Abstract
Migraine and Bipolar Disorder (BPAD) are clinically heterogeneous disorders of the brain with a significant, but complex, genetic component. Epidemiological and clinical studies have demonstrated a high degree of co-morbidity between migraine and BPAD. Several genome-wide linkage studies in BPAD and migraine have shown overlapping regions of linkage on chromosomes, and two functionally similar voltage-dependent calcium channels CACNA1A and CACNA1C have been identified in familial hemiplegic migraine and recently implicated in two whole genome BPAD association studies, respectively. We hypothesized that using migraine co-morbidity to look at subsets of BPAD families in a genetic linkage analysis would prove useful in identifying genetic susceptibility regions in both of these disorders. We used BPAD with co-morbid migraine as an alternative phenotype definition in a re-analysis of the NIMH Bipolar Genetics Initiative wave 4 data set. In this analysis we selected only those families in which at least two members were diagnosed with migraine by a doctor according to patients' reports. Nonparametric linkage analysis performed on 31 families segregating both BPAD and migraine identified a linkage signal on chromosome 4q24 for migraine (but not BPAD) with a peak LOD of 2.26. This region has previously been implicated in two independent migraine linkage studies. In addition we identified a locus on chromosome 20p11 with overlapping elevated LOD scores for both migraine (LOD = 1.95) and BPAD (LOD = 1.67) phenotypes. This region has previously been implicated in two BPAD linkage studies, and, interestingly, it harbors a known potassium dependant sodium/calcium exchanger gene, SLC24A3, that plays a critical role in neuronal calcium homeostasis. Our findings replicate a previously identified migraine linkage locus on chromosome 4 (not co-segregating with BPAD) in a sample of BPAD families with co-morbid migraine, and suggest a susceptibility locus on chromosome 20, harboring a gene for the migraine/BPAD phenotype. Together these data suggest that some genes may predispose to both bipolar disorder and migraine.
Keywords: Migraine, Bipolar disorder, Linkage, Chromosome 4q24, Chromosome 20p11
1. Introduction
Bipolar affective disorder (BPAD [MIM 125480]) is a severe psychiatric illness affecting about 1% of the population with significant morbidity and mortality (World Health Organization, World Health Report, 2002). It is characterized by excessive shifts of mood, including periods of depression and mania. Family, twin and adoption studies strongly indicate a genetic basis for BPAD with heritability of at least 60% (Craddock and Jones, 1999; Smoller and Finn, 2003), multiple genomic regions have been implicated in linkage studies (Craddock and Forty, 2006), though replication has been inconsistent (Segurado et al., 2003). The search for susceptibility genes for BPAD clearly depends on appropriate definitions of the phenotype, and sub-grouping patients with BPAD according to clinical symptoms, long-term course, treatment response or co-morbid psychiatric conditions has been suggested as a fruitful approach for further genetic studies in BPAD (McQueen et al., 2005). However, the correct recognition of the whole clinical spectrum of bipolarity (all genetic predispositions that may in some way produce a BPAD phenotype) may even include a broader range of conditions such as common co-morbid disorders like alcohol and substance abuse, ADHD or migraine headaches.
Migraine (MIM 157300) is a complex, disabling disorder of the brain, characterised by recurrent headache, nausea, emesis, phonophobia and photophobia (Davidoff, 2002). The prevalence of migraine is approximately 18% in women, 6% in men, and 4% in children (Silberstein and Goadsby, 2002). On the basis of clinical manifestations, the International Headache Society (Headache Classification Committee, 2004) has classified migraine into two main subtypes: migraine with aura (MA) and migraine without aura (MO), the latter being three times more common than MA. Migraine prevalence varies by age, sex, ethnic origin, and income. After puberty, prevalence increases more in girls than in boys. Prevalence increases until about age 40 years, then declines. Prevalence is highest in ethnic Caucasians and decreases as household income increases (Silberstein, 2004). Migraine seriously influences quality of life, and the WHO positions migraine among the world's most disabling medical illnesses.
Family and twin studies suggest a significant genetic component in migraine and heritability is estimated between 40 and 65% with MA being the more heritable subtype (Honkasalo et al., 1995; Larsson et al., 1995; Gervil et al., 1999). However, there is a considerable clinical heterogeneity in patients within the headache continuum and even across patients diagnosed with the same sub-type of migraine headaches (i.e. headache severity and frequency) (Anttila et al., 2006). Although family-based linkage studies have identified several chromosomal regions linked to common forms of migraine, there has been little consistency between these studies, and no genes predisposing to the common forms of migraine have been identified (Gardner et al., 1997; Nyholt et al., 1998a,b, 2000; Jones et al., 2001; Carlsson et al., 2002; Wessman et al., 2002; Lea et al., 2002; Björnsson et al., 2003; Soragna et al., 2003; Cader et al., 2003; Nyholt et al., 2005; Russo et al., 2005; Lea et al., 2005; Anttila et al., 2006). However, the detection of three genetic loci for a rare form of familial hemiplegic migraine with a Mendelian inheritance form (FHM1–3, MIMs 141500, 602481, and 609634, respectively) has given a successful model for the identification of migraine associated cellular mechanisms. So far, variants within three genes (two ion channel genes, CACNA1A (MIM 601011) and SCN1A (MIM 182389), and one encoding an ATP-exchanger, ATP1A2 (MIM 182340)) have been found to co-segregate with FHM (Ophoff et al., 1996; Dichgans et al., 2005; De Fusco et al., 2003). All three genes are implicated in ion-transport, and consequences of the mutations can lead to increased extra cellular glutamate and potassium levels, most likely affecting neuronal membrane thresholds and causing hyperexcitability of neurons (Van de Ven et al., 2007). Since the aura and headache symptoms in typical migraine and FHM (except for the hemi-paresis) are similar and most patients with FHM also have typical migraine, FHM is considered a valid model to study molecular mechanisms in the common forms of migraine (Ferrari and Goadsby, 2006) and possibly elucidate the genetic underpinning of migraine.
The co-morbidity between migraine and bipolar disorders is well documented in numerous clinical and epidemiological studies and there are clearly pathophysiological similarities (Endicott, 1989; Merikangas et al., 1990; Breslau and Davis, 1993; Mahmood et al., 1999; Fasmer, 2001; Hirschfeld et al., 2003; Low et al., 2003; Oedegaard and Fasmer, 2005; McIntyre et al., 2006; Dilsaver et al., 2009). Both migraine and bipolar disorders have been linked to disturbances in the serortonergic (Silberstein, 1994; Hargreaves and Shepheard 1999; Hamel, 2007; Mahmood and Silverstone, 2001), dopaminergic (Peroutka, 1997; Emilien et al., 1999) and glutaminergic systems (Vaccaro et al., 2007; Goodwin and Jamison, 2007). Furthermore, there are long-standing observations of alterations in ion distribution in patients with mood disorders (Akiskal and McKinney, 1973), and substantial evidence points towards alterations in sodium and calcium ion-channels as central factors for understanding the pathophysiology of migraine (Pietrobon, 2007; Wessman et al., 2007; Moskowitz et al., 2004; Van de Ven et al., 2007 and bipolar disorders (El-Mallakh and Wyatt, 1995; Rose et al., 1998; El-Mallakh et al., 2003; Askland and Parsons, 2006; Kato, 2008). Also, several pharmacological treatments are successful in the prevention of both disorders, most notably valproate (Mathew et al., 1995; Silberstein, 1996; Bowden et al., 2000), but also lamotrigine and lithium may also, beyond the established effectiveness in bipolar disorder (Bowden, 2002; Bowden et al., 2000), be valuable in migraine treatment (Lampl et al., 2005; Medina and Diamond, 1981).
Hirschfeld et al. (2003) found that the prevalence of migraine was twice as high in persons with bipolar disorder (BPAD I and BPAD II) compared to the general population (15.2% vs. 7%) in an Epidemiological US survey (n = 85358). In a Canadian Community Health Survey (n = 36 984), McIntyre et al. (2006), found that the prevalence of migraine was 24.8% in BPAD I compared to 10.3% in the general population. In addition, they described patients with the BPAD/migraine phenotype as being more severely impaired. Mahmood et al. (1999) found that the prevalence of migraine in BPAD in patients from a mood disorder clinic (n = 117) was 26%, and these patients also seemed to be affected by a more severe variant of the disorder. Fasmer (2001) studied a sample of consecutively admitted inpatients with a current mood disorder (n = 62) and found a strikingly high prevalence of migraine in patients with BPAD II (77%). This finding was later replicated by Low et al. (2003) in a sample of outpatients with bipolar disorder (n = 108), where the prevalence of migraine in the combined bipolar group (BPAD I + II) was 39.7%, with the clearly highest prevalence in the BP II group (64.7%). Recently, Dilsaver et al. (2009), in a study of Latino adults (n = 163) were able to show that having a family history of BPAD is associated with an increased risk of having migraine headache in patients with BPAD. On the other hand, no such association was linked to a family history of unipolar disorder.
The diagnosis and classification of migraine and BPAD are based on the fulfilment of symptomatic criteria, defined by the International Headache Society (IHS) and DSM-IV respectively, and in both cases diagnoses consists of a combination of traits and/or symptoms defining a very broad syndrome. Both migraine and BPAD are disorders where diagnoses are characteristically based on the patient's description of symptoms and no objective, quantitative findings can be measured by laboratory—or visualized by imaging-techniques. Identifying traits or sub-groups that are closer to the molecular background of the disorder than the clinical classification would therefore be particularly helpful in these disorders. Co-morbid migraine is a phenomenon that appears to cluster in BPAD families. It has been suggested that families with different subphenotypes of affective characteristics may have different genetic etiologies, and clinical phenomena such as psychosis, mixed states, rapid cycling, panic disorder, alcoholism, and suicide attempts are considered subphenotypes in genetic BPAD studies (MacQueen et al., 2005). Co-morbid migraine could in the context of BPAD be either a subphenotype (illness-related phenomenon) or even an endophenotype (independent of the illness state), since there could be a basic derangement responsible both for the short-lasting, episodic phenomena seen in patient with migraine and bipolar disorder (migraine, panic attacks, hypomania, mania) and for the longer-lasting disturbances (major depression, affective temperaments), possibly related to an underpinning common genetic disturbance. In summary, since migraine and BPAD both are disorders of the brain with several pathophysiological similarities and a high degree of co-morbidity, we hypothesized that the BPAD/migraine pattern could be a meaningful alternative phenotype/endophenotype for the identification of genetic susceptibility in a genome wide linkage study.
2. Materials and methods
2.1. Subjects
Families for these analyses derived from wave 4 of the NIMH Genetics Initiative for Bipolar Disorder, a multi-center collaborative effort with patient recruitment at 10 sites. Families were first identified through a sib pair with bipolar I disorder, and other first degree relatives were recruited according to availability for the collection of phenotypic and genotypic data for each individual in the family.
Subjects were interviewed with the Diagnostic Interview for Genetic Studies (DIGS) (Nurnberger et al., 1994). At each site, diagnoses were determined through a best-estimate process involving review by two clinicians who did not participate in the interview. We used two models to define BPAD affectation status: Bipolar Narrow, which was defined as BPAD-I, schizoaffective, bipolar-type (SA-BP), or BPAD-II, and Bipolar Broad, which was defined as Bipolar Narrow plus recurrent unipolar depressive disorder (RUDD).
In our linkage analyses, we only included those families in which at least two family members had been diagnosed with migraine by a doctor according to patient report (see migraine phenotype definition), resulting in a sample of 31 families with sufficient phenotypic and genotypic information.
2.2. Migraine phenotype definition
Information regarding migraine was collected from the patients during the clinical interview. Patients were first asked whether they suffered from migraine headaches, and subsequently whether the diagnosis of migraine had been confirmed by a medical doctor. Based on these answers we grouped patients into two groups: Migraine Narrow, which included all patients who had a diagnosis that had been confirmed by a medical doctor, and Migraine Broad, which included Migraine Narrow plus self-reported migraine.
2.3. Co-morbid BPAD/migraine models
In addition to classifying subjects into the BPAD Broad/Narrow and Migraine Broad/Narrow groups, we also identified the individuals who had both BPAD and migraine (either Broad or Narrow) and classified them as into BP-Migraine-Broad (BP-Broad and any migraine diagnosis) or BP-Migraine-Narrow (BP-Broad and migraine diagnosed by a medical doctor) for the chromosomes that showed suggestive linkage for any of the migraine or BPAD-phenotypes. The purpose of this was to observe whether linkage signals (in these BPAD/migraine loaded families) would be driven by the individuals with BPAD or those with migraine or by those who had both disorders.
2.4. Analysis
Genotyping of the wave 4 sample was performed at the Center for Inherited Disease Research (CIDR) through the use of automated fluorescent microsatellite analysis with an ABI 3700 Sequencer and a modification of the Cooperative Human Linkage Center (CHLC) version 9 marker set. A total of 404 microsatellite markers with an average spacing of 9 cM and average heterozygosity of 0.76 were available for analysis following cleaning procedures performed prior to data release to correct Mendelian inheritance errors.
Nonparametric linkage analysis methods were employed using MERLIN (v.1.1.2; Abecasis et al., 2002) to analyze the extent of allele sharing among all affected relative pairs. Marker allele frequencies were estimated among all individuals, the default in MERLIN. Marker order and map positions were obtained from a modified version of the deCODE genetic map (Kong et al., 2004; Nievergelt et al., 2004. Nonparametric linkage (NPL) scores were computed under a linear model and the pairs function at a 1-cM interval across the chromosome starting at the first marker. NPL scores were converted using the Kong-and-Cox function (Kong and Cox, 1997).
These analyses were performed for the Bipolar Narrow, Bipolar Broad, Migraine Narrow and Migraine Broad phenotypes for all 22 chromosomes and the X-chromosome.
Merlin was also used to conduct simulation analyses to estimate the genome-wide significance of the highest linkage peaks in the study. This method involves gene dropping simulations to replace the input data with simulated chromosomes that maintain the family structure, marker spacing, allele frequencies, and missing data patterns present in the original study. 1000 simulations of the entire chromosomes were performed for each phenotype, and nonparametric linkage analyses were run for each of these simulated data sets. Using the traditional method, we recorded the fraction of times a simulated LOD score exceeded the actual LOD score and divided by the number of simulations performed. Genome-wide simulation analyses did not alter the LOD scores or p-values reported in the result section.
3. Results
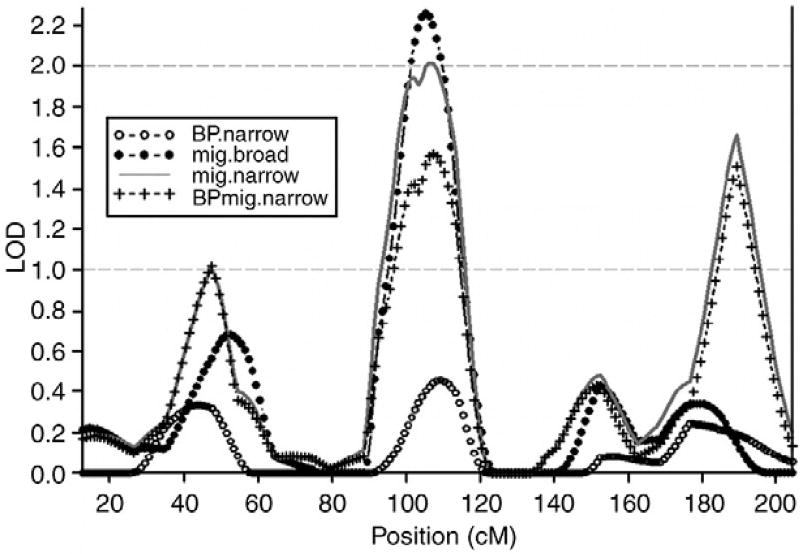
Genome-wide nonparametric linkage analysis was performed for four phenotypes in the 31 families segregating both migraine and bipolar disorder: BP-Narrow, BP-Broad, Migraine Narrow and Migraine Broad. In addition we analysed the BP-Migraine-Broad and BP-Migraine-Narrow phenotypes for the chromosomes with suggestive evidence of linkage for any of the migraine or BPAD phenotypes. Tables 1 and 2 provide a description of the subjects considered affected under these different phenotypic models, all of which had genotypic data and were thus informative for linkage analysis. The results of the genome wide scan revealed the highest linkage peak for the Migraine-Broad phenotype at 105 cM on chromosome 4 with a LOD score of 2.26 (empirical P = 0.001). The nearest marker to this peak is D4S1647 at 103.8 cM and 99.9 Mb. The highest LOD score for the Migraine-Narrow phenotype was seen at the same position (106 cM) with a LOD of 2.02 (empirical P = 0.002). The BP phenotypes did not show linkage to this region and when studying the comorbid BP-Migraine-Broad and BP-Migraine-Narrow groups the LOD scores dropped to 1.68 (empirical P = 0.004) and 1.57 (empirical P = 0.003) respectively, indicating that the linkage signal on chromosome 4 is driven by the migraine phenotype, independently from the BPAD. A region of 20 cM (95 cM to 115 cM) showed LOD scores >1.00 distributed around the maximum LOD at 105 cM, flanked by the markers D4S2361 and D4S2623. This region has previously been significantly linked to migraine in two independent samples from Finland (Wessman et al., 2002) and Iceland (Björnsson et al., 2003), suggesting that our results represent a significant replication of a migraine locus on chromosome 4q24. In an analysis of linkage within individual families, we found that all but 4 of the 31 families had a LOD score >0.2 for both migraine phenotypes at the position around 105 cM (±5 cM), indicating that the linkage signal derives from a common genetic effect shared by most of the families in the sample, increasing the likelihood that this is indeed a migraine related finding.
Table 1.
The study sample: numbers by phenotype definitions.
| Total number of bipolar families with at least two family members with doctor diagnosed migraine | 31 |
| Total number of individuals | 201 |
| Total number of sib-pairs | 105 |
| Number of sib-pairs with BP-Narrow (BPI + SA-BP + BPII) | 98 |
| Number of sib-pairs with BP-Broad (BP-Narrow + RUDD) | 110 |
| Number of sib-pairs with Migraine-Narrow (Migraine diagnosed by MD) | 71 |
| Number of sib-pairs with Migraine-Broad (Migraine-Narrow + self-reported migraine) | 89 |
Table 2.
Co-segregation of migraine and bipolar disorder in the study sib-pairs (n = 105).
| Information regarding diagnosis is missing in one sib |
Only one sib has both diagnoses |
Both sibs have both diagnoses |
|
|---|---|---|---|
| BP-Broad and Migraine Broad | 24 | 50 | 40 |
| BP-Broad and Migraine Narrow | 45 | 58 | 23 |
| BP-Narrow and Migraine Broad | 17 | 49 | 43 |
| BP-Narrow and Migraine Narrow | 38 | 57 | 26 |
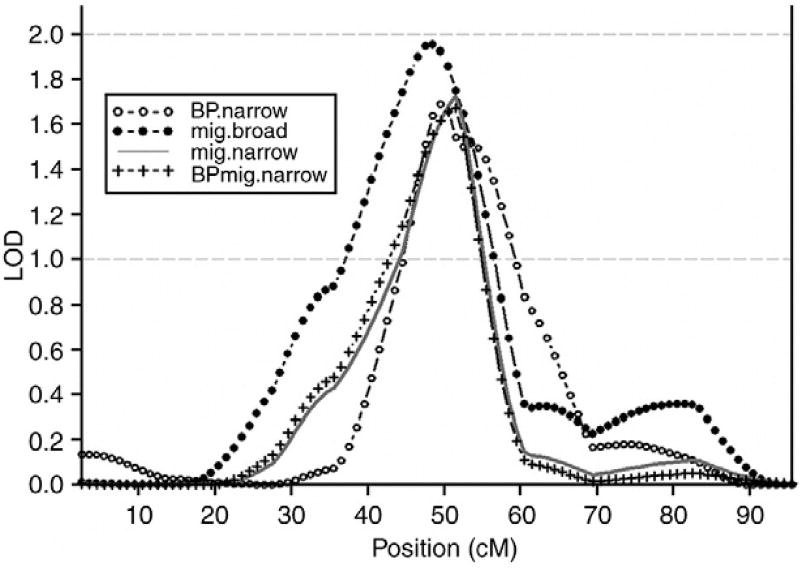
The only other suggestive migraine linkage locus was found at chromosome 20 with a peak between markers D20S470 (44.4 cM, 17.4 Mb) and D20S477 (51.9 cM, 22.4 Mb). The highest LOD score was achieved for the Migraine-Broad phenotype with a LOD of 1.95 (empirical P = 0.002) at 48 cM. Interestingly, this locus also showed nominal linkage for the Migraine-Narrow (LOD = 1.72, empirical P = 0.001 at 51 cM), BP-Narrow (LOD = 1.69, empirical P = 0.001 at 49 cM), BP-Broad (LOD = 1.60, empirical P = 0.003 at 49 cM), BP-Migraine-Broad (LOD = 1.78, empirical P= 0.002 at 48 cM) and BP-Migraine-Narrow (1.67, empirical P = 0.002 at 52 cM) phenotypes suggesting this locus might enclose a genetic overlap in migraine and BPAD. An analysis of linkage within each family showed that 12 out of 31 families had a LOD score >0.2 for both a migraine and a BPAD phenotype at the position around 50 cM (±5 cM), indicating that the linkage signal derives not from a strong signal involving only a few families, but more likely from several families with shared phenotypic and genotypic presentations.
Since the results regarding the BP-Broad, BP-Narrow, BP-Migraine Broad and BP-Migraine Narrow were very similar, only the BP-Narrow and BP-migraine Narrow phenotypes are represented in Figs. 1 and 2, along with the Migraine-Broad and Migraine Narrow phenotypes.
Fig. 1.
The linkage signal on chromosome 4 is a migraine signal. BP.narrow: BPAD I, schizoaffective, bipolar-type (SA-BP), BPAD II. Mig.broad: Migraine self-diagnosed. Mig.narrow: Migraine diagnosed by MD. BPmig.narrow: BPAD, schizoaffective, bipolar-type (SA-BP), BPAD II, RUDD + Migraine diagnose by MD.
Fig. 2.
The linkage signal on chromosome 20 is both a BPAD and migraine signal. BP.narrow: BPAD I, schizoaffective, bipolar-type (SA-BP), BPAD II. Mig. broad: Migraine self-diagnosed. Mig.narrow: Migraine diagnosed by MD. BPmig.narrow: BPAD, schizoaffective, bipolar-type (SA-BP), BPAD II, RUDD + Migraine diagnose by MD.
We also replicated previous evidence of migraine linkage to the X chromosome (Xq24–28) (Nyholt et al., 2000) for our Migraine-Narrow phenotype with a modest LOD score of 1.60 at 156–158 cM (empirical P = 0.003, nearest marker DXS9908). The Migraine-Broad, BP or BP-Migraine phenotypes did not show linkage to the X chromosome. Statistically significant or suggestive linkage was not observed in any other chromosomal region for any of the Migraine, BP phenotypes.
4. Discussion
To our knowledge this is the first genetic study examining the well characterized co-morbidity between migraine and BPAD. We therefore conducted a literature review identifying overlapping linkage findings in migraine and BPAD studies, and these results are displayed in Table 3. As shown here, most reported linkage findings in migraine overlap with reported linkage findings in BPAD. Furthermore, there are BPAD linkage findings that overlap with all the identified genes for FHM (Table 4). At present, several loci, 4q21–q24 (Wessman et al., 2002; Björnsson et al., 2003), 5q21 (Lea et al., 2005), 6p12.2–p21.1 (Carlsson et al., 2002), 11q24 (Cader et al., 2003), 14q21.2–q22.3 (Soragna et al., 2003) with significant evidence of linkage to common forms of migraine have been reported in genome wide linkage analysis, with only the chromosome 4q21–q24 region detected in two independent studies. We here report a replication of the migraine locus on 4q24 in a sample of patients selected for genetic studies of bipolar disorder. Although our sample size was small (31 families, n = 202) the results reached significant evidence for replication of previous findings of linkage for both our migraine models: Migraine Broad (LOD = 2.26) and Migraine Narrow (LOD = 2.02). The nearest marker, D4S1647 (103.8 cM), is the same marker that gave the highest LOD score (4.20) in the Finnish study (Wessman et al., 2002). This study was a genome wide screen of 50 multigenerational, clinically well-defined Finnish families showing intergenerational transmission of MA and included a total of 646 individuals, so they reported the locus at 4q24 as a migraine with aura finding. Soon after, an overlapping locus in a genome wide linkage analysis of patients (103 families, with 289 affected and 518 relatives) with migraine without aura (MO) was reported from Iceland (4q21 (LOD = 2.05; P = 0.001) (Björnsson et al., 2003), suggesting this locus is containing a gene predisposing to typical migraine that contributes to both MA and MO. We have no phenotypic information regarding MA/MO in our study since migraine diagnosis was based on patient's reports. Although we only included families where at least two members reported that their migraine diagnosis had been verified by a medical doctor, this diagnostic approach clearly represents a departure from the ideal. Nonetheless, we find it interesting that we were able to replicate the best documented migraine linkage locus, even when using our simplified migraine diagnosis. The bipolar phenotypes did not show linkage to this region, suggesting that this region harbours a gene for migraine that does not co-segregate with bipolar disorder. Interestingly, Bjornsson and colleagues found that the linkage evidence for this region increased when analyzing females only (LOD = 4.08; P = 7.2 × 10−6), indicating that this region harbours a gene that causes migraine in women. It is well documented that migraine at the population level is about three times more prevalent in women than in men (Silberstein and Goadsby, 2002). However, several studies have found a similar proportion of migraine in men and women with bipolar disorder ((Mahmood et al., 1999): male (25%) and female (27%);(Low et al., 2003): male (31%) and female (44%)), indicating that the prevalence of migraine, in the presence of BPAD, increases more in men than in females. If the migraine locus on 4q21–24 is indeed a female migraine locus it would not be suspected to co-segregate with BPAD since co-morbid migraine in BPAD seems to be gender independent.
Table 3.
Chromosomal regions implicated in migraine linkage studies and findings from bipolar linkage studies that are in close proximity to these regions.
| Migraine linkage studies (year, study description) |
Chromosomal regions implicated in typical migraine linkage studies (study, LOD score/ chromosomes/regions (Mb)) |
Bipolar linkage studies overlapping with regions implicated in typical migraine linkage studies (chromosomes/regions (Mb), study, LOD score) |
BPD linkage studies (year, study description) |
|
|---|---|---|---|---|
| Ligthart et al. (2008) Genome wide 12210 individuals, Dutch. | Nyholt et al. (2005), LOD: 1.53 | 1q23 (160) | 1q42 (232), Hamshere et al. (2005), LOD: 3.54 | Savitz et al. (2007) 9 candidate loci, 47 BPD pedigrees (n = 350). Caucasians of Afrikaner and British origin |
| Anttila et al. (2008) Genome wide, 1675 individuals, Finland and Australia. | Lea et al. (2002), LOD: 3.36 | 1q31 (205) | 1q41 (212), Macgregor et al. (2004), LOD: 2.77 | Jamra et al. (2007) Genome wide interaction scan, 52 BPD families, (n = 448). |
| Wessman et al. (2002), LOD: 1.70 | 1q42 (230) | 1q42 (247), Curtis et al. (2003), LOD: 2.0 | Spanish, Bulgarian and Romany. | |
| Gardner et al. (1997), LOD: 3.51 | 1q31 (205) | 1q25–32 (207), Detera-Wadleigh et al. (1999), LOD: 2.67 | ||
| 1q32 (210), Turecki et al. (1995) | ||||
| Anttila et al. (2006) Genome wide, 50 families MA, trait components, Finland (same as Wessman et al., 2002). | Lea et al. (2005), LOD: 2.28 | 3q29 (198) | 3q28 (193), Zandi et al. (2007), NPL: 2.4 | Zandi et al. (2007) Genome wide, 98 BPD families (n = 428). |
| 3q29 (196), Curtis et al. (2003), HLOD: 2.0 | Goes et al. (2007) Genome wide, mood-incongruent psychotic BPDs, 708 BPD families (n = 2899). NIMH. | |||
| 3q29 (197), Bailer et al. (2002), NPL: 3.74 | Cassidy et al. (2007) Genome wide, 60 BPD families, Ireland. | |||
| 3q27 (191), Kelsoe et al. (2001), LOD: 2.66 | Kerner et al. (2007) Genome wide, waves 1–4 NIMH, psychotic subtype. | |||
| Lea et al. (2005) Genome wide, 92 families, LCA-severe; Australia. | Anttila et al. (2006), HLOD: 4.52 | 4q24 (101) | 4q21 (81), Cassidy et al. (2007), NPL: 2.33 | Jones et al. (2007) Genome wide, 36 BPD families with puerperal psychosis, welcome trust, UK–Irish. |
| Anttila et al. (2006), HLOD: 2.99 | 4q24 (99) | 4q25–q31 (146), Schumacher et al. (2005), NPL: 2.89 | Etain et al. (2006) Genome wide, 87 BPD 1 Sib-pairs with early-AAO. European Collaboratory Study. | |
| Bjørnsson et al. (2003), LOD: 2.05 | 4q28 (130) | 4q12–q21 (70), Lambert et al. (2005), LOD: 3.30 | ||
| Russo et al. (2005) Microsatellite markers of 15q11–q13, 10 families, MA, Italy. | Bjørnsson et al. (2003), LOD: 2.05 | 4q21 (85) | 4p14 (40), Lambert et al. (2005), LOD: 2.17 | |
| Bjørnsson et al. (2003), LOD: | 4p16 (7) | 4p16 (7), Ewald et al. (1998) | Marcheco-Teruel et al. (2006) Genomwide, 6 generation BPD pedigree, Cuba. | |
| Wessman et al. (2002), LOD: 4.20 | 4q24 (99) | 4p16 (11), Ginns et al. (1998), NPL: 4.05 | ||
| 4q23 (99), Detera-Wadleigh et al. (1997) | Tomas et al. (2006) Scan of chr. 17 and 18, 12 BPD families, Baleric Islands. | |||
| 4p16 (7), Blackwood et al. (1996) | ||||
| Nyholt et al. (2005) Genome wide, 756 twin families, LCA-severe, Australia. | Nyholt et al. (2005), LOD: 3.70 | 5q22.1 (110) | Mukherjee et al. (2006) Scan of chr. 18, 211 families with psychosis (BPD or Scz.), India. | |
| Cader et al. (2003) Genomwide, 43 families, MA, Canada. | Nyholt et al. (2005), LOD: 1.22 | 6q16 (94) | 6p22 (20), Cassidy et al. (2007), NPL: 1.88 | Schumacher et al. (2005) Genome wide, 52 BPD families (n = 448). Spanish, Bulgarian and Romany. |
| Carlsson et al. (2002), LOD 5.4 | 6p12.2–21.1 (47) | 6q16.3–22.1(104–112), Dick et al. (2003), LOD: 2.2 | Hamshere et al. (2005) Genome wide, 24 BPD-SCZ pedigrees (n = 68). White from United Kingdom or Ireland. | |
| Soragna et al. (2003) Genome wide, 1 family, MO, Italy. | Nyholt et al. (2005), LOD: 1.77 | 8q23 (93) | 8q21 (87), Kerner et al. (2007) | Lambert et al. (2005) Genome wide, 135 ASP families, Ireland and UK. |
| 8q24 (131), Jones et al. (2007), LOD: 2.03 | McQueen et al. (2005) 11 BPD genomewide linkage scans, 1067 families, North America, Europe, Israel. | |||
| 8q24 (140), Macgregor et al. (2004), LOD: 1.53 | ||||
| 8q24 (134), McQueen et al. (2005), LOD: 3.40 | ||||
| Bjørnsson et al. (2003) Genome wide, 103 families, MO, Iceland. | Anttila et al. (2008), LOD:7.68 and 3.50 | 10q22–23 (82–85) | 10q23 (92), Savitz et al. (2007), NPL: 2.01 | Kealey et al. (2005) 13 markers spanning 14q, 49 BPD families, Ireland. |
| Anttila et al. (2006), HLOD: 2.2 | 10q22 (80) | 10q23 (95), Etain et al. (2006), NPL: 2.25 | Lin et al. (2005) Genome wide, AAO, wave 1–2, NIMH. | |
| Nyholt et al. (2005), LOD: 2.32 | 10q22 (80) | 10q22.–23 (78 cM), Marcheco-teruel et al. (2006), NPL: 9.91 | Macgregor et al. (2004) Genomwide, extended BPD sample (n = 229). | |
| Lea et al. (2002) 8 microsatelite markers spanning 33 cM of chr. 1q31, 3 + 82 families. | Cader et al. (2003), LOD: 5.6) | 11q24 (123) | 11q23, Turecki et al. (1995) | Middleton et al. (2004) Genome wide, 25 BPD families (n = 233). Portuguese |
| Fallin et al. (2004) Genome wide, 41 BPD Ashkenazi Jewish pedigrees. | ||||
| Wessman et al. (2002) Genome wide, 50 families, MA, Finland | Anttila et al. (2006), HLOD: 2.17 | 12q21 (89) | 12q24 (126), Cassidy et al. (2007), 2.20. | Dick et al. (2003) Genome wide, 245 BPD families, NIMH wave 3. |
| 12q24 (124), Curtis et al. (2003), HLOD: 2.8 | Curtis et al. (2003) Genomwide, 7 BPD pedigrees (n = 146). 2 British and 5 Icelandic families | |||
| 12q24 (125), Ewald et al. (2002), LOD: 3.42 | McInnis et al. (2003) Genome wide, 153 BPD pedigrees (n = 909). NIMH, 90% European American Ancestry | |||
| 12q21 (89), Morissette et al. (1999), NPL: 3.92 | ||||
| Carlsson et al. (2002) Genome wide, 1 family, MA, MO, Sweden | Nyholt et al. (2005), LOD: 1.63 | 13q21 (54) | 13q21–33 (92), Goes et al. (2007), LOD: 2.73 | Ewald et al. (2003) 1 BPD family, homozygous by descent, Denmark |
| 13q32 (97), Detera-Wadleigh et al. (1999), MLOD: 3.5 | Willour et al. (2003) Scan chr. 4, 7, 9, 18, 19, 20, 21. 56 BPD families, NIMH. | |||
| 13q31 (95), Kelsoe et al. (2001), LOD: 2.4 | ||||
| Jones et al. (2001) Six chromosome 19p13 markers, 16 families, MA, USA. | Soragna et al. (2003), LOD: 5.25 | 14q21.2–q22.3 (44–56) | 14q24 (69), Cassidy et al. (2007), NPL: 3.27 | Ekholm et al. (2003) Genome wide, 41 BPD families, Finland. |
| 14q21 (51 cM), Marcheco-Teruel et al. (2006), NPL: 3.54 | Ekholm et al. (2002) Genome wide, 41 BPD families, Finland. | |||
| 14q22 (54), Kealey et al. (2005), NPL: 2.72 | ||||
| Anttila et al. (2006), HLOD: 2.14 | 15q14 (32) | 15q13 (25), Edenberg et al. (1997), MOD: 2.37 | Bailer et al. (2002) Genomwide microsatellites, 5 schizophrenia and 3 BPD families, Austria. | |
| Russo et al. (2005), Max LOD: 6.54. | 15q12–13 (23–27) | Ewald et al. (2002) Genome wide, 2 BPD families, Denmark | ||
| Nyholt et al. (2000) 16 microsatellite Xq21-qter chromosome markers, 2 families, MA/MO Australian. | Cader et al. (2003), LOD: 2.22 | 16p12 (26) | 16p13 (4), Savitz et al. (2007), NPL: 1.84 | Dick et al. (2002), chromosomes 3, 5, 15, 16, 17, and 22, 56 families, NIMH |
| 16p13 (6), Jones, LOD: 4.07 | ||||
| 16p13 (12), McInnis et al. (2003), NPL: 3.3 | Cichon et al. (2001) 75 BPD families | |||
| 16p12 (26), Ekholm et al. (2003), Zmax: 2.5 | ||||
| 16p13 (13), Dick et al. (2002), LOD 2.8 | Kelsoe et al. (2001) Genome wide, microsatellite, 20 BPD families, North American. | |||
| 16p13 (13), Edenberg et al. (1997) | ||||
| Nyholt et al. (1998a) 28 microsatellite X chromosome markers, 3 families, MA/MO, Australian. | Anttila et al. (2006), HLOD: 4.65 | 17p13 (9) | Radhakrishna et al. (2001), Genome wide, 1 large Turkish pedigree | |
| Detera-Wadleigh et al. (1999) Genome wide, 22 BPD pedigrees (n = 365). Amish? | ||||
| Nyholt et al. (1998b) 16 microsatellite Chromosome 19 markers, 2 pedigrees, MA/MO, Australia. | Anttila et al. (2006), HLOD: 3.29 | 18q12 (25) | 18p11 (10), Detera-Wadleight et al. (1999), LOD: 2.32 | Morissette et al. (1999) Genome wide, 1 large BPD pedigree (n = 114), Quebec, Canada. |
| Lea et al. (2005), LOD: 2.32, | 18p11 (11) | 18q12 (23), Tomas et al. (2006) | McInnis et al. (1999) Genome wide, NIMH. Wave 1–2. | |
| Wessman et al. (2002), LOD: 2.32 | 18q12 (25) | 18p11 (13), Mukherjee et al. (2006), LOD: 2.02 | Ginns et al. (1998) Genome wide, 25 BPD families, Old Order Amish. | |
| Bjørnsson et al. (2003), LOD: 1.50 | 18q12 (25) | 18p11 (10), Lin et al. (2005), LOD: 2.83 | Ewald et al. (1998) 16 DNA markers 4pter-4p12, 2 BPD families, Denmark. | |
| Bjørnsson et al. (2003), LOD: 1.57 | 18p11 (13) | |||
| Gardner et al. (1997) Microsatellite loci chromosome 19, 1 family, FHM, USA (German/native American) | Nyholt et al. (1998b), MLOD: 2.07 | 19p13 (17) | 19p13.2 (12), Hamshere et al. (2005), LOD: 1.85 | Detera-Wadleight et al. (1997) Scan chr. 4, 7, 9, 18, 19, 20, 21. 97 BPD families, NIMH |
| Jones et al. (2001), LOD: 4.79 | 19p13 (6–7) | 19p13, Polymeropoulos and Schaffer (1996) | ||
| Wessman et al. (2002), LOD: 1.70 | 19p13 (6) | |||
| Ligthart et al. (2008), LOD: 1.85 | 20p11 (16) | 20p12(12) Detera-Wadleight (p<0.05) | Edenberg et al. (1997) Genomic scan, chr. 3,5,15,16,17,22, 97 BPD families, NIMH. | |
| Björnsson et al. (2003), LOD = 1.60 | 20q13 (41) | 20p12 (10), Willour et al. (2003), LOD: 2.38 | Blackwood et al. (1996) Genom search (193 markers), 11 BPD families, Scotland. | |
| 20p11.2–q11.2 (10–48), Radhakrishna et al. (2001) LOD:4.34 | ||||
| Anttila et al. (2006), HLOD: 1.92 | Xp21 (29) | Xq24–26 (128), Ekholm et al. (2002), LOD: 2.78 | Turecki et al. (1995) Case-control, 10 BPD vs. 10 CTR. Brazil. | |
| Nyholt et al. (1998a), NPL: 2.87 | Xq24–28 (148) | Xq24–27 (130), Pekkarinen et al. (1995), LOD: 3.54 | Pekkarinen et al. (1995), 1 BPD pedigree, Finland. | |
| Nyholt et al. (2000), LOD: 2.38 | Xq24–28 (119–133) | Xp22, McInnis et al. (1999), LOD: 2.3 | ||
Table 4.
Identified genes in Familial Hemiplegic Migraine (FHM) and overlapping Bipolar Linkage studies.
| Studies (author, year) | Identified genes (FHM type) | Bipolar linkage studies (author, year, LOD/NPL) |
|---|---|---|
| De Fusco et al. (2003)2 | 1q23 (158), ATP1A2, FHM 2 | 1q23 (158), Fallin et al. (2004), NPL: 2.46 |
| Dichgans et al. (2005) | 2q24 (166), SCN1A, FHM 3 | 2q22–24 (146–167), Jamra et al. (2007) |
| 2q24 (159–184); Zandi et al. (2007), NPL: 2.54. | ||
| 2q22 (145), Middleton et al. (2004), NPL: 3.09 | ||
| 2q21–33 (172), Cichon et al. (2001), LOD: 2.05. | ||
| Ophoff et al. (1996)13 | 19p13 (13), CACNA1A, FMH 1 | 19p13.2 (12), Hamshere et al. (2005), LOD: 1.85 |
Our finding of nominal linkage to the X chromosome for the Migraine-Narrow phenotype with the highest LOD score at position 156–158 cM (LOD = 1.60, nearest marker DXS9908) is interesting since studies by Nyholt and coworkers have implicated a locus on Xq24–28 in two Australian families with migraine, producing a maximum NPL score of 2.87 at marker DXS1123 (148 cM) less than 10 cM away. However, also regarding the X-chromosome, there is no evidence for linkage of our bipolar phenotypes to this region, again possibly indicating that this region harbours a gene putting women at risk for migraine, separately from the gender independent migraine co-segregating with BPAD.
The only suggestive migraine linkage locus that seemed to overlap with the BPAD phenotypes in this sample was located at chromosome 20p11 with a peak LOD between markers D20S470 (44.4 cM, 17.4 Mb) and D20S477 (51.9 cM and 22.4 Mb). Interestingly, this is the same locus that was recently reported for the first time in a Dutch genome wide migraine linkage study (LOD: 1.85 at 41 cM) (Ligthart et al., 2008). Although the highest LOD score was achieved for the Migraine-Broad phenotype with a LOD of 1.95 at 48 cM, linkage was also observed in this same region for the Migraine-Narrow with a LOD of 1.72 at 51 cM, for BP-Narrow with a LOD of 1.69 at 49 cM, and for BP-Broad with a LOD of 1.60 at 49 cM, and for the BP-Migraine-Broad (LOD = 1.78 at 48 cM) and BP-Migraine-Narrow (1.67, at 52 cM) phenotypes, suggesting this locus might enclose a genetic overlap in migraine and BPAD. This region has previously been implicated in BPAD in the first 97 bipolar pedigrees from the NIMH genetics initiative (Detera-Wadleigh et al., 1997) where elevated allele sharing (P<0.05) was identified at marker D20S604 (12.6 Mb), and in the replication pedigree set including 56 additional BPAD pedigrees (Willour et al., 2003) where the finding peaked at marker D20S162 with an allele sharing LOD score of 1.82. When combining these pedigree sets, 20p12 yielded a nonparametric LOD score of 2.38 and the signal peaked between markers D20S162 (10.1 Mb) and D20S604 (Willour et al., 2003). Other evidence for a chromosome 20 bipolar disorder susceptibility locus comes from a genome scan of a large Turkish pedigree with a dominant BPAD phenotype that identified four chromosome 20 polymorphic markers with strong evidence (LOD score of 4.34 at = 0) for linkage to bipolar disorder (Radhakrishna et al., 2001). Haplotype analysis of these data implicated a 42 cM candidate region spanning 20p11.2–q11.2 (markers D20S604 at12 Mb–D20S887 at 47 Mb), and this region overlaps a region, 20q13, that has been implicated in the migraine study by Björnsson et al. (2003), where they found a LOD score of 1.60 at marker D20S96 (41 Mb). In the Turkish pedigree the marker D20S470 (17 Mb) showed the highest LOD score: 4.34, and the same marker was close to the highest LOD scores found in our sample for both migraine and BP phenotypes. Both the US and Turkish studies propose further characterization of this region, suggesting it is a susceptibility target for the identification of a gene responsible for a bipolar phenotype.
Although we found no linkage to the FHM1, FHM2 orFHM3 loci on chromosomes 19p13, 1q23, and 2q24, it is in view of the current evidence that ion channels put together a number of key features in the pathogenesis of migraine and bipolar disorder, interesting that the region most strongly implicated in our linkage study is right on top of a known potassium dependant sodium//calcium exchanger gene, SLC24A3, which is located at approximately 19 Mb on chromosome 20. This gene is abundantly expressed in the brain, with highest levels found in selected thalamic nuclei, in hippocampal CA1 neurons, and in layer IV of the cerebral cortex (Kraev et al., 2001) and it plays a critical role in calcium homeostasis and electrical conduction in neurons (Lytton et al., 2002). Recently, (Sklar et al., 2008) a comparison of the results from a genome-wide association scan in 1461 patients (2008 controls) with BPAD drawn from the Systematic Treatment Enhancement Program for Bipolar Disorder and the University College London sample, and a genome-wide scan of 1868 (2938 controls) patients with BPAD who completed the scan as part of the Wellcome Trust Case–Control Consortium demonstrated concordant signals for SNPs within the voltage-dependent calcium channel, L-type, alpha 1C subunit (CACNA1C) gene. The CACNA1C gene has overlapping functional similarities to the CACNA1A gene that has been identified in FHM1. Both these genes encode an alpha-1 subunit of a voltage-dependant calcium channel. The CACNA1A gene encodes the alpha-1A subunit and is predominantly expressed in neuronal tissue. Mutations in this gene are associated with two neurologic disorders, familial hemiplegic migraine and episodic ataxia 2. The CACNA1C gene encodes the alpha 1C subunit and is primarily expressed in the heart, but also in subthalamic nuclei, amygdale, cingulate and prefrontal cortex. Since genes involved in ion transport, seem to play an important role in both migraine (FHM1–3) and BPAD (Sklar et al., 2008; Baum et al., 2008), it is tempting to postulate that ionic disturbances are relevant in the migraine/BPAD phenotype. In brief, the functional consequences of FHM1 mutations is that channels open at more negative voltages than do normal channels and this “gain-of-function” effect results in increased Ca2+ influx, resulting in enhanced release of glutamate into the synaptic cleft (Van de Ven et al., 2006). In fact, all 3 FHM genes mutations result in increased extracellular glutamate and potassium levels, possibly pointing towards glutamate regulating genes in a molecular basis for our understanding of the pathogenesis of migraine and BPAD.
In most studies no linkage has been found for migraine or BPAD to chromosome 20. This suggests that the chromosome 20 migraine/BPAD susceptibility locus detected in this study is unlikely to be a major locus in common polygenic migraine or BPAD families. However, this could also explain the existence of a phenotypic subgroup with both symptoms of migraine and bipolar disorder, possibly induced by a mutation in an underlying gene (for instance: SLC24A3l). Both migraine and BPAD may be encompassed into the channelopathy concept. Both disorders have an early age of onset, they are paroxysmal, and have similar triggering issues to other ion channel disorders and act in response to similar drugs. Ion channels are of vital significance in excitable cells decisive for background membrane excitability, action potential production and are part of numerous signal transduction paths including those that mediate neurotransmitter release. Mutations in genes involved in ion transport may influence neuronal excitability and electrical transmission and consequently change the activation state of definite neuronal pathways or the excitability of the brain in general, possibly inducing phenomenons like migraine attacks and manic episodes.
In order to study the genetic overlap using the migraine/BPAD phenotype we conducted a literature review of linkage studies in migraine and BPAD comparing overlapping findings. The search was performed in pub med for every chromosome separately with the key words: “Bipolar disorder, genetics, linkage, chromosome 1…X” and “Migraine, genetics, linkage, chromosome 1…X”, Table 3 displays all linkage loci that have been significantly or suggestively found in migraine linkage studies (Gardner et al., 1997; Nyholt et al., 1998a,b, 2000; Jones et al., 2001; Carlsson et al., 2002; Wessman et al., 2002; Lea et al., 2002; Björnsson et al., 2003; Soragna et al., 2003; Cader et al., 2003; Nyholt et al., 2005; Russo et al., 2005; Lea et al., 2005; Anttila et al., 2006), and the bipolar linkage studies (Savitz et al., 2007; Jamra et al., 2007; Zandi et al., 2007; Goes et al., 2007; Cassidy et al., 2007; Kerner et al., 2007; Jones et al., 2007; Etain et al., 2006; Marcheco-Teruel et al., 2006; Tomàs et al., 2006; Mukherjee et al., 2006; Schumacher et al., 2005; Hamshere et al., 2005; Lambert et al., 2005; McQueen et al., 2005; Kealey et al., 2005; Lin et al., 2005; Macgregor et al., 2004; Middleton et al., 2004; Fallin et al., 2004; Curtis et al., 2003; McInnis et al., 2003; Willour et al., 2003; Ewald et al., 2003; Ekholm et al., 2003; Ewald et al., 2002; Ekholm et al., 2002; Bailer et al., 2002; Dick et al., 2002; Cichon et al., 2001; Kelsoe et al., 2001; Radhakrishna et al., 2001; Detera-Wadleigh et al., 1999; Morissette et al., 1999; Ginns et al., 1998; Ewald et al., 1998; Detera-Wadleigh et al., 1997; Edenberg et al., 1997; Blackwood et al., 1996; Turecki et al., 1995; Pekkarinen et al., 1995) that have demonstrated overlapping or closely related linkage to these migraine loci. In addition we compared linkage studies in BPAD to the identified genes in FHM, and the BPAD (Jamra et al., 2007; Zandi et al., 2007; Hamshere et al., 2005; Middleton et al., 2004; Fallin et al., 2004; Cichon et al., 2001) studies with linkage regions encompassing these genes are presented in Table 4. As displayed in Tables 3 and 4, there are several interesting overlapping findings, most notable regarding the findings for 1q23, 1q31, 2q24, 3q29, 4p16, 4q24, 10q22, 14q21–22, 16p12, 18p11, 18q12, 19p13, 20p11.2–q11.2, Xq24–28. This means that our findings on chromosome 4, chromosome 20 and on the X chromosome are in line with what we had expected from our literature review, although only the chromosome 20 locus was associated with both migraine and BPAD. Given the high degree of co-morbidity between migraine and BPAD, the amount of overlapping linkage results is interesting from several perspectives.
1. Linkage signals in BPAD studies could be accounted for by a high prevalence of migraine in BPAD families (30–40%), and therefore represent a “true” migraine signal. 2. Linkage signals in selected migraine pedigrees could be signals that are explained by a high degree of co-morbid affective disorder running in the migraine pedigree considered. 3. Overlapping linkage signals in migraine and BPAD studies could be explained by the existence of shared genes producing symptoms of migraine, BPAD or both. In further analysis, genes involved in ion transport are choice candidates, but other proteins may have direct or indirect interactions with ion channels. Genes involved in mitochondrial function (Kato and Kato, 2000; Welch and Ramadan, 1995), genes which are part of the serotonin, dopamine or glutamate pathways (Silberstein, 1994; Hargreaves and Shepheard, 1999; Hamel, 2007; Mahmood and Silverstone, 2001; Peroutka, 1997; Emilien et al., 1999; Vaccaro et al., 2007; Goodwin and Jamison, 2007), and genes implicated in inflammation (Dickerson et al., 2007; Vanmolkot and de Hoon, 2007) may also contain important migraine/BPAD causing polymorphisms.
Further studies performed with the migraine/BPAD phenotype are needed to confirm whether this symptom constellation can reclassify some patients into a more-homogeneous genetic subgroup. The findings here suggest that information regarding a prevalent comorbid neurological disorder (i.e. migraine) can provide an additional tool for stratifying a bipolar study sample. This should be applicable not only to linkage studies but also whole genome association studies. It is hoped that this approach will facilitate the detection of underlying mutation(s) elucidating the complex relation between migraine and bipolar disorder, and that this approach will help unravel molecular pathways and the development of rational treatment strategies for both disorders.
Acknowledgments
This work was supported by grants from the National Institute of Mental Health (MH073991, MH47612, MH59567, MH68503) and by the Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center. J. Kelsoe is a founder and holds equity in Psynomics, Inc. The terms of this arrangement have been reviewed and approved by UCSD in accordance with its conflict of interest policies.
Data and biomaterials for the NIMH sample were collected as part of 10 projects that participated in the NIMH Bipolar Disorder Genetics Initiative. From 1991–98, the Principal Investigators and Co-Investigators were: Indiana Univ., Indianapolis, IN, U01 MH46282, John Nurnberger, MD, PhD, Marvin Miller, MD, and Elizabeth Bowman, MD; Washington Univ., St. Louis, MO, U01 MH46280, Theodore Reich, MD, Allison Goate, PhD, and John Rice, PhD; Johns Hopkins Univ., Baltimore, MD U01 MH46274, J. Raymond DePaulo, Jr. MD, Sylvia Simpson, MD, MPH, and Colin Stine, PhD; NIMH Intramural Research Program, Clinical Neurogenetics Branch, Bethesda, MD, Elliot Gershon, MD, Diane Kazuba, BA, and Elizabeth Maxwell, M.S.W. From 1999–2003, the Principal Investigators and Co-Investigators were: Indiana Univ., Indianapolis, IN, R01 MH59545, John Nurnberger, MD, PhD, Marvin J. Miller, MD, Elizabeth S. Bowman, MD, N. Leela Rau, MD, P. Ryan Moe, MD, Nalini Samavedy, MD, Rif El-Mallakh, MD (at Univ. of Louisville), Husseini Manji, MD (at Wayne State Univ.), Debra A. Glitz, MD (at Wayne State Univ.), Eric T. Meyer, M.S., Carrie Smiley, RN, Tatiana Foroud, PhD, Leah Flury, M.S., Danielle M. Dick, PhD, Howard Edenberg, PhD; Washington Univ., St. Louis, MO, R01 MH059534, John Rice, Ph.D, Theodore Reich, MD, Allison Goate, PhD, Laura Bierut, MD; Johns Hopkins Univ., Baltimore, MD, R01 MH59533, Melvin McInnis MD, J. Raymond DePaulo, Jr MD Dean F. MacKinnon, MD, Francis M. Mondimore, MD, James B. Potash, MD, Peter P. Zandi, Ph.D, Dimitrios Avramopoulos, and Jennifer Payne; Univ. of Pennsylvania, PA, R01 MH59553, Wade Berrettini MD, PhD; Univ. of California at Irvine, CA, R01 MH60068, William Byerley MD, and Mark Vawter MD; Univ. of Iowa, IA, R01 MH059548, William Coryell MD, and Raymond Crowe MD; Univ. of Chicago, IL, R01 MH59535, Elliot Gershon, MD, Judith Badner PhD, Francis McMahon MD, Chunyu Liu PhD, Alan Sanders MD, Maria Caserta, Steven Dinwiddie MD, Tu Nguyen, Donna Harakal; Univ. of California at San Diego, CA, R01 MH59567, John Kelsoe, MD, Rebecca McKinney, BA; Rush Univ., IL, R01 MH059556, William Scheftner MD, Howard M. Kravitz, D.O., MPH, Diana Marta, BA, Annette Vaughn-Brown, MSN, RN, and Laurie Bederow, MA; NIMH Intramural Research Program, Bethesda, MD, 1Z01MH002810-01, Francis J. McMa-hon, MD, Layla Kassem, PsyD, Sevilla Detera-Wadleigh, Ph.D, Lisa Austin, Ph.D, Dennis L. Murphy, MD.
This work was also sponsored by an unrestricted grant from the legacy of Gerda Meyer Nyquist Guldbrandson and Gerdt Meyer Nyquist, and by a research grant from Helse-Bergen, Norway.
Role of funding source
This research was supported by an unrestricted grant.
Footnotes
NIMH Genetics Initiative for Bipolar Disorder Consortium authors include: J. Nurnberger, Indiana University; W. Byerley, University of California, San Francisco; J. Kelsoe, University of California, San Diego; M. McInnis, University of Michigan; J. Potash, Johns Hopkins; E. Gershon, University of Chicago; W. Scheftner, Rush University Medical Center; W. Coryell, University of Iowa; W. Berrettini, University of Pennsylvania; F. McMahon, National Institute of Mental Health; J. Rice, Washington University.
Conflict of interest
None.
References
- Abecasis GR, Cerny SS, Cookson WO, Cardon LR. Merlin: rapid analysis of dense genetic maps using sparse gene flow trees. Nat. Genet. 2002;30:97–101. doi: 10.1038/ng786. [DOI] [PubMed] [Google Scholar]
- Akiskal HS, McKinney WT. Depressive disorders: toward a unified hypothesis. Science. 1973;182:20–29. doi: 10.1126/science.182.4107.20. [DOI] [PubMed] [Google Scholar]
- Anttila V, Kallela M, Oswell G, Kaunisto MA, Nyholt DR, Hamalainen E, Havanka H, Ilmavirta M, Terwilliger J, Sobel E, Peltonen L, Kaprio J, Farkkila M, Wessman M, Palotie A. Trait components provide tools to dissect the genetic susceptibility of migraine. Am. J. Hum. Genet. 2006;79(1):85–99. doi: 10.1086/504814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anttila V, Nyholt DR, Kallela M, Artto V, Vepsäläinen S, Jakkula E, Wennerström A, Tikka-Kleemola P, Kaunisto MA, Hämäläinen E, Widén E, Terwilliger J, Merikangas K, Montgomery GW, Martin NG, Daly M, Kaprio J, Peltonen L, Färkkilä M, Wessman M, Palotie A. Consistently replicating locus linked to migraine on 10q22–q23. Am. J. Hum. Genet. 2008;85(5):1051–1063. doi: 10.1016/j.ajhg.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askland K, Parsons M. Toward a biaxial model of “bipolar” affective disorders: spectrum phenotypes as the products of neuroelectrical and neurochemical alterations. J. Affect. Disord. 2006;94:15–33. doi: 10.1016/j.jad.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Bailer U, Leisch F, Meszaros K, Lenzinger E, Willinger U, Strobl R, Heiden A, Gebhardt C, Döge E, Fuchs K, Sieghart W, Kasper S, Hornik K, Aschauer HN. Genome scan for susceptibility loci for schizophrenia and bipolar disorder. Biol. Psychiatry. 2002;52(1):40–52. doi: 10.1016/s0006-3223(02)01320-3. [DOI] [PubMed] [Google Scholar]
- Baum AE, Hamshere M, Green E, Cichon S, Rietschel M, Noethen MM, Craddock N, McMahon FJ. Meta-analysis of two genome-wide association studies of bipolar disorder reveals important points of agreement. Mol. Psychiatry. 2008;13(5):466–467. doi: 10.1038/mp.2008.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björnsson A, Gudmundsson G, Gudfinnsson E, Hrafnsdóttir M, Benedikz J, Skúladóttir S, Kristjánsson K, Frigge ML, Kong A, Stefánsson K, Gulcher JR. Localization of a gene for migraine without aura to chromosome 4q21. Am. J. Hum. Genet. 2003;73(5):986–993. doi: 10.1086/378417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwood DH, He L, Morris SW, McLean A, Whitton C, Thomson M, Walker MT, Woodburn K, Sharp CM, Wright AF, Shibasaki Y, St Clair DM, Porteous DJ, Muir WJ. A locus for bipolar affective disorder on chromosome 4p. Nat. Genet. 1996;4:427–430. doi: 10.1038/ng0496-427. [DOI] [PubMed] [Google Scholar]
- Bowden CL. Lamotrigine in the treatment of bipolar disorder. Expert. Opin. Pharmacother. 2002;3(10):1513–1519. doi: 10.1517/14656566.3.10.1513. Review. [DOI] [PubMed] [Google Scholar]
- Bowden CL, Calabrese JR, McElroy SL, Gyulai L, Wassef A, Petty F, Pope HG, Chou JC, Keck PE, Rhodes LJ, Swann AC, Hirschfeld RM, Wozniak PJ. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex Maintenance Study Group. Arch. Gen. Psychiatry. 2000;57(5):481–489. doi: 10.1001/archpsyc.57.5.481. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC. Migraine, physical health and psychiatric disorders: a prospective epidemiologic study in young adults. J. Psychiatr. Res. 1993;27:211–221. doi: 10.1016/0022-3956(93)90009-q. [DOI] [PubMed] [Google Scholar]
- Cader ZM, Noble-Topham S, Dyment DA, Cherny SS, Brown JD, Rice GP, Ebers GC. Significant linkage to migraine with aura on chromosome 11q24. Hum. Mol. Genet. 2003;12(19):2511–2517. doi: 10.1093/hmg/ddg252. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Forsgren L, Nylander PO, Hellman U, Forsman-Semb K, Holmgren G, Holmberg D, Holmberg M. Identification of a susceptibility locus for migraine with and without aura on 6p12.2–p21.1. Neurology. 2002;59(11):1804–1807. doi: 10.1212/01.wnl.0000036617.04943.96. [DOI] [PubMed] [Google Scholar]
- Cassidy F, Zhao C, Badger J, Claffey E, Dobrin S, Roche S, McKeon P. Genome-wide scan of bipolar disorder and investigation of population stratification effects on linkage: support for susceptibility loci at 4q21, 7q36, 9p21, 12q24, 14q24, and 16p13. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2007;144B(6):791–801. doi: 10.1002/ajmg.b.30524. [DOI] [PubMed] [Google Scholar]
- Cichon S, Schumacher J, Müller DJ, Hürter M, Windemuth C, Strauch K, Hemmer S, Schulze TG, Schmidt-Wolf G, Albus M, Borrmann-Hassenbach M, Franzek E, Lanczik M, Fritze J, Kreiner R, Reuner U, Weigelt B, Minges J, Lichtermann D, Lerer B, Kanyas K, Baur MP, Wienker TF, Maier W, Rietschel M, Propping P, Nöthen MM. A genome screen for genes predisposing to bipolar affective disorder detects a new susceptibility locus on 8q. Hum. Mol. Genet. 2001;10(25):2933–2944. doi: 10.1093/hmg/10.25.2933. [DOI] [PubMed] [Google Scholar]
- Craddock N, Jones I. Genetics of bipolar disorder. J. Med. Genet. 1999;36:585–594. doi: 10.1136/jmg.36.8.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock N, Forty L. Genetics of affective (mood) disorders. Eur. J. Hum. Genet. 2006;14:660–667. doi: 10.1038/sj.ejhg.5201549. [DOI] [PubMed] [Google Scholar]
- Curtis D, Kalsi G, Brynjolfsson J, McInnis M, O'Neill J, Smyth C, Moloney E, Murphy P, McQuillin A, Petursson H, Gurling H. Genome scan of pedigrees multiply affected with bipolar disorder provides further support for the presence of a susceptibility locus on chromosome 12q23–q24, and suggests the presence of additional loci on 1p and 1q. Psychiatr. Genet. 2003;13(2):77–84. doi: 10.1097/01.ypg.0000056684.89558.d2. [DOI] [PubMed] [Google Scholar]
- Davidoff RA. Migraine: Manifestations, Pathogenesis, and Management. Oxford University Press; New York: 2002. [Google Scholar]
- De Fusco M, Marconi R, Silvestri L, Atorino L, Rampoldi L, Morgante L, Ballabio A, Aridon P, Casari G. Haploinsufficiency of ATP1A2 encoding the Na+/K+ pump α2 subunit associated with familial hemiplegic migraine type 2. Nat. Genet. 2003;33:192–196. doi: 10.1038/ng1081. [DOI] [PubMed] [Google Scholar]
- Detera-Wadleigh SD, Badner JA, Yoshikawa T, Sanders AR, Goldin LR, Turner G, Rollins DY, Moses T, Guroff JJ, Kazuba D, Maxwell ME, Edenberg HJ, Foroud T, Lahiri D, Nurnberger JI, Stine OC, McMahon F, Meyers DA, MacKinnon D, Simpson S, McInnis M, DePaulo JR, Rice J, Goate A, Gershon ES. Initial genome scan of the NIMH genetics initiative bipolar pedigrees: chromosomes 4, 7, 9, 18, 19, 20, and 21q. Am. J. Med. Genet. 1997;74(3):254–262. doi: 10.1002/(sici)1096-8628(19970531)74:3<254::aid-ajmg4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Detera-Wadleigh SD, Badner JA, Berrettini WH, Yoshikawa T, Goldin LR, Turner G, Rollins DY, Moses T, Sanders AR, Karkera JD, Esterling LE, Zeng J, Ferraro TN, Guroff JJ, Kazuba D, Maxwell ME, Nurnberger JI, Gershon ES. A high-density genome scan detects evidence for a bipolar-disorder susceptibility locus on 13q32 and other potential loci on 1q32 and 18p11.2. Proc. Natl. Acad. Sci. USA. 1999;96(10):5604–5609. doi: 10.1073/pnas.96.10.5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichgans M, Freilinger T, Eckstein G, Babini E, Lorenz-Depiereux B, Biskup S, Ferrari MD, Herzog J, van den Maagdenberg AM, Pusch M, Strom TM. Mutation in the neuronal voltage-gated sodium channel SCN1A in familial hemiplegic migraine. Lancet. 2005;366:371–377. doi: 10.1016/S0140-6736(05)66786-4. [DOI] [PubMed] [Google Scholar]
- Dick DM, Foroud T, Edenberg HJ, Miller M, Bowman E, Rau NL, DePaulo JR, McInnis M, Gershon E, McMahon F, Rice JP, Bierut LJ, Reich T, Nurnberger JJ. Apparent replication of suggestive linkage on chromosome 16 in the NIMH genetics initiative bipolar pedigrees. Am. J. Med. Genet. 2002;114(4):407–412. doi: 10.1002/ajmg.10380. [DOI] [PubMed] [Google Scholar]
- Dick DM, Foroud T, Flury L, Bowman ES, Miller MJ, Rau NL, Moe PR, Samavedy N, El-Mallakh R, Manji H, Glitz DA, Meyer ET, Smiley C, Hahn R, Widmark C, McKinney R, Sutton L, Ballas C, Grice D, Berrettini W, Byerley W, Coryell W, DePaulo R, MacKinnon DF, Gershon ES, Kelsoe JR, McMahon FJ, McInnis M, Murphy DL, Reich T, Scheftner W, Nurnberger JI. Genomewide linkage analyses of bipolar disorder: a new sample of 250 pedigrees from the National Institute of Mental Health Genetics Initiative. Am. J. Hum. Genet. 2003;73(1):107–114. doi: 10.1086/376562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Boronow J, Yolken R. Elevated serum levels of C-reactive protein are associated with mania symptoms in outpatients with bipolar disorder. Prog. Neuro-psychopharmacol. Biol. Psychiatry. 2007;31:952–955. doi: 10.1016/j.pnpbp.2007.02.018. [DOI] [PubMed] [Google Scholar]
- Dilsaver SD, Benazzi F, Oedegaard KJ, Fasmer OB, Akiskal KK, Akiskal HS. Is a family history of bipolar disorder a risk factor for migraine? Psychopathology. 2009;42(2):119–123. doi: 10.1159/000204762. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Foroud T, Conneally PM, Sorbel JJ, Carr K, Crose C, Willig C, Zhao J, Miller M, Bowman E, Mayeda A, Rau NL, Smiley C, Rice JP, Goate A, Reich T, Stine OC, McMahon F, DePaulo JR, Meyers D, Detera-Wadleigh SD, Goldin LR, Gershon ES, Blehar MC, Nurnberger JI. Initial genomic scan of the NIMH genetics initiative bipolar pedigrees: chromosomes 3, 5, 15, 16, 17, and 22. Am. J. Med. Genet. 1997;74(3):238–246. [PubMed] [Google Scholar]
- Ekholm JM, Pekkarinen P, Pajukanta P, Kieseppä T, Partonen T, Paunio T, Varilo T, Perola M, Lönnqvist J, Peltonen L. Bipolar disorder susceptibility region onXq24–q27.1 in Finnish families. Mol. Psychiatry. 2002;7(5):453–459. doi: 10.1038/sj.mp.4001104. [DOI] [PubMed] [Google Scholar]
- Ekholm JM, Kieseppä T, Hiekkalinna T, Partonen T, Paunio T, Perola M, Ekelund J, Lönnqvist J, Pekkarinen-Ijäs P, Peltonen L. Evidence of susceptibility loci on 4q32 and 16p12 for bipolar disorder. Hum. Mol. Genet. 2003;12(15):1907–1915. doi: 10.1093/hmg/ddg199. [DOI] [PubMed] [Google Scholar]
- El-Mallakh RS, Wyatt RJ. The Na,K-ATPase hypothesis for bipolar illness. Biol. Psychiatry. 1995;37:235–244. doi: 10.1016/0006-3223(94)00201-D. [DOI] [PubMed] [Google Scholar]
- El-Mallakh RS, El-Masri MA, Huff MO, Lim XP, Decker S, Levy RS. Intracerebroventricular administration of ouabain as a model of mania in rats. Bipolar. Disord. 2003;5:362–365. doi: 10.1034/j.1399-5618.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- Emilien G, Maloteauw JM, Geurts M, Hoogenberg K, Cragg S. Dopamine receptors—physiological understanding to therapeutic intervention potential. Pharmacol. Ther. 1999;84:133–156. doi: 10.1016/s0163-7258(99)00029-7. [DOI] [PubMed] [Google Scholar]
- Endicott NA. Psychophysiological correlates of “bipolarity”. J. Affect. Disord. 1989;17:47–56. doi: 10.1016/0165-0327(89)90023-2. [DOI] [PubMed] [Google Scholar]
- Etain B, Mathieu F, Rietschel M, Maier W, Albus M, McKeon P, Roche S, Kealey C, Blackwood D, Muir W, Bellivier F, Henry C, Dina C, Gallina S, Gurling H, Malafosse A, Preisig M, Ferrero F, Cichon S, Schumacher J, Ohlraun S, Borrmann-Hassenbach M, Propping P, Abou Jamra R, Schulze TG, Marusic A, Dernovsek ZM, Giros B, Bourgeron T, Lemainque A, Bacq D, Betard C, Charon C, Nöthen MM, Lathrop M, Leboyer M. Genome-wide scan for genes involved in bipolar affective disorder in 70 European families ascertained through a bipolar type I early-onset proband: supportive evidence for linkage at 3p14. Mol. Psychiatry. 2006;11(7):685–694. doi: 10.1038/sj.mp.4001815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald H, Degn B, Mors O, Kruse TA. Support for the possible locus on chromosome 4p16 for bipolar affective disorder. Mol. Psychiatry. 1998;5:442–448. doi: 10.1038/sj.mp.4000420. [DOI] [PubMed] [Google Scholar]
- Ewald H, Flint T, Kruse TA, Mors O. A genome-wide scan shows significant linkage between bipolar disorder and chromosome 12q24.3 and suggestive linkage to chromosomes 1p22–21, 4p16, 6q14–22, 10q26 and 16p13.3. Mol. Psychiatry. 2002;7(7):734–744. doi: 10.1038/sj.mp.4001074. [DOI] [PubMed] [Google Scholar]
- Ewald H, Kruse TA, Mors O. Genome wide scan using homozygosity mapping and linkage analyses of a single pedigree with affective disorder suggests oligogenic inheritance. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2003;120(1):63–71. doi: 10.1002/ajmg.b.20039. [DOI] [PubMed] [Google Scholar]
- Fallin MD, Lasseter VK, Wolyniec PS, McGrath JA, Nestadt G, Valle D, Liang KY, Pulver AE. Genomewide linkage scan for bipolar-disorder susceptibility loci among Ashkenazi Jewish families. Am. J. Hum. Genet. 2004;75(2):204–219. doi: 10.1086/422474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasmer OB. The prevalence of migraine in patients with bipolar and unipolar affective disorders. Cephalalgia. 2001;21:894–899. doi: 10.1046/j.1468-2982.2001.00279.x. [DOI] [PubMed] [Google Scholar]
- Ferrari MD, Goadsby PJ. Migraine as a cerebral ionopathy with abnormal central sensory processing. In: Gilman S, editor. Neurobiology of Disease. Elsevier; New York, NY: 2006. pp. 333–348. [Google Scholar]
- Gardner K, Barmada MM, Ptacek LJ, Hoffman EP. A new locus for hemiplegic migraine maps to chromosome 1q31. Neurology. 1997;49(5):1231–1238. doi: 10.1212/wnl.49.5.1231. [DOI] [PubMed] [Google Scholar]
- Gervil M, Ulrich V, Kaprio J, Olesen J, Russell MB. The relative role of genetic and environmental factors in migraine without aura. Neurology. 1999;53:995–999. doi: 10.1212/wnl.53.5.995. [DOI] [PubMed] [Google Scholar]
- Ginns EI, St Jean P, Philibert RA, Galdzicka M, Damschroder-Williams P, Thiel B, Long RT, Ingraham LJ, Dalwaldi H, Murray MA, Ehlert M, Paul S, Remortel BG, Patel AP, Anderson MC, Shaio C, Lau E, Dymarskaia I, Martin BM, Stubblefield B, Falls KM, Carulli JP, Keith TP, Fann CS, Lacy LG, Allen CR, Hostetter AM, Elston RC, Schork NJ, Egeland JA, Paul SM. A genome-wide search for chromosomal loci linked to mental health wellness in relatives at high risk for bipolar affective disorder among the Old Order Amish. Sci. U. S. A. 1998;95(26):15531–15536. doi: 10.1073/pnas.95.26.15531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goes FS, Zandi PP, Miao K, McMahon FJ, Steele J, Willour VL, Mackinnon DF, Mondimore FM, Schweizer B, Nurnberger JI, Rice JP, Scheftner W, Coryell W, Berrettini WH, Kelsoe JR, Byerley W, Murphy DL, Gershon ES, Bipolar Disorder Phenome Group. Depaulo JR, McInnis MG, Potash JB. Mood-incongruent psychotic features in bipolar disorder: familial aggregation and suggestive linkage to 2p11–q14 and 13q21–33. Am. J. Psychiatry. 2007;164(2):236–247. doi: 10.1176/ajp.2007.164.2.236. [DOI] [PubMed] [Google Scholar]
- Goodwin F, Jamison KR. Manic-depressive Illness. Oxford University Press; New York: 2007. [Google Scholar]
- Hamel E. Serotonin and migraine: biology and clinical implications. Cephalalgia. 2007;27:1295–1300. doi: 10.1111/j.1468-2982.2007.01476.x. [DOI] [PubMed] [Google Scholar]
- Hamshere ML, Bennett P, Williams N, Segurado R, Cardno A, Norton N, Lambert D, Williams H, Kirov G, Corvin A, Holmans P, Jones L, Jones I, Gill M, O'Donovan MC, Owen MJ, Craddock N. Genomewide linkage scan in schizoaffective disorder: significant evidence for linkage at 1q42 close to DISC1, and suggestive evidence at 22q11 and 19p13. Arch. Gen. Psychiatry. 2005;62(10):1081–1088. doi: 10.1001/archpsyc.62.10.1081. [DOI] [PubMed] [Google Scholar]
- Hargreaves RJ, Shepheard SL. Pathophysiology of migraine—new insights. Can. J. Neurol. Sci. 1999;26(Suppl 3):S12–S19. doi: 10.1017/s0317167100000147. [DOI] [PubMed] [Google Scholar]
- Headache Classification Committee. The International Classification of Headache Disorders. 2. Vol. 24. Cephalalgia; 2004. pp. 1–160. [Google Scholar]
- Hirschfeld RM, Calabrese JR, Weissman MM, Reed M, Davies MA, Frye MA, Keck PE, Lewis L, McElroy SL, McNulty JP, Wagner KD. Screening for bipolar disorder in the community. J. Clin. Psychiatry. 2003;64:53–59. doi: 10.4088/jcp.v64n0111. [DOI] [PubMed] [Google Scholar]
- Honkasalo ML, Kaprio J, Winter T, Heikkila K, Sillanpaa M, Koskenvuo M. Migraine and concomitant symptoms among 8167 adult twin pairs. Headache. 1995;35:70–78. doi: 10.1111/j.1526-4610.1995.hed3502070.x. [DOI] [PubMed] [Google Scholar]
- Jamra R, Fuerst R, Kaneva R, Orozco Diaz G, Rivas F, Mayoral F, Gay E, Sans S, Gonzalez MJ, Gil S, Cabaleiro F, Del Rio F, Perez F, Haro J, Auburger G, Milanova V, Kostov C, Chorbov V, Stoyanova V, Nikolova-Hill A, Onchev G, Kremensky I, Jablensky A, Schulze TG, Propping P, Rietschel M, Nothen MM, Cichon S, Wienker TF, Schumacher J. The first genomewide interaction and locus-heterogeneity linkage scan in bipolar affective disorder: strong evidence of epistatic effects between loci on chromosomes 2q and 6q. Am. J. Hum. Genet. 2007;81(5):974–986. doi: 10.1086/521690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KW, Ehm MG, Pericak-Vance MA, Haines JL, Boyd PR, Peroutka SJ. Migraine with aura susceptibility locus on chromosome 19p13 is distinct from the familial hemiplegic migraine locus. Genomics. 2001;78(3):150–154. doi: 10.1006/geno.2001.6665. [DOI] [PubMed] [Google Scholar]
- Jones I, Hamshere M, Nangle JM, Bennett P, Green E, Heron J, Segurado R, Lambert D, Holmans P, Corvin A, Owen M, Jones L, Gill M, Craddock N. Bipolar affective puerperal psychosis: genome-wide significant evidence for linkage to chromosome 16. Am. J. Psychiatry. 2007;64(7):1099–1104. doi: 10.1176/ajp.2007.164.7.1099. [DOI] [PubMed] [Google Scholar]
- Kato T. Role of mitochondrial DNA in calcium signaling abnormality in bipolar disorder. Cell Calcium. 2008;44(1):92–102. doi: 10.1016/j.ceca.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Kato T, Kato N. Mitochondrial dysfunction in bipolar disorder. Bipolar Disord. 2000;2:180–190. doi: 10.1034/j.1399-5618.2000.020305.x. [DOI] [PubMed] [Google Scholar]
- Kealey C, Roche S, Claffey E, McKeon P. Linkage and candidate gene analysis of 14q22–24 in bipolar disorder: support for GCHI as a novel susceptibility gene. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2005;136B(1):75–80. doi: 10.1002/ajmg.b.30192. [DOI] [PubMed] [Google Scholar]
- Kelsoe JR, Spence MA, Loetscher E, Foguet M, Sadovnick AD, Remick RA, Flodman P, Khristich J, Mroczkowski-Parker Z, Brown JL, Masser D, Ungerleider S, Rapaport MH, Wishart WL, Luebbert H. A genome survey indicates a possible susceptibility locus for bipolar disorder on chromosome 22. Proc. Natl. Acad. Sci. USA. 2001;98(2):585–590. doi: 10.1073/pnas.011358498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerner B, Brugman DL, Freimer NB. Evidence of linkage to psychosis on chromosome 5q33–34 in pedigrees ascertained for bipolar disorder. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2007;144B(1):74–78. doi: 10.1002/ajmg.b.30402. [DOI] [PubMed] [Google Scholar]
- Kong A, Cox NJ. Allele-sharing models: LOD scores and accurate linkage tests. Am. J. Hum. Genet. 1997;61:1179–1188. doi: 10.1086/301592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X, Murphy K, Raj T, He C, White PS, Matise TC. A combined linkage-physical map of the human genome. Am. J. Hum. Genet. 2004;75:1143–1148. doi: 10.1086/426405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraev A, Quednau BD, Leach S, Li XF, Dong H, Winkfein R, Perizzolo M, Cai X, Yang R, Philipson KD, Lytton J. Molecular cloning of a third member of the potassium-dependent sodium–calcium exchanger gene family, NCKX3. J. Biol. Chem. 2001;276(25):23161–23172. doi: 10.1074/jbc.M102314200. [DOI] [PubMed] [Google Scholar]
- Lambert D, Middle F, Hamshere ML, Segurado R, Raybould R, Corvin A, Green E, O'Mahony E, Nikolov I, Mulcahy T, Haque S, Bort S, Bennett P, Norton N, Owen MJ, Kirov G, Lendon C, Jones L, Jones I, Holmans P, Gill M, Craddock N. Stage 2 of the Wellcome Trust UK–Irish bipolar affective disorder sibling-pair genome screen: evidence for linkage on chromosomes 6q16–q21, 4q12–q21, 9p21, 10p14–p12 and 18q22. Mol. Psychiatry. 2005;10(9):831–841. doi: 10.1038/sj.mp.4001684. [DOI] [PubMed] [Google Scholar]
- Lampl C, Katsarava Z, Diener HC, Limmroth V. Lamotrigine reduces migraine aura and migraine attacks in patients with migraine with aura. J. Neurol. Neurosurg. Psychiatry. 2005;76(12):1730–1732. doi: 10.1136/jnnp.2005.063750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson B, Bille B, Pedersen NL. Genetic influence in headaches: a Swedish twin study. Headache. 1995;35:513–519. doi: 10.1111/j.1526-4610.1995.hed3509513.x. [DOI] [PubMed] [Google Scholar]
- Lea RA, Shepherd AG, Curtain RP, Nyholt DR, Quinlan S, Brimage PJ, Griffiths LR. A typical migraine susceptibility region localizes to chromosome 1q31. Neurogenetics. 2002;4(1):17–22. doi: 10.1007/s10048-001-0125-1. [DOI] [PubMed] [Google Scholar]
- Lea RA, Nyholt DR, Curtain RP, Ovcaric M, Sciascia R, Bellis C, Macmillan J, Quinlan S, Gibson RA, McCarthy LC, Riley JH, Smithies YJ, Kinrade S, Griffiths LR. A genome-wide scan provides evidence for loci influencing a severe heritable form of common migraine. Neurogenetics. 2005;6(2):67–72. doi: 10.1007/s10048-005-0215-6. [DOI] [PubMed] [Google Scholar]
- Ligthart L, Nyholt DR, Hottenga JJ, Distel MA, Willemsen G, Boomsma DI. A genome-wide linkage scan provides evidence for both new and previously reported loci influencing common migraine. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2008;147B(7):1186–1195. doi: 10.1002/ajmg.b.30749. [DOI] [PubMed] [Google Scholar]
- Lin PI, McInnis MG, Potash JB, Willour VL, Mackinnon DF, Miao K, Depaulo JR, Zandi PP. Assessment of the effect of age at onset on linkage to bipolar disorder: evidence on chromosomes 18p and 21q. Am. J. Hum. Genet. 2005;77(4):545–555. doi: 10.1086/491602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low NC, Du Fort GG, Cervantes P. Prevalence, clinical correlates, and treatment of migraine in bipolar disorder. Headache. 2003;43:940–949. doi: 10.1046/j.1526-4610.2003.03184.x. [DOI] [PubMed] [Google Scholar]
- Lytton J, Li XF, Dong H, Kraev A. K+-dependent Na+/Ca2+ exchangers in the brain. Ann. N. Y. Acad. Sci. 2002;976:382–393. doi: 10.1111/j.1749-6632.2002.tb04765.x. [DOI] [PubMed] [Google Scholar]
- Macgregor S, Visscher PM, Knott SA, Thomson P, Porteous DJ, Millar JK, Devon RS, Blackwood D, Muir WJ. A genome scan and follow-up study identify a bipolar disorder susceptibility locus on chromosome 1q42. Mol. Psychiatry. 2004;12:1083–1090. doi: 10.1038/sj.mp.4001544. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Hajek T, Alda M. The phenotypes of bipolar disorder: relevance for genetic investigations. Mol. Psychiatry. 2005;10:811–826. doi: 10.1038/sj.mp.4001701. [DOI] [PubMed] [Google Scholar]
- Mahmood T, Silverstone T. Serotonin and bipolar disorder. J. Affect. Disord. 2001;66:1–11. doi: 10.1016/s0165-0327(00)00226-3. [DOI] [PubMed] [Google Scholar]
- Mahmood T, Romans S, Silverstone T. Prevalence of migraine in bipolar disorder. J. Affect. Disord. 1999;52:239–241. doi: 10.1016/s0165-0327(98)00082-2. [DOI] [PubMed] [Google Scholar]
- Marcheco-Teruel B, Flint TJ, Wikman FP, Torralbas M, González L, Blanco L, Tan Q, Ewald H, Orntoft T, Kruse TA, Børglum AD, Mors O. A genome-wide linkage search for bipolar disorder susceptibility loci in a large and complex pedigree from the eastern part of Cuba. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2006;141B(8):833–843. doi: 10.1002/ajmg.b.30314. [DOI] [PubMed] [Google Scholar]
- Mathew NT, Saper JR, Silberstein SD. Migraine prophylaxis with divalproex. Arch. Neurol. 1995;52:281–286. doi: 10.1001/archneur.1995.00540270077022. [DOI] [PubMed] [Google Scholar]
- McInnis MG, Dick DM, Willour VL, Avramopoulos D, MacKinnon DF, Simpson SG, Potash JB, Edenberg HJ, Bowman ES, McMahon FJ, Smiley C, Chellis JL, Huo Y, Diggs T, Meyer ET, Miller M, Matteini AT, Rau NL, DePaulo JR, Gershon ES, Badner JA, Rice JP, Goate AM, Detera-Wadleigh SD, Nurnberger JJ, Reich T, Zandi PP, Foroud TM. Genome-wide scan and conditional analysis in bipolar disorder: evidence for genomic interaction in the National Institute of Mental Health genetics initiative bipolar pedigrees. Biol. Psychiatry. 2003;54(11):1265–1273. doi: 10.1016/j.biopsych.2003.08.001. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Konarski JZ, Wilkins K, Bouffard B, Soczynska JK, Kennedy SH. The prevalence and impact of migraine headache in bipolar disorder: results from the Canadian Community Health Survey. Headache. 2006;46(6):973–982. doi: 10.1111/j.1526-4610.2006.00469.x. [DOI] [PubMed] [Google Scholar]
- McQueen MB, Devlin B, Faraone SV, Nimgaonkar VL, Sklar P, Smoller JW, Abou Jamra R, Albus M, Bacanu SA, Baron M, Barrett TB, Berrettini W, Blacker D, Byerley W, Cichon S, Coryell W, Craddock N, Daly MJ, Depaulo JR, Edenberg HJ, Foroud T, Gill M, Gilliam TC, Hamshere M, Jones I, Jones L, Juo SH, Kelsoe JR, Lambert D, Lange C, Lerer B, Liu J, Maier W, Mackinnon JD, McInnis MG, McMahon FJ, Murphy DL, Nothen MM, Nurnberger JI, Pato CN, Pato MT, Potash JB, Propping P, Pulver AE, Rice JP, Rietschel M, Scheftner W, Schumacher J, Segurado R, Van Steen K, Xie W, Zandi PP, Laird NM. Combined analysis from eleven linkage studies of bipolar disorder provides strong evidence of susceptibility loci on chromosomes 6q and 8q. Am. J. Hum. Genet. 2005;77(4):582–595. doi: 10.1086/491603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JL, Diamond S. Cyclical migraine. Arch. Neurol. 1981;38:343–344. doi: 10.1001/archneur.1981.00510060045005. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Angst J, Isler H. Migraine and psychopathology. Arch. Gen. Psychiatry. 1990;47:849–853. doi: 10.1001/archpsyc.1990.01810210057008. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Pato MT, Gentile KL, Morley CP, Zhao X, Eisener AF, Brown A, Petryshen TL, Kirby AN, Medeiros H, Carvalho C, Macedo A, Dourado A, Coelho I, Valente J, Soares MJ, Ferreira CP, Lei M, Azevedo MH, Kennedy JL, Daly MJ, Sklar P, Pato CN. Genomewide linkage analysis of bipolar disorder by use of a high-density single-nucleotide-polymorphism (SNP) genotyping assay: a comparison with microsatellite marker assays and finding of significant linkage to chromosome 6q22. Am. J. Hum. Genet. 2004;74(5):886–897. doi: 10.1086/420775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morissette J, Villeneuve A, Bordeleau L, Rochette D, Laberge C, Gagné B, Laprise C, Bouchard G, Plante M, Gobeil L, Shink E, Weissenbach J, Barden N. Genome-wide search for linkage of bipolar affective disorders in a very large pedigree derived from a homogeneous population in quebec points to a locus of major effect on chromosome 12q23–q24. Am. J. Med. Genet. 1999;88(5):567–587. doi: 10.1002/(sici)1096-8628(19991015)88:5<567::aid-ajmg24>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Moskowitz MA, Bolay H, Dalkara T. Deciphering migraine mechanisms: clues from familial hemiplegic migraine genotypes. Ann. Neurol. 2004;55:276–280. doi: 10.1002/ana.20035. [DOI] [PubMed] [Google Scholar]
- Mukherjee O, Meera P, Ghosh S, Kubendran S, Kiran K, Manjunath KR, Subhash MN, Benegal V, Brahmachari SK, Majumder PP, Jain S. Evidence of linkage and association on 18p11.2 for psychosis. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2006;141B(8):868–873. doi: 10.1002/ajmg.b.30363. [DOI] [PubMed] [Google Scholar]
- Nievergelt CM, Smith DW, Kohlenberg JB, Schork NJ. Large-scale integration of human genetic and physical maps. Genome. Res. 2004;14:1199–1205. doi: 10.1101/gr.1475304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurnberger JI, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T, Miller M, Bowman ES, DePaulo JR, Cloninger CR, Robinson G, Moldin S, Gershon ES, Maxwell E, Guroff JJ, Kirch D, Wynne D, Berg K, Tsuang MT, Faraone SV, Pepple JR. Diagnostic interview for genetic studies: rationale, unique features, and training. Arch. Gen. Psychiat. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- Nyholt DR, Dawkins JL, Brimage PJ, Goadsby PJ, Nicholson GA, Griffiths LR. Evidence for an X-linked genetic component in familial typical migraine. Hum. Mol. Genet. 1998a;7(3):459–463. doi: 10.1093/hmg/7.3.459. [DOI] [PubMed] [Google Scholar]
- Nyholt DR, Lea RA, Goadsby PJ, Brimage PJ, Griffiths LR. Familial typical migraine: linkage to chromosome 19p13 and evidence for genetic heterogeneity. Neurology. 1998b;50(5):1428–1432. doi: 10.1212/wnl.50.5.1428. [DOI] [PubMed] [Google Scholar]
- Nyholt DR, Curtain RP, Griffiths LR. Familial typical migraine: significant linkage and localization of a gene to Xq24–28. Hum. Genet. 2000;107(1):18–23. doi: 10.1007/s004390000329. [DOI] [PubMed] [Google Scholar]
- Nyholt DR, Morley KI, Ferreira MA, Medland SE, Boomsma DI, Heath AC, Merikangas KR, Montgomery GW, Martin NG. Genomewide significant linkage to migrainous headache on chromosome 5q21. Am. J. Hum. Genet. 2005;77(3):500–512. doi: 10.1086/444510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oedegaard KJ, Fasmer OB. Is migraine in unipolar depressed patients a bipolar spectrum trait? J. Affect. Disord. 2005;84:233–242. doi: 10.1016/j.jad.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Ophoff RA, Terwindt GM, Vergouwe MN, van Eijk R, Oefner PJ, Hoffman SM, Lamerdin JE, Mohrenweiser HW, Bulman DE, Ferrari M, Haan J, Lindhout D, van Ommen GJ, Hofker MH, Ferrari MD, Frants RR. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell. 1996;87:543–552. doi: 10.1016/s0092-8674(00)81373-2. [DOI] [PubMed] [Google Scholar]
- Pekkarinen P, Terwilliger J, Bredbacka PE, Lönnqvist J, Peltonen L. Evidence of a predisposing locus to bipolar disorder on Xq24–q27.1 in an extended Finnish pedigree. Genome Res. 1995;5(2):105–115. doi: 10.1101/gr.5.2.105. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ. Dopamine and migraine. Neurology. 1997;49:650–656. doi: 10.1212/wnl.49.3.650. [DOI] [PubMed] [Google Scholar]
- Pietrobon D. Familial hemiplegic migraine. Neurotherapeutics. 2007;4:274–284. doi: 10.1016/j.nurt.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Schaffer AA. Scanning the genome with 1772 microsatellite markers in search of a bipolar disorder susceptibility gene. Mol. Psychiatry. 1996;1(5):404–407. [PubMed] [Google Scholar]
- Radhakrishna U, Senol S, Herken H, Gucuyener K, Gehrig C, Blouin JL, Akarsu NA, Antonarakis SE. An apparently dominant bipolar affective disorder (BPAD) locus on chromosome 20p11.2–q11.2 in a large Turkish pedigree. Eur. J. Hum. Genet. 2001;(1):39–44. doi: 10.1038/sj.ejhg.5200584. [DOI] [PubMed] [Google Scholar]
- Rose AM, Mellett BJ, Valdes R, Kleinman JE, Herman, Li R, El-Mallakh RS. Alpha2 isoform of the Na,K-adenosine triphosphatase is reduced in temporal cortex of bipolar individuals. Biol. Psychiatry. 1998;44:892–897. doi: 10.1016/s0006-3223(97)00440-x. [DOI] [PubMed] [Google Scholar]
- Russo L, Mariotti P, Sangiorgi E, Giordano T, Ricci I, Lupi F, Chiera R, Guzzetta F, Neri G, Gurrieri F. A new susceptibility locus for migraine with aura in the 15q11–q13 genomic region containing three GABA-A receptor genes. Am. J. Hum. Genet. 2005;76(2):327–333. doi: 10.1086/427521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Cupido CL, Ramesar RK. Preliminary evidence for linkage to chromosome 1q31–32, 10q23.3, and 16p13.3 in a South African cohort with bipolar disorder. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2007;144B(3):383–387. doi: 10.1002/ajmg.b.30461. [DOI] [PubMed] [Google Scholar]
- Schumacher J, Kaneva R, Jamra RA, Diaz GO, Ohlraun S, Milanova V, Lee YA, Rivas F, Mayoral F, Fuerst R, Flaquer A, Windemuth C, Gay E, Sanz S, González MJ, Gil S, Cabaleiro F, del Rio F, Perez F, Haro J, Kostov C, Chorbov V, Nikolova-Hill A, Stoyanova V, Onchev G, Kremensky I, Strauch K, Schulze TG, Nürnberg P, Gaebel W, Klimke A, Auburger G, Wienker TF, Kalaydjieva L, Propping P, Cichon S, Jablensky A, Rietschel M, Nöthen MM. Genomewide scan and fine-mapping linkage studies in four European samples with bipolar affective disorder suggest a new susceptibility locus on chromosome 1p35–p36 and provides further evidence of loci on chromosome 4q31 and 6q24. Am. J. Hum. Genet. 2005;77(6):1102–1111. doi: 10.1086/498619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segurado R, Detera-Wadleigh SD, Levinson DF, Lewis CM, Gill M, Nurnberger JI, Craddock N, DePaulo JR, Baron M, Gershon ES. Genome scan meta-analysis of schizophrenia and bipolar disorder, part III: bipolar disorder. Am. J. Hum. Genet. 2003;73:49–62. doi: 10.1086/376547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein SD. Serotonin (5-HT) and migraine. Headache. 1994;34:408–417. doi: 10.1111/j.1526-4610.1994.hed3407408.x. [DOI] [PubMed] [Google Scholar]
- Silberstein SD. Divalproex sodium in headache: literature review and clinical guidelines. Headache. 1996;36:547–555. doi: 10.1046/j.1526-4610.1996.3609547.x. [DOI] [PubMed] [Google Scholar]
- Silberstein SD. Migraine pathophysiology and its clinical implications. Cephalalgia. 2004;24(Suppl 2):2–7. doi: 10.1111/j.1468-2982.2004.00892.x. [DOI] [PubMed] [Google Scholar]
- Silberstein SD, Goadsby PJ. Migraine: preventive treatment. Cephalalgia. 2002;22:491–512. doi: 10.1046/j.1468-2982.2002.00386.x. [DOI] [PubMed] [Google Scholar]
- Sklar P, Smoller JW, Fan J, Ferreira MA, Perlis RH, Chambert K, Nimgaonkar VL, McQueen MB, Faraone SV, Kirby A, de Bakker PI, Ogdie MN, Thase ME, Sachs GS, Todd-Brown K, Gabriel SB, Sougnez C, Gates C, Blumenstiel B, Defelice M, Ardlie KG, Franklin J, Muir WJ, McGhee KA, MacIntyre DJ, McLean A, VanBeck M, McQuillin A, Bass NJ, Robinson M, Lawrence J, Anjorin A, Curtis D, Scolnick EM, Daly MJ, Blackwood DH, Gurling HM, Purcell SM. Whole-genome association study of bipolar disorder. Mol. Psychiatry. 2008;13(6):558–569. doi: 10.1038/sj.mp.4002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoller JW, Finn CT. Family, twin, and adoption studies of bipolar disorder. Am. J. Med. Genet. 2003;60:497–502. doi: 10.1002/ajmg.c.20013. [DOI] [PubMed] [Google Scholar]
- Soragna D, Vettori A, Carraro G, Marchioni E, Vazza G, Bellini S, Tupler R, Savoldi F, Mostacciuolo ML. A locus for migraine without aura maps on chromosome 14q21.2–q22.3. Am. J. Hum. Genet. 2003;72(1):161–167. doi: 10.1086/345298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomàs C, Cañellas F, Rodríguez V, Picornell A, Lafau O, Nadal M, Roca M, Serrano MJ, Castro JA, Ramon MM. Genetic linkage study for bipolar disorders on chromosomes 17 and 18 in families with a high expression of mental illness from the Balearic Islands. Psychiatr. Genet. 2006;16(4):145–151. doi: 10.1097/01.ypg.0000218614.42762.b0. [DOI] [PubMed] [Google Scholar]
- Turecki G, Smith M, Mari JJ. Type I bipolar disorder associated with a fragile site on chromosome 1. Am. J. Med. Genet. 1995;60(3):179–182. doi: 10.1002/ajmg.1320600302. [DOI] [PubMed] [Google Scholar]
- Vaccaro M, Riva C, Tremolizzo L, Longoni M, Aliprandi A, Agostoni E, Rigamonti A, Leone M, Bussone G, Ferrarese C. Platelet glutamate uptake and release in migraine with and without aura. Cephalalgia. 2007;27(1):35–40. doi: 10.1111/j.1468-2982.2006.01234.x. [DOI] [PubMed] [Google Scholar]
- Van de Ven RC, Kaja S, Plomp JJ, Frants RR, van den Maagdenberg AM, Ferrari MD. Genetic models of migraine. Arch. Neurol. 2007;64(5):643–646. doi: 10.1001/archneur.64.5.643. [DOI] [PubMed] [Google Scholar]
- Vanmolkot FH, de Hoon JN. Increased C-reactive protein in young adult patients with migraine. Cephalalgia. 2007;27:843–846. doi: 10.1111/j.1468-2982.2007.01324.x. [DOI] [PubMed] [Google Scholar]
- Welch KM, Ramadan NM. Mitochondria, magnesium and migraine. J. Neurol. Sci. 1995;4(1–2):9–14. doi: 10.1016/0022-510x(95)00196-1. Review. [DOI] [PubMed] [Google Scholar]
- Wessman M, Kallela M, Kaunisto MA, Marttila P, Sobel E, Hartiala J, Oswell G, Leal SM, Papp JC, Hämäläinen E, Broas P, Joslyn G, Hovatta I, Hiekkalinna T, Kaprio J, Ott J, Cantor RM, Zwart JA, Ilmavirta M, Havanka H, Färkkilä M, Peltonen L, Palotie A. A susceptibility locus for migraine with aura, on chromosome 4q24. Am. J. Hum. Genet. 2002;70(3):652–662. doi: 10.1086/339078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessman M, Terwindt GM, Kaunisto MA, Palotie A, Ophoff RA. Migraine: a complex genetic disorder. Lancet Neurol. 2007;6:521–532. doi: 10.1016/S1474-4422(07)70126-6. [DOI] [PubMed] [Google Scholar]
- Willour VL, Zandi PP, Huo Y, Diggs TL, Chellis JL, MacKinnon DF, Simpson SG, McMahon FJ, Potash JB, Gershon ES, Reich T, Foroud T, Nurnberger JI, DePaulo JR, McInnis MG. Genome scan of the fifty-six bipolar pedigrees from the NIMH genetics initiative replication sample: chromosomes 4, 7, 9, 18, 19, 20, and 21. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2003;121B(1):21–27. doi: 10.1002/ajmg.b.20051. [DOI] [PubMed] [Google Scholar]
- World Health Organization. World Health Report 2002 [Google Scholar]
- Zandi PP, Badner JA, Steele J, Willour VL, Miao K, MacKinnon DF, Mondimore FM, Schweizer B, McInnis MG, DePaulo JR, Gershon E, McMahon FJ, Potash JB. Genome-wide linkage scan of 98 bipolar pedigrees and analysis of clinical covariates. Mol. Psychiatry. 2007;12(7):630–639. doi: 10.1038/sj.mp.4002027. [DOI] [PubMed] [Google Scholar]