Abstract
Magnetic resonance elastography (MRE) is an MRI-based noninvasive technique for quantitatively assessing tissue stiffness. The hypothesis of this study is that stiffness increases with portal pressure. We further hypothesized that the rate of stiffness change with pressure would be larger in liver tissue treated to simulate the stiffening effects of fibrosis. In agreement with our hypothesis, the formalin-treated livers were stiffer than the untreated livers, and in both groups the liver stiffness increased with portal venous pressure. The rate of stiffness change with portal pressure was significantly greater after formalin treatment. In this study, we have developed an ex vivo liver model incorporating portal venous pressure variations and observed significant changes in liver stiffness due to portal pressure. This model could be useful for understanding and investigating the changes in the static and dynamic components of liver stiffness.
Keywords: magnetic resonance elastography, liver, ex vivo, porcine model, portal hypertension, formalin
Introduction
Hepatic fibrosis is a common consequence of liver diseases caused by viruses, fat, alcohol, drugs and other risk factors. Magnetic resonance elastography (MRE) is an MRI-based noninvasive technique for quantitatively assessing tissue stiffness [1]. Studies have shown that MRE-assessed liver stiffness can be used to assess hepatic fibrosis, which systematically increases with disease progress, and that MRE offers a safer, less expensive and potentially more accurate alternative to invasive liver biopsy for determining hepatic fibrosis [2]. It has consistently been shown that liver MRE has excellent diagnostic accuracy for assessing hepatic fibrosis. Receiver operating characteristic (ROC) analysis has provided evidence that MR elastography can discriminate between patients with moderate and severe fibrosis and those with mild fibrosis [3, 4]. However, some studies, including those performed using ultrasound-based transient elastography, have found a discordance between liver stiffness and the biopsy-proven fibrosis stage [5, 6]. This could be caused by confounding factors such as inflammation and portal hypertension, which can elevate intrahepatic resistance and tissue tension via increased portal pressure [7–9]. It is possible that in order to more accurately assess hepatic fibrosis in patients with chronic liver disease, it may be helpful to consider two components of liver stiffness: a static component reflecting the extent of fibrosis and a dynamic, pressure-dependent stiffness component. Yin et al. reported a postprandial increase in hepatic stiffness in patients with hepatic fibrosis when compared with normal volunteers which may indicate that the portal pressure has a greater influence on liver stiffness in fibrotic livers than in normal ones [10]. Wallihan et al. and Serai et al. reported separately that patients with congenital heart disease palliated with the Fontan procedure had abnormal liver stiffness and a long Fontan duration resulted in a greater liver stiffness than a shorter duration; however, it was unknown if the increase in stiffness values was due to fibrosis or venous congestion [11–12]. Furthermore, an vivo animal study has shown that liver stiffness has a dynamic component that increases significantly with increases in portal pressure [13]. A possible explanation is that increasing intravascular pressure creates volumetric strain, causing increasing stiffness due to elastic nonlinearity of the tissue.
Direct portal pressure measurements in vivo can be inaccurate due to calibration variations and possible misplacement of the catheters. In this study, we developed an ex vivo liver model where portal pressure was well controlled and investigated the effect of portal pressure on liver stiffness before and after formalin treatment. The hypothesis of this study is that stiffness increases with portal pressure. We further hypothesized that the rate of stiffness change with pressure would be larger in liver tissue treated to simulate the stiffening effects of fibrosis.
2. Materials and methods
2.1 Ex vivo porcine liver portal pressure model
Six whole pig livers were harvested from six healthy domestic pigs in a local butcher shop (#1 and 2) and in an animal lab in our institute (#3–6). Pigs 1 and 2 were male, about 8 months old and 130 kg. Pigs 3–6 were female, about 3–5 months old and 60 kg.
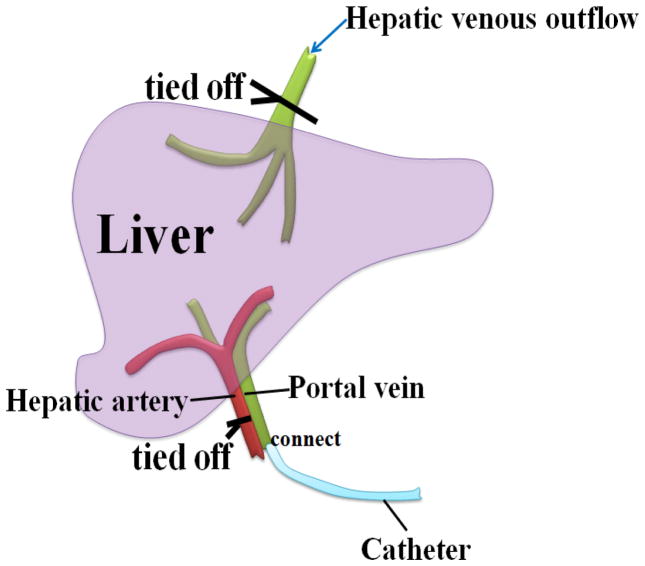
A catheter was inserted in the portal vein and the hepatic artery was tied. The catheter was connected to a saline bag hanging from an IV pole about 2 meters high. Each liver was perfused with 2L of saline (0.9% sodium chloride solution) to wash the blood out; we saw saline and blood flow out from the second hepatic portal. After the perfusion, the hepatic venous outflow (hepatic vein) was closed. The setup is shown in Fig. 1.
Fig. 1.
Liver specimen setup. The catheter was inserted in the portal vein and the hepatic artery was tied off.
The livers were kept at room temperature (22 °C) and MRE scans were performed within 6 hours of the harvest. The portal venous pressure was controlled by adjusting the height of the saline bag to 0, 20, 40, and 80 cmH2O. At each height, we waited 8 minutes for the pressure to stabilize before each MRE scan.
After the untreated livers were scanned, each specimen was immersed in a tank of 10% neutral buffered formalin (NBF, Fisher Scientific Company L.L.C., Kalamazoo, MI, USA) for 3–4 days. The MRE exams were repeated in all livers after the formalin treatment at the same 4 portal pressures (0, 20, 40, and 80 cmH2O) at room temperature.
2.2 Magnetic Resonance Elastography Acquisition
MRE scans were performed in a 1.5T whole-body MRI scanner (Signa HDxt, GE Healthcare, Milwaukee, WI, USA) with a four-channel torso coil. During the MRE scans, the liver was placed in a plastic container with a passive driver positioned on top of the liver; the driver was vibrating at 60 Hz continuously during the MRE acquisitions.
A two-shot multislice EPI MRE sequence was used for imaging the wave propagation in the liver. Data acquisition parameters included the following: 44.8-cm field of view (FOV), axial imaging plane, right-left frequency-encoding direction, 3.5-mm slice thickness, 72×72 image acquisition matrix, 128×128 reconstruction matrix, 32 slices, 3 time offsets, TR/TE = 1067/36.6 ms, parallel imaging acceleration factor = 1, motion sensitivity = 37.8 μm/π radians, 3 motion-encoding gradient (MEG) sensitization directions, receiver bandwidth = 250.00 kHz, total scan time = 1.5 minutes.
Images of tissue stiffness (elastograms) were calculated from the MRE wave data by first calculating the vector curl of MRE wave data, and then using the curl data to calculate tissue stiffness using a 3D direct inversion of the Helmholtz wave equation with 20 evenly spaced 3D directional filters incorporating a fourth-order Butterworth bandpass filter. The cut-off frequencies of the directional filters (0.001 and 24 cycles/FOV) were used to remove undesired low-frequency wave information due to background phase artifacts, longitudinal waves, and bulk motion, and to remove high-frequency noise.
ROIs were drawn manually in the entire hepatic parenchyma excluding major blood vessels in each slice, keep 2 pixels away from the edges and exclude the first and last 2 slices. Average stiffness values were measured for each slice; mean and standard deviation values of the average values were reported for the whole liver.
2.3 Statistical Analysis
A least-squares linear regression analysis was performed to calculate correlation coefficients between liver stiffness and portal pressure before and after formalin treatment. The slope of the linear fit for each liver was compared before and after formalin treatment using a paired Student’s t test. A p value of less than 0.05 indicates statistically significant difference. A correlation coefficient of greater than 0.8 indicates a strong correlation and a coefficient of less than 0.5 indicates a weak correlation. Statistical software (JMP 8.0; SAS, Cary, NC, USA) was used in this study.
3. Results
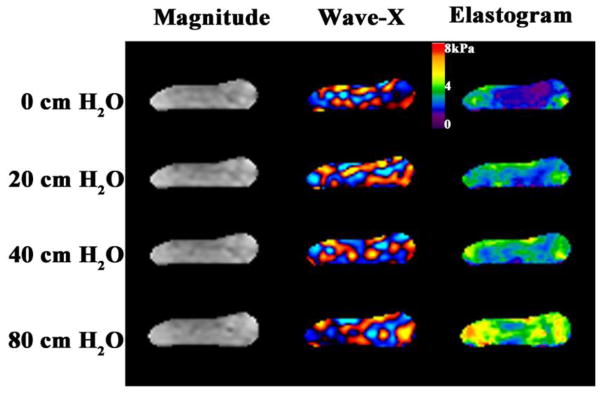
MRE data were showed from one of the six ex vivo livers at the four pressures (0, 20, 40, and 80 cmH2O) before (Figs. 2) and after formalin treatment, respectively. We observed that the wavelength of the shear waves and the liver stiffness increased with increased hydrostatic pressure with and without treatment for all six livers.
Fig. 2.
Fig. 2 shows the magnetization magnitude, one component of the MRE wave data, and the shear stiffness map (elastogram) of one liver at the four pressure levels (0, 20, 40, and 80 cm hydrostatic pressure (cmH2O)) before being immersed in formalin. The liver stiffness increased with increased hydrostatic pressure.
Before formalin treatment, the mean stiffness of the 6 livers was 2.63± 0.34 kPa at 0 cmH2O (baseline), 2.79 ± 0.48 kPa at 20 cmH2O, 3.03 ± 0.54 kPa at 40 cmH2O and 3.58 ± 0.65 kPa at 80 cmH2O. After the formalin treatment, the mean liver stiffness was 7.60 ± 1.25 kPa at 0 cmH2O (baseline), 7.87 ± 1.44 kPa at 20 cmH2O, 8.93 ± 2.13 kPa at 40 cmH2O and 10.00 ± 2.22 kPa at 80cmH2O.
The linear regression analysis showed that the liver stiffness was highly correlated to the hydrostatic pressure (Fig. 3) and the correlation coefficients (R) were greater than 0.8 for all livers, both before and after formalin treatment. The slope of the fits of liver stiffness versus pressure (kPa/cmH20) increased significantly after the formalin treatment for all livers. The range of the slopes was 0.0049 to 0.0174 (before) versus 0.0143 to 0.0528 (after). The t-test showed a significant difference between the means of the slopes: mean (± sd) = 0.0121 ± 0.0050 (before) versus 0.0316 ± 0.0157 (after), p =0.0419.
Fig. 3.
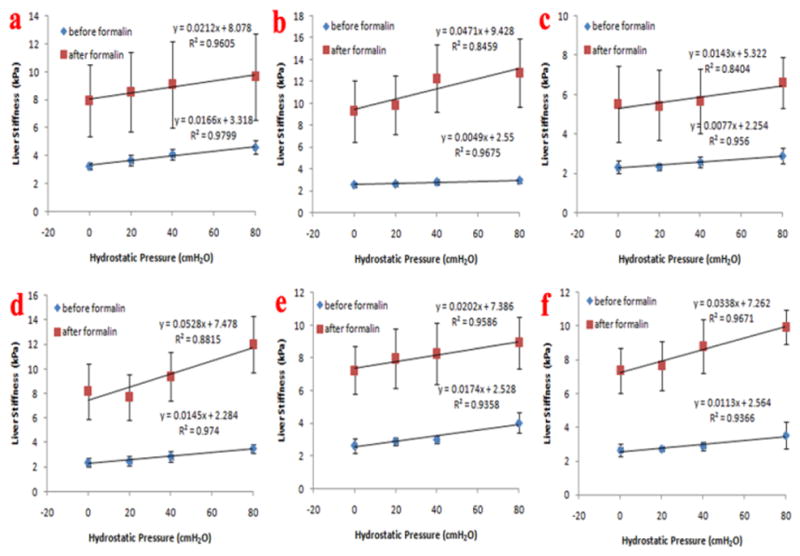
Fig. 3 shows the linear regression analysis for all six livers. The liver stiffness increased after the formalin treatment. The liver stiffness correlated very well with portal pressure for each liver (R>0.8). The linear regression analysis results show that the slope of the fits was larger for the formalin-treated livers than for the untreated livers.
4 Discussion
In this study, we observed that the six ex vivo fresh porcine livers all increased in stiffness systematically with an increase in portal venous pressure, firstly. The hepatic tissue is a biphasic system: a solid tissue matrix and a fluid-filled vascular tree, which create a static component and a dynamic component to liver mechanical properties [13–16]. Previous studies have shown that liver fibrosis changes the intracellular matrix of liver tissue, resulting in the elevation of the static component of liver stiffness. On the other hand, changes in the dynamic component of liver stiffness have also been observed in both human and animal in vivo studies [10, 13]. For example, liver stiffness increased with portal pressure, with correlation coefficients (R) from 0.59 to 0.97, in three in vivo pigs [13]. However, the direct portal pressure measurement used in vivo [13] could be inaccurate because of variations in the calibrations and possible misplacement of the catheters. In our ex vivo model, the portal pressure was well controlled by the height of the saline bag and was consistent throughout the six ex vivo livers. We found that liver stiffness increased with portal pressure and the correlation coefficient (R) was 0.935 to 0.979 in the six livers.
In this study, we changed the static component of liver stiffness by immersing the livers into formalin, secondly. The baseline liver stiffness was significantly greater after formalin treatment in all six livers. By doing this, we were able to investigate the effect of portal pressure on the liver stiffness in livers that already had elevated baseline stiffness values.
Studies have shown that formalin fixation may alter the intrinsic structural properties of the liver [17]. The use of 10% neutral buffed formalin can prevent extensive swelling or shrinkage of tissues, and just stiffen the tissues during fixation [18].
Our study showed that after formalin treatment, the baseline liver stiffness increased by about 3 times. In addition, the liver stiffness still increased with increasing portal venous pressure for all six livers (correlation coefficients (R): 0.840 to 0.967).
Our study also found that the slope of liver stiffness versus pressure increased significantly after the formalin treatment for all livers (p =0.0419). Before formalin treatment, the slope ranged from 0.0049 to 0.0174, mean (± sd) = 0.0121 ± 0.0050. After formalin treatment, the slope ranged from 0.0143 to 0.0528, mean (± sd) = 0.0316 ± 0.0157. A reasonable explanation for this observation is that formalin fixation increases the elastic nonlinearity of hepatic tissue. These results are in agreement with a previous patient study, where mean postprandial liver stiffness augmentation increased progressively with increased fibrosis [10].
The limitations of this study include the small sample size and potential thrombus formed in the ex vivo liver model. In our model, we closed the hepatic artery and hepatic venous outflow to maintain the portal venous pressure in the liver created by hanging the saline bag at different heights. However there was always some leaking from unclosed vessels or damaged hepatic tissues due to the harvesting of the liver, which could cause pressure variations during the experiments. A thrombus in the ex vivo livers would cause heterogeneous pressure distribution and heterogeneous tissue stiffness in the liver. In our ex vivo liver model preparation, we took care to wash thrombi from the livers using a saline flush before each experiment. Finally, we did not have a catheter based pressure sensor to validate the portal pressures set by the different saline bag height.
In conclusion, we have developed an ex vivo liver model incorporating portal venous pressure variations and observed significant changes in liver stiffness due to portal pressure. The liver stiffness increased with portal venous pressure and the rate of change was significantly greater after formalin treatment when the baseline liver stiffness was elevated. This model could be useful for understanding and investigating the changes in the static and dynamic components of liver stiffness that occur due to diseases related to abnormal hepatic blood flow and extracellular matrix remodeling, such as venous congestion or cirrhosis induced portal hypertension.
Acknowledgments
This study was supported by grants from National Institutes of Health (NIH) (Contract grant number: EB001981); and Clinical medical science and technology project of Jiangsu Province (Contract grant number: BL2012044).
The authors thank Diane M. Sauter and Joseph M. Kreidermacher for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Muthupillai R, Lomas DJ, Rossman PJ, Greenleaf JF, Manduca A, Ehman RL. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science. 1995;269:1854–1857. doi: 10.1126/science.7569924. [DOI] [PubMed] [Google Scholar]
- 2.Binkovitz LA, El-Youssef M, Glaser KJ, Yin M, Binkovitz AK, Ehman RL. Pediatric MR elastography of hepatic fibrosis: principles, technique and early clinical experience. Pediatric radiology. 2012;42:402–409. doi: 10.1007/s00247-011-2298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin M, Talwalkar JA, Glaser KJ, et al. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin Gastroenterol Hepatol. 2007;5:1207–1213. e2. doi: 10.1016/j.cgh.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huwart L, Sempoux C, Vicaut E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology. 2008;135:32–40. doi: 10.1053/j.gastro.2008.03.076. [DOI] [PubMed] [Google Scholar]
- 5.Wong VW, Vergniol J, Wong GL, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454–462. doi: 10.1002/hep.23312. [DOI] [PubMed] [Google Scholar]
- 6.Calvaruso V, Camma C, Di Marco V, et al. Fibrosis staging in chronic hepatitis C: analysis of discordance between transient elastography and liver biopsy. Journal of viral hepatitis. 2010;17:469–474. doi: 10.1111/j.1365-2893.2009.01199.x. [DOI] [PubMed] [Google Scholar]
- 7.Arena U, Vizzutti F, Corti G, et al. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology. 2008;47:380–384. doi: 10.1002/hep.22007. [DOI] [PubMed] [Google Scholar]
- 8.Sagir A, Erhardt A, Schmitt M, Häussinger D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology. 2008;47:592–595. doi: 10.1002/hep.22056. [DOI] [PubMed] [Google Scholar]
- 9.Guturu P, Shah V. New insights into the pathobiology of portal hypertension. Hepatol Res. 2009;39:1016–1019. doi: 10.1111/j.1872-034X.2009.00553.x. [DOI] [PubMed] [Google Scholar]
- 10.Yin M, Talwalkar JA, Glaser KJ, et al. Dynamic postprandial hepatic stiffness augmentation assessed with MR elastography in patients with chronic liver disease. AJR Am J Roentgenol. 2011;197:64–70. doi: 10.2214/AJR.10.5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallihan DB, Podberesky DJ, Marino BS, Sticka JS, Serai S. Relationship of MR elastography determined liver stiffness with cardiac function after Fontan palliation. J Magn Reson Imaging. 2014;40:1328–1335. doi: 10.1002/jmri.24496. [DOI] [PubMed] [Google Scholar]
- 12.Serai SD, Wallihan DB, Venkatesh SK, et al. Magnetic resonance elastography of the liver in patients status-post fontan procedure: feasibility and preliminary results. Congenit Heart Dis. 2014;9:7–14. doi: 10.1111/chd.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yin M, Kolipaka A, Woodrum DA, et al. Hepatic and splenic stiffness augmentation assessed with MR elastography in an in vivo porcine portal hypertension model. J Magn Reson Imaging. 2013;38:809–815. doi: 10.1002/jmri.24049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nedredal GI, Yin M, McKenzie T, et al. Portal hypertension correlates with splenic stiffness as measured with MR elastography. J Magn Reson Imaging. 2011;34:79–87. doi: 10.1002/jmri.22610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talwalkar JA, Yin M, Venkatesh S, et al. Feasibility of in vivo MR elastographic splenic stiffness measurements in the assessment of portal hypertension. AJR Am J Roentgenol. 2009;193:122–127. doi: 10.2214/AJR.07.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirsch S, Guo J, Reiter R, et al. Towards compression-sensitive magnetic resonance elastography of the liver: sensitivity of harmonic volumetric strain to portal hypertension. J Magn Reson Imaging. 2014;39:298–306. doi: 10.1002/jmri.24165. [DOI] [PubMed] [Google Scholar]
- 17.Xu L, Chen J, Yin M, et al. Assessment of stiffness changes in the ex vivo porcine aortic wall using magnetic resonance elastography. Magn Reson Imaging. 2012;30:122–127. doi: 10.1016/j.mri.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chesnick IE, Mason JT, O’Leary TJ, Fowler CB. Elevated Pressure Improves the Rate of Formalin Penetration while Preserving Tissue Morphology. J Cancer. 2010;1:178–183. doi: 10.7150/jca.1.178. [DOI] [PMC free article] [PubMed] [Google Scholar]