Abstract
Objective
To examine the temporal associations, within-day and day-to-day, between pain, fatigue, depressed mood, and cognitive function in multiple sclerosis (MS).
Design
Repeated-measures study involving seven days of ecological momentary assessment (EMA) of symptoms five times a day. Multilevel mixed models were used to analyze data.
Setting
Community.
Participants
Ambulatory adults (N=107) with MS.
Interventions
Not applicable.
Main Outcome Measure(s)
EMA of pain, fatigue, depressed mood, and cognitive function rated on a 0–10 scale.
Results
Fatigue and pain were linked within-day such that higher pain was associated with higher subsequent fatigue (B=0.09, p=0.04; likewise, higher fatigue was associated with higher pain in the following time frame (B=0.05, p=0.04). Poorer perceived cognitive function preceded increased subsequent pain (B=0.08, p=0.007) and fatigue (B=0.10, p=0.01) within-day. Depressed mood was not temporally linked with other symptoms. In terms of day-to-day effects, a day of higher fatigue related to decreased next day fatigue (B=−0.16, p=0.01), and a day of higher depressed mood related to increased depressed mood the next day (B=0.17, p=0.01). There were no cross-symptom associations from one day to the next.
Conclusions
Findings provide new insights on how common symptoms in MS relate to each other and vary within and over days. Pain and fatigue show evidence of a dynamic bidirectional relation over the course of a day, and worsening of perceived cognitive function preceded worsening of both pain and fatigue. Most temporal associations between symptoms occur within the course of a day, with relatively little carry-over from one day to the next.
Keywords: multiple sclerosis, pain, fatigue, depressed mood, cognitive function, ecological momentary assessment
Multiple sclerosis (MS), a progressive neurological disorder,1–3 is often associated with a complex symptoms burden, including chronic pain4–7, fatigue8–13, depression14–16, and cognitive dysfunction17–22. As there is no cure for MS, efforts to stabilize the disease with disease-modifying therapies and treatments to manage comorbid symptoms and improve functioning are the mainstays of MS patient care.
There is growing recognition that the confluence of symptoms, rather than any single symptom, determines quality of life, functional ability, and efficacy of symptom-specific interventions in MS23–25. Despite decades of research, our understanding of the complex nature of MS-related symptoms is limited. Research examining associations between commonly comorbid symptoms in MS is increasing. For instance, studies that have considered multiple MS symptoms have consistently identified pain, fatigue, depressed mood23, 26–30, and cognitive problems24, 31 as symptoms that cluster together. Although these studies have advanced our understanding of how symptoms correlate in MS, a number of questions about the nature of these symptoms remain. For example, an overreliance on cross-sectional research has limited our knowledge of how symptoms are experienced in the daily lives of people with MS. With the exception of three papers examining the daily variability in fatigue31–33, little is known about the variability or stability of symptoms or about dynamic temporal associations between symptoms in the daily lives of persons with MS.
We have demonstrated that daily within-person symptom variability differs depending on the symptom, with fatigue showing the most variability relative to pain, depressed mood, and cognitive function (see companion paper34). To build on this finding and increase our understanding of the covariation of symptoms from moment-to-moment and day-to-day in MS, this study used ecological momentary assessment (EMA), to assess symptoms (pain, fatigue, depressed mood, and cognitive function) in real-time at five daily intervals over seven days. One benefit to this methodology, in addition to improving data reliability, is that it allows for the examination of temporal associations between symptoms in MS.
The goal of this analysis was to examine the within-person temporal associations between real-time self-reported symptoms of pain, fatigue, depressed mood, and cognitive function on two time scales: 1) moment-to-moment within a day; and 2) from one day to the next. Within-day, we expected that pain would be associated with later fatigue (consistent with the common patient complaint that pain is tiring) and that fatigue and depressed mood would be positively associated (consistent with recent findings33).
Methods
Participants
Ambulatory adults with clinically definite MS (confirmed by medical record) were recruited. Inclusion criteria were: 1) ≥ 18 years of age; 2) able to speak and read English at a 6th grade level 3) able to ambulate with minimal assistance (e.g. use of a cane/walker allowed). Exclusion criteria were: 1) MS exacerbation within the past 30 days; 2) an atypical sleep/wake pattern (e.g. sleeping during the day due to shift work); 3) diagnosis of rheumatological disease or fibromyalgia; 4) adjustments in disease-modifying therapy regimens during study.
Study Procedures
This was a repeated-measures observational study that utilized a combination of recall surveys, EMA of symptoms, objectively measured physical activity (accelerometer), and end-of-day diaries. This paper focuses on survey and EMA measures. Further study details can be found in our companion papers34, 35. Data were collected at [masked] between October 2014 and March 2016. Institutional Review Board approval was granted prior to study initiation. A total of 108 participants were were recruited in an around the community of [masked] through doctor referrals, flyers placed in clinical and community locations, electronic medical records, existing participant registries, and via web-based promotion [masked]. Records on numbers of participants recruited by each means were not retained. Eligibility screening was conducted over the telephone. When electronic medical records for volunteers were accessible, they were checked for MS diagnosis; in other cases copies of medical records confirming MS diagnosis were obtained at the volunteers request from his/her physician.
Eligible volunteers came to the lab where they underwent informed consent, completed a baseline survey battery, and received training on how to complete daily on-line diaries and use the PRO-Diarya (CamNTech, Cambridge, United Kingdom), a wrist-worn accelerometer enhanced with a user interface for entry of self-report data. At the end of the lab visit, participants were given a PRO-Diary, a logbook (for noting problems/exceptions/context), and a pre-paid box for returning the PRO-Diary. The day following the lab visit began the 7-day home monitoring period, during which participants wore the PRO-Diary on the non-dominant wrist continuously, except while bathing or swimming. The PRO-Diary was used to collect and store physical activity and time-stamped EMA data until it was returned to the lab where data were downloaded. Participants logged pain, fatigue, depression, and cognitive function five times a day: upon waking, 11AM, 3PM, 7PM, and bedtime. An audible alarm alerted participants to enter ratings at 11AM, 3PM, and 7PM, and participant-initiated ratings at wake and bed times. At the end of the home monitoring period, participants mailed the PRO-Diary to the lab and were compensated commensurate with the number of days for which they provided data.
Measures
Baseline Measures
Self-report measures of demographic (age, race, sex, educational level/total years of education, and employment status) and clinical (time since MS diagnosis) data were collected. MS diagnosis and subtype were retrieved from participants’ medical records.
Ecological Momentary Assessments
As outlined in the companion paper, four EMA items were developed and demonstrated good convergent validity with standard surveys administered daily during the home monitoring period34.
Pain intensity was assessed with the question: “What is your level of pain right now?” rated on a numerical rating scale from 0 = “no pain” to 10 = “worst pain imaginable.” Fatigue was assessed with the question: “What is your level of fatigue right now?” rated on a scale from 0 = “no fatigue” to 10 = “extremely severe fatigue.” Depressed mood was assessed with the question: “What is your level of depression right now?” on a scale from 0 = “Not at all depressed” to 10 = “Extremely depressed.”
Perceived Cognitive Function was assessed with the question: “What is your level of cognitive functioning right now?” on a scale from 0 = “Good: my thinking is sharp and quick” to 10= “Bad: my thinking is very difficult or slow.”
Data Analyses
Preliminary Data Analyses
Descriptive statistics were generated to examine data distribution. Pearson correlation analyses were used to examine associations between aggregated EMA symptom variables. Missing data rates were calculated. Sample means of EMA ratings were graphed for each time point to depict moment-to-moment levels and variability in each symptom.
Primary Data Analyses
Multilevel random effects modeling (MLM) was applied to examine the temporal association between EMA symptoms. This statistical approach utilizes as many data points as possible by retaining cases with missing within-person data and allows for modelling of random effects, which is based on the premise that the data represents a random sample of a larger range of possible values. Prior to conducting analyses, the dataset was arranged so that associations between EMA symptoms in the previous and next time points could be examined. EMA variables were centered to create person-centered deviation scores, such that the centered value indicated the momentary change (for moment-to-moment analyses) or daily change (for day-to-day analyses) relative to each person’s own weekly average32,33. Centering in this manner allows for examination of within-person and between-person variance separately36, 37. SAS PROC MIXED software was used to simultaneously model between-person and within-person variance and account for the auto-correlation between adjacent observations. Four MLMs for momentary (within-day) associations, one for each EMA symptom, were constructed. In each case, variables of interest were all four EMA symptom ratings from the previous time point (including the previous rating for the criterion symptom). All momentary MLMs were run within-day. Similarly, four MLMs for day-to-day associations, one for each symptom, were constructed. In each case, variables of interest were the daily average of EMA symptom ratings (centered) from the previous day. A person’s average level of each EMA symptom was included in all models to account for between-person differences in symptom levels. Clinical and demographic variables (age, sex, MS duration, MS subtype) that were deemed clinically-relevant to symptom experience were included as covariates and retained in the models regardless of statistical significance. Categorical covariates were dummy coded38; one dummy code each was created for sex (male=reference) and MS subtype (relapsing remitting MS (RRMS) was contrasted with all progressive types, collapsed into a single reference category). To determine effect size, we calculated the amount of shared variance (pseudo-R2) between momentary symptoms that showed significant associations39. Statistical tests were performed using SASb version 9.4 (SAS Institute, Cary, NC, USA).
Results
As reported in companion papers34, 35, 108 volunteers enrolled and completed baseline measures. One person withdrew prior to beginning home monitoring; data from 107 individuals were analyzed. As shown in Table 1, average age was approximately 45 years and time since MS diagnosis was about 9.5 years. Most participants were white and female, and nearly three-quarters were characterized as having RRMS. Generally, symptom burden, as indicated by weekly-averaged EMA ratings, was relatively mild in this sample; scores for EMA fatigue were highest, but are still in the “mild” range. Importantly, this sample was found to be similar to other, larger MS research samples in terms of sample-average scores on standardized symptom surveys34. Out of a possible 35 EMA data points per person, 83.4% (3145/3745) of the data were complete.
Table 1.
Descriptive statistics for demographic and baseline study variables (N = 107)
| Mean (SD) | Min-Max | |
|---|---|---|
|
|
||
| Age | 45.16 (11.73) | 23 − 67 |
| MS Duration (years) | 9.49 (8.36) | <1 − 44 |
| EMA Fatigue | 3.41 (1.94) | 0 − 8.10 |
| EMA Pain | 2.44 (1.74) | 0 − 6. |
| EMA Depressive Symptoms | 1.20 (1.62) | 0 − 8.37 |
| EMA Cognitive Problems | 1.78 (1.76) | 0 − 8.25 |
| N (%) | ||
| Sex (Female) | 74 (69.2%) | |
| MS Subtype | ||
| Relapsing Remitting | 78 (72.9) | |
| Primary Progressive | 14 (13.1) | |
| Progressive Relapsing | 2 (1.9) | |
| Secondary Progressing | 13 (12.1) | |
| Unknown | ||
| Race | ||
| White | 88 (82.2%) | |
| Black | 10 (10.7%) | |
| Asian | 6 (5.6%) | |
| Native | 2 (1.9%) | |
| Biracial (Black/White) | 1 (0.9%) | |
| Hispanic | 1 (0.9%) | |
| Employment | ||
| Employed Full-time | 41 (38.3%) | |
| Employed Part-Time | 19 (17.8%) | |
| Unemployed | 47 (43.9%) | |
| Education | ||
| Some high school | 1 (0.9%) | |
| HS grad/GED | 13 (12.1%) | |
| Vocational or Tech school | 2 (1.9%) | |
| Some College | 26 (24.3%) | |
| College Grad | 41 (38.3%) | |
| Graduate school/Prof school | 24 (22.4%) | |
Note. EMA variables are person-averaged (aggregated across the week of home monitoring for each person).
Correlations between aggregated EMA symptoms ratings (Table 2.) were all statistically significant and in the moderate (r ≥0.30) to large (r ≥0.50) effect size range. In absolute terms, the correlation between pain and fatigue was highest (r = 0.70) and the correlation between pain and depression was lowest (r = 0.39).
Table 2.
Bivariate Pearson correlations between person-averaged (aggregated) ecological momentary assessment (EMA) ratings of pain, fatigue, depressive symptoms, and cognitive problems.
| Pain | Depressive Symptoms | Cognitive Problems | |
|---|---|---|---|
|
|
|||
| Fatigue | 0.70 | 0.48 | 0.66 |
| Pain | – | 0.39 | 0.55 |
| Depressive Symptoms | – | 0.53 | |
Note. All p < 0.001; N = 107.
As can be seen in Figures 1–4, EMA symptom ratings were relatively low. Fatigue was the most and depressed the least variable within-person and fatigue showed the greatest “diurnal effect” with ratings rising across the day to peak levels at night.
Figures 1.
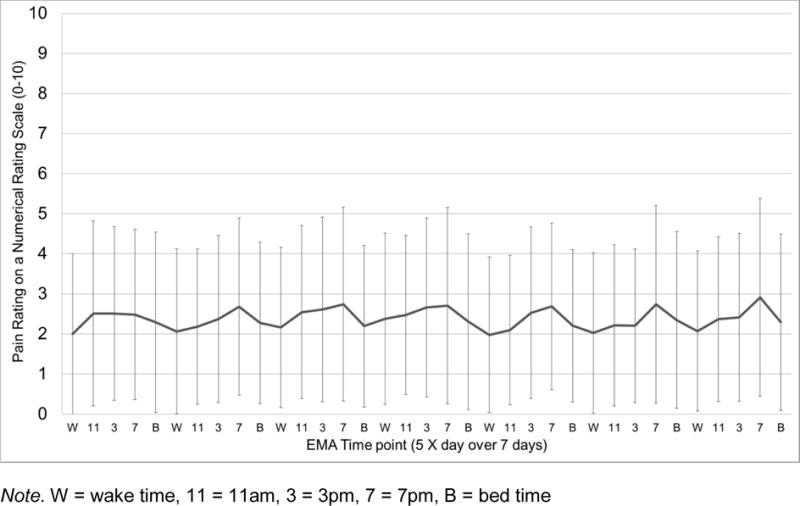
Sample mean of momentary pain intensity levels (NRS 0–10) across the 35 EMA time points (5 X day for 7 days). Error bars depict standard deviations.
Figures 4.
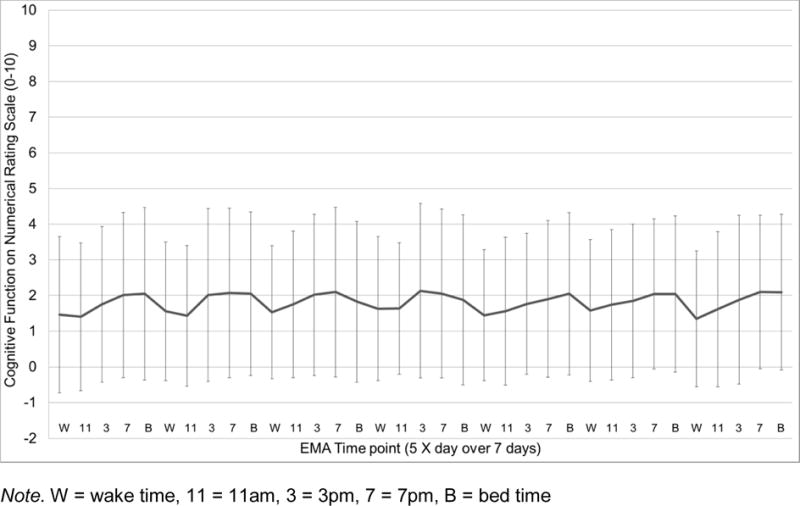
Sample mean of momentary perceived cognitive function (NRS 0–10) across the 35 EMA time points (5 X day for 7 days). Error bars depict standard deviations.
Within-person moment-to-moment temporal associations amongst symptoms
MLM results examining the within-day associations between later symptom ratings and earlier symptom ratings (Table 3.) indicate significant associations between aggregate symptom scores (between-person levels) and momentary symptoms for a single symptom (e.g. a person with higher pain in general reported higher momentary pain). The only cross-symptom association for aggregated symptoms was for pain; those with higher average pain reported lower momentary fatigue (B=−0.09, p=0.004). MLMs also showed strong associations between ratings of a single symptom at different time points for all four symptoms; that is, pain at one time point was strongly related to pain at the next time point. The findings support the notion that there are temporal associations across symptoms, even when controlling for the criterion symptom in the previous time point. Specifically, greater pain ratings were preceded by worse fatigue (B=0.05, p=0.04) and cognitive function (B=0.08, p=0.007), which collectively accounted for 9% of the within-person variance in momentary pain. Similarly, two symptoms were significantly associated with subsequent fatigue, pain (B=0.09, p=0.04) and cognitive function (B=0.10, p=0.01) were associated with greater fatigue in the next time frame and, together, accounted for 5.8% of within-person variance in momentary fatigue. Notably, depressed mood was only associated with previous depressed mood. Similarly, cognitive function was only associated with previous cognitive symptoms; although there was a trend for greater fatigue to relate to later cognitive function (B=0.05, p=0.06).
Table 3.
Within-day (moment-to-moment) temporal associations between symptoms, controlling for age, sex, MS subtype, time sinceMS diagnosis, and average symptom levels: Results from results random effects multilevel models
| Random Effects | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Pain | Fatigue | Depressive Symptoms | Cognitive Problems | |||||||||
| Est. | SE | P | Est. | SE | p | Est. | SE | p | Est. | SE | p | |
|
|
||||||||||||
| AR(1) | 0.06 | 0.05 | 0.19 | −0.01 | 0.03 | 0.74 | −0.16 | 0.04 | <0.001 | 0.02 | 0.04 | 0.56 |
| Residual | 1.58 | 0.05 | <0.001 | 3.05 | 0.09 | <0.001 | 0.93 | 0.03 | <0.001 | 1.32 | 0.04 | <0.001 |
| Fixed Effects | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Pain (df = 2088) |
Fatigue (df = 2087) |
Depressive Symptoms (df = 2072) |
Cognitive Problems (df = 2086) |
||||||||||
| B | SE | P | B | SE | p | B | SE | p | B | SE | p | ||
|
|
|||||||||||||
| Between-Person Variables (time invariant) df = 98 | |||||||||||||
| Intercept | −0.19 | 0.19 | 0.31 | −0.16 | 0.18 | 0.37 | −0.07 | 0.08 | 0.38 | −0.16 | 0.14 | 0.25 | |
| Female | 0.10 | 0.07 | 0.14 | 0.11 | 0.08 | 0.17 | 0.01 | 0.03 | 0.68 | 0.06 | 0.06 | 0.34 | |
| MS Type | 0.02 | 0.09 | 0.86 | 0.01 | 0.08 | 0.89 | 0.02 | 0.03 | 0.60 | 0.07 | 0.06 | 0.23 | |
| Age | 0.003 | 0.003 | 0.37 | 0.006 | 0.003 | 0.04 | 0.0008 | 0.001 | 0.55 | 0.002 | 0.002 | 0.33 | |
| MS Duration | −0.004 | 0.003 | 0.28 | 0.008 | 0.004 | 0.03 | 0.003 | 0.002 | 0.85 | −0.003 | 0.003 | 0.37 | |
| Pain* | 1.01 | 0.02 | <0.001 | −0.09 | 0.03 | 0.004 | 0.003 | 0.01 | 0.80 | 0.003 | 0.03 | 0.92 | |
| Fatigue* | 0.03 | 0.03 | 0.39 | 1.07 | 0.03 | <0.001 | 0.02 | 0.02 | 0.37 | −0.03 | 0.03 | 0.35 | |
| Depressive* | −0.02 | 0.02 | 0.39 | −0.04 | 0.03 | 0.06 | 1.04 | 0.01 | <0.001 | 0.05 | 0.03 | 0.14 | |
| Cognitive* | 0.03 | 0.11 | 0.91 | 0.06 | 0.03 | 0.06 | 0.007 | 0.03 | 0.80 | 1.05 | 0.02 | <0.001 | |
| Within-Person Predictor Variables – Symptoms (previous timepoint) | |||||||||||||
| Δ Pain | 0.20 | 0.03 | <0.001 | 0.09 | 0.04 | 0.04 | −0.008 | 0.02 | 0.62 | −0.001 | 0.03 | 0.96 | |
| Δ Fatigue | 0.05 | 0.03 | 0.04 | 0.21 | 0.03 | <0.001 | 0.006 | 0.01 | 0.61 | 0.05 | 0.02 | 0.06 | |
| Δ Depressive | 0.07 | 0.05 | 0.20 | 0.13 | 0.08 | 0.09 | 0.46 | 0.04 | <0.001 | 0.07 | 0.05 | 0.13 | |
| Δ Cognitive | 0.08 | 0.03 | 0.007 | 0.10 | 0.04 | 0.01 | 0.007 | 0.03 | 0.80 | 0.19 | 0.04 | <0.001 | |
Note. Est. = covariance parameter estimate; B = unstandardized beta; SE = standard error; * = person-average of EMA values over the week-long home monitoring period; Δ = person-centered variable representing deviation (change) from a persons average. An (AR) autoregressive matrix was used to model the error variance. Female = Male was reference category; MS Type = relapsing remitting MS subtype, progressive subtypes was reference category
Within-person day-to-day temporal associations amongst symptoms
MLM results examining next-day associations between symptom ratings from previous day symptom ratings (Table 4.) showed associations between average and momentary levels of a symptom (e.g., higher average pain was related to higher daily pain), but no cross-symptom associations. In terms of day-to-day analyses, there were no cross-symptom associations from one day to the next. Interestingly, fatigue showed a negative day-to-day association such that a day of higher fatigue was associated with lower fatigue the next day (B=−0.16, p=0.01; 2.2% of within-person variance). In contrast, a day of higher depressed mood was associated with greater depressed mood the following day (B=0.17, p=0.01; 3.7% of within-person variance). Notably, neither pain nor cognitive function related to next day ratings of the same symptom.
Table 4.
Day-to-day temporal associations between symptoms, controlling for age, sex, MS subtype, time since MS diagnosis, and average symptoms levels: Results from results random effects multilevel models.
| Random Effects | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Pain | Fatigue | Depressive Symptoms | Cognitive Problems | ||||||||||
| Est. | SE | P | Est. | SE | p | Est. | SE | p | Est. | SE | p | ||
|
|
|||||||||||||
| AR(1) | 0.06 | 0.06 | 0.31 | −0.008 | 0.07 | 0.91 | −0.14 | 0.08 | 0.06 | 0.04 | 0.06 | 0.57 | |
| Residual | 0.68 | 0.04 | <0.001 | 0.88 | 0.05 | <0.001 | 0.59 | 0.04 | <0.001 | 0.47 | 0.03 | <0.001 | |
| Fixed Effects | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Pain (df = 508) |
Fatigue (df = 507) |
Depressive Symptoms (df = 507) |
Cognitive Problems (df = 507) |
|||||||||
| B | SE | P | B | SE | p | B | SE | p | B | SE | p | |
|
|
||||||||||||
| Between-Person Variables (time invariant) df = 98 | ||||||||||||
| Intercept | −0.05 | 0.11 | 0.63 | 0.007 | 0.09 | 0.93 | 0.06 | 0.07 | 0.44 | −0.02 | 0.12 | 0.88 |
| Female | −0.01 | 0.05 | 0.82 | 0.02 | 0.05 | 0.78 | −0.03 | 0.04 | 0.43 | −0.03 | 0.04 | 0.53 |
| MS Type | 0.07 | 0.05 | 0.22 | 0.04 | 0.05 | 0.40 | 0.04 | 0.03 | 0.19 | 0.04 | 0.07 | 0.57 |
| Age | 0.004 | 0.002 | 0.80 | −0.002 | 0.02 | 0.27 | −0.002 | 0.01 | 0.22 | 0.0005 | 0.002 | 0.82 |
| MS Duration | 0.02 | 0.03 | 0.45 | 0.006 | 0.003 | 0.03 | 0.002 | 0.002 | 0.34 | 0.002 | 0.002 | 0.32 |
| Pain* | 1.03 | 0.02 | <0.001 | 0.008 | 0.01 | 0.55 | 0.02 | 0.02 | 0.24 | −0.001 | 0.02 | 0.94 |
| Fatigue* | −0.03 | 0.02 | 0.25 | 1.00 | 0.02 | <0.001 | 0.003 | 0.02 | 0.86 | −0.01 | 0.02 | 0.48 |
| Depressive* | −0.008 | 0.02 | 0.61 | −0.02 | 0.01 | 0.06 | 1.02 | 0.01 | <0.001 | −0.006 | 0.02 | 0.73 |
| Cognitive* | 0.02 | 0.02 | 0.54 | 0.02 | 0.02 | 0.23 | −0.01 | 0.01 | 0.33 | 1.03 | 0.04 | <0.001 |
| Within-Person Predictor Variables – Symptoms (previous day) | ||||||||||||
| Δ Pain | 0.08 | 0.06 | 0.18 | 0.08 | 0.07 | 0.22 | 0.0006 | 0.04 | 0.99 | 0.04 | 0.05 | 0.46 |
| Δ Fatigue | −0.003 | 0.06 | 0.95 | −0.16 | 0.06 | 0.01 | −0.03 | 0.05 | 0.53 | −0.04 | 0.04 | 0.35 |
| Δ Depressive | −0.005 | 0.07 | 0.95 | 0.07 | 0.06 | 0.26 | 0.17 | 0.07 | 0.01 | 0.07 | 0.04 | 0.13 |
| Δ Cognitive | 0.02 | 0.05 | 0.64 | 0.08 | 0.06 | 0.21 | −0.04 | 0.08 | 0.67 | 0.05 | 0.06 | 0.48 |
Note. Est. = covariance parameter estimate; B = unstandardized beta; SE = standard error; * = person-average of EMA values over the week-long home monitoring period; Δ = person-centered variable representing deviation (change) from a person’s average. An (AR) autoregressive matrix was used to model the error variance. Female = Male was reference category; MS Type = relapsing remitting MS subtype, progressive subtypes was reference category
Discussion
In this first-of-its-kind study to examine temporal associations between four of the most common symptoms of MS, results suggest that cross-symptom associations exhibit more robust temporal associations over shorter within-day time frames than across consecutive days. For within-day, moment-to-moment associations, pain and fatigue showed a bi-directional relationship; greater pain was associated with greater subsequent fatigue, and greater fatigue related to greater subsequent pain. This finding, paired with a strong between-person correlation between average levels of pain and fatigue (r=0.70), suggest a substantial link between pain and fatigue in MS. Conversely, depressed mood did not demonstrate significant temporal associations with other symptoms; this, coupled with the relatively modest between-person correlations between depressive and other symptoms (r’s range 0.39–0.53) suggest that depressed mood is largely independent from short-term within-person covariation with other symptoms in MS. It is possible that the relatively low levels and low within-person variability of depressed mood34 compared to the other symptoms partially explain the lack of daily associations. However, results for cognitive function, which were also quite low and stable in this sample, demonstrated that higher momentary cognitive problem ratings were associated with later increases in both pain and fatigue.
Links between cognition and pain have been demonstrated in experimental and imaging research40–46; although most have explored how pain disrupts cognitive performance41–46, or how pain-specific cognitive processes (e.g. attention) can either augment or attenuate pain40, one study demonstrated immediate but short-lived changes in fatigue and pain following a cognitively-demanding task in an osteoarthritis sample47. Cognitive problems have typically been seen as a consequence of pain and fatigue; in contrast, our data implicate worse than average perceived cognitive function as a sort of “leading indicator” of later increases in pain and fatigue. Potential mechanisms of these associations include shared central nervous system processes underlying fluctuations in pain, fatigue, and cognition, and behavioral mechanisms, such as poorer attentional control and/or cognitive/emotional self-regulation related to declines in cognitive function, which could contribute to later worsening of pain and fatigue.
Significant correlations between average symptom levels are consistent with previous cross-sectional research concluding that pain, fatigue, and depressive and cognitive symptoms often cluster together in ms4,23,26,27,29,30,48–50. Taken together, these findings underscore the importance of considering both between-person and within-person processes; for example, although the extant data support the hypothesis that people who report higher depressed mood also report higher pain, these data suggest that when a person feels particularly depressed, they are not more likely to experience a subsequent increase in pain on a typical day.
There were fewer temporal associations between symptoms across consecutive days, suggesting that for the most part, chronic MS symptoms are not particularly influenced by symptom carryover from the previous day. There are two notable exceptions. First, a day of higher than average fatigue was associated withlower fatigue the next day. Interestingly, this finding is highly consistent with our previous data that showed large within-person variability in fatigue and the notion that fatigue is dynamic day-to-day34. In contrast, depressed mood, which we found to be relatively stable for a person34, showed a positive association across days; that is, a day of higher depressed mood related to greater depressed mood the following day. Pain and cognitive function were not associated with same-symptom levels the next day and there were no cross-symptom associations across days. Several factors could explain the observed day-to-day associations, and lack of significant symptom carryover across days. In terms of fatigue, it is possible that individuals curb or pace activity the day after a day of especially high fatigue, resulting in relatively lower fatigue the following day. Sleep could also play a key role in the day-to-day symptom experience (including the lack of finding for pain and cognitive function), and warrants further exploration. For example, the possibility exists that individuals may prioritize sleep on days of particularly high fatigue, which in turn result in lower fatigue the next day. In contrast, sleep may be less restful after a day of feeling unusually depressed; which could in turn maintain or even exacerbate depressed mood the following day. Such relationships warrant further exploration.
Study Limitations
This study examined a subset of common MS symptoms, which were chosen based on their response to behavioral intervention. Future examination of other consequential MS symptoms (e.g., weakness, balance problems) is needed. Study sample characteristics, including predominantly white and female participants, exclusion of non-ambulatory individuals, and modest symptom burden may limit our ability to generalize findings to the broader MS population; however, this study sample was found to be similar to other larger study samples in terms of symptom burden as measured by standardized recall surveys51,52. As we note in the companion paper, the reasons for higher symptom ratings on surveys compared to EMA is not clear, and warrants further study34. EMA data collection can take many forms53, with inter-assessment intervals of different length, frequency, and schedule (i.e. fixed/variable). Temporal associations between symptoms may be different depending on time-frame and schedule. Findings from this study warrant replication and further examination. Although we employed the most appropriate statistical approach available, the use of multiple independent MLMs is limited by the fact that fitting these models separately does not allow correlations between the errors and the models assume equally-spaced EMA time points. Effect sizes of moment-to-moment associations between symptoms were small in absolute terms. However, the significance of these finding must be evaluated in the context of their relevance in the day-to-day lives of those who live with MS54,55 and with the understanding that in studies of momentary associations, there may be a larger cumulative effect of even “minuscule” variance values over time54.
Conclusions
Symptoms experienced by people with MS are dynamic, and exhibit distinct cross-symptom temporal associations within-but not across-days. Although causal pathways between symptoms cannot be elucidated from these data, results indicate that temporal associations between symptoms may be unique to specific sets of symptoms. Although symptoms in MS are often thought to “cluster together”, our data suggest a greater degree of specificity in terms of which and in what order symptoms are experienced on a moment-to-moment and day-to-day basis. Improved clarity about the dynamics and associations between symptoms as they are experienced in daily life could improve assessment and treatment of these MS symptoms.
Figures 2.
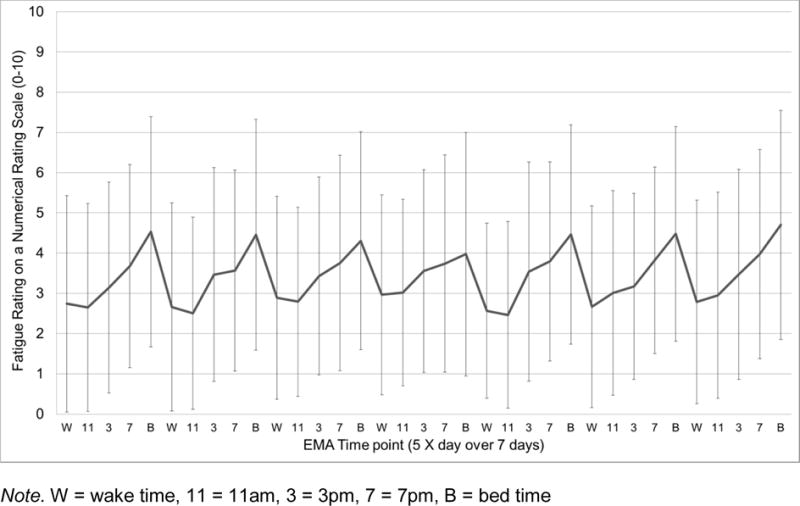
Sample mean of momentary fatigue intensity levels (NRS 0–10) across the 35 EMA time points (5 X day for 7 days). Error bars depict standard deviations.
Figures 3.
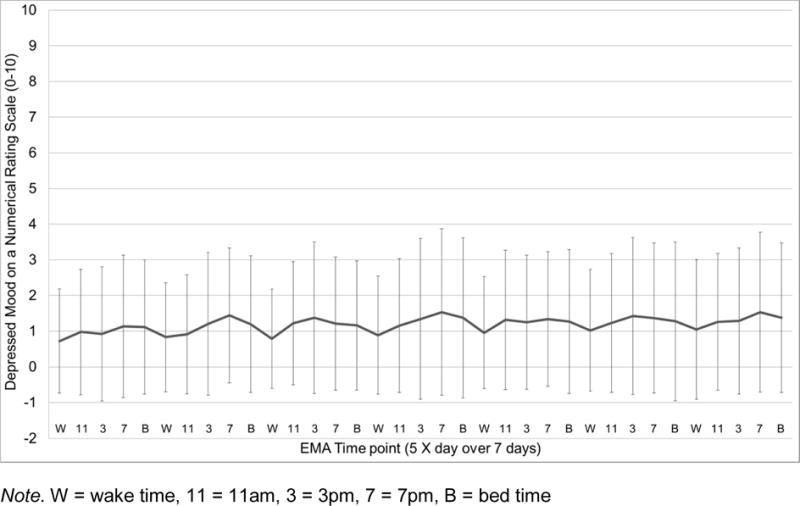
Sample mean of momentary depressed mood (NRS 0–10) across the 35 EMA time points (5 X day for 7 days). Error bars depict standard deviations.
Acknowledgments
Sources of Funding: Research reported in this publication was supported by the National Institute of Nursing Research of the National Institutes of Health under award number R03NR014515; PI: Kratz. Dr. Kratz was supported during manuscript preparation by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (award number 1K01AR064275). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The manuscript submitted does not contain information about medical device(s).
Abbreviations
- EMA
ecological momentary assessment
- MS
multiple sclerosis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors have no conflicts of interest to declare.
SUPPLIERS:
CamNtech Ltd., Upper Pendrill Court, Ermine Street North, Papworth Everard, Cambridge, CB23 3UY, United Kingdom
SAS, SAS Campus Drive, Building T, Cary, NC 27513
References
- 1.Hauser SL, Oksenberg JR. The neurobiology of multiple sclerosis: genes, inflammation, and neurodegeneration. Neuron. 2006 Oct 5;52(1):61–76. doi: 10.1016/j.neuron.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Noseworthy JH. Progress in determining the causes and treatment of multiple sclerosis. Nature. 1999 Jun 24;399(6738 Suppl):A40–47. doi: 10.1038/399a040. [DOI] [PubMed] [Google Scholar]
- 3.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000 Sep 28;343(13):938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 4.Ehde DM, Gibbons LE, Chwastiak L, Bombardier CH, Sullivan MD, Kraft GH. Chronic pain in a large community sample of persons with multiple sclerosis. Mult Scler. 2003 Dec;9(6):605–611. doi: 10.1191/1352458503ms939oa. [DOI] [PubMed] [Google Scholar]
- 5.Ehde DM, Osborne TL, Hanley MA, Jensen MP, Kraft GH. The scope and nature of pain in persons with multiple sclerosis. 2006 Oct;Mult Scler;12(5):629–638. doi: 10.1177/1352458506071346. [DOI] [PubMed] [Google Scholar]
- 6.Ehde DM, Osborne TL, Jensen MP. Chronic pain in persons with multiple sclerosis. Phys Med Rehabil Clin N Am. 2005 May;16(2):503–512. doi: 10.1016/j.pmr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Hirsh AT, Turner AP, Ehde DM, Haselkorn JK. Prevalence and impact of pain in multiple sclerosis: physical and psychologic contributors. Arch Phys Med Rehabil. 2009 Apr;90(4):646–651. doi: 10.1016/j.apmr.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kos D, Kerckhofs E, Nagels G, D’Hooghe MB, Ilsbroukx S. Origin of fatigue in multiple sclerosis: review of the literature. Neurorehabil Neural Repair. 2008 Jan-Feb;22(1):91–100. doi: 10.1177/1545968306298934. [DOI] [PubMed] [Google Scholar]
- 9.Krupp LB. Fatigue in multiple sclerosis: definition, pathophysiology and treatment. CNS Drugs. 2003;17(4):225–234. doi: 10.2165/00023210-200317040-00002. [DOI] [PubMed] [Google Scholar]
- 10.Krupp LB, Alvarez LA, LaRocca NG, Scheinberg LC. Fatigue in multiple sclerosis. Arch Neurol. 1988 Apr;45(4):435–437. doi: 10.1001/archneur.1988.00520280085020. [DOI] [PubMed] [Google Scholar]
- 11.Krupp LB, Christodoulou C. Fatigue in multiple sclerosis. Curr Neurol Neurosci Rep. 2001 May;1(3):294–298. doi: 10.1007/s11910-001-0033-7. [DOI] [PubMed] [Google Scholar]
- 12.Krupp LB, Serafin DJ, Christodoulou C. Multiple sclerosis-associated fatigue. Expert Rev Neurother. 2010 Sep;10(9):1437–1447. doi: 10.1586/ern.10.99. [DOI] [PubMed] [Google Scholar]
- 13.MacAllister WS, Krupp LB. Multiple sclerosis-related fatigue. Phys Med Rehabil Clin N Am. 2005 May;16(2):483–502. doi: 10.1016/j.pmr.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Joy JE, Johnston RB. Multiple Sclerosis: Current Status and Strategies for the Future. National Academies Press: The National Academies; 2001. [PubMed] [Google Scholar]
- 15.Hadjimichael O, Kerns RD, Rizzo MA, Cutter G, Vollmer T. Persistent pain and uncomfortable sensations in persons with multiple sclerosis. Pain. 2007 Jan;127(1–2):35–41. doi: 10.1016/j.pain.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Khan F, Pallant J. Chronic pain in multiple sclerosis: prevalence, characteristics, and impact on quality of life in an Australian community cohort. J Pain. 2007 Aug;8(8):614–623. doi: 10.1016/j.jpain.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Amato MP, Ponziani G, Siracusa G, Sorbi S. Cognitive dysfunction in early-onset multiple sclerosis: a reappraisal after 10 years. Arch Neurol. 2001 Oct;58(10):1602–1606. doi: 10.1001/archneur.58.10.1602. [DOI] [PubMed] [Google Scholar]
- 18.Bagert B, Camplair P, Bourdette D. Cognitive dysfunction in multiple sclerosis: natural history, pathophysiology and management. CNS Drugs. 2002;16(7):445–455. doi: 10.2165/00023210-200216070-00002. [DOI] [PubMed] [Google Scholar]
- 19.Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008 Dec;7(12):1139–1151. doi: 10.1016/S1474-4422(08)70259-X. [DOI] [PubMed] [Google Scholar]
- 20.Huijbregts SC, Kalkers NF, de Sonneville LM, de Groot V, Reuling IE, Polman CH. Differences in cognitive impairment of relapsing remitting, secondary, and primary progressive MS. Neurology. 2004 Jul 27;63(2):335–339. doi: 10.1212/01.wnl.0000129828.03714.90. [DOI] [PubMed] [Google Scholar]
- 21.Kesselring J, Klement U. Cognitive and affective disturbances in multiple sclerosis. J Neurol. 2001 Mar;248(3):180–183. doi: 10.1007/s004150170223. [DOI] [PubMed] [Google Scholar]
- 22.Lazeron RH, Boringa JB, Schouten M, et al. Brain atrophy and lesion load as explaining parameters for cognitive impairment in multiple sclerosis. Mult Scler. 2005 Oct;11(5):524–531. doi: 10.1191/1352458505ms1201oa. [DOI] [PubMed] [Google Scholar]
- 23.Motl RW, McAuley E. Symptom cluster as a predictor of physical activity in multiple sclerosis: preliminary evidence. J Pain Symptom Manage. 2009 Aug;38(2):270–280. doi: 10.1016/j.jpainsymman.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Motl RW, Weikert M, Suh Y, Dlugonski D. Symptom cluster and physical activity in relapsing-remitting multiple sclerosis. Res Nurs Health. 2010 Oct;33(5):398–412. doi: 10.1002/nur.20396. [DOI] [PubMed] [Google Scholar]
- 25.Kraft GH, Johnson KL, Yorkston K, et al. Setting the agenda for multiple sclerosis rehabilitation research. Mult Scler. 2008 Nov;14(9):1292–1297. doi: 10.1177/1352458508093891. [DOI] [PubMed] [Google Scholar]
- 26.Alschuler KN, Ehde DM, Jensen MP. The co-occurrence of pain and depression in adults with multiple sclerosis. Rehabil Psychol. 2013 May;58(2):217–221. doi: 10.1037/a0032008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alschuler KN, Ehde DM, Jensen MP. Co-occurring depression and pain in multiple sclerosis. Phys Med Rehabil Clin N Am. 2013 Nov;24(4):703–715. doi: 10.1016/j.pmr.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amtmann D, Askew RL, Kim J, et al. Pain affects depression through anxiety, fatigue, and sleep in multiple sclerosis. Rehabil Psychol. 2015 Feb;60(1):81–90. doi: 10.1037/rep0000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McAuley E, White SM, Rogers LQ, Motl RW, Courneya KS. Physical activity and fatigue in breast cancer and multiple sclerosis: psychosocial mechanisms. Psychosom Med. 2010 Jan;72(1):88–96. doi: 10.1097/PSY.0b013e3181c68157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Motl RW, McAuley E, Wynn D, Suh Y, Weikert M, Dlugonski D. Symptoms and physical activity among adults with relapsing-remitting multiple sclerosis. J Nerv Ment Dis. 2010 Mar;198(3):213–219. doi: 10.1097/NMD.0b013e3181d14131. [DOI] [PubMed] [Google Scholar]
- 31.Heine M, van den Akker LE, Blikman L, et al. Real-Time Assessment of Fatigue in Patients With Multiple Sclerosis: How Does It Relate to Commonly Used Self-Report Fatigue Questionnaires? Arch Phys Med Rehab. 2016 Nov;97(11):1887–1894. doi: 10.1016/j.apmr.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 32.Kim E, Lovera J, Schaben L, Melara J, Bourdette D, Whitham R. Novel method for measurement of fatigue in multiple sclerosis: Real-Time Digital Fatigue Score. J Rehabil Res Dev. 2010;47(5):477–484. doi: 10.1682/jrrd.2009.09.0151. [DOI] [PubMed] [Google Scholar]
- 33.Powell DJ, Liossi C, Schlotz W, Moss-Morris R. Tracking daily fatigue fluctuations in multiple sclerosis: ecological momentary assessment provides unique insights. J Behav Med. 2017 Mar 09; doi: 10.1007/s10865-017-9840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.masked; Ecological momentary assessment of pain, fatigue, depressive and cognitive symptoms reveals significant daily variability in multiple sclerosis. Arch Phys Med Rehab. doi: 10.1016/j.apmr.2017.07.002. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.masked; How do pain, fatigue, depressive, and cognitive symptoms relate to well-being and social and physical functioning in the daily lives of in individuals with multiple sclerosis? Arch Phys Med Rehab. doi: 10.1016/j.apmr.2017.07.004. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Enders CK, Tofighi D. Centering predictor variables in cross-sectional multilevel models: a new look at an old issue. Psychol Methods. 2007 Jun;12(2):121–138. doi: 10.1037/1082-989X.12.2.121. [DOI] [PubMed] [Google Scholar]
- 37.Enders CK. Centering predictors and contextual effects. In: Scott MA, Simonoff JS, Marx BD, editors. The SAGE Handbook of Multilevel Modeling. Thousand Oaks, CA: Sage Publications Inc; 2013. pp. 89–107. [Google Scholar]
- 38.Cohen J, Cohen P, West SG, Aiken LS. Applied Multiple Regression/Correlation Analyses for the Behavioral Sciences. Third. Mahwah, NJ: Lawrence Erlbaum Associates; 2003. [Google Scholar]
- 39.Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York, NY: Oxford University Press; 2003. [Google Scholar]
- 40.Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nature reviews. Neuroscience. 2013 Jul;14(7):502–511. doi: 10.1038/nrn3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lorenz J, Bromm B. Event-related potential correlates of interference between cognitive performance and tonic experimental pain. Psychophysiology. 1997 Jul;34(4):436–445. doi: 10.1111/j.1469-8986.1997.tb02387.x. [DOI] [PubMed] [Google Scholar]
- 42.Lorenz J, Beck H, Bromm B. Cognitive performance, mood and experimental pain before and during morphine-induced analgesia in patients with chronic non-malignant pain. Pain. 1997 Dec;73(3):369–375. doi: 10.1016/S0304-3959(97)00123-1. [DOI] [PubMed] [Google Scholar]
- 43.Eccleston C, Crombez G. Pain demands attention: a cognitive-affective model of the interruptive function of pain. Psychol Bull. 1999 May;125(3):356–366. doi: 10.1037/0033-2909.125.3.356. [DOI] [PubMed] [Google Scholar]
- 44.Remy F, Frankenstein UN, Mincic A, Tomanek B, Stroman PW. Pain modulates cerebral activity during cognitive performance. Neuroimage. 2003 Jul;19(3):655–664. doi: 10.1016/s1053-8119(03)00146-0. [DOI] [PubMed] [Google Scholar]
- 45.Seminowicz DA, Davis KD. Pain enhances functional connectivity of a brain network evoked by performance of a cognitive task. Journal of neurophysiology. 2007 May;97(5):3651–3659. doi: 10.1152/jn.01210.2006. [DOI] [PubMed] [Google Scholar]
- 46.Erpelding N, Davis KD. Neural underpinnings of behavioural strategies that prioritize either cognitive task performance or pain. Pain. 2013 Oct;154(10):2060–2071. doi: 10.1016/j.pain.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 47.Kratz AL, Schepens SL, Murphy SL. Effects of cognitive task demands on subsequent symptoms and activity in adults with symptomatic osteoarthritis. Am J Occup Ther. 2013 Nov-Dec;67(6):683–691. doi: 10.5014/ajot.2013.008540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Connor AB, Schwid SR, Herrmann DN, Markman JD, Dworkin RH. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain. 2008 Jul;137(1):96–111. doi: 10.1016/j.pain.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 49.Arnett PA, Barwick FH, Beeney JE. Depression in multiple sclerosis: Review and theoretical proposal. J Int Neuropsych Soc. 2008 Sep;14(5):691–724. doi: 10.1017/S1355617708081174. [DOI] [PubMed] [Google Scholar]
- 50.Braley TJ, Segal BM, Chervin RD. Obstructive sleep apnea and fatigue in patients with multiple sclerosis. Journal of clinical sleep medicine : JCSM: official publication of the American Academy of Sleep Medicine. 2014 Feb 15;10(2):155–162. doi: 10.5664/jcsm.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989 Oct;46(10):1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 52.Amtmann D, Bamer AM, Johnson KL, et al. A comparison of multiple patient reported outcome measures in identifying major depressive disorder in people with multiple sclerosis. J Psychosom Res. 2015 Dec;79(6):550–557. doi: 10.1016/j.jpsychores.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 53.Stone AA, Shiffman S. Ecological momentary assessment (EMA) in behavioral medicine. nn Behav Med. 1994;16(3):199–202. [Google Scholar]
- 54.Abelson RP. A variance explanation paradox: When a little is a lot. Psychological Bulletin. 1985;97(1):129–133. [Google Scholar]
- 55.Cohen J. Statistical Power Analyses for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1987. [Google Scholar]


