Abstract
The pathogenesis of Sjögren’s syndrome has not been elucidated. There has been evidence that genetics play an important role in the development of this disease from earlier studies. However, till now only a number of genes have been identified to be associated with SS, and these have only a weak or moderate effect. In this review we summarize the findings of the genetics studies and emphasize the need of large multicenter projects that will increase the sample sizes to provide more meaningful associations, as is the case in other common autoimmune diseases.
Introduction
By the late 1940’s, the idea of Sjögren’s syndrome (SS) having an association with genetics, being primarily a women affecting disease, and existing as a systemic disorder had been recognized [1]. Thirty years later, the association of specific human leukocyte antigens (HLA) and autoimmune diseases had been accepted leading to the first published relationship between immune response genes and SS pathogenesis. [2–4]. In the early 80’s, studies on (HLA) antigens disclosed genetic connections and dissimilarities between primary SS; the disease occurring alone in the absence of other concomitant autoimmune diseases [5]. At present, the HLA locus remains the strongest genetic variant to SS predisposition, while the rest of the genetic variants have been shown to have feeble to reasonable influences [6].
Although SS is the 2nd most prevalent autoimmune disease, it has maintained a constant pattern of slow progress in genetics studies when compared to rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and Type 1 Diabetes. The approach taken in SS research has been focusing on candidate genes that have been linked to other autoimmune diseases. Additionally, most of these studies have been limited to small cohorts in focused populations making it difficult to replicate. [7, 8]. The current era of next generation sequencing and accessible tools such as genome wide association studies allow scientists to fill the gaps in genetics and epigenetics that are involved in the lack of regulation in both the innate and adaptive immune system in SS patients [9, 10]. The present time is promising to further examine the environmental roles which have also been in question in SS. Viruses have been the factors declared most in terms of environmental activators among other contributing activators; these include Epstein-Barr virus (EBV), Human T-lymphotropic virus, HBV, retrovirus, HCV, Coxsackie virus [11–15].
In the field of SS, there have been numerous candidate gene approach studies using linkage disequilibrium and RFLP analysis and two major Genome-Wide Association Studies (GWAS). The candidate gene studies have identified several genes associated with SS, but the large scale GWAS have identified additional new loci as genetic risk factors. There is a very wide genomic variability in human genomes. GWAS allows for a simultaneous massive; in parallel screening for genomic loci that might be associated with a specific phenotype. The disadvantage of GWAS is inherent to the impressive human genomic variability, and for statistically significant results to be obtained. Massive numbers of DNAs have to be screened, and these DNAs need to be from the same racial background. Additionally, a further problem faced by GWAS for SS association is the heterogeneity of this disease. SS diagnosis encompasses a spectrum of similar expressed diseases and stratification of patients in distinct categories which is not only difficult, but also must maintain increased sample size requirements to be able to reach statistical and clinical significance.
The first GWAS study by Silvis group at the Oklahoma Medical Research Foundation, analyzed 10,000 samples from SS and healthy controls, all from European descent [16]. This seminal study identified association genes involved in both the innate and the adaptive immunity. The strongest associations were identified with the HLA region, but six additional loci surpassed the Genome Wide Significant (GWS) threshold of p < 5 × 10−8. These loci were IRF5-TNPO3, STAT4, IL12A, FAM167A-BLK, DDX6-CXCR5 and TNIP1. Importantly, 29 more loci were identified as having a suggestive association (p< 5 × 10−5). The loci included TNFAIP3, PTTG1, PRDM1, DGKQ, FCGR2A, IRAK1BP1 among others.
The second large scale GWAS study, analyzed DNA from Han Chinese SS and controls [17]. The discovery cohort was comprised by 542 cases and 1050 controls, while the validation/replication stages used 1303 cases and 2727 control samples. While this study identified in the Han Chinese some of the previously discovered associations in Europeans, (STAT4, TNFAIP3 and the MHC), it also identified for the first time GTF2I as a susceptibility locus for SS. Interestingly, the IRF5 polymorphism (as well as other immune genes) were not significant in the Han Chinese study. These differences suggest that the 2 examined populations may have different risk associated genes for SS and make the examination of SS subjects from other descent compelling.
One conclusion from these 2 studies is the lack of identification of genes with strong associations with disease (OR > 2.5) and till now, none of the statistically significant polymorphisms alter the coding sequences. An interesting observation is that none of the identified genes are directly involved in the functional machinery of the SS targeted organs (lachrymal and salivary glands). Larger studies, using diverse technologies to minimize potential biases, should shed light to this observation and help better delineate the roles of the epithelial and immune cells in SS pathogenesis.
For the rest of this review, we summarize the global studies examining SS susceptibility to their associated HLA alleles and we summarize the findings of the non-antigen presenting genes identified as implicated in SS either through the gene candidate approach or through GWAS.
Sjögren’s syndrome predisposition to HLA
The cell surface antigen presenting proteins which are encoded by the HLA genes have been considered comprehensively in regards to autoimmune diseases. In Table 1, we show the summary of the worldwide HLA studies in primary SS patients. The first U.S.A studies associated with HLA antigens and disease condition were conducted in the mid-70’s., at the National Institute of Health. Gershwin reported that the occurrence of HLA-B8 among 24 SS patients was 58% compare to 21% in controls. [18]. This study was followed by more studies on American Caucasians and the primary associations included HLA-Dw3, HLA-B8, HLA-DRw3, HLA-DR3, DRw52 and B lymphocyte immune response associated antigens. [2–5, 19, 20]. The first comprehensive study on men in SS was published in 1986, which reported DRw52 as being strongly associated with men and primary Sjögren’s, nevertheless HLA-B8 and HLA-DR3 which at the time had been the best mutual locus seen in women; was not statically different from the control group in comparison to the male patients. [20].
Table 1.
| Country of Origin/Population | HLA Alleles Connotation | Method/Patients/Controls | Reference/Year |
|---|---|---|---|
| U.S.A./American Caucasian | HLA-B8 | microcytotoxicity assay/24/1,205 | Gershwin et al./1975 |
| U.S.A./American Caucasian | HLA-Dw3 | microcytotoxicity assay/19/91 | Chused et al./1977 |
| U.S.A./American Caucasian | HLA-Dw3-HLA-B8 | microdroplet lymphocyte cytotoxicity technique/19/96 | Fye et al./1978 |
| U.S.A./American Caucasian | B lymphocytes immune response associated (Ia) antigen | microdroplet lymphocyte cytotoxicity technique/24/184 | Moutsopoulos et al./1978 |
| U.S.A./American Caucasian | HLA-DRw3-HLA-B8 | microcytotoxicity assay/22/184 | Moutsopoulos et al./1979 |
| U.S.A./American Caucasian | HLA-DRw3-HLA-B8 | microcytotoxicity assay/25/27 | Mann and Moutsopoul os/1979 |
| U.S.A./American Caucasian | DRw52 | microcytotoxicity assay/36 male/69 females/626 controls | Molina et al./1986 |
| U.S.A./American Caucasian | HLA-DRB1*0301-DRB3*0101-DQA1*0501 -DQB1*0201 | PCR amplification of genomic DNA using oligonucleotide primer/75/135 | Kang et al./1993 |
| Japan/Japanese population | DRB1*0405-DRB4*0101-DQA1*0301-DQB1*0401 | PCR amplification of genomic DNA using oligonucleotide primer/33/49 | Kang et al./1993 |
| Japan/Japanese population | DRw53 | complement-dependent microcytotoxicity method/21/114 | Moriuchi et al./1986 |
| China/Chinese population | DRB1*0803-DQA1*0103-DQB1*0601 | PCR amplification of genomic DNA using oligonucleotide primer/45/42 | Kang et al./1993 |
| PCR-SSP technique/70/136 | Wang et al./1997 | ||
| Mexico/Mexican population | HLA-DRB1*01:01 and HLA-B*35:01 | Allele SEQR Sequenced Based Typing Kits/28 pSS, 30 sSS, 96 connective tissue disease w/o SS patients/234 controls |
Hernández-Molina et al./2015 |
| Colombia/Mestizo Colombian population | HLADRB1*0301-DQB1*0201 | polymerase chain reaction techniques/75/76 | Anaya et al./2002 |
| Israel/Israeli Jewish/Greek | DQA1*001-DQA1*0201-DQB1*0501-Jewish/DQA1*0501-Greek | sequence-specific oligonucleotide probe technique/Jewish (17/258) & Greek (22/54) | Roitberg-Tambur et al./1993 |
| Greece/Greek population | DR-5 | NIH microlymphocytotoxicity test/46/172 | Papasteriade s et al./1988 |
| Greece/Greek population | DRB1*0301 | polymerase chain reaction & and hybridization with sequence-specific oligonucleotide probes/55/246 | Manoussakis et al./2004 |
| Spain/Spanish population | HLA-Cw7, HLA-DR3 and HLA-DR11 | Serological Technique/30/256 | Portales et al./1994 |
| France/French population | DRBI*1501*-0301-DQB1*0201*-0602 | PCR-based methodologies/42/200 | Jean et al./1998 |
| France/French population | HLA-DRB1*03 and DQB1*02 | microlymphocytotoxicity method with monoclonal antibodies & polymerase chain reaction single-strand oligonucleotide reverse dot-blot kits/149/222 | Gottenberg et al./2003 |
| Italy/Italian population | DR3 | microlymphocytotoxicity method/28/62 | Vitali et al./1986 |
| Denmark/Danish population | HLA-Dw2 | microlymphocytotoxic method/32/-- | Manthorpe et al./1981 |
| Denmark/Danish population | DQA1*0501-DQB1*0201-DQA1*0301 | microlymphocytotoxic method/19/-- | Morling et al./1991 |
| Finland/Finnish population | DRB1*0301, DQA1*0501, ANR DQB1*0201 | 2-step polymerase chain reaction (PCR)/20/9 | Kerttula et al./1996 |
| Norway/Norwegian Caucasian population | DRB1*0301 | polymerase chain reaction (PCR) using sequence-specific primers (SSP)/29/181 | Nakken et al./2001 |
| Norway/Norwegian Caucasian population | DRB1*03-DQB1*02-DQA1*0501 | oligonucleotide hybridization of enzymatically amplified DNA/31/64 | Bolstad et al./2001 |
| United Kingdom/British Caucasian | DR-3 & DRw52 | PCR-based methodologies/41/100 | Pease et al./1989 |
| Australia/Australian population | DR3-DQA1*0501-DQB1*02 | polymerase chain reaction sequence-specific oligonucleotide/80/164 | Rishmueller et al./1998 |
By the 90s, studiesexamining HLA associations comparing different ethnic groups was published [21] and more followed since. The results revealed that in North American Caucasian the predicted haplotype was HLA-DRB1*0301-DRB3*0101-DQA1*0501 -DQB1*0201, in Japanese (HLA-DRBl*O405-DRB4*0101 -DQA1*0301-DQB1*0401), an additional study added DRw53to the list in Japanese SS patients [22]; and in Chinese SS patients (DRB1*0803-DQAl*0103-DQB1*0601). A different study examining Chinese SS patients found HLA-DR3, DR52 and DR2 to be significantly higher than their control patients [23] There results emphasized that there is no single class II allele associated with primary SS [21]. In 2013 one of the 1st GWAS study examining Chinese patients endorsed HLA class II as a significant region in Han Chinese SS patients [17]
In Americas, outside of the U.S.A, Mexico and Columbia are among the countries that have examined HLA alleles associations in SS. By means of a High-Resolution HLA analysis the study on Mexican (SS) patients revealed HLA-DRB1*01:01 and HLA-B*35:01 as the highest prevalence compared to controls, confirming HLA connotation to primary SS, and with association to the production of anti-Ro/SSA [24]. In the Columbian population, HLA-DRB1*0301-DQB1*0201 associations were significant in the samples with advanced histopathological features. In addition they verified HLADRB1*0301-DQB1*0201 to be linked with autoantibody production [25].
Utilizing a sequence-specific oligonucleotide probe; Roitberg-Tambur et al examined the HLA genes that contribute to the predisposition of SS; in a cohort of patients with Jewish Israel descent and Greeks, non- Jewish patients in 1993. The authors confirmed HLA-DQA1*001-DQA1*0201-DQB1*0501 in Israelite Jews and in Greeks HLA-DQA1*050 [26]. Five years prior, HLA-DR5 was found to be one of the susceptibility genes in SS Greek patients [27], and in 2004 an extensive study examining Sjögren’s syndrome associated with Systemic Lupus Erythematosus revealed that in these patients a high frequency of the HLA-DRB1*0301 allele was present [28].
Significant associations were also found in primary SS patients from southern Spain with HLA-Cw7, HLA-DR3 and HLA-DR11 [29]. In the French population, the leading association determined to be HLA-DRB1*15-DRB1*0301, but it was also observed that the DRB1*0301 haplotype was associated with the DPB1* 0201 and TNF-a2 alleles in SS patients [30], However, a second study found HLA-DRB1*03 and DQB1*02 associated exclusively with the presence of anti-SSA and/or anti-SSB antibodies in a French SS patient cohort [31]. A study examining a primary SS Italian cohort identified DR3 to be correlated with autoantibodies and extraglandular indices [32].
Two studies from Denmark observing the role HLA alleles and SS, the 1st study found HLA-Dw2 to be significantly increased [33], and the second study found DQA1*0501-DQB1*0201-DQA1*0301 to have positive association [34]. In a Finnish study the significant haplotypes were HLA-DRB1*0301-DQA1*0501-DQB1*0201[35]. Two Norwegian studies published in the same year (2001) showed HLA alleles as having association with SS patients with anti-SSA and/or anti-SSB, the alleles identified were DRB1*03-DQB1*02-DQA1*0501 [36] and DRB1*0301 and DRB3*0101 [37]. Among other countries which have examined HLA allele and it association to SS are United Kingdom (HLA-DR3, and HLA-DRw52) [38], Hungary (HLA-DQB1*0201-DRB1*03-DQB1*0501)[39] and Australia DR3-DQA1*0501-DQB1*02 which haplotype were primarily associated to La/Ro ribonucleoproteins [40].
Non-Antigen Presenting Risk Genes
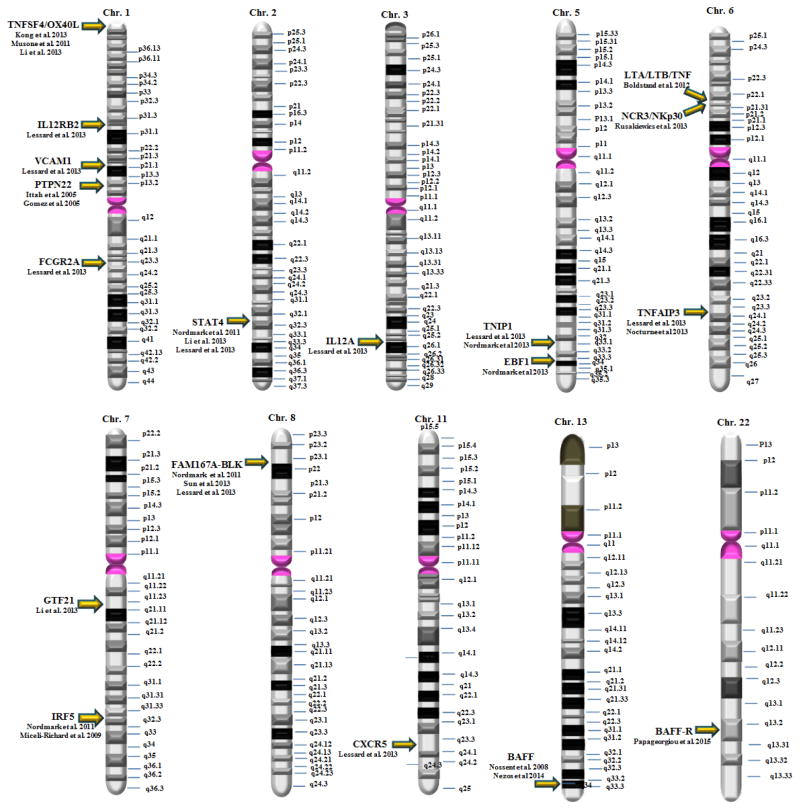
There is a small number of risk genes that fall outside the antigen presenting clusters, that have been examined mainly in small cohorts, with the exemption of the 2 GWAS studies previously mentioned. In Figure 1, we summarize these genes within their chromosomal localization. Overall the identified genes seem to be falling in the following categories based on their function:
Figure 1.
A summary of the chromosomal localization of the genes identified in single candidate genes or GWAS studies. No discernible patterns are observed and no genes expressed in the X chromosome have been identified so far.
Genes Associated with Interferon Signature: Interferon Regulatory Factor 5 (IRF5), Signal Transducer and Activator of Transcription 4 (STAT4), Interleukin 12A (IL12A) and Natural Cytotoxicity Triggering Receptor 3 (NCR3)
Genes Associated with B and T-Cell function: B-Lymphocyte Kinase (BLK), B-cell activating factor (BAFF), Early B-Cell Factor 1(EBF1), General Transcription Factor IIi (GTF2I), C-X-C chemokine receptor type 5 (CXCR5), Tumor Necrosis Factor Superfamily Member 4 (TNFSF4), TNF-Alpha Induced Protein 3 (TNFAIP3), TNFAIP3-Interacting Protein 1 (TNIP1), Lymphotoxin-α (LTA), C-C motif chemokine 11 (CCL11).
Other categories: Serotonin Transporter (HTT, Solute Carrier Family 6 Member 4)
These genes have been extensively discussed in other reviews. Here we briefly discuss a few genes involved in the aforementioned categories.
Interferon regulatory factor 5
The Interferon regulatory factor 5 (IRF5) is the gene shown to have the strongest genetic association in SS but also has been implicated in other autoimmune diseases such as systemic lupus erythematosus, inflammatory bowel disease, rheumatoid arthritis, multiple sclerosis and systemic sclerosis [41–45]. This gene encodes for a transcription factor regulating a number of homeostatic processes but also immunity, inflammation, antiviral defenses and tumor pathogenesis [46–48]. IRF5 is expressed on a number of cell types, including white blood cells and epithelial cells.
IRF5 is a multi-domain 60–63 kDa protein with an amino-terminal DNA-binding domain, an association domain and a transcriptional co-activator binding domain on the C-terminus [48, 49]. All these domains are required for the transcriptional function of this gene which occurs, after an initial activation, through serine phosphorylation that leads to dimerization, nuclear translocation and binding to gene promoters along with other transcription factors. The most well characterized function of IRF5 and the one likely most relevant in SS is its role in the production of Type-I IFNs and proinflammatory cytokines such as IL-12, IL-17, IL-23 and TNFα [50, 51].
Signal Transducer and Activator of Transcription 4
The signal transducer and activator of transcription 4 (STAT4) is one of the very first genes discovered to have a polymorphism associated with SS. More specifically, Korman et al, showed that the intronic rs7574865 T allele was more common in SS patients (P=0.01)[29]. Prior to SS, STAT4 was shown to be associated with RA and SLE [52].
STAT4 is a member of the STAT family of transcription factors that upon phosphorylation, they dimerize and translocate to the nucleus to initiate the transcription of a number of genes. IL17 and interferon have been shown to be induced by the translocation of STAT4, guiding T helper cells toward the proinflammatory T-helper type 1 and T-helper type 17 lineages, while the activation of STAT4 is induced by IL12, IL23 and Type I interferons.
The specifics about how the functional variant of rs7574865 is involved in SS are not known. It is possible that the rate of transcription is altered by this nucleotide change by alteration of binding of histones in that area[52].
Interleukin 12A
The interleukin 12A (IL12A) gene encodes for a subunit of the Interleukin 12 (IL12). IL12 is one of the cytokines involved in the induction of IFN-γ, in a T-cell independent matter. It has been shown to be critical for the differentiation of Th1 and Th2 cells. Additionally, IL12 is regulated by IRF5. Additionally, STAT4 mediates IL12 response in lymphocytes.
A total of 7 variants of IL12A have met genome wide significance and the rs485497 variant in the promoter region of the gene has been shown to significantly influence IL12A transcript expression [16].
Tumor necrosis factor receptor-1
Tumor necrosis factor receptor-1 (TNFR1) is a ubiquitously expressed receptor that initiates most of the cellular TNF-associated signaling. This signaling pathway has been very well characterized and it leads to the activation and nuclear translocation of NF-κB, c-Fos and c-Jun. These transcription factors lead to the induction of a number of pro-inflammatory and anti-apoptotic genes [53, 54].
TNFR1 knockout mice have also shown an important role of this protein in immunity as it protects against bacterial infections and viruses. [55, 56]. In SS, soluble TNFR1 has been shown to be highly expressed in salivary glands and serum [57]. Similar findings have been observed in in rheumatoid arthritis [58]. The importance of this receptor in autoimmune diseases is also shown by the successful treatment of rheumatoid arthritis and inflammatory bowel disease with anti-TNF biologics. Both of these diseases present with markedly elevated TNF levels which mediates its effects primarily through TNFR1.
A recent study examined the association of a polymorphism in TNFR1 promoter with clinical characteristics of SS patients in Mexico [59]. This polymorphism (TNFR1-383 A>C) has previously been associated with other autoimmune diseases. In this study, SS patients with A>C genotype showed high levels of C-Reactive Protein (P = 0.045) and Rheumatoid Factor (p = 0.04). No statistically significant genotypic and allelic frequencies were observed in this study between SS and controls.
Family with sequence similarity 167 member A-B-lymphoid tyrosine kinase
Associated with autoimmune diseases, a single (SNP -rs2736340) within the Family with sequence similarity 167 member A–B lymphoid tyrosine kinase (FAM167A-BLK) has been linked with systemic lupus erythematosus [60–64], rheumatoid arthritis [65–68], Kawasaki disease [69], primary antiphospholipid syndrome (APS) [70] in addition to SS [71]. Zhou et al performed a meta-analysis study showing this specific (SNP-rs2736340) as having increased susceptibility in European, North Americans, and Asians, yet not Africans [72]. BLK is involved in B-Cell development, differentiation and signaling. Although the functional role of this SNP has not been identified, it has been suggested that it might lead to an upregulation of B cells including auto-reactive B cells that are key factors in the propagation and severity of autoimmune diseases
Serotonin transporter
The Serotonin transporter (5-HTT or SLC6A4) is a gene coding for a membrane protein that transports serotonin in a sodium- and chloride-dependent manner from synaptic spaces into presynaptic neurons. Platelet serotonin levels (PSL) have been shown to be decreased not only in SS [73]; but also in other autoimmune diseases such as rheumatoid arthritis, and systemic sclerosis [74, 75]. Recently, Markeljevic et al showed that in a subset of SS patients the presence of variants of the 5-HTT gene contributes to decreased PSL. This is one of the few studies looking for associations of genes that might not be directly linked to the immune and autoimmune genes and might be interesting to explore for a number of neurological and psychological findings in the SS population [76].
Discussion
With the exception of HLA loci, the genetic variations that have been discovered for SS have a weak, or at best a moderate effect with low Odds Ratios, even if there are a lot of clinical evidences that there is a genetic component in SS. It is commonly accepted that the vast majority of SNPs underlie the various degrees of susceptibility to a specific disease. SS being a complex disease with diverse phenotypes is probably manifested by a coordinated altered function of sets of SNPs, possibly influenced by a number of epigenomic factors. Viral and microbial infections have been long discussed it the context of autoimmunity. Several mechanisms have been suggested of how infections can cause an autoimmune condition, among them molecular mimicry, epitome spreading and cryptic antigens. These mechanisms do not require an active infection and can lead to autoimmunity a long time after the initial infection, that might also be subclinical. The potential interplay between infections and SNPs will be hard to discern, but the understanding of the function of the identified SNPs will need to be take into consideration as many epigenetic parameters as possible.
Large cohort studies are clearly needed to assess better role the role of genes in the development of SS. Since SS encompasses a spectrum of similarly presenting diseases, it will also be very important the examined samples to be fully phenotyped so that the associations can reach statistical and clinical significance in relevance to their position in the spectrum of this diverse disease. This could also allow for a more precise exploration of the environmental and epigenetic aspects that also contribute to the development of SS by identifying the pathways altered on the specific disease phenotypes. With new sequence technologies, analysis platforms and with large scale collaborative projects future genetic studies in SS are promising and their deliverables will certainly help in our understanding of its pathophysiology.
Highlights.
With the exception of HLA loci, the genetic variations that have been discovered for Sjogren’s syndrome have a weak, or at best a moderate effect with low Odds Ratios
There is a need for large scale studies with complete phenotypic data to redefine the wide spectrum of diseases encompassing Sjogren’s syndrome
Acknowledgments
Work in the authors’ laboratory is supported by the Intramural Research Program of the NIH, NIDCR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coverdale H. Some unusual cases of Sjogren’s syndrome. The British journal of ophthalmology. 1948;32:669–673. [PMC free article] [PubMed] [Google Scholar]
- 2.Chused TM, Kassan SS, Opelz G, Moutsopoulos HM, Terasaki PI. Sjogren’s syndrome association with HLA-Dw3. The New England journal of medicine. 1977;296:895–897. doi: 10.1056/NEJM197704212961602. [DOI] [PubMed] [Google Scholar]
- 3.Fye KH, Terasaki PI, Michalski JP, Daniels TE, Opelz G, Talal N. Relationshipp of HLA-Dw3 and HLA-B8 to Sjogren’s syndrome. Arthritis and rheumatism. 1978;21:337–342. doi: 10.1002/art.1780210308. [DOI] [PubMed] [Google Scholar]
- 4.Moutsopoulos HM, Chused TM, Johnson AH, Khudsen B, Mann DL. B lymphocyte antigens in sicca syndrome. Science. 1978;199:1441–1442. doi: 10.1126/science.415366. [DOI] [PubMed] [Google Scholar]
- 5.Moutsopoulos HM, Mann DL, Johnson AH, Chused TM. Genetic differences between primary and secondary sicca syndrome. The New England journal of medicine. 1979;301:761–763. doi: 10.1056/NEJM197910043011405. [DOI] [PubMed] [Google Scholar]
- 6.Nezos A, Mavragani CP. Contribution of Genetic Factors to Sjogren’s Syndrome and Sjogren’s Syndrome Related Lymphomagenesis. Journal of immunology research. 2015;2015:754825. doi: 10.1155/2015/754825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ice JA, Li H, Adrianto I, Lin PC, Kelly JA, Montgomery CG, Lessard CJ, Moser KL. Genetics of Sjogren’s syndrome in the genome-wide association era. Journal of autoimmunity. 2012;39:57–63. doi: 10.1016/j.jaut.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jonsson R, Bolstad AI, Brokstad KA, Brun JG. Sjogren’s syndrome--a plethora of clinical and immunological phenotypes with a complex genetic background. Annals of the New York Academy of Sciences. 2007;1108:433–447. doi: 10.1196/annals.1422.046. [DOI] [PubMed] [Google Scholar]
- 9.Altorok N, Coit P, Hughes T, Koelsch KA, Stone DU, Rasmussen A, Radfar L, Scofield RH, Sivils KL, Farris AD, Sawalha AH. Genome-wide DNA methylation patterns in naive CD4+ T cells from patients with primary Sjogren’s syndrome. Arthritis & rheumatology. 2014;66:731–739. doi: 10.1002/art.38264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miceli-Richard C, Wang-Renault SF, Boudaoud S, Busato F, Lallemand C, Bethune K, Belkhir R, Nocturne G, Mariette X, Tost J. Overlap between differentially methylated DNA regions in blood B lymphocytes and genetic at-risk loci in primary Sjogren’s syndrome. Annals of the rheumatic diseases. 2016;75:933–940. doi: 10.1136/annrheumdis-2014-206998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox RI, Pearson G, Vaughan JH. Detection of Epstein-Barr virus-associated antigens and DNA in salivary gland biopsies from patients with Sjogren’s syndrome. Journal of immunology. 1986;137:3162–3168. [PubMed] [Google Scholar]
- 12.Igoe A, Scofield RH. Autoimmunity and infection in Sjogren’s syndrome. Current opinion in rheumatology. 2013;25:480–487. doi: 10.1097/BOR.0b013e32836200d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sipsas NV, Gamaletsou MN, Moutsopoulos HM. Is Sjogren’s syndrome a retroviral disease? Arthritis research & therapy. 2011;13:212. doi: 10.1186/ar3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tincani A, Andreoli L, Cavazzana I, Doria A, Favero M, Fenini MG, Franceschini F, Lojacono A, Nascimbeni G, Santoro A, Semeraro F, Toniati P, Shoenfeld Y. Novel aspects of Sjogren’s syndrome in 2012. BMC medicine. 2013;11:93. doi: 10.1186/1741-7015-11-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallo A, Jang SI, Ong HL, Perez P, Tandon M, Ambudkar I, Illei G, Alevizos I. Targeting the Ca(2+) Sensor STIM1 by Exosomal Transfer of Ebv-miR-BART13-3p is Associated with Sjogren’s Syndrome. EBioMedicine. 2016;10:216–226. doi: 10.1016/j.ebiom.2016.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lessard CJ, Li H, Adrianto I, Ice JA, Rasmussen A, Grundahl KM, Kelly JA, Dozmorov MG, Miceli-Richard C, Bowman S, Lester S, Eriksson P, Eloranta ML, Brun JG, Goransson LG, Harboe E, Guthridge JM, Kaufman KM, Kvarnstrom M, Jazebi H, Cunninghame Graham DS, Grandits ME, Nazmul-Hossain AN, Patel K, Adler AJ, Maier-Moore JS, Farris AD, Brennan MT, Lessard JA, Chodosh J, Gopalakrishnan R, Hefner KS, Houston GD, Huang AJ, Hughes PJ, Lewis DM, Radfar L, Rohrer MD, Stone DU, Wren JD, Vyse TJ, Gaffney PM, James JA, Omdal R, Wahren-Herlenius M, Illei GG, Witte T, Jonsson R, Rischmueller M, Ronnblom L, Nordmark G, Ng WF, Mariette X, Anaya JM, Rhodus NL, Segal BM, Scofield RH, Montgomery CG, Harley JB, Sivils KL. Variants at multiple loci implicated in both innate and adaptive immune responses are associated with Sjogren’s syndrome. Nature genetics. 2013;45:1284–1292. doi: 10.1038/ng.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Zhang K, Chen H, Sun F, Xu J, Wu Z, Li P, Zhang L, Du Y, Luan H, Li X, Wu L, Li H, Wu H, Li X, Li X, Zhang X, Gong L, Dai L, Sun L, Zuo X, Xu J, Gong H, Li Z, Tong S, Wu M, Li X, Xiao W, Wang G, Zhu P, Shen M, Liu S, Zhao D, Liu W, Wang Y, Huang C, Jiang Q, Liu G, Liu B, Hu S, Zhang W, Zhang Z, You X, Li M, Hao W, Zhao C, Leng X, Bi L, Wang Y, Zhang F, Shi Q, Qi W, Zhang X, Jia Y, Su J, Li Q, Hou Y, Wu Q, Xu D, Zheng W, Zhang M, Wang Q, Fei Y, Zhang X, Li J, Jiang Y, Tian X, Zhao L, Wang L, Zhou B, Li Y, Zhao Y, Zeng X, Ott J, Wang J, Zhang F. A genome-wide association study in Han Chinese identifies a susceptibility locus for primary Sjogren’s syndrome at 7q11.23. Nature genetics. 2013;45:1361–1365. doi: 10.1038/ng.2779. [DOI] [PubMed] [Google Scholar]
- 18.Gershwin ME, Terasaki I, Graw R, Chused TM. Increased frequency of HL-A8 in Sjogren’s syndrome. Tissue antigens. 1975;6:342–346. doi: 10.1111/j.1399-0039.1975.tb00653.x. [DOI] [PubMed] [Google Scholar]
- 19.Mann DL, Moutsopoulos HM. HLA DR alloantigens in different subsets of patients with Sjogren’s syndrome and in family members. Annals of the rheumatic diseases. 1983;42:533–536. doi: 10.1136/ard.42.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molina R, Provost TT, Arnett FC, Bias WB, Hochberg MC, Wilson RW, Alexander EL. Primary Sjogren’s syndrome in men. Clinical, serologic, and immunogenetic features. The American journal of medicine. 1986;80:23–31. doi: 10.1016/0002-9343(86)90044-6. [DOI] [PubMed] [Google Scholar]
- 21.Kang HI, Fei HM, Saito I, Sawada S, Chen SL, Yi D, Chan E, Peebles C, Bugawan TL, Erlich HA, et al. Comparison of HLA class II genes in Caucasoid, Chinese, and Japanese patients with primary Sjogren’s syndrome. Journal of immunology. 1993;150:3615–3623. [PubMed] [Google Scholar]
- 22.Moriuchi J, Ichikawa Y, Takaya M, Shimizu H, Uchiyama M, Sato K, Tsuji K, Arimori S. Association between HLA and Sjogren’s syndrome in Japanese patients. Arthritis and rheumatism. 1986;29:1518–1521. doi: 10.1002/art.1780291215. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Jiang M, Qiu C. Study on the relationship between primary Sjogren syndrome and HLA-DRbeta gene. Zhonghua nei ke za zhi. 1997;36:398–401. [PubMed] [Google Scholar]
- 24.Hernandez-Molina G, Vargas-Alarcon G, Rodriguez-Perez JM, Martinez-Rodriguez N, Lima G, Sanchez-Guerrero J. High-resolution HLA analysis of primary and secondary Sjogren’s syndrome: a common immunogenetic background in Mexican patients. Rheumatology international. 2015;35:643–649. doi: 10.1007/s00296-014-3143-7. [DOI] [PubMed] [Google Scholar]
- 25.Anaya JM, Correa PA, Mantilla RD, Arcos-Burgos M. TAP, HLA-DQB1, and HLA-DRB1 polymorphism in Colombian patients with primary Sjogren’s syndrome. Seminars in arthritis and rheumatism. 2002;31:396–405. doi: 10.1053/sarh.2002.32557. [DOI] [PubMed] [Google Scholar]
- 26.Roitberg-Tambur A, Friedmann A, Safirman C, Markitziu A, Ben-Chetrit E, Rubinow A, Moutsopoulos HM, Stavropoulos E, Skopouli FN, Margalit H, et al. Molecular analysis of HLA class II genes in primary Sjogren’s syndrome. A study of Israeli Jewish and Greek non-Jewish patients. Human immunology. 1993;36:235–242. doi: 10.1016/0198-8859(93)90130-s. [DOI] [PubMed] [Google Scholar]
- 27.Papasteriades CA, Skopouli FN, Drosos AA, Andonopoulos AP, Moutsopoulos HM. HLA-alloantigen associations in Greek patients with Sjogren’s syndrome. Journal of autoimmunity. 1988;1:85–90. doi: 10.1016/0896-8411(88)90079-0. [DOI] [PubMed] [Google Scholar]
- 28.Manoussakis MN, Georgopoulou C, Zintzaras E, Spyropoulou M, Stavropoulou A, Skopouli FN, Moutsopoulos HM. Sjogren’s syndrome associated with systemic lupus erythematosus: clinical and laboratory profiles and comparison with primary Sjogren’s syndrome. Arthritis and rheumatism. 2004;50:882–891. doi: 10.1002/art.20093. [DOI] [PubMed] [Google Scholar]
- 29.Garcia Portales R, Belmonte Lope MA, Camps Garcia MT, Ocon Sanchez P, Alonso Ortiz A, Guil Garcia M, de Ramon Garrido E. Immunogenetics of the Sjogren’s syndrome in southern Spain. Anales de medicina interna. 1994;11:56–61. [PubMed] [Google Scholar]
- 30.Jean S, Quelvennec E, Alizadeh M, Guggenbuhl P, Birebent B, Perdriger A, Grosbois B, Pawlotsky PY, Semana G. DRB1*15 and DRB1*03 extended haplotype interaction in primary Sjogren’s syndrome genetic susceptibility. Clinical and experimental rheumatology. 1998;16:725–728. [PubMed] [Google Scholar]
- 31.Gottenberg JE, Busson M, Loiseau P, Cohen-Solal J, Lepage V, Charron D, Sibilia J, Mariette X. In primary Sjogren’s syndrome. HLA class II is associated exclusively with autoantibody production and spreading of the autoimmune response. Arthritis and rheumatism. 2003;48:2240–2245. doi: 10.1002/art.11103. [DOI] [PubMed] [Google Scholar]
- 32.Vitali C, Tavoni A, Rizzo G, Neri R, D’Ascanio A, Cristofani R, Bombardieri S. HLA antigens in Italian patients with primary Sjogren’s syndrome. Annals of the rheumatic diseases. 1986;45:412–416. doi: 10.1136/ard.45.5.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manthorpe R, Morling N, Platz P, Ryder LP, Svejgaard A, Thomsen M. HLA-D antigen frequencies in Sjogren’s syndrome. Differences between the primary and secondary form. Scandinavian journal of rheumatology. 1981;10:124–128. doi: 10.3109/03009748109095284. [DOI] [PubMed] [Google Scholar]
- 34.Morling N, Andersen V, Fugger L, Georgsen J, Halberg P, Oxholm P, Odum N, Svejgaard A. Immunogenetics of rheumatoid arthritis and primary Sjogren’s syndrome: DNA polymorphism of HLA class II genes. Disease markers. 1991;9:289–296. [PubMed] [Google Scholar]
- 35.Kerttula TO, Collin P, Polvi A, Korpela M, Partanen J, Maki M. Distinct immunologic features of Finnish Sjogren’s syndrome patients with HLA alleles DRB1*0301, DQA1*0501, and DQB1*0201. Alterations in circulating T cell receptor gamma/delta subsets. Arthritis and rheumatism. 1996;39:1733–1739. doi: 10.1002/art.1780391017. [DOI] [PubMed] [Google Scholar]
- 36.Bolstad AI, Wassmuth R, Haga HJ, Jonsson R. HLA markers and clinical characteristics in Caucasians with primary Sjogren’s syndrome. The Journal of rheumatology. 2001;28:1554–1562. [PubMed] [Google Scholar]
- 37.Nakken B, Jonsson R, Brokstad KA, Omholt K, Nerland AH, Haga HJ, Halse AK. Associations of MHC class II alleles in Norwegian primary Sjogren’s syndrome patients: implications for development of autoantibodies to the Ro52 autoantigen. Scandinavian journal of immunology. 2001;54:428–433. doi: 10.1046/j.1365-3083.2001.00993.x. [DOI] [PubMed] [Google Scholar]
- 38.Pease CT, Shattles W, Charles PJ, Venables PJ, Maini RN. Clinical, serological, and HLA phenotype subsets in Sjogren’s syndrome. Clinical and experimental rheumatology. 1989;7:185–190. [PubMed] [Google Scholar]
- 39.Kovacs A, Endreffy E, Petri I, Kovacs L, Pokorny G. HLA class II allele polymorphism in Hungarian patients with primary Sjogren’s syndrome. Scandinavian journal of rheumatology. 2006;35:75–76. doi: 10.1080/03009740500287517. [DOI] [PubMed] [Google Scholar]
- 40.Rischmueller M, Lester S, Chen Z, Champion G, Van Den Berg R, Beer R, Coates T, McCluskey J, Gordon T. HLA class II phenotype controls diversification of the autoantibody response in primary Sjogren’s syndrome (pSS) Clinical and experimental immunology. 1998;111:365–371. doi: 10.1046/j.1365-2249.1998.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dawidowicz K, Allanore Y, Guedj M, Pierlot C, Bombardieri S, Balsa A, Westhovens R, Barrera P, Alves H, Teixeira VH, Petit-Teixeira E, van de Putte L, van Riel P, Prum B, Bardin T, Meyer O, Cornelis F, Dieude P, Ecraf The interferon regulatory factor 5 gene confers susceptibility to rheumatoid arthritis and influences its erosive phenotype. Annals of the rheumatic diseases. 2011;70:117–121. doi: 10.1136/ard.2010.129171. [DOI] [PubMed] [Google Scholar]
- 42.Dideberg V, Kristjansdottir G, Milani L, Libioulle C, Sigurdsson S, Louis E, Wiman AC, Vermeire S, Rutgeerts P, Belaiche J, Franchimont D, Van Gossum A, Bours V, Syvanen AC. An insertion-deletion polymorphism in the interferon regulatory Factor 5 (IRF5) gene confers risk of inflammatory bowel diseases. Human molecular genetics. 2007;16:3008–3016. doi: 10.1093/hmg/ddm259. [DOI] [PubMed] [Google Scholar]
- 43.Dieguez-Gonzalez R, Calaza M, Perez-Pampin E, de la Serna AR, Fernandez-Gutierrez B, Castaneda S, Largo R, Joven B, Narvaez J, Navarro F, Marenco JL, Vicario JL, Blanco FJ, Fernandez-Lopez JC, Caliz R, Collado-Escobar MD, Carreno L, Lopez-Longo J, Canete JD, Gomez-Reino JJ, Gonzalez A. Association of interferon regulatory factor 5 haplotypes, similar to that found in systemic lupus erythematosus, in a large subgroup of patients with rheumatoid arthritis. Arthritis and rheumatism. 2008;58:1264–1274. doi: 10.1002/art.23426. [DOI] [PubMed] [Google Scholar]
- 44.Reksten TR, Lessard CJ, Sivils KL. Genetics in Sjogren Syndrome. Rheumatic diseases clinics of North America. 2016;42:435–447. doi: 10.1016/j.rdc.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Tang L, Chen B, Ma B, Nie S. Association between IRF5 polymorphisms and autoimmune diseases: a meta-analysis. Genetics and molecular research: GMR. 2014;13:4473–4485. doi: 10.4238/2014.June.16.6. [DOI] [PubMed] [Google Scholar]
- 46.Byrne AJ, Weiss M, Mathie SA, Walker SA, Eames HL, Saliba D, Lloyd CM, Udalova IA. A critical role for IRF5 in regulating allergic airway inflammation. Mucosal immunology. 2016 doi: 10.1038/mi.2016.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marsili G, Perrotti E, Remoli AL, Acchioni C, Sgarbanti M, Battistini A. IFN Regulatory Factors and Antiviral Innate Immunity: How Viruses Can Get Better. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research. 2016;36:414–432. doi: 10.1089/jir.2016.0002. [DOI] [PubMed] [Google Scholar]
- 48.Ryzhakov G, Eames HL, Udalova IA. Activation and function of interferon regulatory factor 5. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research. 2015;35:71–78. doi: 10.1089/jir.2014.0023. [DOI] [PubMed] [Google Scholar]
- 49.Lazzari E, Jefferies CA. IRF5-mediated signaling and implications for SLE. Clinical immunology. 2014;153:343–352. doi: 10.1016/j.clim.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 50.Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N, Hussell T, Feldmann M, Udalova IA. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nature immunology. 2011;12:231–238. doi: 10.1038/ni.1990. [DOI] [PubMed] [Google Scholar]
- 51.Krausgruber T, Saliba D, Ryzhakov G, Lanfrancotti A, Blazek K, Udalova IA. IRF5 is required for late-phase TNF secretion by human dendritic cells. Blood. 2010;115:4421–4430. doi: 10.1182/blood-2010-01-263020. [DOI] [PubMed] [Google Scholar]
- 52.Korman BD, Kastner DL, Gregersen PK, Remmers EF. STAT4: genetics, mechanisms, and implications for autoimmunity. Curr Allergy Asthma Rep. 2008;8:398–403. doi: 10.1007/s11882-008-0077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 54.Puimege L, Libert C, Van Hauwermeiren F. Regulation and dysregulation of tumor necrosis factor receptor-1. Cytokine & growth factor reviews. 2014;25:285–300. doi: 10.1016/j.cytogfr.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 55.Belisle SE, Tisoncik JR, Korth MJ, Carter VS, Proll SC, Swayne DE, Pantin-Jackwood M, Tumpey TM, Katze MG. Genomic profiling of tumor necrosis factor alpha (TNF-alpha) receptor and interleukin-1 receptor knockout mice reveals a link between TNF-alpha signaling and increased severity of 1918 pandemic influenza virus infection. Journal of virology. 2010;84:12576–12588. doi: 10.1128/JVI.01310-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choi S, Park YS, Koga T, Treloar A, Kim KC. TNF-alpha is a key regulator of MUC1, an anti-inflammatory molecule, during airway Pseudomonas aeruginosa infection. American journal of respiratory cell and molecular biology. 2011;44:255–260. doi: 10.1165/rcmb.2009-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Szodoray P, Alex P, Brun JG, Centola M, Jonsson R. Circulating cytokines in primary Sjogren’s syndrome determined by a multiplex cytokine array system. Scandinavian journal of immunology. 2004;59:592–599. doi: 10.1111/j.0300-9475.2004.01432.x. [DOI] [PubMed] [Google Scholar]
- 58.Valle Y, Padilla-Gutierrez JR, Torres-Carrillo NM, Ledezma-Lozano IY, Corona-Sanchez EG, Vazquez-Del Mercado M, Rangel-Villalobos H, Gamez-Nava JI, Gonzalez-Lopez L, Munoz-Valle JF. The -383A>C TNFRI polymorphism is associated with soluble levels and clinical activity in rheumatoid arthritis. Rheumatology international. 2010;30:655–659. doi: 10.1007/s00296-009-1049-6. [DOI] [PubMed] [Google Scholar]
- 59.Fletes-Rayas AL, Palafox-Sanchez CA, Munoz-Valle JF, Orozco-Barocio G, Navarro-Hernandez RE, Oregon-Romero E. TNFR1-383 A>C polymorphism association with clinical manifestations in primary Sjogren’s syndrome patients. Genetics and molecular research: GMR. 2016;15 doi: 10.4238/gmr.15024177. [DOI] [PubMed] [Google Scholar]
- 60.Elghzaly AA, Metwally SS, El-Chennawi FA, Elgayaar MA, Mosaad YM, El-Toraby EE, Hegab MM, Ibrahim SM. IRF5, PTPN22, CD28, IL2RA, KIF5A, BLK and TNFAIP3 genes polymorphisms and lupus susceptibility in a cohort from the Egypt Delta; relation to other ethnic groups. Human immunology. 2015;76:525–531. doi: 10.1016/j.humimm.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 61.Guthridge JM, Lu R, Sun H, Sun C, Wiley GB, Dominguez N, Macwana SR, Lessard CJ, Kim-Howard X, Cobb BL, Kaufman KM, Kelly JA, Langefeld CD, Adler AJ, Harley IT, Merrill JT, Gilkeson GS, Kamen DL, Niewold TB, Brown EE, Edberg JC, Petri MA, Ramsey-Goldman R, Reveille JD, Vila LM, Kimberly RP, Freedman BI, Stevens AM, Boackle SA, Criswell LA, Vyse TJ, Behrens TW, Jacob CO, Alarcon-Riquelme ME, Sivils KL, Choi J, Joo YB, Bang SY, Lee HS, Bae SC, Shen N, Qian X, Tsao BP, Scofield RH, Harley JB, Webb CF, Wakeland EK, James JA, Nath SK, Graham RR, Gaffney PM. Two functional lupus-associated BLK promoter variants control cell-type- and developmental-stage-specific transcription. American journal of human genetics. 2014;94:586–598. doi: 10.1016/j.ajhg.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hom G, Graham RR, Modrek B, Taylor KE, Ortmann W, Garnier S, Lee AT, Chung SA, Ferreira RC, Pant PV, Ballinger DG, Kosoy R, Demirci FY, Kamboh MI, Kao AH, Tian C, Gunnarsson I, Bengtsson AA, Rantapaa-Dahlqvist S, Petri M, Manzi S, Seldin MF, Ronnblom L, Syvanen AC, Criswell LA, Gregersen PK, Behrens TW. Association of systemic lupus erythematosus with C8orf13-BLK and ITGAM-ITGAX. The New England journal of medicine. 2008;358:900–909. doi: 10.1056/NEJMoa0707865. [DOI] [PubMed] [Google Scholar]
- 63.Jarvinen TM, Hellquist A, Zucchelli M, Koskenmies S, Panelius J, Hasan T, Julkunen H, D’Amato M, Kere J. Replication of GWAS-identified systemic lupus erythematosus susceptibility genes affirms B-cell receptor pathway signalling and strengthens the role of IRF5 in disease susceptibility in a Northern European population. Rheumatology. 2012;51:87–92. doi: 10.1093/rheumatology/ker263. [DOI] [PubMed] [Google Scholar]
- 64.Namjou B, Ni Y, Harley IT, Chepelev I, Cobb B, Kottyan LC, Gaffney PM, Guthridge JM, Kaufman K, Harley JB. The effect of inversion at 8p23 on BLK association with lupus in Caucasian population. PloS one. 2014;9:e115614. doi: 10.1371/journal.pone.0115614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deshmukh HA, Maiti AK, Kim-Howard XR, Rojas-Villarraga A, Guthridge JM, Anaya JM, Nath SK. Evaluation of 19 autoimmune disease-associated loci with rheumatoid arthritis in a Colombian population: evidence for replication and gene-gene interaction. The Journal of rheumatology. 2011;38:1866–1870. doi: 10.3899/jrheum.110199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Freudenberg J, Lee HS, Han BG, Shin HD, Kang YM, Sung YK, Shim SC, Choi CB, Lee AT, Gregersen PK, Bae SC. Genome-wide association study of rheumatoid arthritis in Koreans: population-specific loci as well as overlap with European susceptibility loci. Arthritis and rheumatism. 2011;63:884–893. doi: 10.1002/art.30235. [DOI] [PubMed] [Google Scholar]
- 67.Gregersen PK, Amos CI, Lee AT, Lu Y, Remmers EF, Kastner DL, Seldin MF, Criswell LA, Plenge RM, Holers VM, Mikuls TR, Sokka T, Moreland LW, Bridges SL, Jr, Xie G, Begovich AB, Siminovitch KA. REL, encoding a member of the NF-kappaB family of transcription factors, is a newly defined risk locus for rheumatoid arthritis. Nature genetics. 2009;41:820–823. doi: 10.1038/ng.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Orozco G, Eyre S, Hinks A, Bowes J, Morgan AW, Wilson AG, Wordsworth P, Steer S, Hocking L, Thomson W, Worthington J, Barton A. Study of the common genetic background for rheumatoid arthritis and systemic lupus erythematosus. Annals of the rheumatic diseases. 2011;70:463–468. doi: 10.1136/ard.2010.137174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jin XQ, Liu P, Zhang QP. Genetic susceptibility in children with incomplete Kawasaki disease. Zhongguo dang dai er ke za zhi = Chinese journal of contemporary pediatrics. 2015;17:663–667. [PubMed] [Google Scholar]
- 70.Yin H, Borghi MO, Delgado-Vega AM, Tincani A, Meroni PL, Alarcon-Riquelme ME. Association of STAT4 and BLK, but not BANK1 or IRF5, with primary antiphospholipid syndrome. Arthritis and rheumatism. 2009;60:2468–2471. doi: 10.1002/art.24701. [DOI] [PubMed] [Google Scholar]
- 71.Sun F, Xu J, Wu Z, Li P, Chen H, Su J, You X, Li M, Zhao Y, Tian X, Li Y, Zhang F. Polymorphisms in the FAM167A-BLK, but not BANK1, are associated with primary Sjogren’s syndrome in a Han Chinese population. Clinical and experimental rheumatology. 2013;31:704–710. [PubMed] [Google Scholar]
- 72.Zhou Y, Li X, Wang G, Li X. Association of FAM167A-BLK rs2736340 Polymorphism with Susceptibility to Autoimmune Diseases: A Meta-Analysis. Immunological investigations. 2016;45:336–348. doi: 10.3109/08820139.2016.1157812. [DOI] [PubMed] [Google Scholar]
- 73.Sarac H, Markeljevic J, Mokrovic G, Erdeljic V, Bozina N, Cicin-Sain L. Platelet serotonin in primary Sjogren’s syndrome: level and relation with disease activity. Journal of neuroimmunology. 2012;251:87–89. doi: 10.1016/j.jneuroim.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 74.Klimiuk PS, Grennan A, Weinkove C, Jayson MI. Platelet serotonin in systemic sclerosis. Annals of the rheumatic diseases. 1989;48:586–589. doi: 10.1136/ard.48.7.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meyerhoff J, Dorsch CA. Decreased platelet serotonin levels in systemic lupus erythematosus. Arthritis and rheumatism. 1981;24:1495–1500. doi: 10.1002/art.1780241207. [DOI] [PubMed] [Google Scholar]
- 76.Markeljevic J, Sarac H, Bozina N, Henigsberg N, Simic M, Cicin Sain L U consortium, UKPSsS Registry. Serotonin transporter gene polymorphisms: Relation with platelet serotonin level in patients with primary Sjogren’s syndrome. Journal of neuroimmunology. 2015;282:104–109. doi: 10.1016/j.jneuroim.2015.04.002. [DOI] [PubMed] [Google Scholar]