (r = −0.168, N = 74, P = 0.15), and thus, age was not included in our model.
Table 5.
Top hits from association test for common SNPs (MAF > 5%).
| CHR | SNP | BP | N | Allele frequency |
Beta | STAT | P-value | Power |
|---|---|---|---|---|---|---|---|---|
| 26 | MT-73 | 73 | 74 | 0.45 | −3.184 | −1.594 | 0.12 | 0.62 |
| 26 | MT-16256 | 16,256 | 74 | 0.11 | −4.202 | −1.307 | 0.2 | 0.46 |
| 26 | MT-16519 | 16,519 | 74 | 0.28 | −2.945 | −1.33 | 0.19 | 0.47 |
3.2.2. Haplogroup analysis
Sixty-eight unique haplogroups were found in our sample set. Association was tested between mtDNA phylogenetic groups and weight change (%). None of the groups tested (i.e. H-HV-V, J-T, and K-U) was significantly associated with the phenotype (see Table 6).
Table 6.
Results from association analysis between mitochondrial DNA groups and weight change (%).
| Haplogroup | Beta | P-value | % Weight gain (SD, K) |
|---|---|---|---|
| H-HV-V | −1.878 | 0.35 | H-HV-V: 2.88% (8.54, 35) other: 4.75% (8.71, 39) |
| J-T | 0.611 | 0.79 | J-T: 4.33% (9.31, 18) other: 3.72% (8.48, 56) |
| K-U | 1.112 | 0.64 | K-U: 4.72% (9.02, 17) other: 3.61% (8.57, 57) |
4. Discussion
In this study, we examined the hypothesis that nuclear-encoded mitochondrial genes are associated with AIWG. The gene-set analysis did not show enrichment of mitochondrial variants to be associated with weight change (%). However, the analysis of genes individually revealed 30 nominally significant for the phenotype, of which three were replicated in an independent sample.
The most interesting finding was with the gene CLPB, which is a member of the ATP-ases (AAA+) superfamily. It cooperates with Hsp70 in the disaggregation of (misfolded) protein aggregates. Although the protein function is not completely understood in humans, variants in CLPB appear to disrupt the integrity of mitochondrial membrane by allowing abnormal protein aggregation (Kanabus et al., 2015).
PARL is a mitochondrial intermembrane Rhomboid protease. Its function is associated with maintenance of mitochondrial morphology and apoptosis. Reduced PARL protein levels was associated with diabetes, possibly due to an imbalance between mitochondrial biogenesis and degradation, which in turn reduces mitochondrial mass and alters mitochondrial dynamics (Civitarese et al., 2010). Mitochondrial dynamics (i.e., mitochondrial fusion, fission, and motility) regulates important processes including mitochondrial morphology, mitophagy, mtDNA stability, ROS generation and cellular stress response. It has been shown that disturbance in the integrity of mitochondrial membranes and mitochondrial dynamics promotes mitochondrial dysfunction, which leads to a variety of metabolic stresses and disorders (Nasrallah and Horvath, 2014).
ACAD10 is involved in the beta-oxidation of fatty acids in mitochondria. Variants in ACAD10were associated with type-2 diabetes and insulin resistance in Pima Indians, possibly due to impaired lipid oxidation and/or increased adipocytes size (Bian et al., 2010). These two abnormalities are correlated with insulin resistance and increased risk for type-2 diabetes (Weyer et al., 2000). Thus, variants described for two of the genes identified in our study have been associated with metabolic alteration linked to mitochondrial dysfunction.
This study also investigated the association between weight change (%) and variants in mtDNA which is generally excluded from routine GWAS analyses. To the best of our knowledge, this is the first study to date to examine this hypothesis. We did not observe any significant association of both common and rare variants with the phenotype. However, we identified variants exclusively in the group with ≥7% weight gain, which should serve as stimulus for future AIWG studies.
There are a number of limitations with the current study. Our findings were derived from a modest sample size of only 74 subjects: While we carefully selected patients to be removed from the sample based on trial medication, and previous use of psychotropic medication, we did not have enough power to detect polymorphisms of small/moderate effects. Adherence to prescribed medication was also a potential confound. The CATIE sample also has considerable diagnostic heterogeneity, and was collected across >57 sites. Additionally, since the CATIE DNA sample was derived from cell lines, it is possible that passage effect partly contributed to sample heterogeneity. Furthermore, there may be potential differences between blood and brain in terms of mitochondrial variants. However, for mtDNA homoplasmic variants, we have previously reported 100% concordant sequencing results between blood and different brain regions (Sequeira et al., 2012).
In summary, this study identified nuclear-encoded mitochondrial genes conferring risk for AIWG. Proteins involved in mitochondrial membranes and dynamics appeared to be candidates worthy of further examination in studies of this type. The mtDNA analysis showed limitations mainly due to the fact that CATIE sample was not designed for this type of study (AIWG). However, we were able to produce preliminary data to be further explored in larger samples. The analysis of the mitochondrial genes encoded by the nucleus and mtDNA as presented in this study holds the potential to shed light on the role of mitochondria in the antipsychotic-induced side effects and related phenotypes. Replication in larger samples and/or other ethnic groups will permit a better understanding of the role of mitochondria in AIWG.
Supplementary Material
Acknowledgments
We are greatly indebted to the participating subjects. This work was funded by AFP Innovation Fund.
JLK, DJM, CCZ, AKT, and VFG: patent application: “Compositions and Methods for the Treatment and Prevention of Antipsychotic Medication–Induced Weight Gain”. JLK, DJM, CCZ: patent application: “Genetic Markers for Antipsychotic Induced Weight Gain and Methods for Use Thereof”. DJM is supported by the Canadian Institutes of Health Research (CIHR Operating Grant MOP 142192), the National Institutes of Health (R01MH085801), and the Centre for Addiction and Mental Health Foundation (Joanne Murphy Professorship). Past funding which have contributed to this project include a Brain & Behaviour Research (NARSAD) Independent Investigator Award, the Michael Smith New Investigator Salary Prize for Research in Schizophrenia (CIHR) and an Early Researcher Award by the Ministry of Research and Innovation of Ontario. Dr. Vawter's work on mitochondria and psychiatric disorders is supported by NIMH (R21MH099440-01 and R01MH085801). Dr. Vawter also receives support from the Pritzker Neuropsychiatric Disorders Research Consortium. (University of California, Irvine; PDNRF-28409). Dr. Kennedy has also received speaker honoraria and expenses from Eli Lilly, Novartis and Shire, and consultant honoraria and expenses from Roche.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.schres.2017.06.046.
Contributors
V.F.G. designed the study, performed experiments, data analysis, and wrote the manuscript. K.M. performed data analysis and wrote first draft of manuscript. H.R. performed bioinformatics analysis. A.B.C., A.K.T., and C.C.Z. performed data analysis. A.K.T, M.M., D.J.M. performed data curation. B.R. performed libraries for sequencing. M.P.V. supervised mtDNA sequencing and performed data cleaning and quality control. J.L.K. supervised the study. All co-authors contributed with writing review & editing.
Disclosures
KM, ABC, BR, MM, and RH declare no conflict of interest.
References
- Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, Weiden PJ. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am. J. Psychiatry. 1999;156(11):1686–1696. doi: 10.1176/ajp.156.11.1686. [DOI] [PubMed] [Google Scholar]
- Anderson CA, Pettersson FH, Clarke GM, Cardon LR, Morris AP, Zondervan KT. Data quality control in genetic case-control association studies. Nat. Protoc. 2010;5(9):1564–1573. doi: 10.1038/nprot.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian L, Hanson RL, Muller YL, Ma L, Investigators, M. Kobes S, Knowler WC, Bogardus C, Baier LJ. Variants in ACAD10 are associated with type 2 diabetes, insulin resistance and lipid oxidation in Pima Indians. Diabetologia. 2010;53(7):1349–1353. doi: 10.1007/s00125-010-1695-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl EJ, Tiwari AK, Zai CC, Nurmi EL, Chowdhury NI, Arenovich T, Sanches M, Goncalves VF, Shen JJ, Lieberman JA, Meltzer HY, Kennedy JL, Muller DJ. Genome-wide association study on antipsychotic-induced weight gain in the CATIE sample. Pharmacogenomics J. 2016 Aug.16(4):352–356. doi: 10.1038/tpj.2015.59. [DOI] [PubMed] [Google Scholar]
- Civitarese AE, MacLean PS, Carling S, Kerr-Bayles L, McMillan RP, Pierce A, Becker TC, Moro C, Finlayson J, Lefort N, Newgard CB, Mandarino L, Cefalu W, Walder K, Collier GR, Hulver MW, Smith SR, Ravussin E. Regulation of skeletal muscle oxidative capacity and insulin signaling by the mitochondrial rhomboid protease PARL. Cell Metab. 2010;11(5):412–426. doi: 10.1016/j.cmet.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke GM, Anderson CA, Pettersson FH, Cardon LR, Morris AP, Zondervan KT. Basic statistical analysis in genetic case-control studies. Nat. Protoc. 2011;6(2):121–133. doi: 10.1038/nprot.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaneau O, Zagury JF, Marchini J. Improved whole-chromosome phasing for disease and population genetic studies. Nat. Methods. 2013;10(1):5–6. doi: 10.1038/nmeth.2307. [DOI] [PubMed] [Google Scholar]
- Gebhardt S, Theisen FM, Haberhausen M, Heinzel-Gutenbrunner M, Wehmeier PM, Krieg JC, Kuhnau W, Schmidtke J, Remschmidt H, Hebebrand J. Body weight gain induced by atypical antipsychotics: an extension of the monozygotic twin and sib pair study. J. Clin. Pharm. Ther. 2010;35(2):207–211. doi: 10.1111/j.1365-2710.2009.01084.x. [DOI] [PubMed] [Google Scholar]
- Genomes Project, C. Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA, Abecasis GR. A global reference for human genetic variation. Nature. 2015;526(7571):68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves VF, Zai CC, Tiwari AK, Brandl EJ, Derkach A, Meltzer HY, Lieberman JA, Muller DJ, Sun L, Kennedy JL. A hypothesis-driven association study of 28 nuclear-encoded mitochondrial genes with antipsychotic-induced weight gain in schizophrenia. Neuropsychopharmacology. 2014;39(6):1347–1354. doi: 10.1038/npp.2013.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves VF, S W, Crowley JJ, Vawter M, Saxena R, Bulik CM, Yilmaz Z, Hultman C, Sklar P, Kennedy JL, Sullivan PF, Knight J. Examining the Role of Common and Rare Mitochondrial Variants in Schizophrenia (under review) 2017 doi: 10.1371/journal.pone.0191153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Cai Q, Samuels DC, Ye F, Long J, Li CI, Winther JF, Tawn EJ, Stovall M, Lahteenmaki P, Malila N, Levy S, Shaffer C, Shyr Y, Shu XO, Boice JD., Jr The use of next generation sequencing technology to study the effect of radiation therapy on mitochondrial DNA mutation. Mutat. Res. 2012;744(2):154–160. doi: 10.1016/j.mrgentox.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie B, Fuchsberger C, Stephens M, Marchini J, Abecasis GR. Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nat. Genet. 2012;44(8):955–959. doi: 10.1038/ng.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionita-Laza I, Lee S, Makarov V, Buxbaum JD, Lin X. Sequence kernel association tests for the combined effect of rare and common variants. Am. J. Hum. Genet. 2013;92(6):841–853. doi: 10.1016/j.ajhg.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan SD, Konner AC, Bruning JC. Sensing the fuels: glucose and lipid signaling in the CNS controlling energy homeostasis. Cell. Mol. Life Sci. 2010;67(19):3255–3273. doi: 10.1007/s00018-010-0414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn RS, Sommer IE, Murray RM, Meyer-Lindenberg A, Weinberger DR, Cannon TD, O'Donovan M, Correll CU, Kane JM, van Os J, Insel TR. Schizophrenia. Nature reviews. Disease primers. 2015;1:15067. doi: 10.1038/nrdp.2015.67. [DOI] [PubMed] [Google Scholar]
- Kanabus M, Shahni R, Saldanha JW, Murphy E, Plagnol V, Hoff WV, Heales S, Rahman S. Bi-allelic CLPB mutations cause cataract, renal cysts, nephrocalcinosis and 3-methylglutaconic aciduria, a novel disorder of mitochondrial protein disaggregation. J. Inherit. Metab. Dis. 2015;38(2):211–219. doi: 10.1007/s10545-015-9813-0. [DOI] [PubMed] [Google Scholar]
- Kloss-Brandstatter A, Weissensteiner H, Erhart G, Schafer G, Forer L, Schonherr S, Pacher D, Seifarth C, Stockl A, Fendt L, Sottsas I, Klocker H, Huck CW, Rasse M, Kronenberg F, Kloss FR. Validation of next-generation sequencing of entire mitochondrial genomes and the diversity of mitochondrial DNA mutations in oral squamous cell carcinoma. PLoS One. 2015;10(8):e0135643. doi: 10.1371/journal.pone.0135643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw CA, Mooij JM, Heskes T, Posthuma D. MAGMA: generalized gene-set analysis of GWAS data. PLoS Comput. Biol. 2015;11(4):e1004219. doi: 10.1371/journal.pcbi.1004219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lett TA, Wallace TJ, Chowdhury NI, Tiwari AK, Kennedy JL, Muller DJ. Pharmacogenetics of antipsychotic-induced weight gain: review and clinical implications. Mol. Psychiatry. 2012;17(3):242–266. doi: 10.1038/mp.2011.109. [DOI] [PubMed] [Google Scholar]
- Li B, Krishnan VG, Mort ME, Xin F, Kamati KK, Cooper DN, Mooney SD, Radivojac P. Automated inference of molecular mechanisms of disease from amino acid substitutions. Bioinformatics. 2009;25(21):2744–2750. doi: 10.1093/bioinformatics/btp528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N. Engl. J. Med. 2005 Sep 22;353(12):1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- Maciukiewicz M, V M, Tiwari AK, Fonseka TM, Freeman N, Kennedy JL, Rotzinger S, Foster JA, Kennedy SH, Muller DJ. Genome-wide association studies of placebo and duloxetine response in major depressive disorder. The pharmacogenomics journal. 2017 doi: 10.1038/tpj.2017.29. (in press) [DOI] [PubMed] [Google Scholar]
- MacNeil RR, Muller DJ. Genetics of common antipsychotic-induced adverse effects. Molecular neuropsychiatry. 2016;2(2):61–78. doi: 10.1159/000445802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuffin P, Owen MJ, Farmer AE. Genetic basis of schizophrenia. Lancet. 1995;346(8976):678–682. doi: 10.1016/s0140-6736(95)92285-7. [DOI] [PubMed] [Google Scholar]
- Morrison WGJ. Quanto 1.1: a computer program for power and sample size calculations for genetic-epidemiology studies. Internet 2006 [Google Scholar]
- Muller DJ, Kennedy JL. Genetics of antipsychotic treatment emergent weight gain in schizophrenia. Pharmacogenomics. 2006;7(6):863–887. doi: 10.2217/14622416.7.6.863. [DOI] [PubMed] [Google Scholar]
- Muller DJ, Chowdhury NI, Zai CC. The pharmacogenetics of antipsychotic-induced adverse events. Curr. Opin. Psychiatry. 2013;26(2):144–150. doi: 10.1097/YCO.0b013e32835dc9da. [DOI] [PubMed] [Google Scholar]
- Nasrallah CM, Horvath TL. Mitochondrial dynamics in the central regulation of metabolism. Nature reviews. Endocrinology. 2014;10(11):650–658. doi: 10.1038/nrendo.2014.160. [DOI] [PubMed] [Google Scholar]
- Nyholt DR. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am. J. Hum. Genet. 2004;74(4):765–769. doi: 10.1086/383251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L, Freitas F, Fernandes V, Pereira JB, Costa MD, Costa S, Maximo V, Macaulay V, Rocha R, Samuels DC. The diversity present in 5140 human mitochondrial genomes. Am. J. Hum. Genet. 2009;84(5):628–640. doi: 10.1016/j.ajhg.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L, Soares P, Radivojac P, Li B, Samuels DC. Comparing phylogeny and the predicted pathogenicity of protein variations reveals equal purifying selection across the global human mtDNA diversity. Am. J. Hum. Genet. 2011;88(4):433–439. doi: 10.1016/j.ajhg.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira A, Martin MV, Rollins B, Moon EA, Bunney WE, Macciardi F, Lupoli S, Smith EN, Kelsoe J, Magnan CN, van Oven M, Baldi P, Wallace DC, Vawter MP. Mitochondrial mutations and polymorphisms in psychiatric disorders. Front. Genet. 2012;3:103. doi: 10.3389/fgene.2012.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari AK, Brandl EJ, Zai CC, Goncalves VF, Chowdhury NI, Freeman N, Lieberman JA, Meltzer HY, Kennedy JL, Muller DJ. Association of orexin receptor polymorphisms with antipsychotic-induced weight gain. World. J. Biol. Psychiatry. 2016;17(3):221–229. doi: 10.3109/15622975.2015.1076173. [DOI] [PubMed] [Google Scholar]
- Ucok A, Gaebel W. Side effects of atypical antipsychotics: a brief overview. World psychiatry: official journal of the World Psychiatric Association. 2008;7(1):58–62. doi: 10.1002/j.2051-5545.2008.tb00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. Mitochondrial DNA sequence variation in human evolution and disease. Proc. Natl. Acad. Sci. U. S. A. 1994;91(19):8739–8746. doi: 10.1073/pnas.91.19.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. A mitochondrial bioenergetic etiology of disease. J. Clin. Invest. 2013;123(4):1405–1412. doi: 10.1172/JCI61398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissensteiner H, Forer L, Fuchsberger C, Schopf B, Kloss-Brandstatter A, Specht G, Kronenberg F, Schonherr S. mtDNA-server: next-generation sequencing data analysis of human mitochondrial DNA in the cloud. Nucleic Acids Res. 2016;44(W1):W64–W69. doi: 10.1093/nar/gkw247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia. 2000;43(12):1498–1506. doi: 10.1007/s001250051560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.