Abstract
Rationale
Recent research has established the imidazoline I2 receptor as a promising target for the development of novel analgesics. However, despite an increasing understanding of imidazoline I2 receptor-mediated behavioral effects, little is known about post-I2-receptor signaling mechanisms.
Objective
This study examined the effects of several inhibitors of Ca2+ signaling mechanisms on two behavioral effects of the prototypical imidazoline I2 receptor ligand 2-BFI.
Methods
The von Frey filament test was used to examine the antinociceptive effects of 2-BFI in complete Freund’s adjuvant (CFA)-induced inflammatory pain in rats. A two-lever drug discrimination paradigm in which rats were trained to discriminate 5.6 mg/kg (i.p.) 2-BFI from its vehicle was used to examine the discriminative stimulus effects of 2-BFI.
Results
The L-type Ca2+ channel blockers verapamil and nimodipine, the calmodulin antagonist W-7, and the internal Ca2+ release inhibitor ryanodine all attenuated the antinociceptive effects of 2-BFI. Oxycodone- and acetaminophen-induced antinociception were unaffected by pretreatment with the Ca2+ channel blockers. Rats learned to reliably discriminate 5.6 mg/kg 2-BFI from saline. The I2 receptor agonists BU224, RS45041, tracizoline, and CR4056 all fully substituted for 5.6 mg/kg 2-BFI while idazoxan, S22687, 2,5-Dimethoxy-4-methylamphetamine (DOM), and phenyzoline produced partial or no substitution. Verapamil, nimodipine, and W-7 did not alter the discriminative stimulus effects of 2-BFI.
Conclusion
These results indicate that the antinociceptive effects of 2-BFI involve intracellular Ca2+ elevation and/or downstream Ca2+/calmodulin signaling, whereas the discriminative stimulus effects of 2-BFI are mediated by a distinct, independent mechanism.
Keywords: Imidazoline I2 receptor, antinociception, drug discrimination, calcium channel blocker, rats
Introduction
Chronic pain remains one of the largest healthcare problems, affecting over 100 million adult Americans each day (NIH 2013). Though historically relied upon as first-line pain therapies, currently available analgesics like opioids are poorly suited to long term pain management due to analgesic tolerance, dependence, abuse, and overdose. A recent survey found that interest from both top-tier publishers and the pharmaceutical industry failed to increase in any pain topic between 2005–2015 (Kissin 2015). This, combined with the current trend of opioid epidemic, and the lack of mechanistically novel painkillers developed in the past 50 years (Kissin 2010), strongly support the dire need of developing novel and effective analgesics.
Preclinical investigations of the imidazoline I2 receptor (I2R) have revealed promising results. Initially discovered over 20 years ago, recent studies have demonstrated that the I2R is an effective target in several rodent chronic pain models, and its activation also enhances opioid antinociception while preventing some opioid use-related side effects (Li et al. 2014a; Siemian et al. 2016b; Thorn et al. 2016). I2Rs are thought to exist primarily as allosteric inhibitory binding sites on the enzymes monoamine oxidase (MAO) A and B, but also exist in lower amounts on other proteins, one of which is thought to be brain creatine kinase (Kimura et al. 2009). However, because the I2R has not been cloned nor have any signaling pathways been characterized, recent studies primarily focused on I2R-related in vivo effects instead of understanding the receptor and post-receptor mechanisms. Thus, current efforts have been directed at understanding the post-receptor signaling mechanisms of the I2R so as to better design pain management strategies in the future.
One hypothesis for I2R functioning originates from the observation that imidazoline compounds increased cytoplasmic Ca2+ concentration via blockade of ATP-sensitive K+-channels and perhaps other ion channels in pancreatic β-cells (Rustenbeck et al. 1995). Although these effects were thought to be mediated by a different subtype of imidazoline receptor (Eglen et al. 1998; Morgan and Chan 2001), another study observed that the prototypical I2R ligand 2-(2-benzofuranyl)-2-imidazoline hydrochloride (2-BFI) reversibly stimulated locus coeruleus cells in slice electrophysiology, which was modulated by ATP-sensitive K+ channel openers or blockers (Ugedo et al. 1998). This raises the possibility that some behavioral effects of I2R-selective compounds may involve intracellular Ca2+ signaling.
This study examined the antinociceptive effects of the selective I2R agonist 2-BFI alone and in combination with several inhibitors of Ca2+ signaling pathways using the von Frey filament test of mechanical nociception in adult male rats with complete Freund’s adjuvant (CFA)-induced inflammatory pain. In a separate experiment, rats were trained to discriminate 2-BFI from its vehicle. The discriminative stimulus effects of 2-BFI alone and in combination with several Ca2+ signaling inhibitors were examined to determine the specificity of these pharmacological manipulations on I2R-mediated behaviors.
Methods
Subjects
Male Sprague-Dawley rats (Harlan, Indianapolis, IN) approximately 12 weeks old and weighing approximately 250 g at the experiment onset were individually housed on a 12/12-hour light/dark cycle with behavioral experiments conducted during the light period. Subjects had free access to water, except during test sessions. Rats used in pain tests had free access to standard rodent chow in their home cages and were randomly assigned to different treatment groups (n = 6–7 per group). Rats used in the drug discrimination experiment (n = 8) were provided with restricted access to food after their daily sessions, such that their bodyweights were maintained at approximately 90% of their free-feeding counterparts. Animals were maintained and experiments were conducted in accordance with guidelines of the International Association for the Study of Pain (Zimmermann 1983) and with the 2011 Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, National Academy of Sciences, Washington, DC), and were approved by the Institutional Animal Care and Use Committee, University at Buffalo, the State University of New York (Buffalo, NY).
Induction of inflammatory pain
Inflammatory pain was induced by CFA inoculation as previously described (Li et al., 2014). Briefly, 0.1 ml of CFA (Sigma-Aldrich, St. Louis, MO) containing approximately 0.05 mg of Mycobacterium butyricum dissolved in paraffin oil was injected in the right hind foot pad of rats while under isoflurane anesthesia (2% isoflurane mixed with 100% oxygen). Sufficient anesthesia was determined by the loss of righting and toe-pinch reflexes.
Mechanical hyperalgesia
Mechanical nociception was measured using von Frey filaments consisting of calibrated filaments (1.4–26 g; North Coast Medical, Morgan Hill, CA). Rats (n = 6–7 per group) were placed in elevated plastic chambers with a wire mesh floor (IITC Life Science Inc., Woodland Hills, CA) and allowed to habituate prior to testing. Filaments were applied perpendicularly to the medial plantar surface of the hind paw from below the mesh floor in an ascending order of filament force, beginning with the lowest filament (1.4 g). A filament was applied until buckling occurred (i.e., filament bent at its rated force and further application would not generate higher force) and maintained for approximately 2 seconds. Mechanical thresholds (expressed as percentage of maximum possible effect (% MPE), see Data Analysis) correspond to the lowest force that elicited a behavioral response (withdrawal of the hind paw) in at least 2 out of 3 applications. Tests were performed using cumulatively-dosed multiple-cycle procedures, in which a 20-min inter-injection interval was used and the behavioral measurements were taken immediately prior to drug administrations. These cycles continued until at least 80% MPE was achieved or up to a dose that could be safely studied (17.8 mg/kg 2-BFI). Forces larger than 26 g would physically elevate the non-CFA-treated paw and did not reflect pain-like behavior. For the experiments examining antagonism of 2-BFI-induced antinociception, control 2-BFI dose-effect curves were first established for all rats. Two days following the control 2-BFI test, Ca2+ signaling inhibitors were administered 10 min before re-establishing the 2-BFI dose-effect curve. It was previously shown that CFA-induced hypersensitivity remained stable over a period of weeks and repeated treatment with I2R ligands does not alter the baseline hypersensitivity status (Li et al., 2014; Thorn et al., 2015). Experimenters were blind to the treatments, and they received extensive training with this procedure to ensure accurate judgment of paw withdrawal responses and minimize experimenter bias.
Drug Discrimination
Drug-discrimination studies were performed in commercially available two-lever operant chambers located within sound-attenuating, ventilated enclosures (Coulbourn Instruments Inc., Allentown, Pennsylvania, USA) as described previously (Qiu et al., 2014; Qiu et al., 2015). Data were collected through an interface using Graphic State 3.03 software (Coulbourn Instruments Inc., Whitehall, Pennsylvania, USA). A modification of previously described training protocols was used (Li et al., 2008; Qiu et al., 2015). Rats were trained to discriminate 5.6 mg/kg 2-BFI injected intraperitoneally (i.p.) from saline in a multiple-cycle cumulative-dosing procedure. Each cycle consisted of a 10 min timeout during which the chamber was dark and responses had no programmed consequence, followed by a 5 min response period, during which a house light and a cue light above each lever were illuminated and signaled availability of reinforcers. Ten consecutive responses (fixed ratio [FR] 10) on the correct lever resulted in food delivery (45 mg; BioServ Inc., Frenchtown, New Jersey, USA). The correct lever was predetermined by an injection (e.g., right, saline; left, 2-BFI). Response periods ended after 5 min or after delivery of 10 food pellets, whichever occurred first. Training began with single-cycle sessions, in which saline or 5.6 mg/kg 2-BFI was administered i.p. 10 min before the start of the session. Sessions were conducted seven days per week according to a double alternation schedule (e.g., saline, saline, drug, drug). Rats had to achieve at least 90% of the total responses on the correct lever for five consecutive or six out of seven consecutive sessions to progress to multiple cycle training. For multiple-cycle training, some training days consisted of two cycles in which either the saline lever or the 2-BFI lever was active during both cycles, depending on the injection given 10 min prior to the initiation of the training sessions. On other training days, one to three saline training cycles preceded the administration of 2-BFI and two 2-BFI training cycles. Other training sessions included five saline training cycles. This training protocol minimizes the subjects’ lever bias and allow for roughly comparable frequencies of food reinforcement on both levers during training. These protocols were varied non-systematically; rats needed to pass two consecutive sessions (one saline training session and one 2-BFI training session) by responding at least 90% on the correct lever during each active period before each test. Test sessions lasted up to five cycles and were identical to training sessions except that ten consecutive responses on either lever delivered a food pellet. During test sessions, saline or test drugs were administered 10 min before the start of the first cycle, followed by increasing doses of 2-BFI during the first minute of each subsequent cycle up to doses that occasioned greater than 80% responding on the 2-BFI-appropriate lever or reduced the rate of responding to less than 20% that of control responding (see Data Analysis). In either case, rats were removed from the test chambers and the test session was finished. Control 2-BFI dose-effect curves were periodically established throughout the study to ensure the performance stability of 2-BFI discrimination. In substitution studies, compounds with known I2R activity (BU224, 4-chloro-2-(imidazolin-2-yl)isoindoline (RS45041), CR4056, tracizoline, S22687, phenyzoline and idazoxan) (Brown et al. 1995; Ferrari et al. 2011; Pigini et al. 1997; Qiu et al. 2015) were tested to examine the generality of the discriminative stimulus effects of 2-BFI. A serotonin 5-HT2A receptor agonist 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane (DOM) was tested as a negative control to examine the pharmacological specificity of 2-BFI discriminative stimulus.
Data Analysis
Antinociceptive effects were quantified for each animal as % MPE based on the paw withdrawal thresholds (PWT) according to the following formula: % MPE = (post-drug PWT – pre-drug PWT)/(pre-CFA PWT – pre-drug PWT) × 100%. To construct antinociceptive dose-effect curves, % MPEs were averaged within each group and plotted as a function of dose. In Fig. 1, each control dose-effect curve in the three left panels represents the pooled data from two groups (twelve rats) because this was a within group design. Dose-effect curves in the presence of an antagonist represent the data collected from six of those twelve rats. For all other pain data, dose-effect curves represent data from six or seven rats. Repeated measures one- or two-way ANOVA (2-BFI dose × treatment), with treatment entered as the within-subject factor, followed by Bonferroni’s multiple comparison test were used to determine the statistical significances in pain experiments. In the analysis to determine whether 2-BFI-induced antinociception was stable during a second administration, the number of tests instead of treatment was used as a factor in the two-way ANOVA analysis.
Figure 1.
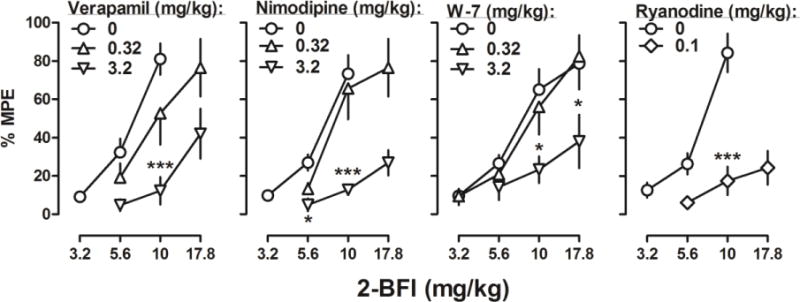
Percentage of the maximum possible effects of 2-BFI alone (n = 12, except right panel n = 7) and in combination with verapamil (left, n = 6), nimodipine (middle left, n = 6), W-7 (middle right, n = 6), and ryanodine (right, n = 7) on CFA-induced mechanical nociception. Ordinates: mean (± SEM) percentage of maximum possible effects; abscissa: 2-BFI dose (mg/kg, i.p.). *P < 0.05, ***p < 0.001.
For drug discrimination studies, two sets of data were collected for each test: (a) the percentage of responses on the 2-BFI-associated lever, calculated by dividing the number of responses on the 2-BFI-associated lever by the number of total responses on either lever and multiplying by 100; and (b) response rate, which was plotted as the percentage of the control rate. The rates were calculated by dividing the responses made on both levers by the duration of the response period in seconds. When a rat responded at a rate less than 20% of the no-treatment control rate, the percentage of responses on the 2-BFI-associated lever for that rat at that dose or higher doses was not included for further analysis. The response rate data for that dose were included for statistical analysis. In this case, no data were available for subsequent cycles because the session was finished. For substitution and combination studies, group ED50 values (± 95% confidence limits [CL]) were calculated from individual ED50 values determined via linear regression. Response rate data were analyzed using one- or two-way ANOVA because the existence of omitted values prevented from repeated measures analysis.
Drugs
2-(2-benzofuranyl)-2-imidazoline hydrochloride (2-BFI) hydrochloride, BU224 hydrochloride, S22687, tracizoline oxalate, phenyzoline, and CR4056 were synthesized according to published procedures (Ferrari et al. 2011; Ishihara and Togo 2007; Pigini et al. 1997). RS45041 was kindly provided by National Institute of Mental Health’s Chemical Synthesis and Drug Supply program (Bethesda, MD, USA). Idazoxan hydrochloride, verapamil hydrochloride, nimodipine, and acetaminophen were purchased from Sigma-Aldrich (Laramie, WY). W-7 hydrochloride and ryanodine were purchased from Tocris Bioscience (Bristol, UK). DOM was provided by Research Technology Branch, National Institute of Drug Abuse (Rockville, MD). All drugs were dissolved in 0.9% saline except otherwise noted. Verapamil and nimodipine were dissolved in 20% DMSO and 10% Alkamuls EL-620 (Novecare, Cranbury, NJ) in saline. Acetaminophen, W-7 and ryanodine were dissolved in 20% DMSO in saline. CR4056 was suspended in 5% Tween 80 and sonicated before use. Doses of drugs are expressed in terms of their salt form, and all drugs were administered i.p. in a volume of 1 ml/kg.
Results
Before CFA treatment, the PWT (± SEM) for all rats was 24.7 ± 0.5 g in the von Frey test. CFA injection in the hind paw produced a decrease in PWT to 5.7 ± 0.2 g when measured 24 h later, similar to values found in previous reports (Siemian et al. 2016a; Siemian et al. 2016b). When the data of the rats’ first tests were pooled together, one-way ANOVA revealed that a significant main effect of 2-BFI treatment on PWT (F (3, 192) = 190.5, p < 0.0001). The antinociceptive dose-effect curve of 2-BFI was not significantly different when retested in one group of rats 3 d later (data not shown). Two-way ANOVA revealed main effects of 2-BFI dose (F (2, 20) = 26.07, p < 0.0001) but not number of test (F (1, 20) = 0.21, p = 0.66). No Ca2+ signaling inhibitor influenced PWT when administered alone (data not shown).
When studied in combination with 2-BFI, each study compound dose-dependently attenuated the antinociceptive effects of 2-BFI (Fig. 1). For 3.2 mg/kg verapamil, two-way ANOVA revealed a significant verapamil × 2-BFI interaction (F (1, 10) = 11.13, p = 0.0075) with significant main effects of 2-BFI dose (F (1, 10) = 8.28, p =0.0164) and verapamil treatment (F (1, 10) = 52.41, p < 0.0001), whereas for 0.32 mg/kg verapamil, two-way ANOVA revealed significant main effects of 2-BFI dose (F (1, 10) = 11.36, p =0.0071) but not verapamil treatment (F (1, 10) = 3.24, p =0.10). For 3.2 mg/kg nimodipine, two-way ANOVA revealed a significant nimodipine × 2-BFI interaction (F (1, 10) = 6.34, p =0.03) with significant main effects of 2-BFI dose (F (1, 10) = 23.01, p = 0.0007) and nimodipine treatment (F (1, 10) = 47.21, p < 0.0001), whereas for 0.32 mg/kg nimodipine, two-way ANOVA revealed significant main effects of 2-BFI dose (F (1, 10) = 34.44, p = 0.0002) but not nimodipine treatment (F (1, 10) = 0.01, p = 0.94). For 3.2 mg/kg W-7, two-way ANOVA revealed a significant W-7 × 2-BFI interaction (F (1, 10) = 11.38, p =0.0071) with significant main effects of 2-BFI dose (F (1, 10) = 13.47, p = 0.0043) and W-7 treatment (F (1, 10) = 11.38, p = 0.0071), whereas for 0.32 mg/kg W-7, two-way ANOVA revealed significant main effects of 2-BFI dose (F (3, 20) = 16.65, p < 0.0001) but not W-7 treatment (F (1, 20) = 0.16, p = 0.69). For 0.1 mg/kg ryanodine, two-way ANOVA revealed a significant ryanodine × 2-BFI interaction (F (1, 10) = 15.45, p = 0.0028) with significant main effects of 2-BFI dose (F (1, 10) = 26.98, p = 0.0004) and ryanodine treatment (F (1, 10) = 51.10, p < 0.0001). Results of Bonferroni’s post-tests are shown as asterisks in Fig. 1.
In contrast, the antinociceptive effects of pharmacologically distinct analgesics were not significantly affected by verapamil or nimodipine (Fig. 2). When oxycodone was tested, two-way ANOVA revealed significant main effects of oxycodone dose but not verapamil (F (1, 15) = 0.00, p = 0.96) or nimodipine (F (1, 15) = 0.63, p = 0.44) treatment. Likewise, when acetaminophen was tested, two-way ANOVA revealed a significant main effect of acetaminophen dose (F (2, 15) = 57.35, p < 0.0001) but not verapamil treatment (F (1, 15) = 4.49, p = 0.06), although post hoc analysis did reveal a significant reduction of acetaminophen-induced antinociception at the dose of 100 mg/kg.
Figure 2.
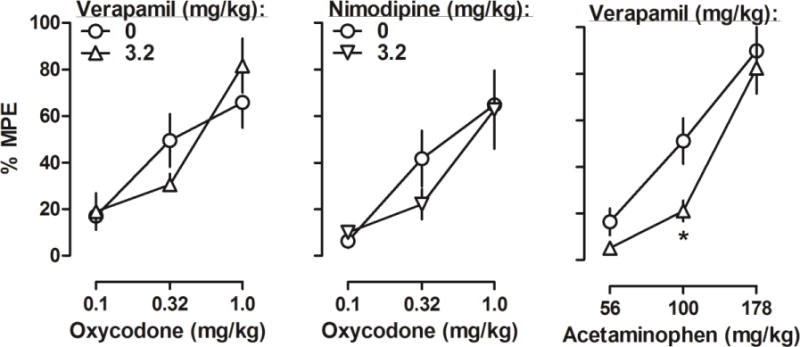
Percentage of the maximum possible effects of oxycodone alone and in combination with large doses of verapamil (top left, n = 6) or nimodipine (bottom left, n = 6), and acetaminophen alone and in combination with verapamil (right, n = 6) on CFA-induced mechanical nociception. Ordinates: mean (± SEM) percentage of maximum possible effects; abscissa: oxycodone or acetaminophen dose (mg/kg, i.p.).
In the drug discrimination experiment, rats met test criteria after an average of 42 training sessions (range = 26–57). Saline produced less than 5% 2-BFI-associated lever responding (upper panels, Fig. 3, circles above “V”), whereas 2-BFI dose-dependently increased responding on the 2-BFI-associated lever up to a maximum of 100% at a dose of 10 mg/kg. The ED50 value (± 95% CL) for 2-BFI substitution was 2.48 (1.63, 3.12) mg/kg. At the doses studied, 2-BFI did not significantly alter the response rate. Four known I2 receptor ligands BU224, RS45041, CR4056 and tracizoline all dose-dependently increased and, at the largest dose studied, produced > 80% 2-BFI-associated lever responding (Fig. 3). The ED50 values (± 95% CL) for BU224, RS45041, CR4056 and tracizoline to substitute for 2-BFI discrimination were 2.58 (1.76, 3.21) mg/kg, 2.30 (0.49, 3.18) mg/kg, 5.83 (2.11, 8.49) mg/kg, and 32.14 (15.91, 45.10) mg/kg, respectively. Overall, the four I2 receptor ligands did not significantly alter the operant response rate at the doses that produced greater than 80% 2-BFI-associated lever responding. However, CR4056 at the dose of 10 mg/kg and tracizoline at the dose of 56 mg/kg significantly reduced the response rate as compared to the control condition (bottom left, Fig. 3). Up to the doses that significantly reduced the response rate, S22687 and phenyzoline, two compounds that reportedly bind to I2 receptors, produced < 40% 2-BFI-associated lever responding (right, Fig. 3). The hallucinogenic phenethylamine DOM produced a maximum of 25% 2-BFI-associated lever responding up to a dose (1 mg/kg) that nearly eliminated the operant responding and the effect was not dose-dependent. Idazoxan produced a maximum of 66% 2-BFI-associated lever responding up to a dose (10 mg/kg) that significantly reduced the rate of responding.
Figure 3.
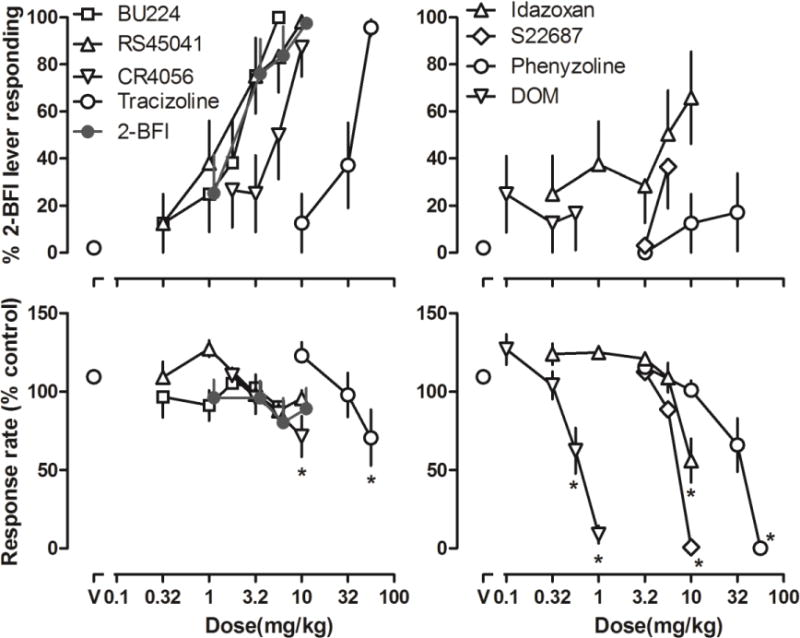
Discriminative stimulus effects of 2-BFI in rats discriminating 5.6 mg/kg 2-BFI from its vehicle (n = 8). Top: substitution profiles of I2 receptor agonists. Bottom: response rate. Ordinates: top, percentage of 2-BFI-appropropriate lever responding; bottom, response rate as compared to no treatment control. Abscissa: doses (mg/kg). *P < 0.05 as compared to “V”.
Verapamil, nimodipine, or W-7 alone at the dose of 3.2 mg/kg occasioned less than 10% responding on the 2-BFI-associated lever (top, Fig. 4, triangles above “V”). None of these three compounds significantly altered the ED50 values (± 95% CL) of 2-BFI discrimination dose-effect curve. The ED50 value of 2-BFI dose-effect curve without drug pretreatment was 2.14 (1.37, 3.36) mg/kg, while the ED50 values of 2-BFI dose-effect curve in combination with verapamil, nimodipine, or W-7 pretreatments were 1.39 (0.66, 2.93) mg/kg, 2.03 (0.80, 5.20) mg/kg, and 2.35 (1.24, 4.44) mg/kg, respectively. Two-way ANOVA analysis of the response rate data found that only 3.2 mg/kg W-7 (F (1, 70) = 10.23, p = 0.0021) produced a significant main effect on the response rate, although Bonferroni’s post-tests revealed no significant difference in response rates at any individual data point.
Figure 4.
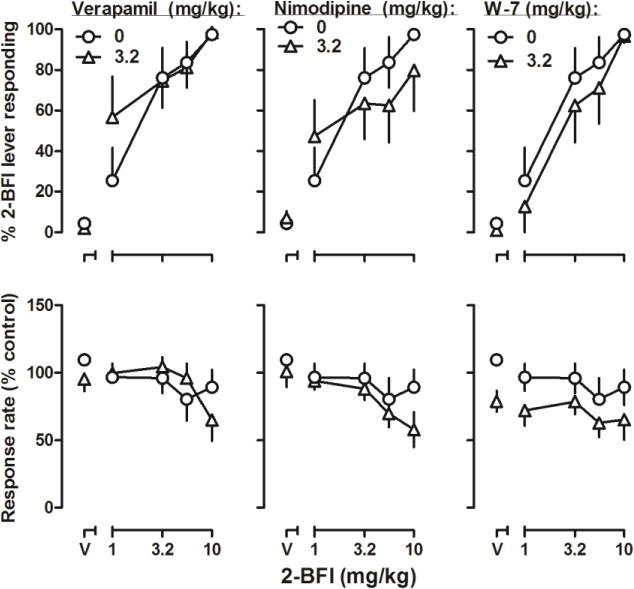
Effects of 2-BFI alone and in combination with large doses of verapamil (left), nimodipine (middle), or W-7 (right) in rats discriminating 5.6 mg/kg 2-BFI from its vehicle. See Figure 3 for other details.
Discussion
The primary finding of this study was that inhibitors of Ca2+ signaling pathways antagonized mechanical antinociception induced by an I2R agonist in a rat model of chronic inflammatory pain. The study compounds were nimodipine and verapamil (blockers of L-type Ca2+ channels), W-7 (a calmodulin antagonist), and ryanodine (an inhibitor of Ca2+ release from intracellular Ca2+ stores). Pretreatment with any one of these compounds at sufficient doses significantly antagonized the antinociceptive effects of the prototypical I2R agonist 2-BFI. In contrast, pretreatment with nimodipine, verapamil, or W-7 did not alter the discriminative stimulus effects of 2-BFI in a separate group of rats trained to discriminate 2-BFI from its vehicle. These data support the hypothesis that different behavioral effects (e.g., antinociception, discriminative stimulus) of 2-BFI and related I2 receptor agonists are mediated either by distinct post-receptor signaling pathways or by distinct populations of I2Rs. Such findings will increase the understanding of I2R pharmacology and may help guide the development of novel I2R ligands toward specifically producing therapeutically important (e.g., analgesic) effects without having other unwanted effects. Given the recent opioid epidemic, such new analgesics, which are unrelated to opioids, will help dampening opioid abuse and addiction, particularly in individuals with chronic pain.
CFA-induced inflammatory pain is a well-described and widely used animal model of chronic pain. Inoculation of CFA into the hind paw induces a rapid and persistent hypersensitivity to a range of stimuli (Li et al. 2014a; Nagakura et al. 2003), making it an appropriate model with which to study potential analgesics. When administered alone to CFA-treated rats, 2-BFI produced a dose-dependent PWT increase in the von Frey test, consistent with previous reports (Siemian et al. 2016a; Thorn et al. 2015). Since ATP-sensitive K+ channel blockade with a net effect of cytoplasmic Ca2+ elevation had been proposed as a mechanism related to I2R function (Rustenbeck et al. 1995; Ugedo et al. 1998), we examined whether interruptions of this purported pathway would alter I2R agonist-induced antinociception. We initially studied nimodipine and verapamil, two L-type Ca2+ channel blockers which inhibit Ca2+ influx and decrease intracellular Ca2+ level. We observed that pretreatment with either drug significantly attenuated the antinociceptive effect of 2-BFI. The Ca2+ binding messenger protein calmodulin is required for most intracellular Ca2+-dependent signaling pathways, and perhaps even for Ca2+ increases after depolarization (Sitges and Talamo 1993). Thus, we next examined the effect of the calmodulin inhibitor W-7, which also caused a significant attenuation in 2-BFI-induced antinociception. Finally, since ryanodine receptors, which mediate Ca2+ efflux from intracellular stores, are activated by physical coupling with L-type Ca2+ channels and also by Ca2+ elevation (Fabiato 1983; Fleischer and Inui 1988), we studied the effect of ryanodine, an inhibitor of intracellular Ca2+ store release, which significantly attenuated 2-BFI-induced antinociception. These results suggest that I2R-mediated antinociception relies upon cytoplasmic Ca2+ elevation and possibly also upon the activation of downstream calmodulin-dependent pathways.
I2 receptor agonists produce a number of behavioral and physiological effects: antinociception (Li 2017; Li et al. 2014b), hypothermia (Thorn et al. 2012) and discriminative stimulus effects (Jordan et al. 1996; Qiu et al. 2015). In order to test the generality of the observed findings, we tested the role of Ca2+ signaling in mediating the discriminative stimulus effects of 2-BFI. Several imidazoline I2 receptor agonists have been studied as discriminative stimuli, including 2-BFI (Jordan et al. 1996; Qiu et al. 2014; Qiu et al. 2015). Consistent with the literature, we found that rats acquired 5.6 mg/kg 2-BFI discrimination at a similar rate as previous studies. For example, in a study that trained rats to discriminate 7 mg/kg 2-BFI from its vehicle and all rats acquired the discrimination after 44 – 64 training sessions (Jordan et al. 1996). The I2 receptor ligand BU224 fully substituted for 2-BFI, which is consistent with previous reports (MacInnes and Handley 2002). RS45041, CR4056 and tracizoline, which bind to I2 receptors with high selectivity over many other receptors including α2 adrenoceptors and imidazoline I1 receptors (Brown et al. 1995; Ferrari et al. 2011; Pigini et al. 1997) also all fully substituted for 5.6 mg/kg 2-BFI. In rats discriminating 10 mg/kg CR4056 from its vehicle, 2-BFI produced a maximum of 75% CR4056-associated lever responding (Qiu et al. 2014). This symmetrical substitution suggests that both compounds share similar pharmacological mechanisms in producing their discriminative stimulus effects. The discriminative stimulus effects of 2-BFI have been extensively characterized (Jordan et al. 1996; MacInnes and Handley 2002) which demonstrated that its discriminative stimulus effect is pharmacological specific to I2 receptor activity. In order to further examine the pharmacological specificity of 2-BFI discrimination, we also tested a 5-HT2A receptor agonist DOM. Not surprisingly, DOM failed to produce significant 2-BFI-associated lever responding despite the fact that DOM itself produces robust discriminative stimulus effect (Li et al. 2008; Li et al. 2009). S22687 is a compound that binds to I2 receptors and is also a potent dopamine releaser (Barrot et al. 2000). S22687 only produced approximately 40% 2-BFI-associated lever responding. It is unknown why S22687 did not fully substitute for 2-BFI-induced discriminative stimulus given its high binding affinity at I2 receptors. One possibility could be that its non-I2 receptor (e.g., dopamine releaser) activity disrupted the operant responding before reaching effective doses at I2 receptors (Barrot et al. 2000). Phenyzoline is a selective I2 receptor agonist (Qiu et al. 2015) and in rats discriminating 32 mg/kg phenyzoline from its vehicle in a previous study, 2-BFI partially substituted for phenyzoline (Qiu et al. 2015). However, in the present study phenyzoline failed to produce significant substitution to 5.6 mg/kg 2-BFI. 2-BFI and phenyzoline share some in vivo effects (e.g., hypothermia and antinociception) (Thorn et al. 2012; Thorn et al. 2015). However, in CFA-treated rats, combinations of 2-BFI and the opioid oxycodone produced additive antinociceptive interactions while phenyzoline and oxycodone produced synergic interactions, suggesting that the effects of these I2R ligands may differ under certain conditions (Thorn et al. 2015). The fact that 2-BFI partially substitutes for phenyzoline while phenyzoline fails to substitute for 2-BFI suggests that these compounds share some pharmacological mechanisms but differ in others.
Given the observed effective blockade of 2-BFI-induced antinociceptive effects by various intracellular Ca2+ signaling inhibitors, we examined the effects of the same inhibitors on 2-BFI discrimination. It was hypothesized that if I2R agonists produce antinociceptive and discriminative stimulus effects through a common mechanism, then drugs which blocked the antinociceptive effect of 2-BFI should also block its discriminative stimulus effect. Surprisingly, in contrast to the results from the pain studies, none of the Ca2+ signaling inhibitors significantly altered the discriminative stimulus effects of 2-BFI. As such, pretreatments with verapamil, nimodipine, or W-7 at doses which markedly attenuated 2-BFI-induced antinociception all failed to alter the dose-effect curve of 2-BFI discrimination. This suggests that the antinociceptive and discriminative stimulus effects of 2-BFI are mediated through distinct mechanisms. Our previous studies demonstrated that antinociception induced by several I2R ligands is sensitive to idazoxan, an I2R antagonist, and that while several I2R ligands substitute for each other in drug discrimination (Qiu et al. 2014; Qiu et al. 2015), idazoxan does not antagonize their discriminative stimulus effects but instead fully substitutes for the training drugs. In agreement with the previous findings, here idazoxan partially substituted for 2-BFI discrimination. Together, these results suggest that 2-BFI and other I2R agonists may produce antinociceptive and discriminative stimulus effects via two distinct mechanisms, one that is sensitive to idazoxan and involves intracellular Ca2+ signaling mechanisms and another that is not. The sparse information in the literature makes it difficult to speculate whether these two mechanisms are a result of different receptor subtypes, receptor populations or even different post-receptor signaling pathways. This puzzle awaits further investigation.
Ca2+ and its corresponding signaling pathways are vital to many physiological events, and it is important to test whether the observed effects of Ca2+ signaling involvement is somewhat specific to I2R agonists or a general disruption of intracellular Ca2+ balance which could impact all drugs. In the present study, we found that verapamil or nimodipine did not alter the opioid oxycodone- or acetaminophen-induced antinociception in the same model of CFA-induced mechanical hypersensitivity. These results suggest that the modulation of Ca2+ signaling to attenuate 2-BFI-induced antinociceptive effect is pharmacologically specific. It remains unclear regarding the intracellular signaling cascades how I2 receptor activation leads to intracellular Ca2+ signaling modulation. One possibility may involve monoamine activity. It is believed that I2Rs represent a novel allosteric modulating site on MAO and biochemical studies support this notion. I2R agonists such as 2-BFI, BU224 and CR4056 all inhibit MAO activity and elevate monoamine levels (Ferrari et al. 2011; Nutt et al. 1995; Ugedo et al. 1999). Monoamines are stored in vesicles in axonal terminals, and released when an action potential causes the axon to depolarize via Ca2+ elevation (Matthews 1996). Inhibiting axonal depolarization and monoamine release via Ca2+ channel blockade or internal Ca2+-release inhibition would thus be expected to produce the observed antagonist-like effects as seen in this study (Fig. 1) if 2-BFI-induced antinociception were mediated through elevated monoamine release. Similarly, Ca2+-bound calmodulin is important for vesicle exocytosis (Di Giovanni et al. 2010; Morel and Poea-Guyon 2015) and is thought to promote vesicle-SNARE protein binding as well as vesicle pool replenishment (Lipstein et al. 2013). Thus, calmodulin inhibition could exert a powerful modulatory effect on a drug target which involves increased levels and release of vesicular monoamines. This speculation remains to be determined in future studies.
In summary, this is the first study to demonstrate that two behavioral effects of the prototypical I2R agonist 2-BFI are mediated by two distinct pharmacological mechanisms. The results provide in vivo functional evidence that I2R populations and/or signaling pathways are heterogeneous and include at least two distinct components, one of which is sensitive to idazoxan blockade and Ca2+ signaling inhibition while the other is unrelated to Ca2+ signaling.
Acknowledgments
This work was supported by the National Institute on Drug Abuse of the National Institutes of Health [Grant R01DA034806] and by a grant from the National Natural Science Foundation of China [Grant 81373390]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of interest
The authors declare that they have no competing interests.
References
- Barrot M, Rettori MC, Guardiola-Lemaitre B, Jarry C, Le Moal M, Piazza PV. Interactions between imidazoline binding sites and dopamine levels in the rat nucleus accumbens. Eur J Neurosci. 2000;12:4547–51. [PubMed] [Google Scholar]
- Brown CM, MacKinnon AC, Redfern WS, Williams A, Linton C, Stewart M, Clague RU, Clark R, Spedding M. RS-45041-190: a selective, high-affinity ligand for I2 imidazoline receptors. Br J Pharmacol. 1995;116:1737–44. doi: 10.1111/j.1476-5381.1995.tb16656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giovanni J, Iborra C, Maulet Y, Leveque C, El Far O, Seagar M. Calcium-dependent regulation of SNARE-mediated membrane fusion by calmodulin. The Journal of biological chemistry. 2010;285:23665–75. doi: 10.1074/jbc.M109.096073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eglen RM, Hudson AL, Kendall DA, Nutt DJ, Morgan NG, Wilson VG, Dillon MP. ‘Seeing through a glass darkly’: casting light on imidazoline ‘I’ sites. Trends in pharmacological sciences. 1998;19:381–90. doi: 10.1016/s0165-6147(98)01244-9. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. The American journal of physiology. 1983;245:C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Ferrari F, Fiorentino S, Mennuni L, Garofalo P, Letari O, Mandelli S, Giordani A, Lanza M, Caselli G. Analgesic efficacy of CR4056, a novel imidazoline-2 receptor ligand, in rat models of inflammatory and neuropathic pain. J Pain Res. 2011;4:111–25. doi: 10.2147/JPR.S18353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer S, Inui M. Regulation of muscle contraction and relaxation in heart. Progress in clinical and biological research. 1988;273:435–50. [PubMed] [Google Scholar]
- Ishihara M, Togo H. Direct oxidative conversion of aldehydes and alcohols to 2-imidazolines and 2-oxazolines using molecular iodine. Tetrahedron. 2007;63:1474–1480. [Google Scholar]
- Jordan S, Jackson HC, Nutt DJ, Handley SL. Discriminative stimulus produced by the imidazoline I2 site ligand, 2-BFI. J Psychopharmacol. 1996;10:273–8. doi: 10.1177/026988119601000403. [DOI] [PubMed] [Google Scholar]
- Kimura A, Tyacke RJ, Robinson JJ, Husbands SM, Minchin MC, Nutt DJ, Hudson AL. Identification of an imidazoline binding protein: creatine kinase and an imidazoline-2 binding site. Brain research. 2009;1279:21–8. doi: 10.1016/j.brainres.2009.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissin I. The development of new analgesics over the past 50 years: a lack of real breakthrough drugs. Anesthesia and analgesia. 2010;110:780–9. doi: 10.1213/ANE.0b013e3181cde882. [DOI] [PubMed] [Google Scholar]
- Kissin I. Scientometrics of drug discovery efforts: pain-related molecular targets. Drug design, development and therapy. 2015;9:3393–404. doi: 10.2147/DDDT.S85633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Thorn DA, Qiu Y, Peng B-W, Zhang Y. Antihyperalgesic effects of imidazoline I(2) receptor ligands in rat models of inflammatory and neuropathic pain. Brit J Pharmacol. 2014;171:1580–1590. doi: 10.1111/bph.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX. Imidazoline I2 receptors: An update. Pharmacol Ther. 2017 doi: 10.1016/j.pharmthera.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Rice KC, France CP. Discriminative stimulus effects of 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane in rhesus monkeys. J Pharmacol Exp Ther. 2008;324:827–33. doi: 10.1124/jpet.107.130625. [DOI] [PubMed] [Google Scholar]
- Li JX, Unzeitig A, Javors MA, Rice KC, Koek W, France CP. Discriminative stimulus effects of 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane (DOM), ketanserin, and (R)-(+)-{alpha}-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl]-4-pipidinemetha nol (MDL100907) in rats. J Pharmacol Exp Ther. 2009;331:671–9. doi: 10.1124/jpet.109.157560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipstein N, Sakaba T, Cooper BH, Lin KH, Strenzke N, Ashery U, Rhee JS, Taschenberger H, Neher E, Brose N. Dynamic control of synaptic vesicle replenishment and short-term plasticity by Ca(2+)-calmodulin-Munc13-1 signaling. Neuron. 2013;79:82–96. doi: 10.1016/j.neuron.2013.05.011. [DOI] [PubMed] [Google Scholar]
- MacInnes N, Handley SL. Characterization of the discriminable stimulus produced by 2-BFI: effects of imidazoline I(2)-site ligands, MAOIs, beta-carbolines, agmatine and ibogaine. Br J Pharmacol. 2002;135:1227–34. doi: 10.1038/sj.bjp.0704579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G. Neurotransmitter release. Annual review of neuroscience. 1996;19:219–33. doi: 10.1146/annurev.ne.19.030196.001251. [DOI] [PubMed] [Google Scholar]
- Morel N, Poea-Guyon S. The membrane domain of vacuolar H(+)ATPase: a crucial player in neurotransmitter exocytotic release. Cellular and molecular life sciences: CMLS. 2015;72:2561–73. doi: 10.1007/s00018-015-1886-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan NG, Chan SL. Imidazoline binding sites in the endocrine pancreas: can they fulfil their potential as targets for the development of new insulin secretagogues? Current pharmaceutical design. 2001;7:1413–31. doi: 10.2174/1381612013397366. [DOI] [PubMed] [Google Scholar]
- Nagakura Y, Okada M, Kohara A, Kiso T, Toya T, Iwai A, Wanibuchi F, Yamaguchi T. Allodynia and hyperalgesia in adjuvant-induced arthritic rats: time course of progression and efficacy of analgesics. The Journal of pharmacology and experimental therapeutics. 2003;306:490–7. doi: 10.1124/jpet.103.050781. [DOI] [PubMed] [Google Scholar]
- NIH. Pain in America NINDS Chronic Pain Information Page, Bethesda, MD 2013 [Google Scholar]
- Nutt DJ, French N, Handley S, Hudson A, Husbands S, Jackson H, Jordan S, Lalies MD, Lewis J, Lione L, et al. Functional studies of specific imidazoline-2 receptor ligands. Annals of the New York Academy of Sciences. 1995;763:125–39. doi: 10.1111/j.1749-6632.1995.tb32397.x. [DOI] [PubMed] [Google Scholar]
- Pigini M, Bousquet P, Carotti A, Dontenwill M, Giannella M, Moriconi R, Piergentili A, Quaglia W, Tayebati SK, Brasili L. Imidazoline receptors: qualitative structure-activity relationships and discovery of tracizoline and benazoline. Two ligands with high affinity and unprecedented selectivity. Bioorg Med Chem. 1997;5:833–41. doi: 10.1016/s0968-0896(97)00009-6. [DOI] [PubMed] [Google Scholar]
- Qiu Y, He XH, Zhang Y, Li JX. Discriminative stimulus effects of the novel imidazoline I(2) receptor ligand CR4056 in rats. Sci Rep. 2014;4:6605. doi: 10.1038/srep06605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Zhang Y, Li JX. Discriminative stimulus effects of the imidazoline I2 receptor ligands BU224 and phenyzoline in rats. Eur J Pharmacol. 2015;749:133–41. doi: 10.1016/j.ejphar.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustenbeck I, Kowalewski R, Herrmann C, Dickel C, Ratzka P, Hasselblatt A. Effects of imidazoline compounds on cytoplasmic Ca2+ concentration and ATP-sensitive K+ channels in pancreatic B-cells. Experimental and clinical endocrinology & diabetes: official journal, German Society of Endocrinology [and] German Diabetes Association. 1995;103(Suppl 2):42–5. doi: 10.1055/s-0029-1211393. [DOI] [PubMed] [Google Scholar]
- Siemian JN, Li J, Zhang Y, Li JX. Interactions between imidazoline I2 receptor ligands and acetaminophen in adult male rats: antinociception and schedule-controlled responding. Psychopharmacology. 2016a;233:873–82. doi: 10.1007/s00213-015-4166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemian JN, Obeng S, Zhang Y, Zhang Y, Li JX. Antinociceptive Interactions between the Imidazoline I2 Receptor Agonist 2-BFI and Opioids in Rats: Role of Efficacy at the mu-Opioid Receptor. The Journal of pharmacology and experimental therapeutics. 2016b;357:509–19. doi: 10.1124/jpet.116.232421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitges M, Talamo BR. Sphingosine, W-7, and trifluoperazine inhibit the elevation in cytosolic calcium induced by high K+ depolarization in synaptosomes. Journal of neurochemistry. 1993;61:443–50. doi: 10.1111/j.1471-4159.1993.tb02144.x. [DOI] [PubMed] [Google Scholar]
- Thorn DA, An XF, Zhang Y, Pigini M, Li JX. Characterization of the hypothermic effects of imidazoline I(2) receptor agonists in rats. Br J Pharmacol. 2012;166:1936–45. doi: 10.1111/j.1476-5381.2012.01894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn DA, Siemian JN, Zhang Y, Li JX. Anti-hyperalgesic effects of imidazoline I2 receptor ligands in a rat model of inflammatory pain: interactions with oxycodone. Psychopharmacology (Berl) 2015;232:3309–18. doi: 10.1007/s00213-015-3983-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn DA, Zhang Y, Li JX. Effects of the imidazoline I receptor agonist 2-BFI on the development of tolerance and behavioral/physical dependence to morphine in rats. Br J Pharmacol. 2016;173:1363–72. doi: 10.1111/bph.13435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugedo L, Pineda J, Martin-Ruiz R, Ruiz-Ortega JA, Artigas F. Imidazoline-induced inhibition of firing rate of 5-HT neurons in rat dorsal raphe by modulation of extracellular 5-HT levels. Annals of the New York Academy of Sciences. 1999;881:365–8. doi: 10.1111/j.1749-6632.1999.tb09382.x. [DOI] [PubMed] [Google Scholar]
- Ugedo L, Pineda J, Ruiz-Ortega JA, Martin-Ruiz R. Stimulation of locus coeruleus neurons by non-I1/I2-type imidazoline receptors: an in vivo and in vitro electrophysiological study. Br J Pharmacol. 1998;125:1685–94. doi: 10.1038/sj.bjp.0702255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]


