Abstract
Objective
To determine whether applying controlled resistance forces to the legs during the swing phase of gait may improve the efficacy of treadmill training compared to applying controlled assistance forces in children with cerebral palsy (CP).
Design
Randomized controlled study.
Setting
Research unit of rehabilitation hospital.
Participants
Children with spastic CP (n = 23, average age 10.6 years old, ranged from 6–14, GMFCS levels: I to IV).
Interventions
Participants were randomly assigned to receive controlled assistance (n=11) or resistance (n=12) loads applied to the legs at the ankle. Participants underwent robotic treadmill training 3 times a week for 6 weeks (18 sessions). A controlled swing assistance/resistance load was applied to both legs starting from toe-off to mid-swing phase of gait during training.
Main outcome measures
Outcome measures consisted of overground walking speed, 6 minute walking distance, and GMFM scores, and were assessed pre, post 6 weeks of training, and 8 weeks after the end of training.
Results
Following 6 weeks of treadmill training for participants from the resistance training group, fast walking speed and 6 minute walking distance significantly improved (18% and 30% increases, respectively), and 6 minute walking distance was still significantly greater than baseline (35% increase) 8 weeks after the end of training. In contrast, overground gait speed and 6 minute walking distance had no significant changes after robotic assistance training.
Conclusion
Results from the current study indicated that robotic resistance treadmill training is more effective than assistance training in improving locomotor function in children with CP.
MeSH keywords: Locomotion, treadmill training, cerebral palsy, resistance force, assistance force, child, therapy
Attaining walking ability is often an important functional goal for children with cerebral palsy (CP). Of the children who are diagnosed with CP, up to 90% of them have difficulty walking [1, 2]. Reduced walking speed and endurance are two of the main functional problems [3, 4]. Ambulation plays a central role in healthy bone development [5] and cardiopulmonary endurance [6, 7] and children who are able to ambulate are more accomplished in activities of daily living and social roles than children who use a wheelchair [8]. The development of independent gait and efficiency of walking are often the focus of therapeutic interventions for children with CP.
The use of treadmill training has demonstrated improvement in walking capacity in some children with CP [9, 10]. For instance, results have demonstrated improvements in gait velocity [9, 11], endurance [9], and the Gross Motor Function Classification System (GMFCS) measures [10, 12]. However, while statistically significant improvements in walking capacity with treadmill training have been shown, it remains unclear whether therapeutic effects of such training are maximized, and further evidence is needed to support treadmill training in children with CP [13].
Results from motor learning studies indicated that active training is more effective than passive training in improving the efficacy of motor training [14]. Thus, we postulated that active engagement from children with CP might improve the efficacy of locomotor training. We proposed that applying a resistance force to leg swing may force them to be more actively involved because they need to generate additional joint torques to counteract the load. In addition, applying a resistance force to leg swing may produce a deviation in step kinematics, i.e., increase the kinematic errors, which is supported by previous studies in individuals post stroke [15] [16] and spinal cord injury [17]. Error augmentation may accelerate motor learning during treadmill training [18] [19, 20], resulting in improvement in efficacy of locomotor training in children with CP.
On the other hand, providing leg assistance force may facilitate leg swing, which imitates the way that physical therapist provides leg assistance during treadmill training, and improve locomotor function in children with CP through use-dependent motor learning mechanisms [21], but it may reduce the level of active engagement of children with CP. To date, there are no randomized controlled studies that have directly compared leg resistance vs. assistance during treadmill training in children with CP. The purpose of this study was to assess functional changes after resistance vs. assistance treadmill training in children with CP. We hypothesized that children with CP from both groups would show improvements in locomotor function, which was assessed using walking speed and 6 minute walking distance, although greater improvements were expected after resistance than that after assistance training.
Methods
Participants
Participants were recruited through the outpatient of the Rehabilitation Institute of Chicago between 2011 and 2014. Specifically, 92 children were contacted through phone calls, 69 children were excluded, and 23 children with (11 males and 12 females) CP were recruited to participate in this study, see Figure 1. The average age of participants was 10.6 years old (ranged from 6–14). Their Gross Motor Function Classification System level ranged from I–IV (I (1), II (11), III (8), IV (3)).
Figure 1.
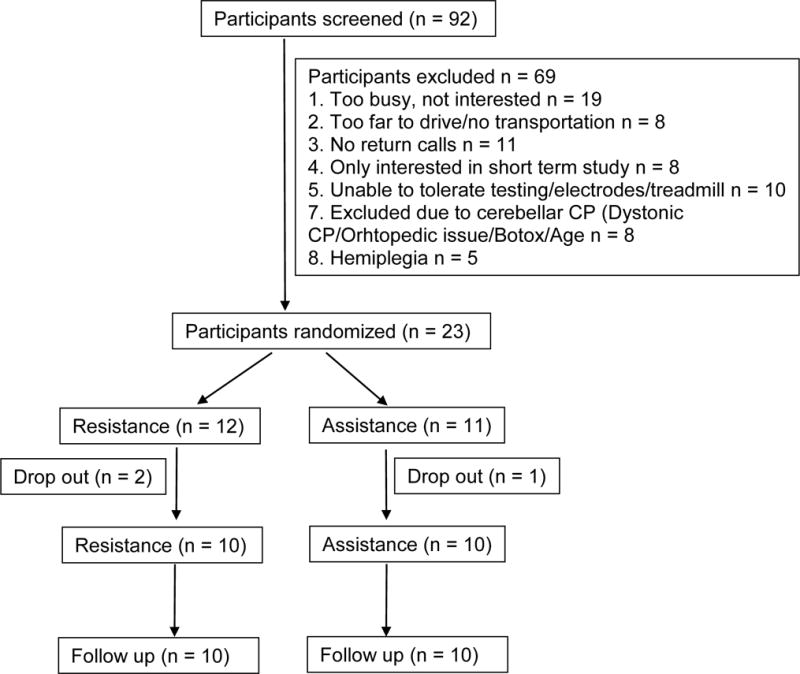
Flowchart of participants’ enrollment and randomization.
Inclusion criteria: 1) a diagnosis of bilateral spastic CP attributed to complications of prematurity, intracranial hemorrhage and periventricular leukomalacia according to the definition of Bax [22]; 2) aged 4 to 14 years old; 3) without botulinum toxin treatment and orthopedic surgery or neurosurgery within 6 months before the onset of the training; 4) Gross Motor Function Classification System (GMFCS) level ranged from I to IV; 5) able to signal pain, fear or discomfort reliably; 6) with mild scoliosis (Cobb angle < 20 °); 7) passive range of motion within functional limits; 8) were able to follow instructions on behavior tests.
Exclusion criteria included severe lower extremity contractures, fractures, osseous instabilities, osteoporosis, severe disproportional bone growth, unhealed skin lesions in the lower extremities, thromboembolic diseases, cardiovascular instability, and aggressive or self-harming behaviors.
All procedures were approved by the Institutional Review Board of the Northwestern University. Medical clearance for participation was requested from the primary physician of each participant. Written consent was obtained from all participants and their parents.
Apparatus
A custom designed cable-driven robotic gait training system (CaLT) was used to apply controlled resistance or assistance to both legs during treadmill walking, which was reported previously [23]. In brief, the cable-driven robotic system consists of 4 DC motors and cable spools, see Figure 2. Two motors and cable spools located in the front of the treadmill were used to apply a controlled assistance force to the legs at ankle, and two motors located at the back of the treadmill were used to apply a resistance force to the legs. The ankle trajectory signals were measured using a custom designed 3D position detector. The ankle position signals were used by the operator to control the timing and magnitude of loading at targeted phases of gait. An adaptive control algorithm was used to automatically adjust the amount of the resistance/assistance load based on the motor performance of the participants [23]. The operator controlled the robotic system via a user interface that was programmed in LabVIEW (National Instruments, Austin, TX).
Figure 2.
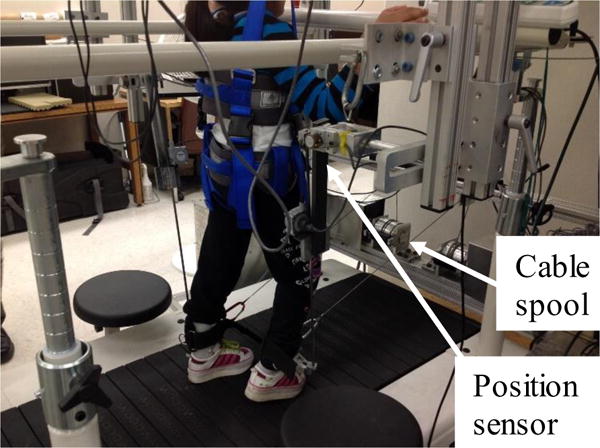
Experimental setup for the robotic assistance/assistance treadmill training. Two motors and cable spools are attached to a fixed frame located in the front of the treadmill, and are used to apply a controlled assistance force to the legs at ankle, and two are attached to a frame located at the back of the treadmill, and are used to apply a resistance force to the legs. A computer is used to control the coordinated movement of 4 motors.
Protocol
Participants were stratified according to initial gait speed and were randomly assigned to receive controlled assistance or resistance loads applied to both legs at the ankle. The treadmill speed was set at the comfortable speed of each participant on a treadmill and an overhead harness was used to provide body weight support as needed to prevent knee buckling and toe drag. Participants walked on a treadmill for 40 minutes, adjusted based on their tolerance on the activity, excluding set up time in each training session. Training sessions took place 3 times a week for 6 weeks. For participants who were assigned to the assistance training group, a load that assisted swing was applied to both legs starting from toe-off to mid-swing phase of gait. For participants who were assigned to the resistance training group, a controlled resistance load was applied to both legs. Both the treadmill speed and resistance/assistance load were gradually increased during the course of 6 weeks of treadmill training. Body weight support was provided in 8 participants (3 in the resistance and 5 in the assistance groups, respectively) with the average support was 11.4 ± 4.2 % of body weight at session 1, and 8.1 ± 6.2 % of body weight at session 18. The rating of perceived exertion (RPE) [24], which was used to quantify the perceived training intensity, was monitored during the course of training and the targeted RPE was 11 to 16.
Outcome measures
Primary outcome measures included participant’s self-selected and fast overground walking velocity collected on a 10m instrumented walkway (GAITRite, CIR Systems Inc, Sparta, NJ) [25, 26] and averaged across 3 trials, and walking distance assessed through the 6-minute timed walk [27]. Secondary outcome measures included clinical assessments of motor function. Specifically, the dimensions D (standing) and E (walking, running, jumping) of the Gross Motor Function Measure (GMFM-66) were assessed by licensed physical therapists [28, 29]. Physical function was measured using Pediatric Outcomes Data Collection Instrument (PODCI) [30]. In addition, muscle tone of major knee joint muscle groups was assessed clinically using the Modified Ashworth Scale (0–4; [31]), which was conducted before the GMFM and 6-minute walking tests.
Data analysis
A power analysis was conducted based on results from our previous pilot study, which indicated that with a power of 0.8 and an alpha of 0.05, 11 participants per group would be required. Baseline characteristics and training parameters were compared between the two test groups using paired t tests, and Wilcoxon rank sum tests, as appropriate, with data normality checked using the Shapiro-Wilk test. Repeated measures ANOVAs were used for all parametric measures for within group comparison. If a significant difference was detected, Tukey-Kramer post-hoc tests were conducted to determine which conditions were different from each other. Modified Ashworth Scale (MAS) scores were analyzed using Friedman tests with post-hoc Wilcoxon tests. Changes in primary outcomes (i.e., gait speed and endurance) were calculated by subtracting the baseline value from the values obtained at post and follow up tests, and analyzed using repeated-measures ANOVA with main factor of treatment (resistance vs. assistance training), and repeated for time (post and follow up tests). Statistical significance for all tests was set at p < 0.05.
Results
Twenty participants completed all the training and assessment sessions with 3 participants dropping out (due to poor attendance, the child’s request, and participation in another study, respectively). At baseline, there were no significant differences between the two groups in age, overground walking speed, 6 minute walk distance, and GMFM scores (see Table 1). Treadmill training speed, distance, and time were gradually increased for both groups (p < 0.01) during the course of training with no significant difference noted between the two groups. In addition, the training intensity, which was quantified using RPE, had no significant difference between the two groups, see Table 2.
Table 1.
Characteristics of participants for baseline comparisons.
| Characteristics | Resistance | Assistance | p |
|---|---|---|---|
| Age (y) | 10.6 ± 3.0 | 10.8 ± 2.3 | 0.93 |
| Gender (M/F) | 6/6 | 5/6 | |
| Race (white/other) | |||
| African American | 1 | 1 | |
| Asian | 1 | 0 | |
| Hispanic | 4 | 5 | |
| White | 6 | 5 | |
| Extremity distribution | |||
| Diplegia | 7 | 7 | |
| Quadriplegia | 5 | 4 | |
| GMFCS | |||
| I | 1 | 0 | |
| II | 5 | 5 | |
| III | 5 | 4 | |
| IV | 1 | 2 | |
| Ankle braces | |||
| None | 0 | 2 | |
| BAFO/SMO | 12 | 9 | |
| GMFM | |||
| Total score | 63.6 ± 8.6 | 60.8 ± 9.1 | 0.33 |
| Dimension D | 25.8 ± 8.0 | 22.8 ± 11.3 | 0.18 |
| Dimension E | 32.3 ± 18.2 | 30.4 ± 18.0 | 0.64 |
| Self-selected gait speed (m/s) | 0.64 ± 0.28 | 0.57 ± 0.23 | 0.35 |
| Fast walking gait speed (m/s) | 1.00 ± 0.35 | 0.88 ± 0.34 | 0.28 |
| 6-minute walking distance (m) | 277.2 ± 111.9 | 228.3 ± 117.8 | 0.27 |
| MAS | 0.67 ± 0.53 | 0.95 ± 0.72 | 0.40 |
Abbreviations: GMFCS, Gross Motor Function Classification System; GMFM, Gross Motor Function Measure; BAFO, Bilateral Ankle Foot Orthosis; SMO, Supra-Malleolar Orthosis; MAS, Modified Ashworth Scale (t tests and Wilcoxon rank sum tests were used for comparison between two groups).
Table 2.
Training paradigms, including treadmill speed, time, and training intensity at session 1, session 9, and session 18 for robotic resistance vs. assistance treadmill training groups.
| Resistance training | Assistance training | p | |
|---|---|---|---|
| Session1 | |||
| Speed (m/s) | 0.42 ± 0.15 | 0.42 ± 0.19 | 0.95 |
| Distance (km) | 0.80 ± 0.31 | 0.85 ± 0.43 | 0.78 |
| Time (min) | 31 ± 2 | 33 ± 3 | 0.15 |
| RPE | 12.9 ± 2.9 | 11.8 ± 1.6 | 0.27 |
| Peak force (N) | 11.4 ± 2.6 | 13.2 ± 2.9 | 0.18 |
| Session 9 | |||
| Speed (m/s) | 0.51 ± 0.19 | 0.49 ± 0.16 | 0.85 |
| Distance (km) | 1.13 ± 0.48 | 1.11 ± 0.45 | 0.95 |
| Time (min) | 36 ± 3 | 37 ± 4 | 0.58 |
| RPE | 11.1 ± 1.1 | 12.2 ± 1.6 | 0.23 |
| Peak force (N) | 13.3 ± 4.1 | 16.4 ± 2.2 | 0.08 |
| Session18 | |||
| Speed (m/s) | 0.55 ± 0.18 | 0.53 ± 0.22 | 0.86 |
| Distance (km) | 1.13 ± 0.42 | 1.28 ± 0.57 | 0.98 |
| Time (min) | 39 ± 3 | 39 ± 3 | 0.93 |
| RPE | 12.0 ± 1.8 | 11.3 ± 1.5 | 0.19 |
| Peak force (N) | 14.8 ± 5.2 | 18.2 ± 3.2 | 0.15 |
Abbreviations: RPE, rating of perceived exertion (t tests were used for comparison between two groups).
Robotic resistance treadmill training improved walking function in children with CP. Specifically, fast walking speed significantly increased after robotic resistance treadmill training [F (2, 9) =4.12, p = 0.03, ANOVA], Figure 3. Post-hoc tests indicated a significant increase from baseline to post testing (mean increase 18%, IQR, 2%–31%, p = 0.01), although no significant difference between baseline and follow up testing (increase 17%, IQR, −6%–17%, p = 0.13). Self-selected walking speed tended to increase (mean increase 32%, IQR, −8%–44%), but this was not significant due to large variability across participants [F (2, 9) = 1.66, p = 0.21]. In addition, 6 minute walking distance significantly increased after resistance training [F (2, 9) = 10.04, p = 0.001]. Post-hoc tests indicated a significant increase from baseline to post testing (increase 30%, IQR, 4%–45%, p = 0.01), and from baseline to follow up testing (increase 35%, IQR, 6%–64%, p = 0.001).
Figure 3.
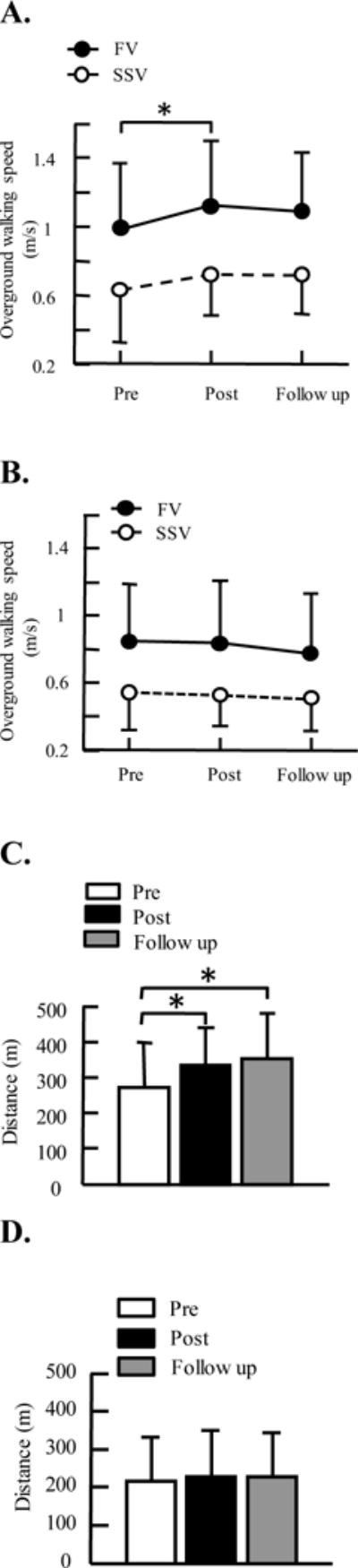
Average of self-selected, fast walking velocities, 6 minute walking distance pre, post 6 weeks of robotic treadmill training and 8 weeks after the end of treadmill training, i.e., follow up. A. overground walking speed for participants from the resistance treadmill training group; B. overground walking speed for participants from the assistance treadmill training group; C. 6 minute walking distance for participants from the resistance training group; D. 6 minute walking distance for participants from the assistance training group. Three trials were tested for walking speed and averaged across participants for each group. Error bars indicate standard deviation of each gait parameter. SSV, self-selected velocity; FV, fast velocity. * indicates significant difference, p < 0.05.
In contrast, robotic assistance treadmill training induced modest changes in walking function in children with CP. Specifically, both fast and self-selected walking speeds had no significant changes after assistance training (mean increase 0.4%, IQR, 0%–10%, F (2, 9) = 1.85, p = 0.19, and increase 2%, IQR, −12%–20%, F (2, 9) = 0.51, p = 0.6, for fast and self-selected walking speeds, respectively), Figure 3. In addition, 6 minute walking distance had no significant change after assistance training (increase 4%, IQR, −6%–16%, F (2, 9) = 0.48, p = 0.63).
Greater functional gains were observed for participants who underwent resistance training than those who underwent assistance training. Specifically, changes in self-selected walking speed, fast walking speed, and 6 minute walking distance after treadmill training were significantly greater for participants from the resistance group than that from the assistance group [F (1,1) = 5.36, p = 0.03, F (1,1) = 10, p = 0.003, and F (1,1) = 12.23, p = 0.001, for self-selected walking speed, fast walking speed, and 6 minute walking distance, respectively], Figure 4. We observed no significant differences in changes of walking function between the post test and follow up test (p = 0.75 for self-selected walking speed, p = 0.33 for fast walking speed, and p = 0.73 for 6 minute walking distance). GMFM scores significantly changed after resistance training [F (2, 9) = 5.21, p = 0.02]. Post-hoc test indicated significant differences between post testing and follow up testing scores (p = 0.02), although there was no significant difference between pre testing vs. post testing scores (p = 0.89), and pre testing vs. follow up testing scores (p = 0.05). In contrast, GMFM scores had no significant changes after assistance training [F (2, 9) = 0.49, p = 0.69], Table 3. In addition, step cadence during fast walking significantly increased after resistance training [F (2, 9) = 6.11, p = 0.01]. A post-hoc test indicated significant differences in step cadence pre vs. post training (p = 0.01), although no significant difference between pre vs. follow up tests (p = 0.06), see Table 4. Other spatial-temporal gait parameters had no significant changes for both groups (p > 0.05).
Figure 4.
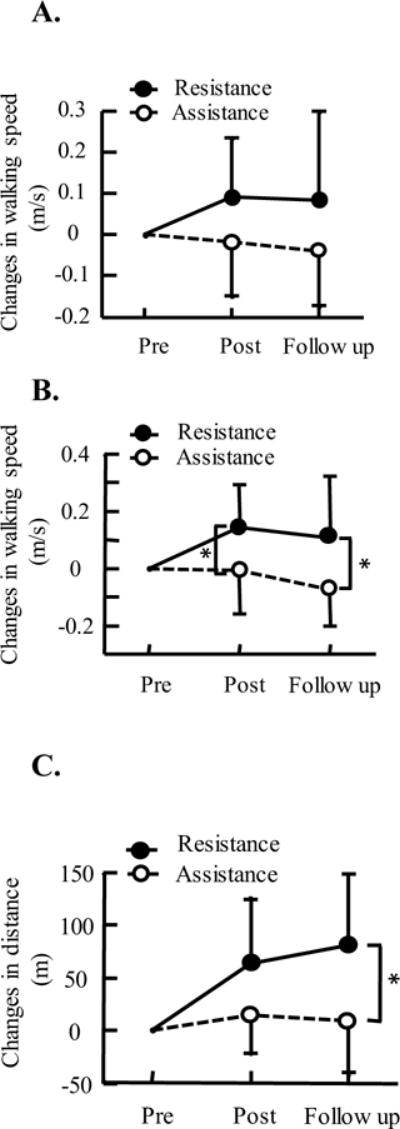
Changes in walking self-selected walking speeds, A, fast walking speed, B, and 6-minute walking distance, C, after robotic resistance/assistance treadmill training, and 8 weeks after the end of training, i.e., follow up test. Data shown in the figure are the mean and standard deviation of walking speeds and distance across participants. * indicates significant difference, p < 0.05.
Table 3.
Self-selected, fast walking speeds, and 6-minute walking distance, GMFM scores, Modified Ashworth Scale (MAS), and the Pediatric Outcomes Data Collection Instrument (PODCI) after robotic resistance and assistance treadmill training.
| Robotic resistance training | Robotic assistance training | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | FU | p | Pre | Post | FU | p | |
| FV (m/s) | 0.98 ± 0.39 | 1.13 ± 0.38 | 1.09 ± 0.35 | 0.01* | 0.84 ± 0.34 | 0.84 ± 0.37 | 0.77 ± 0.36 | 0.19 |
| SSV (m/s) | 0.63 ± 0.30 | 0.72 ± 0.24 | 0.71 ± 0.22 | 0.22 | 0.54 ± 0.22 | 0.52 ± 0.18 | 0.50 ± 0.19 | 0.61 |
| 6minWT(m) | 272.7 ± 113.0 | 336.3 ± 104.9 | 353.9 ± 125.8 | 0.001* | 216.3 ± 116.8 | 230.1 ± 119.2 | 224.7 ± 118.7 | 0.63 |
| GMFM | ||||||||
| Total score | 63.7±8.7 | 63.4±8.2 | 64.9±9.4 | 0.02* | 60.0±9.2 | 59.8±9.6 | 60.3±9.4 | 0.69 |
| Dimensi on D | 25.1±8.6 | 26.0±6.9 | 27.3±7.1 | 0.18 | 21.7±11. 3 | 21.8±11. 7 | 20.8±11. 4 | 0.62 |
| Dimensi on E | 31.4±19. 5 | 30.5±19. 2 | 32.5±19. 8 | 0.11 | 29.2±18. 5 | 28.3±17. 7 | 28.8±19. 5 | 0.35 |
| MAS | 0.65±0.5 7 | 0.83±0.6 6 | 0.63±0.3 9 | 0.97 | 0.85±0.6 7 | 0.68±0.5 6 | 0.87±0.5 5 | 0.20 |
| PODCI (self) | 23.0±17. 2 | 23.0±18. 0 | 24.5±12. 2 | 0.84 | 23.0±23. 6 | 19.5±12. 1 | 24.0±16. 0 | 0.74 |
| PODCI (parent) | 7.5±16.2 | 19.1±15. 5 | 19.0±16. 8 | 0.002* | 7.9±22.8 | 9.8±16.4 | 3.9±24.5 | 0.83 |
indicates significant difference (ANOVAs and Friedman tests were used for within group comparison).
Abbreviations: FU, Follow Up; FV, Fast Velocity; SSV, Self-Selected Velocity; 6minWT, 6-minute Walking Test.
Table 4.
Spatial-temporal gait parameters pre, post robotic resistance and assistance treadmill training.
| Resistance training | Assistance training | |||||||
|---|---|---|---|---|---|---|---|---|
| pre | post | follow up | p | pre | post | follow up | p | |
| Fast walking | ||||||||
| Step length (m) | 0.47± 0.10 | 0.50± 0.09 | 0.49± 0.09 | 0.26 | 0.42± 0.10 | 0.42± 0.09 | 0.40± 0.10 | 0.45 |
| Stride length (m) | 0.94± 0.20 | 1.00± 0.18 | 0.98± 0.18 | 0.15 | 0.84± 0.20 | 0.84± 0.18 | 0.84± 0.22 | 0.99 |
| Cadence (step/minute) | 120.3 ±25.5 | 136.5 ±29.0 | 132.0 ±28.4 | 0.01* | 119.2 ±40.2 | 120.2 ±41.3 | 116.9 ±44.3 | 0.86 |
| Swing time (%) | 38.9± 4.8 | 39.8± 3.8 | 40.0± 3.2 | 0.61 | 40.1± 5.6 | 41.6± 4.9 | 40.6± 7.2 | 0.72 |
| Normal walking | ||||||||
| Step length (m) | 0.39± 0.11 | 0.43± 0.09 | 0.42± 0.08 | 0.16 | 0.34± 0.09 | 0.35± 0.08 | 0.35± 0.08 | 0.83 |
| Stride length (m) | 0.78± 0.22 | 0.86± 0.18 | 0.85± 0.16 | 0.16 | 0.71± 0.19 | 0.71± 0.16 | 0.70± 0.16 | 0.98 |
| Cadence (step/minute) | 93.2± 26.3 | 100.6 ±21.8 | 100.7 ±20.8 | 0.22 | 91.4± 27.8 | 86.8± 20.5 | 83.9± 23.8 | 0.21 |
| Swing time (%) | 33.6± 4.7 | 35.7± 1.9 | 36.5± 2.0 | 0.11 | 36.1± 4.8 | 37.4± 8.7 | 38.2± 7.7 | 0.78 |
indicates significant difference (ANOVAs were used for within group comparison).
Discussion
Applying a controlled swing resistance force to the legs during robotic treadmill training induced significant improvements in walking function of children with CP. In contrast, applying an assistance to legs during treadmill training only induced modest changes in walking function. Further, greater functional gains in walking were obtained for participants from the resistance group than those from the assistance group.
In this study, greater improvements in walking function were observed for children who underwent resistance training than those who underwent assistance training. One possible reason for the differences in functional gains may be that children underwent resistance training were more actively engaged in the locomotor training session than those who underwent assistance training. Specifically, for children who were assigned the resistance training group, the resistance load applied to both legs during swing phase may force participants to generate additional joint torque to counteract the load and move the leg forward during treadmill training, which may require participants to increase voluntary activation through enhanced supraspinal input to the motoneuron pool and/or increase motoneuron excitability [32].
In contrast, for children who underwent assistance training, the central nervous system may adapt to the assistance force applied to the leg(s) during the swing phase of gait by reducing the motor output of the leg muscles [33], probably due to optimization of the energy cost [34]. Thus, a leg assistance force that is too large may encourage passive rather than active training. As a consequence, the training effect could be suboptimal. This is also consistent with a previous study, which indicated that only a modest increase in the gait speed of children with CP was observed after robotic training in which a passive guidance force was applied to both legs [35]. However, results from a previous study in adults with stroke indicated that assistance and resistance training induced comparable walking functional gains [18]. One possible reason may be due to the age (or the motivation level) difference of participants between the two studies.
In addition, results from this study also suggest that repeated exposure to force perturbations during walking may induce a prolonged retention of increased stride length in children with CP. Results from previous studies suggests that the neural system may adapt to the resistance force, and show an aftereffect consisting of an increased stride length when the resistance force is removed [36], which is consistent with individuals post stroke [15, 37], and humans with spinal cord injury [38, 39]. While the aftereffect after one session of swing resistance training is generally short lived, results from this study suggest that repeated exposure to a swing resistance force through long-term training, such as 18 sessions of training in this case, may induce an accumulation of increased stride length through a repeated adaptation and de-adaptation process [40], which is consistent with previous results from studies involving humans with spinal cord injury [19], and individuals post stroke using a split-belt paradigm [20]. In addition, repeated exposure to leg resistance force may enhance muscle activation of hip flexors, which may facilitate leg swing, but may have a modest impact on hip abductors, which are identified as key muscles in maintaining mediolateral balance of standing leg during walking [41]. As a result, we observed a greater increase in cadence but a smaller increase in step length due to modest change in single leg support time after resistance training.
To our knowledge, this is the first study to show the differences in walking functional gains in children with CP after robotic treadmill training with robotic resistance vs. assistance force. The functional gains obtained after robotic resistance treadmill training are comparable or even greater than that from previous studies using treadmill training. For instance, the functional gain in walking speed (i.e., 0.15 m/s, although < 0.17m/s, minimal clinical importance difference, MCID) [42] after robotic resistance training was greater/comparable with that after treadmill training with applied manual assistance force (0.01–0.07 m/s) [9, 43], or with robotic assistance using the pediatric Lokomat (i.e., 0.02m/s) [35], which is comparable with functional gains obtained after assistance training (i.e., −0.02 m/s), and after robotic training using the GaitTrainer (i.e., 0.12 m/s) [44]. Similarly, the functional gain in 6 minute walking distance after resistance training (81.2m > 61.5m, MCID) was greater than that after treadmill training with manual assistance (i.e., −25.0 −19.8 m) [9, 43], which is comparable to functional gains obtained after assistance training (i.e., 13.9 m), and after robotic training using the GaitTrainer (i.e., 69m) [44].
The results from this study may have some clinical applications for improving locomotor function in children with CP. For instance, in order to improve the efficacy of locomotor training, physical therapists may consider applying a resistance force to the legs, and refrain from applying a manual assistance force (that is too large) to legs during locomotor training in order to increase the intensity and/or active involvement of children with CP.
This study had several limitations. For instance, the sample size was small due to the challenges of participant recruitment. In addition, the assessing physical therapists were not blinded to the group assignment, which may have potentially biased the results. We also do not know whether a robotic resistance treadmill training paradigm is more effective than a conventional treadmill training paradigm, and we do not know whether the cognitive abilities of subject had an impact on the results. In addition, the reliability of GAITRite data from some low functional children with CP was relatively low [25], which might impact the results. We also do not know whether the level of resistance/assistance load applied in this paradigm is optimized. Further studies are needed to optimize this resistance training paradigm.
Conclusions
Applying a resistance force to the legs during robotic treadmill training is more effective than applying an assistance force in improving walking function in children with CP. This suggests that active involvement of children with CP during locomotor training is crucial for improving the efficacy of treadmill training. In addition, repeated exposure to force perturbations induces a prolonged retention of improved stride length in children with CP.
Highlights.
Children with CP underwent 6 weeks (3 times/week) of robotic treadmill training
An assistance/resistance load was applied to both legs to help/hinder swing phase
Resistance is more effective than assistance training in improving walking function
Acknowledgments
We thank Drs. Sheng-Che Yen and Feng Wei, for their assistance during data collection. We thank Ms. Jill Landry for her comments and suggestions for this manuscript. This study was supported by NIH, 1R21HD066261.
Abbreviations
- CP
Cerebral palsy
- GMFCS
Gross Motor Function Classification System
- DC
Direct current
- RPE
Rating of perceived exertion
- GMFM
Gross Motor Function Measure
- PODCI
Pediatric Outcomes Data Collection Instrument
- ANOVA
Analysis of variance
- MAS
Modified Ashworth Scale
- MCID
Minimal clinical importance difference
- FV
Fast velocity
- SSV
Self-selected velocity
- BAFO
Bilateral Ankle Foot Orthosis
- SMO
Supra-Malleolar Orthosis
- FU
Follow up
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Suppliers:
GaitRite, CIR Systems Inc. 12 Cork Hill Road, Bldg #2, Franklin, NJ, 07416 Woodway, WOODWAY USA, Inc. W229 N591 Foster Ct., Waukesha, WI, 53186
References
- 1.Pharoah PO, et al. Epidemiology of cerebral palsy in England and Scotland. Arch Dis Child Fetal Neonatal Ed. 1998;79(1):1984–9. F21–5. doi: 10.1136/fn.79.1.f21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hutton JL, Pharoah PO. Effects of cognitive, motor, and sensory disabilities on survival in cerebral palsy. Arch Dis Child. 2002;86(2):84–9. doi: 10.1136/adc.86.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duffy CM, et al. Energy consumption in children with spina bifida and cerebral palsy: a comparative study. Dev Med Child Neurol. 1996;38(3):238–43. doi: 10.1111/j.1469-8749.1996.tb15085.x. [DOI] [PubMed] [Google Scholar]
- 4.Bjornson KF, et al. Walking activity patterns in youth with cerebral palsy and youth developing typically. Disabil Rehabil. 2014;36(15):1279–84. doi: 10.3109/09638288.2013.845254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilmshurst S, et al. Mobility status and bone density in cerebral palsy. Arch Dis Child. 1996;75(2):164–5. doi: 10.1136/adc.75.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chien LY, et al. Health-related quality of life among 3–4-year-old children born with very low birthweight. J Adv Nurs. 2006;56(1):9–16. doi: 10.1111/j.1365-2648.2006.03974.x. [DOI] [PubMed] [Google Scholar]
- 7.Gorter H, et al. Changes in endurance and walking ability through functional physical training in children with cerebral palsy. Pediatr Phys Ther. 2009;21(1):31–7. doi: 10.1097/PEP.0b013e318196f563. [DOI] [PubMed] [Google Scholar]
- 8.Lepage C, Noreau L, Bernard PM. Association between characteristics of locomotion and accomplishment of life habits in children with cerebral palsy. Phys Ther. 1998;78(5):458–69. doi: 10.1093/ptj/78.5.458. [DOI] [PubMed] [Google Scholar]
- 9.Dodd KJ, Foley S. Partial body-weight-supported treadmill training can improve walking in children with cerebral palsy: a clinical controlled trial. Dev Med Child Neurol. 2007;49(2):101–5. doi: 10.1111/j.1469-8749.2007.00101.x. [DOI] [PubMed] [Google Scholar]
- 10.Schindl MR, et al. Treadmill training with partial body weight support in nonambulatory patients with cerebral palsy. Arch Phys Med Rehabil. 2000;81(3):301–6. doi: 10.1016/s0003-9993(00)90075-3. [DOI] [PubMed] [Google Scholar]
- 11.Provost B, et al. Endurance and gait in children with cerebral palsy after intensive body weight-supported treadmill training. Pediatr Phys Ther. 2007;19(1):2–10. doi: 10.1097/01.pep.0000249418.25913.a3. [DOI] [PubMed] [Google Scholar]
- 12.Cherng RJ, et al. Effect of treadmill training with body weight support on gait and gross motor function in children with spastic cerebral palsy. Am J Phys Med Rehabil. 2007;86(7):548–55. doi: 10.1097/PHM.0b013e31806dc302. [DOI] [PubMed] [Google Scholar]
- 13.Damiano DL, DeJong SL. A systematic review of the effectiveness of treadmill training and body weight support in pediatric rehabilitation. J Neurol Phys Ther. 2009;33(1):27–44. doi: 10.1097/NPT.0b013e31819800e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lotze M, et al. Motor learning elicited by voluntary drive. Brain. 2003;126(Pt 4):866–72. doi: 10.1093/brain/awg079. [DOI] [PubMed] [Google Scholar]
- 15.Yen SC, Schmit BD, Wu M. Using swing resistance and assistance to improve gait symmetry in individuals post-stroke. Hum Mov Sci. 2015;42:212–24. doi: 10.1016/j.humov.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savin DN, et al. Poststroke hemiparesis impairs the rate but not magnitude of adaptation of spatial and temporal locomotor features. Neurorehabil Neural Repair. 2013;27(1):24–34. doi: 10.1177/1545968311434552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yen SC, Landry JM, Wu M. Size of kinematic error affects retention of locomotor adaptation in human spinal cord injury. J Rehabil Res Dev. 2013;50(9):1187–200. doi: 10.1682/JRRD.2012.09.0175. [DOI] [PubMed] [Google Scholar]
- 18.Wu M, et al. Robotic resistance/assistance training improves locomotor function in individuals poststroke: a randomized controlled study. Arch Phys Med Rehabil. 2014;95(5):799–806. doi: 10.1016/j.apmr.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu M, et al. Repeat Exposure to Leg Swing Perturbations During Treadmill Training Induces Long-Term Retention of Increased Step Length in Human SCI A Pilot Randomized Controlled Study. Am J Phys Med Rehabil. 2016 doi: 10.1097/PHM.0000000000000517. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Reisman DS, et al. Repeated split-belt treadmill training improves poststroke step length asymmetry. Neurorehabil Neural Repair. 2013;27(5):460–8. doi: 10.1177/1545968312474118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sawaki L, et al. Effects of somatosensory stimulation on use-dependent plasticity in chronic stroke. Stroke. 2006;37(1):246–7. doi: 10.1161/01.STR.0000195130.16843.ac. [DOI] [PubMed] [Google Scholar]
- 22.Bax M, et al. Proposed definition and classification of cerebral palsy, April 2005. Dev Med Child Neurol. 2005;47(8):571–6. doi: 10.1017/s001216220500112x. [DOI] [PubMed] [Google Scholar]
- 23.Wu M, et al. A cable-driven locomotor training system for restoration of gait in human SCI. Gait Posture. 2011;33(2):256–60. doi: 10.1016/j.gaitpost.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 24.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2(2):92–8. [PubMed] [Google Scholar]
- 25.Graser JV, Letsch C, Hedel HJ van. Reliability of timed walking tests and temporo-spatial gait parameters in youths with neurological gait disorders. BMC Neurol. 2016;16:15. doi: 10.1186/s12883-016-0538-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chrysagis N, Skordilis EK, Koutsouki D. Validity and clinical utility of functional assessments in children with cerebral palsy. Arch Phys Med Rehabil. 2014;95(2):369–74. doi: 10.1016/j.apmr.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 27.Andersson C, Asztalos L, Mattsson E. Six-minute walk test in adults with cerebral palsy. A study of reliability. Clin Rehabil. 2006;20(6):488–95. doi: 10.1191/0269215506cr964oa. [DOI] [PubMed] [Google Scholar]
- 28.Russell DJ, et al. The gross motor function measure: a means to evaluate the effects of physical therapy. Dev Med Child Neurol. 1989;31(3):341–52. doi: 10.1111/j.1469-8749.1989.tb04003.x. [DOI] [PubMed] [Google Scholar]
- 29.Russell DJ, et al. Improved scaling of the gross motor function measure for children with cerebral palsy: evidence of reliability and validity. Phys Ther. 2000;80(9):873–85. [PubMed] [Google Scholar]
- 30.Daltroy LH, et al. The POSNA pediatric musculoskeletal functional health questionnaire: report on reliability, validity, and sensitivity to change. Pediatric Outcomes Instrument Development Group. Pediatric Orthopaedic Society of North America. J Pediatr Orthop. 1998;18(5):561–71. doi: 10.1097/00004694-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67(2):206–7. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 32.Ekblom MM. Improvements in dynamic plantar flexor strength after resistance training are associated with increased voluntary activation and V-to-M ratio. J Appl Physiol (1985) 2010;109(1):19–26. doi: 10.1152/japplphysiol.01307.2009. [DOI] [PubMed] [Google Scholar]
- 33.Wu M, et al. Kinematic and EMG Responses to Pelvis and Leg Assistance Force during Treadmill Walking in Children with Cerebral Palsy. Neural Plast. 2016;2016:5020348. doi: 10.1155/2016/5020348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reinkensmeyer DJ, et al. Slacking by the human motor system: computational models and implications for robotic orthoses. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:2129–32. doi: 10.1109/IEMBS.2009.5333978. [DOI] [PubMed] [Google Scholar]
- 35.Druzbicki M, et al. Functional effects of robotic-assisted locomotor treadmill thearapy in children with cerebral palsy. J Rehabil Med. 2013;45(4):358–63. doi: 10.2340/16501977-1114. [DOI] [PubMed] [Google Scholar]
- 36.Tang R, et al. Small size of error induce a longer retention of locomotor adaptation in children with cerebral palsy. Journal of Neuralphysiology. 2016 In revision. [Google Scholar]
- 37.Savin DN, Morton SM, Whitall J. Generalization of improved step length symmetry from treadmill to overground walking in persons with stroke and hemiparesis. Clin Neurophysiol. 2014;125(5):1012–20. doi: 10.1016/j.clinph.2013.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yen SC, et al. Locomotor adaptation to resistance during treadmill training transfers to overground walking in human SCI. Exp Brain Res. 2012;216(3):473–82. doi: 10.1007/s00221-011-2950-2. [DOI] [PubMed] [Google Scholar]
- 39.Houldin A, Luttin K, Lam T. Locomotor adaptations and aftereffects to resistance during walking in individuals with spinal cord injury. J Neurophysiol. 2011;106(1):247–58. doi: 10.1152/jn.00753.2010. [DOI] [PubMed] [Google Scholar]
- 40.Reisman DS, Bastian AJ, Morton SM. Neurophysiologic and rehabilitation insights from the split-belt and other locomotor adaptation paradigms. Phys Ther. 2010;90(2):187–95. doi: 10.2522/ptj.20090073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacKinnon CD, Winter DA. Control of whole body balance in the frontal plane during human walking. J Biomech. 1993;26(6):633–44. doi: 10.1016/0021-9290(93)90027-c. [DOI] [PubMed] [Google Scholar]
- 42.Norman G. The effectiveness and effects of effect sizes. Adv Health Sci Educ Theory Pract. 2003;8(3):183–7. doi: 10.1023/a:1026090406201. [DOI] [PubMed] [Google Scholar]
- 43.Willoughby KL, et al. Efficacy of partial body weight-supported treadmill training compared with overground walking practice for children with cerebral palsy: a randomized controlled trial. Arch Phys Med Rehabil. 2010;91(3):333–9. doi: 10.1016/j.apmr.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 44.Smania N, et al. Improved gait after repetitive locomotor training in children with cerebral palsy. Am J Phys Med Rehabil. 2011;90(2):137–49. doi: 10.1097/PHM.0b013e318201741e. [DOI] [PubMed] [Google Scholar]


