Abstract
Giardia lamblia is one of the most common infectious protozoans in the world. Giardia rarely causes severe life-threatening diarrhea, and may even have a slight protective effect in this regard, but it is a major contributor to malnutrition and growth faltering in children in the developing world. Giardia infection also appears to be a significant risk factor for post-infectious irritable bowel and chronic fatigue syndromes. In this review we highlight recent work focused on the impact of giardiasis and the mechanisms that contribute to the various outcomes of this infection, including changes in the composition of the microbiota, activation of immune responses, and immunopathology.
Keywords: Giardia, Giardiasis, Immunity, Microbiota, Immunopathology
THE IMPACT OF GIARDIASIS
Giardia lamblia (syn. G. duodenalis, G. intestinalis) is a protozoan parasite that infects both humans and other mammals through the ingestion of parasite-contaminated water or food [1, 2]. This parasite is divided into 8 genetic groups, termed assemblages A-H. Assemblages A and B are the main assemblages found to infect humans [2], although a recent report has described human infections with assemblage E [3]. The Giardia lifecycle has two distinct phases: a vegetative trophozoite and an infective cyst that is resistant to harsh environmental conditions. Cysts are transmitted via the fecal-oral route and an excystation process begins after reaching the stomach. Each cyst will release an excyzoite, which can generate four trophozoites following two rounds of division. The trophozoites will colonize the host's intestinal tract, particularly the upper intestine or duodenum and replicate via asexual binary fission. Eventually, some trophozoites initiate the encystation process, migrating towards the lower intestine where they are shed from the hosts to the outside environment as infective cysts [1].
Human Giardia infections (giardiasis) are found globally, with prevalence ranging between 20–30% in the developing world and 3–7% in industrialized nations [4, 5]. Unfortunately, no protective vaccines are available for human use and drug therapy options have varying efficacies [6, 7]. Although Giardia infections typically resolve within a few weeks, infections may also last for several months as chronic infections.
Symptoms can present as diarrhea, cramps, nausea, intestinal malabsorption, and reduction in brush-border dissacharidases, though asymptomatic infections appear to be very common. Several reports have noted an association between Giardia infections and food sensitivities and allergies, possibly because food antigens may be able to translocate outside of the intestinal lumen during infection [8, 9]. Additionally, Giardia exposure may lead to increased prevalence of perceived food intolerance in humans [10], although the cause for this reaction was not identified. The concept of food antigen translocation is supported by various reports of increased intestinal epithelial barrier permeability during infection [5, 11–14]. The Global Enteric Multicenter Study (GEMS) reported that Giardia infections appear to reduce the incidence of severe diarrheal disease in children in the developing world [15]. The reason for this is not well understood, though it could be attributed to several factors including development of cross-protective immune responses, reduced inflammatory response or altered composition of the microbiota. Nevertheless, Giardia was found to be the 4th most common pathogen found in stools of children younger than 1 year of age and the second most common pathogen among children between 1 and 2 years of age [16]. In the latest report from the Interactions of Malnutrition and Enteric Infections: Consequences for Child Health and Development Project (MAL-ED) project, Giardia detection was associated with reduced height and weight measurements by children of age 2 [17], though stunting and wasting were reported to be somewhat variable between infection studies [18].
Giardia infections can also lead to post-infectious syndromes. Recent work has shown that irritable bowel syndrome (IBS) and chronic fatigue syndrome (CFS) develop years after this parasite has been eliminated [19]. Recently a potential mechanism for post-infectious IBS has been examined using a neonatal rat infection model. Infections led to visceral hypersensitivity in the jejunum and rectum of rats 50 days post infection and were associated with villus atrophy, crypt hyperplasia and increased immune cell numbers. This study also reported the translocation of commensal bacteria across the epithelial barrier and increased expression of neuronal c-fos, which is also associated with post-infectious IBS [20].
The aim of this review is to highlight recent work that focus on the impact of giardiasis on the intestinal microbiota and examine mechanisms that may contribute to the development of immune responses and the various outcomes of this infection (Figure 1). We will first discuss the interactions between Giardia and the intestinal microbiota, reviewing recent reports that describe shifts in microbial composition and the effects on infection outcomes. Next, we describe the role of immune cells and protective host mechanisms that aid in Giardia protection and provide several directions of future research. Lastly, the mechanisms behind Giardia-induced immunopathology are discussed.
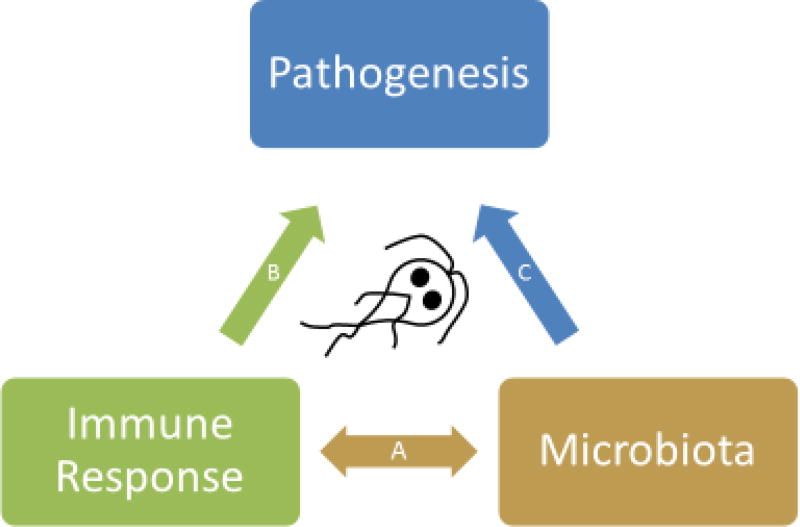
Figure 1. A model for the intersection between Immune Response, Microbiota, and Pathogenesis during Giardia infections.
A) Immune response and composition of the intestinal microbiota are highly linked to each other. The composition of the microbiota influences T cell development (e.g. segmented filamentous bacteria promote Th17 responses while Clostridia promote regulatory T cell development). Conversely, immune responses modulate the microbiota composition through numerous effector mechanisms such as anti-microbial peptides, mucous, nitric oxide (NO) and IgA.
B) Several elements of Giardia pathogenesis have been shown to depend on immune responses activated by infection. These include anti-parasitic effects of anti-microbial peptides, NO and IgA. Smooth muscle hypercontractility and intestinal hypermotility also depend on adaptive immune responses as well as mast cell responses, and disaccahridase deficiency is driven by activated CD8+ T cells.
C) Commensal microbiota also contribute to the pathology observed during, and possibly after, Giardia infections. In addition to contributing to activation of the immune response during infections, dysbiosis of the gut microbiome may also directly mediate some of the nutrient malabsorption observed during infections and also the long-term sequelae, such as post-infectious irritable bowel syndrome.
GIARDIA INTERACTIONS WITH INTESTINAL COMMENSAL MICROBIOTA
There are rare (and controversial) instances of invasive Giardia infections [21], however, Giardia parasites are typically extracellular organisms that do not invade cells lining the small intestine and instead remain attached to the microvilli within the intestinal lumen where they proliferate [1]. As such, it is not surprising that interactions occur between this parasite and commensal intestinal microbiota. Singer and Nash first reported that infection outcomes in the adult mouse model of giardiasis were dependent on the source of animals [22]. Mice purchased from Taconic Farms were resistant to Giardia infections while mice of the same strain from Jackson Laboratories were susceptible. Treatment with antibiotics made mice from either vendor susceptible to infection, while co-housing animals made them all resistant, indicating that the composition of microbes within the gut determined susceptibility to this parasitic infection [22].
In addition to susceptibility to infection, intestinal microbiota may also affect pathogenesis [23]. A group in Brazil reported that duodenal microbes are able to stimulate Giardia pathogenicity. Conventional, germ-free, or germ-free mice that were reconstituted with duodenal microbes from five patients with symptomatic giardiasis were infected with G. lamblia trophozoites. Infected conventional mice showed the greatest signs of intestinal pathology among the three groups, with the reconstituted germ-free mice showing moderate pathology. Interestingly, infected germ-free mice did not develop intestinal pathology as compared to the other groups, suggesting a need for intestinal microbes to stimulate pathology [24]. Parasite genetics may also play a role in infection susceptibility and outcomes. Murine infections using the WB strain (assemblage A) of Giardia require the use of a pre-infection antibiotic treatment to render mice susceptible to infection, whereas the GS strain (assemblage B) of Giardia do not require antibiotic treatment and readily infect mice [25]. This report describes higher cytokines levels in mice infected with WB, when compared to GS-infected mice, and also the loss of disaccharidase impairment in WB-infected mice, though it is now known that intestinal microbiota are important for Giardia-induced disaccharidase deficiency in vivo [26].
Several recent studies have also highlighted the interactions between Giardia and intestinal microbes. Exposure of lab strains of Escherichia coli or human intestinal microbiota to Giardia was able to convert the bacteria to a toxic state that is deadly to Caenorhabditis elegans [27]. Moreover, the same group demonstrated that Giardia caused not only microbial dysbiosis in co-cultures with human mucosal microbial biofilms, but also increased virulence of commensal microbiota towards human epithelial cells in vitro; this report also described increased levels of inflammatory markers in humanized germ-free mice that were reconstituted with human intestinal microbes that had been exposed to Giardia products, but not the living parasite [28]. These Giardia-altered biofilms were capable of inducing immunopathology (intestinal permeability) that was dependent on microbial community disruption caused by Giardia secretory proteases. As nutrient malnourishment and Giardia infection in humans both likely contribute to decreased immune function [29], a recent report has examined the effects of protein malnourishment on murine Giardia infections. Mice fed a protein deficient diet presented with persistent infections, an alteration in the composition of duodenal microbes, and enhanced growth (weight) impairment when infected with Giardia [30]. Taken together, these reports suggest that Giardia have significant interactions with the intestinal microbiota, which may lead to changes in infection outcome and immunopathology.
Non-invasive microbial probiotic therapies have been examined as a possible avenue to aid in the resolution of infections. It was found in vitro that the proliferative ability of the WB (assemblage A) strain of Giardia was inhibited when trophozoites were exposed to supernatants of Lactobacillus johnsonii culture medium [31] and confirmed in vivo in gerbils that evaded WB colonization when treated with Lactobacilli [32]. The cause for this toxicity to Giardia trophozoites was recently attributed to the generation of deconjugated bile salts produced by L. johnsonii [33]. Additionally, in mouse models of G. lamblia infections, probiotic administration of Enterococcus faecium and Lactobacillus casei both lessened parasite burdens and induced a stronger antibody immune response [34] and reduced parasite-mediated mucosal damage [35]. Giardia-mediated immunopathology may also be alleviated with probiotic treatments. Lactobacillus rhamnosus treatments in infected mice increased levels of anti-oxidants and brush-border disaccharidases, while decreasing levels of oxidants in the small intestine [36].
Since microbiota were shown to influence the outcome of infection, our lab and the Dawson group conducted the first culture-independent assessment of the intestinal microbiota during Giardia infections and found that infections influence both the architecture and abundance of the intestinal microbiota in the small intestine [37]. While this report emphasizes data using Giardia-infected mice that were pre-treated with antibiotics, and although antibiotic treatment itself causes a shift in the microbiota composition, the overall shift in microbial ecology was still present in infected mice that had not been treated with antibiotics. A decrease in the diversity and abundance of the obligate anaerobic Firimicutes taxa occurred, while an increase in the diversity of the taxa of aerobic Rhodocyclaceae, Moraxellaceae, Flavobacteriales, and Comomonadaceae was reported [37]. These enriched taxa are all considered to be metabolically flexible, and prosper with increased oxygen tension, lipid availability, and competition for arginine. Thus, Giardia colonization may lead to a metabolic shift among the intestinal microbial community, and this altered microbiota then could contribute to the symptoms present during giardiasis or aid in protection.
Much of what we know about the human intestinal microbiota comes from fecal samples, which limits identification of microbial composition in a specific region of the intestine. Furthermore, if biopsy samples are used, they were most likely extracted from patients with gastrointestinal diseases that may already contain perturbed microbiota communities. One study comparing the intestinal microbial composition of obese humans with normal weighted individuals described the dominance of Firmicutes and Actinobacteria in all samples taken from gastric tubes inserted in the gut [38]. However, this result must be further examined as the data was provided from 12 year-frozen samples and were taken from a study that was not designed to examine the intestinal microbiota composition [38]. The two latest studies identifying the taxa of duodenal microbiota during murine Giardia infections both use samples extracted from intestinal segments for sequencing rather than fecal samples [30, 37]. However, bacterial community analysis derived from fecal samples of non-antibiotic treated, infected mice were indistinguishable when compared to uninfected control mice [30]. Accordingly, if possible, future microbial studies should use samples isolated from intestinal segments rather than fecal samples to provide accurate results.
PROTECTIVE IMMUNITY TOWARDS GIARDIA
Innate Immunity
Intestinal Epithelial Cells
During infections, the immune system’s first line of defense is the natural surface barrier (i.e. skin and mucous membranes) (Figure 2). Intestinal epithelial cells (IECs) express pattern-recognition receptors (PRRs) that enable them to sense the environment within the intestines and are important in directing immune responses when pathogens are detected. In Toll-like receptor (TLR) signaling-deficient mice or antibiotic-treated mice, intestinal inflammation was severely exacerbated during dextran sulfate sodium (DSS) induced colitis, thus implicating TLRs and microbes in maintaining intestinal homeostasis [39]. However, we are unaware of any reports describing the phenotype of Giardia infected MyD88-deficient mice which lack signaling via TLRs. Aley et al. [40] were the first to report that antimicrobial peptides produced by specialized IECs, specifically the α-defensins cryptdin-2 and -3, reduced Giardia viability in vitro [40]. However, in vivo infection studies suggest redundant host mechanisms are active during colonization, as metalloproteinase-7 (MMP-7) deficient mice, which do not produce any active α-defensins, have only minor deficits in control of Giardia muris [41] or G. lamblia infections [42]. However, mice lacking both MMP-7 and inducible nitric oxide synthase (NOS2), exhibited a significant defect in infection control [42].
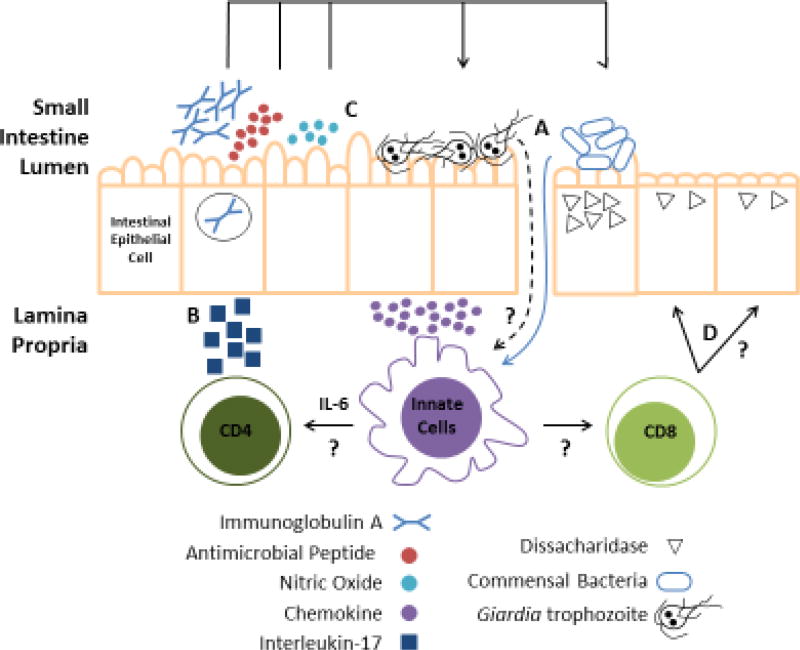
Figure 2. The multiple roles of intestinal epithelial cells during Giardia infections.
The intestinal epithelium normally provides a secure barrier against invading microbes. (A) During Giardia colonization of the small intestine, permeability increases resulting in the translocation of intact microbes and/or microbial antigens into the lamina propria. Innate immune cells (e.g., macrophages and dendritic cells) become activated and initiate the downstream adaptive responses. Which host receptors and parasite ligands serve to activate these responses are unclear, and other than IL-6, the cytokines which drive development of adaptive responses are unknown. (B) Adaptive responses include IgA production by B cells, IL-17 production by CD4+ Th17 cells and activation of CD8+ T cells, which contribute to protection or immunopathology as described in the text. In response to IL-17, intestinal epithelial cells (IECs) secrete antimicrobial peptides and transport IgA into the lumen where they can aid in Giardia protection, but also impact the commensal microbes. (C) Additionally, in response to Giardia, IECs can produce nitric oxide to aid in protective immunity and also release chemokines to recruit innate immune cells. (D) Activation of CD8+ T cells induces pathological symptoms including microvilli shortening and reduced dissacharidase activity through an unknown mechanism.
Nitric oxide (NO) is produced from L-arginine by the three forms of nitric oxide synthase (NOS): neuronal NOS (NOS1), inducible NOS (NOS2), and endothelial NOS (NOS3). NO has been reported to have inhibitory effects on Giardia growth, differentiation, encystation, and excystation in vitro [43]. However, arginine consumption by Giardia in vitro was able to reduce NO production by differentiated CaCo-2 IECs [44]. Furthermore, NOS2 is not uniquely required for parasite clearance in vivo [42]. Instead, NOS1 is more important than NOS2 and NOS3 for Giardia immunity as it contributes to parasite clearance and intestinal motility changes during infection [45, 46].
Several studies have examined IEC responses to Giardia and parasite mechanisms to subvert these responses. IECs may help initiate immune responses against Giardia through production of chemokines. Microarray analysis revealed that exposure of human colon carcinoma, CaCo2 cells, to Giardia resulted in increased expression of chemokines, including CXCL1-3, CCL2, and CCL20 [47]. In contrast, a cathepsin B-like cysteine protease secreted by Giardia can modulate immune responses by degrading CXCL8, a neutrophil attractant [48]. In addition to reducing NO production, consumption of arginine by Giardia was shown to reduce IEC proliferation [44], and infection reduced intestinal epithelial barrier function [11, 12, 14, 49]. It is reasonable to assume that this disruption is partly mediated by Giardia proteins and enzymes through excretory/secretory pathways [50]. One report suggested that parasite proteases aid in parasite adhesion to IECs in a rat intestinal cell culture model [51]. Additionally, brief Giardia exposure to human epithelial cell cultures also triggers the release of three enzymes that participate in host immunoregulation and Giardia metabolism: arginine deiminase (ADI), ornithine carbamoyl transferase (OCT) and enolase [52]. Taken together, it is becoming increasingly clear that the interactions between host and parasite dictate colonization outcomes during infection and is a topic that should be studied further.
Complement
The first role for the complement system in protective Giardia immunity was described by Hill et al. [53] when trophozoites were exposed to human sera extracted from patients with no history of giardiasis. Parasite killing was greater in groups exposed to sera rather than controls, and killing was lost when complement components were blocked, suggesting parasite killing was complement-dependent. Furthermore, this killing was dependent on structures found on the parasite’s surface, as trophozoites treated with neuraminidase or trypsin did not activate the complement pathway [53]. While this paper attributed complement activation to the alternative pathway, the lectin pathway had not yet been identified. The lectin pathway is initiated through the binding of mannose binding lectin (MBL), a C-type lectin, to carbohydrates such as mannose and N-acetylglucosamine (GlcNAc) that are commonly found on pathogens [54], including surface proteins of Giardia trophozoites [55, 56]. It was shown in vitro that MBL is able to bind to trophozoites and cause cell lysis through the complement system [57]. More recently, Li et al. [58] reported a delay in the clearance of Giardia parasites and recruitment of mast cells to the small intestine in mice lacking MBL or complement component C3a receptor (C3aR). Furthermore, mice lacking C3aR also exhibited significantly reduced T-cell responses against parasite antigens following infection but IgA levels within the small intestine were unaffected [58]. Taken together, these results indicate a role for the complement system in not only activating but also mediating downstream immune responses during infection.
Mast Cells
Mast cells have been found to play a significant role during the immune response towards Giardia and are recruited to the small intestine in significant numbers during infection [59, 60]. Both mast cell-deficient mice (c-kitw/wv) and mice treated with c-kit blocking antibody failed to control Giardia. Furthermore, IgA production was also effected as c-kit-deficient mice failed to make parasite-specific IgA [60]. These cells have also been shown to contribute to pathological condition of intestinal hypermotility, which will be discussed in the immunopathology section.
Neutrophils and Eosinophils
Neutrophils are phagocytic cells that are often the first immune cells recruited to the site of infection. Currently, it is uncertain if neutrophils provide significant contributions to parasite clearance during Giardia infections. One report noted an increase in neutrophil numbers in the intestinal lamina propria during infection in vivo, due to an influx of microbes. However, the staining method used to detect neutrophils (chloroacetate esterase activity) may also identify mast cells [14]. Functionally, human peripheral blood neutrophils that were incubated in vitro with Giardia trophozoites and human hyperimmune serum increased production of reactive oxygen species (ROS) suggesting an oxidative response occurred [61]. Interestingly, this oxidative response decreased with the addition of anti-CR1 and anti-CR3 blocking antibody suggesting an important role for complement in this cellular response [61]. Giardia is capable of using NADH oxidase and NADH peroxidase to render ROS less harmful [62]. It is unclear if ROS is effective in parasite killing in vivo, although a recent in vitro study reported that Giardia was susceptible to IEC-mediated ROS [63].
Several groups reported an increase in the number of eosinophils, granulocytes involved in host protection against parasites, during Giardia infection [29, 64,65]. Recurrent giardiasis has been associated to chronic eosinophilia [66], along with increased IL-5 concentrations within blood serum in humans [67]. One report described a critical role for eosinophils in the production of IgA in an IL-1β dependent manner [68]. Thus, during Giardia infections, it is possible that these cells are not only aiding in the production of IgA but also aiding the in the activation of other IL-1β-sensitive cells, such as group 3 innate lymphoid cells (ILC3s), Th17 cells, [69] or γδ T-cells [70].
Mononuclear Phagocytic Cells (Macrophages and Dendritic Cells)
Intestinal macrophages and dendritic cells are antigen presenting cells that are found throughout the intestine and are known for their phagocytic activity. These cells must be able to distinguish very rapidly between invasive pathogens or innocuous commensals or food, and are partly responsible for maintaining a balance between inflammation and tolerance within the gut. Recently, our lab has identified an increase in a population of intestinal macrophages, following infection, that expresses both NOS2 and arginase 1 (ARG1) [71]. While NOS2 is normally a marker for inflammatory M1 macrophages and ARG1 is a marker for alternatively activated M2 macrophages, expression of both is more similar to myeloid derived suppressor cells (MDSCs) associated with certain tumor microenvironments [72]. Recruitment of MDSCs in other infections is thought to reduce inflammatory responses and may contribute to the development of chronic infections [73–75]
Dendritic cells (DCs) are necessary for proper parasite clearance during infection. DCs have been shown to be a significant source of IL-6 during infection and IL-6 deficient mice have a defect in parasite control [76–78]. However, it is unclear how DCs recognize Giardia parasites directly and how DC activation contributes to development of adaptive responses against this parasite. One study, using recombinant Giardia immunoglobulin binding protein (BiP) suggests that DCs are activated by TLR4, but the relevance of this in vivo is unclear [79]. Total Giardia extract increased expression of the costimulatory molecules, CD80 and CD86, on murine bone marrow derived DCs, yet it did not invoke a strong cytokine response [80]. This weak cytokine response was also seen in human monocyte-derived DCs [81] exposed to Giardia. Interestingly, both studies [80, 81] showed that DC exposure to lipopolysaccharide (LPS) and Giardia resulted in an increase in IL-10 production; Kamda and Singer [80] further showed that blocking IL-10R and phosphoinositide-3 kinase (PI-3K) restores production of IL-12, which suggests that Giardia may activate PI-3K in DCs. This reduced production of IL-12 may restrict development of Th1 cells.
Adaptive Immunity
T cells
It is well established that CD4+ T cells are essential for the clearance of Giardia since T cell-deficient mice cannot control infections, whereas B cell-deficient mice can eliminate parasites [82, 83]. T cells were found to provide distinct antibody-independent, but T cell-dependent, immune mechanisms that lead to elimination of the parasite [83]. CD8+ T cells are not required for parasite clearance, as β2m-deficent mice which lack class I MHC expression and are devoid of CD8+ T cells are able to rapidly control infection [83]; however, it appears that CD8+ cells are required for reduction of intestinal disaccharidase activity [84], as will be discussed in the immunopathology section.
CD4+ T cells typically specialize in the types of cytokines that they produce. For example, Th1 cells produce interferon (IFN)-γ, while Th2 cells produce IL-4, among others. Though both IFN-γ and IL-4 are increased in sera from humans infected with Giardia [67, 85], there is no substantial defect in parasite clearance when IFN-γ and IL-4-deficient female mice are infected [83]. It is becoming increasingly evident that IL-17, within the context of a Giardia infection, is essential for protective immunity [86]. This pro-inflammatory cytokine is produced by Th17 cells that require both IL-6 and TGF-β for their development. IL-6-deficent mice fail to clear infections and also lack IL-17 expression [87], which suggests Th17 cells are required for parasite clearance in an IL-6 dependent manner. Additionally, it would be interesting to understand if mast cell-derived IL-6 is critical to Th17 cell development. Li et al. [88] found a reduction in IL-17 responses in the absence of signaling via C3aR, which suggest that a decrease in mast cell recruitment also correlates with decreased IL-17 production. In human patients that have recovered from Giardia, IL-17 has been found to be upregulated when effector memory CD4+ T-cell are restimulated with Giardia antigen [89]; in addition, several studies [25, 87, 90, 91] reported the upregulation of IL-17 during murine or bovine infections, and delayed parasite clearance in IL-17 knockout mice has also been reported [87, 90]. Moving forward it will be interesting to understand the effector mechanisms triggered by IL-17 and the signaling mechanisms leading to activation of IL-17 production during giardiasis. For example, are the activation signals for Th17 development dependent on direct recognition of parasite molecules or do defects in the epithelial barrier permit components of the intestinal microbiome to activate immune responses? Additionally, it would be important to understand if Th17 cells are involved in any immunopathology or long lasting post-infectious diseases such as, irritable bowel syndrome or chronic fatigue syndrome [19].
IgA
Humans with immunoglobulin deficiencies develop chronic giardiasis and it is well known that Giardia generate large amounts of IgA in response to infection [92–94]. As such, IgA-mediated protection has been examined to elucidate mechanisms that control parasite infections. B-cells within the intestinal lamina propria (LP) are responsible for the production of IgA – the most abundant antibody within mucosal secretions [95]. IECs transport IgA into the lumen using a polymeric immunoglobulin receptor (pIgR) where it can then bind to various microbial (commensal or pathogenic) surfaces to prevent adhesion to IECs [96]. Langford et al. [97]reported a defect in parasite clearance when IgA-deficient mice were infected with G. muris; furthermore plgR-deficient mice, which fail to transport IgA into the intestinal lumen, also developed chronic infections when infected with G. muris [97]. However, G. lamblia infections do not seem to be as sensitive to IgA as no difference in parasite clearance was detected in pIgR-deficient [98] or B-cell deficient mice [83]. Recently, a report linked IL-17 signaling to the transport of IgA into the lumen of the intestine, as fecal, but not serum, IgA diminished in infected IL-17-deficient mice [87]. This difference in sensitivity to IgA between Giardia species is not fully understood and may be dependent on several factors such as antigenic surface variation, susceptibility to other antimicrobial factors (e.g., complement or NO), and Giardia infection kinetics (G. muris infections typically peak one to two weeks later than G. lamblia in adult mice).
IMMUNOPATHOLOGY
Mast Cells and Hypermotility
Severe cramping is a commonly reported symptom of giardiasis in humans. Similarly, infections with Giardia in mice lead to changes in intestinal motility [45], and subsequent reports linked enhanced smooth muscle contractility to mast cell responses [99]. The neurotransmitter cholecystokinin (CCK) was found to significantly increase muscle contractions when longitudinal muscle from infected mice was exposed to CCK ex vivo. This effect could be blocked with either the addition of ketotifen, which inhibits mast cell degranulation, or pre-treatment of tissues with compound 48/80, which depletes mast cells of their granule contents, indicating that CCK increases muscle contractility through mast cell degranulation. Enhanced muscle contractility in conjunction with NOS1-mediated muscle relaxation leads to the promotion of intestinal propulsion and hypermotility [99]. Hsu et al., [100] also describe an increase in CCK levels in the colon of Giardia infected mice, although blockade of CCK receptors did not ameliorate the visceral hypersensitivity induced by infection [101]. In human Giardia infections, CCK was found to be elevated among a small number of patients with symptomatic giardiasis [102]. CCK is released by enteroendocrine cells located in the small intestine and signals the presence of fat in the lumen. This CCK normally leads to luminal bile release to aid in fat solubilization and absorption, although bile is readily used by Giardia trophozoites during growth. As such, parasite-mediated CCK release should be advantageous for the parasite during infection.
CD8+ T-cells and Intestinal Malabsorption
Another hallmark symptom of giardiasis is the shortening of microvilli lining the gut, which results in a reduction in dissacharidase activity [103, 104]. This pathological disruption has been reported in both human infections and in animal infection models [105, 106] and leads to reduced nutrient absorption. Scott et al. [84] showed that athymic nude mice, lacking T cells, failed to exhibit microvilli shortening or reduced dissacharidase activity after infection. Moreover, adoptive transfers of CD8+ T cells, but not CD4+ T cells, from infected donors into uninfected nude mice produced both microvilli shortening and reduced dissacharidase activity [84]. Furthermore, neither infected CD4−/− nor β2m−/− mice (which lack surface expression of major histocompatibility complex I (MHC I) and are deficient in CD8+ T cells) exhibited reduced dissacharidase activity [25]. This is consistent with CD8+ T cells mediating this pathology and CD4+ T cells being important for development of the CD8+ T cell response.
While CD8+ T-cells are responsible for this immunopathology, events leading to the activation of these cells are not well understood. Recently, we demonstrated that this effect is dependent on intestinal microbiota as antibiotic treatments reduced activation of CD8+ T cells and dissacharidase deficiency during infections [26]. Furthermore, this effect also appears to be Giardia strain dependent [25]. As Giardia can alter intestinal microbial communities [37] it would be interesting to understand the link between intestinal microbial diversity, Giardia, and immune cells leading to subsequent immunopathology.
Malnutrition Cycle
Chronic giardiasis (recurring incidents of diarrhea) appears to be dependent on several factors that lead to a cyclical system. In low and middle-income countries, malnourishment and Giardia infections in humans both likely contribute to decreased immune function [29] and further nutrient malabsorption due to intestinal damage [107]. In a murine malnutrition model, protective Giardia cytokines and eosinophil recruitment were reduced in mice that were fed a low protein diet prior to infection with Giardia cysts [29]. Micronutrients appear to be important in Giardia protection as children with higher concentrations of vitamin A presented with fewer detectable parasites and zinc deficiency led to increased Giardia susceptibility [108]; additionally, vitamin A supplementation in Brazilian children decreased Giardia susceptibility [109].
Past studies examining an association between Giardia and human growth seem to vary [18, 110–114]. Yet in the past several years, it has become apparent that Giardia infections that occur early in childhood (earlier than age 2) are associated with reduced height and weight attainment [17, 115], with a critical window of preventing infection between birth and 6 months of life having the greatest impact on growth faltering [115]. This window may be critical due to the establishment and shaping of the composition of intestinal microbiota that provide essential amino acids and vitamins used for growth [116]. As such, these two recent studies suggest that the prevention of exposure to this parasite is more important in preventing long-term growth deficiencies rather than early intervention therapies, such as the use of metronidazole [117]. Moreover, the cause for the variation in studies examining the association between Giardia and growth development may be due to the presence (or absence) of very early childhood Giardia infections since acquired childhood protection may only limit the severity of giardiasis rather than provide full protection [115].
Concluding Remarks: Looking Ahead
Future research highlighting the interaction between the intestinal microbiota and immune system will be of great importance to the field of Giardia pathogenesis (see Outstanding Questions). Several studies have identified mechanisms whereby intestinal bacteria can influence the development of Th17 and Treg immune responses [118][119][120][121]. Intestinal microbiota clearly impact colonization and pathogenesis of Giardia and recent work highlights potential influences on the anti-Giardia immune response as well. At steady state, there is complex crosstalk between microbes and host cells and it is becoming increasingly evident that Giardia infections have the capability to perturb microbial homeostasis, causing microbial dysbiosis. Since Giardia infections can lead to post-infectious IBS and CFS, it will be interesting to understand the long-lasting effects of Giardia-induced microbial dysbiosis in promoting these postinfectious disease syndromes. Finally, since many advances in our understanding of protective Giardia immunity have been discovered using murine infection models or human cell culture models, additional emphasis on studies of immune responses in human infections are needed. The greater challenge for our field resides in translating these advances towards humans systems to generate novel therapeutics that promote human health.
OUTSTANDING QUESTIONS BOX.
What are the main drivers of protective immunity during Giardia infections? Specifically what drives production of IL-17 and how does this cytokine exert its protective effects in humans?
What are the effects of co-infections on Giardia pathogenesis in humans? And on the pathogenesis of other enteric pathogens?
What are the long-term consequences of Giardia-induced microbial dysbiosis?
How can current advances in Giardia research be translated to human systems?
TRENDS BOX.
Giardia infections can perturb intestinal microbial diversity and lead to an increase in the abundance of aerobic microbial taxa
Giardiasis immunopathology is driven by the presence of intestinal microbiota and activation of immune responses
Interleukin-17 is a cytokine that appears to be essential for protective immunity during giardiasis
Giardia infections can lead to post-infectious syndromes, including malnutrition, irritable bowel syndrome and chronic fatigue syndrome.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adam RD. Biology of Giardia lamblia. Clin Microbiol Rev. 2001;14:447–475. doi: 10.1128/CMR.14.3.447-475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monis PT, et al. Variation in Giardia: towards a taxonomic revision of the genus. Trends Parasitol. 2009;25:93–100. doi: 10.1016/j.pt.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Zahedi A, et al. Molecular typing of Giardia duodenalis in humans in Queensland - first report of Assemblage E. Parasitology. 2017:1–8. doi: 10.1017/S0031182017000439. [DOI] [PubMed] [Google Scholar]
- 4.Roxstrom-Lindquist K, et al. Giardia immunity--an update. Trends Parasitol. 2006;22:26–31. doi: 10.1016/j.pt.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Halliez MC, Buret AG. Extra-intestinal and long term consequences of Giardia duodenalis infections. World J Gastroenterol. 2013;19:8974–8985. doi: 10.3748/wjg.v19.i47.8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ansell BR, et al. Drug resistance in Giardia duodenalis. Biotechnol Adv. 2015;33:888–901. doi: 10.1016/j.biotechadv.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Leitsch D. Drug Resistance in the Microaerophilic Parasite Giardia lamblia. Curr Trop Med Rep. 2015;2:128–135. doi: 10.1007/s40475-015-0051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Prisco MC, et al. Association between giardiasis and allergy. Ann Allergy Asthma Immunol. 1998;81:261–265. doi: 10.1016/s1081-1206(10)62823-2. [DOI] [PubMed] [Google Scholar]
- 9.Di Prisco MC, et al. Possible relationship between allergic disease and infection by Giardia lamblia. Ann Allergy. 1993;70:210–213. [PubMed] [Google Scholar]
- 10.Litleskare S, et al. Perceived food intolerance and irritable bowel syndrome in a population 3 years after a giardiasis-outbreak: a historical cohort study. BMC Gastroenterol. 2015;15:164. doi: 10.1186/s12876-015-0393-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teoh DA, et al. Giardia lamblia rearranges F-actin and alpha-actinin in human colonic and duodenal monolayers and reduces transepithelial electrical resistance. J Parasitol. 2000;86:800–806. doi: 10.1645/0022-3395(2000)086[0800:GLRFAA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 12.Buret AG, et al. Giardia lamblia disrupts tight junctional ZO-1 and increases permeability in non-transformed human small intestinal epithelial monolayers: effects of epidermal growth factor. Parasitology. 2002;125:11–19. doi: 10.1017/s0031182002001853. [DOI] [PubMed] [Google Scholar]
- 13.Chin AC, et al. Strain-dependent induction of enterocyte apoptosis by Giardia lamblia disrupts epithelial barrier function in a caspase-3-dependent manner. Infection and immunity. 2002;70:3673–3680. doi: 10.1128/IAI.70.7.3673-3680.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen TL, et al. Persistent gut barrier damage and commensal bacterial influx following eradication of Giardia infection in mice. Gut Pathog. 2013;5:26. doi: 10.1186/1757-4749-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotloff KL, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 16.Platts-Mills JA, et al. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED) Lancet Glob Health. 2015;3:e564–575. doi: 10.1016/S2214-109X(15)00151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rogawski ET, et al. Determinants and Impact of Giardia Infection in the First 2 Years of Life in the MAL-ED Birth Cohort. Journal of the Pediatric Infectious Diseases Society. 2017;6:153–160. doi: 10.1093/jpids/piw082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartelt LA, Sartor RB. Advances in understanding Giardia: determinants and mechanisms of chronic sequelae. F1000Prime Rep. 2015;7:62. doi: 10.12703/P7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanevik K, et al. Giardia-specific cellular immune responses in post-giardiasis chronic fatigue syndrome. BMC Immunol. 2017;18:5. doi: 10.1186/s12865-017-0190-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halliez MC, et al. Giardia duodenalis induces paracellular bacterial translocation and causes postinfectious visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol. 2016;310:G574–585. doi: 10.1152/ajpgi.00144.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reynoso-Robles R, et al. The invasive potential of Giardia intestinalis in an in vivo model. Sci Rep. 2015;5:15168. doi: 10.1038/srep15168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singer SM, Nash TE. The Role of Normal Flora in Giardia lamblia Infections in Mice. J Infect Dis. 2000;181:1510–1512. doi: 10.1086/315409. [DOI] [PubMed] [Google Scholar]
- 23.Bär AK, et al. The Interplay of Host Microbiota and Parasitic Protozoans at Mucosal Interfaces: Implications for the Outcomes of Infections and Diseases. PLoS Negl Trop Dis. 2015;9:e0004176. doi: 10.1371/journal.pntd.0004176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torres MF, et al. Influence of bacteria from the duodenal microbiota of patients with symptomatic giardiasis on the pathogenicity of Giardia duodenalis in gnotoxenic mice. J Med Microbiol. 2000;49:209–215. doi: 10.1099/0022-1317-49-3-209. [DOI] [PubMed] [Google Scholar]
- 25.Solaymani-Mohammadi S, Singer SM. Host Immunity and Pathogen Strain Contribute to Intestinal Disaccharidase Impairment following Gut Infection. Journal of immunology. 2011;187:3769–3775. doi: 10.4049/jimmunol.1100606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keselman A, et al. The Microbiota Contributes to CD8+ T Cell Activation and Nutrient Malabsorption following Intestinal Infection with Giardia duodenalis. Infection and immunity. 2016;84:2853–2860. doi: 10.1128/IAI.00348-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerbaba TK, et al. Giardia duodenalis-induced alterations of commensal bacteria kill Caenorhabditis elegans: a new model to study microbial-microbial interactions in the gut. Am J Physiol Gastrointest Liver Physiol. 2015;308:G550–561. doi: 10.1152/ajpgi.00335.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beatty JK, et al. Giardia duodenalis induces pathogenic dysbiosis of human intestinal microbiota biofilms. Int J Parasitol. 2017;47:311–326. doi: 10.1016/j.ijpara.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Bartelt LA, et al. Persistent G. lamblia impairs growth in a murine malnutrition model. J Clin Invest. 2013;123:2672–2684. doi: 10.1172/JCI67294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartelt LA, et al. Cross-modulation of pathogen-specific pathways enhances malnutrition during enteric co-infection with Giardia lamblia and enteroaggregative. Escherichia coli PLoS Pathog. 2017;13(7):e1006471. doi: 10.1371/journal.ppat.1006471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perez PF, et al. Inhibition of Giardia intestinalis by extracellular factors from Lactobacilli: an in vitro study. Appl Environ Microbiol. 2001;67:5037–5042. doi: 10.1128/AEM.67.11.5037-5042.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Humen MA, et al. Lactobacillus johnsonii La1 antagonizes Giardia intestinalis in vivo. Infection and immunity. 2005;73:1265–1269. doi: 10.1128/IAI.73.2.1265-1269.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Travers MA, et al. Deconjugated Bile Salts Produced by Extracellular Bile-Salt Hydrolase-Like Activities from the Probiotic Lactobacillus johnsonii La1 Inhibit Giardia duodenalis In vitro Growth. Front Microbiol. 2016;7:1453. doi: 10.3389/fmicb.2016.01453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benyacoub J, et al. Enterococcus faecium SF68 enhances the immune response to Giardia intestinalis in mice. J Nutr. 2005;135:1171–1176. doi: 10.1093/jn/135.5.1171. [DOI] [PubMed] [Google Scholar]
- 35.Shukla G, et al. Effect of Lactobacillus casei as a probiotic on modulation of giardiasis. Dig Dis Sci. 2008;53:2671–2679. doi: 10.1007/s10620-007-0197-3. [DOI] [PubMed] [Google Scholar]
- 36.Goyal N, et al. Lactobacillus rhamnosus GG antagonizes Giardia intestinalis induced oxidative stress and intestinal disaccharidases: an experimental study. World J Microbiol Biotechnol. 2013;29:1049–1057. doi: 10.1007/s11274-013-1268-6. [DOI] [PubMed] [Google Scholar]
- 37.Barash NR, et al. Giardia Alters Commensal Microbial Diversity throughout the Murine Gut. Infection and immunity. 2017;85:e00948–16. doi: 10.1128/IAI.00948-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Angelakis E, et al. A Metagenomic Investigation of the Duodenal Microbiota Reveals Links with Obesity. PLoS One. 2015;10:e0137784. doi: 10.1371/journal.pone.0137784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rakoff-Nahoum S, et al. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Aley SB, et al. Killing of Giardia lamblia by cryptdins and cationic neutrophil peptides. Infection and immunity. 1994;62:5397–5403. doi: 10.1128/iai.62.12.5397-5403.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eckmann L. Mucosal defences against Giardia. Parasite Immunol. 2003;25:259–270. doi: 10.1046/j.1365-3024.2003.00634.x. [DOI] [PubMed] [Google Scholar]
- 42.Tako EA, et al. Transcriptomic Analysis of the Host Response to Giardia duodenalis Infection Reveals Redundant Mechanisms for Parasite Control. MBio. 2013;4:e00660–00613. doi: 10.1128/mBio.00660-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eckmann L, et al. Nitric oxide production by human intestinal epithelial cells and competition for arginine as potential determinants of host defense against the lumen-dwelling pathogen Giardia lamblia. Journal of immunology. 2000;164:1478–1487. doi: 10.4049/jimmunol.164.3.1478. [DOI] [PubMed] [Google Scholar]
- 44.Stadelmann B, et al. Arginine consumption by the intestinal parasite Giardia intestinalis reduces proliferation of intestinal epithelial cells. PLoS One. 2012;7:e45325. doi: 10.1371/journal.pone.0045325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li E, et al. Neuronal Nitric Oxide Synthase Is Necessary for Elimination of Giardia lamblia Infections in Mice. Journal of immunology. 2006;176:516–521. doi: 10.4049/jimmunol.176.1.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andersen YS, et al. Adaptive immunity-dependent intestinal hypermotility contributes to host defense against Giardia spp. Infection and immunity. 2006;74:2473–2476. doi: 10.1128/IAI.74.4.2473-2476.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roxstrom-Lindquist K, et al. Giardia lamblia-Induced Changes in Gene Expression in Differentiated Caco-2 Human Intestinal Epithelial Cells. Infection and immunity. 2005;73:8204–8208. doi: 10.1128/IAI.73.12.8204-8208.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cotton JA, et al. Giardia duodenalis cathepsin B proteases degrade intestinal epithelial interleukin-8 and attenuate interleukin-8-induced neutrophil chemotaxis. Infection and immunity. 2014;82:2772–2787. doi: 10.1128/IAI.01771-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buret AG. Mechanisms of epithelial dysfunction in giardiasis. Gut. 2007;56:316–317. doi: 10.1136/gut.2006.107771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Carvalho TB, et al. Protease activity in extracellular products secreted in vitro by trophozoites of Giardia duodenalis. Parasitol Res. 2008;104:185–190. doi: 10.1007/s00436-008-1185-z. [DOI] [PubMed] [Google Scholar]
- 51.Rodriguez-Fuentes GB, et al. Giardia duodenalis: analysis of secreted proteases upon trophozoite-epithelial cell interaction in vitro. Mem Inst Oswaldo Cruz. 2006;101:693–696. doi: 10.1590/s0074-02762006000600020. [DOI] [PubMed] [Google Scholar]
- 52.Ringqvist E, et al. Release of metabolic enzymes by Giardia in response to interaction with intestinal epithelial cells. Mol Biochem Parasitol. 2008;159:85–91. doi: 10.1016/j.molbiopara.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hill DR, et al. Susceptibility of Giardia lamblia trophozoites to the lethal effect of human serum. Journal of immunology. 1984;132:2046–2052. [PubMed] [Google Scholar]
- 54.Thiel S. Complement activating soluble pattern recognition molecules with collagen-like regions, mannan-binding lectin, ficolins and associated proteins. Mol Immunol. 2007;44:3875–3888. doi: 10.1016/j.molimm.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 55.Ward HD, et al. Biology of Giardia lamblia. Detection of N-acetyl-D-glucosamine as the only surface saccharide moiety and identification of two distinct subsets of trophozoites by lectin binding. J Exp Med. 1988;167:73–88. doi: 10.1084/jem.167.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ratner DM, et al. Changes in the N-glycome, glycoproteins with Asn-linked glycans, of Giardia lamblia with differentiation from trophozoites to cysts. Eukaryot Cell. 2008;7:1930–1940. doi: 10.1128/EC.00268-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Evans-Osses I, et al. Involvement of lectin pathway activation in the complement killing of Giardia intestinalis. Biochem Biophys Res Commun. 2010;395:382–386. doi: 10.1016/j.bbrc.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 58.Li E, et al. Complement Activation by Giardia Parasites through the Lectin Pathway Contributes to Mast Cell Responses and Parasite Control. Infection and immunity. 2016;84:1092–1099. doi: 10.1128/IAI.00074-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hardin JA, et al. Mast cell hyperplasia and increased macromolecular uptake in an animal model of giardiasis. J Parasitol. 1997;83:908–912. [PubMed] [Google Scholar]
- 60.Li E, et al. Mast Cell-Dependent Control of Giardia lamblia Infections in Mice. Infection and immunity. 2004;72:6642–6649. doi: 10.1128/IAI.72.11.6642-6649.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arbo A, et al. Opsonic requirements for the respiratory burst of neutrophils against Giardia lamblia trophozoites. Arch Med Res. 2006;37:465–473. doi: 10.1016/j.arcmed.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 62.Brown DM, et al. Anaerobic bacterial metabolism in the ancient eukaryote Giardia duodenalis. Int J Parasitol. 1998;28:149–164. doi: 10.1016/s0020-7519(97)00172-0. [DOI] [PubMed] [Google Scholar]
- 63.Ma’ayeh SY, et al. Transcriptional profiling of Giardia intestinalis in response to oxidative stress. International Journal for Parasitology. 2015;45:925–938. doi: 10.1016/j.ijpara.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 64.Jimenez JC, et al. Systemic and mucosal responses to oral administration of excretory and secretory antigens from Giardia intestinalis. Clin Diagn Lab Immunol. 2004;11:152–160. doi: 10.1128/CDLI.11.1.152-160.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rothenberg ME, et al. Gastrointestinal eosinophils in health and disease. Adv Immunol. 2001;78:291–328. doi: 10.1016/s0065-2776(01)78007-8. [DOI] [PubMed] [Google Scholar]
- 66.Canonne D, et al. Wells’ syndrome associated with recurrent giardiasis. Br J Dermatol. 2000;143:425–427. doi: 10.1046/j.1365-2133.2000.03675.x. [DOI] [PubMed] [Google Scholar]
- 67.Matowicka-Karna J, et al. IFN-gamma, IL-5, IL-6 and IgE in patients infected with Giardia intestinalis. Folia Histochem Cytobiol. 2009;47:93–97. doi: 10.2478/v10042-009-0013-3. [DOI] [PubMed] [Google Scholar]
- 68.Jung Y, et al. IL-1beta in eosinophil-mediated small intestinal homeostasis and IgA production. Mucosal Immunol. 2015;8:930–942. doi: 10.1038/mi.2014.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Coccia M, et al. IL-1beta mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4(+) Th17 cells. J Exp Med. 2012;209:1595–1609. doi: 10.1084/jem.20111453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sutton CE, et al. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 71.Maloney J, et al. Macrophages expressing arginase 1 and nitric oxide synthase 2 accumulate in the small intestine during Giardia lamblia infection. Microbes Infect. 2015;17:462–467. doi: 10.1016/j.micinf.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang C, et al. Accumulation of myeloid-derived suppressor cells in the lungs during Pneumocystis pneumonia. Infection and immunity. 2012;80:3634–3641. doi: 10.1128/IAI.00668-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heim CE, et al. Myeloid-derived suppressor cells contribute to Staphylococcus aureus orthopedic biofilm infection. Journal of immunology. 2014;192:3778–3792. doi: 10.4049/jimmunol.1303408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pallett LJ, et al. Metabolic regulation of hepatitis B immunopathology by myeloid-derived suppressor cells. Nature medicine. 2015;21:591–600. doi: 10.1038/nm.3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou P, et al. Role of interleukin-6 in the control of acute and chronic Giardia lamblia infections in mice. Infection and immunity. 2003;71:1566–1568. doi: 10.1128/IAI.71.3.1566-1568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bienz M, et al. Interleukin-6-deficient mice are highly susceptible to Giardia lamblia infection but exhibit normal intestinal immunoglobulin A responses against the parasite. Infection and immunity. 2003;71:1569–1573. doi: 10.1128/IAI.71.3.1569-1573.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kamda JD, et al. Giardia duodenalis: dendritic cell defects in IL-6 deficient mice contribute to susceptibility to intestinal infection. Exp Parasitol. 2012;130:288–291. doi: 10.1016/j.exppara.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee HY, et al. Giardia lamblia binding immunoglobulin protein triggers maturation of dendritic cells via activation of TLR4-MyD88-p38 and ERK1/2 MAPKs. Parasite Immunol. 2014;36:627–646. doi: 10.1111/pim.12119. [DOI] [PubMed] [Google Scholar]
- 80.Kamda JD, Singer SM. Phosphoinositide 3-kinase-dependent inhibition of dendritic cell interleukin-12 production by Giardia lamblia. Infection and immunity. 2009;77:685–693. doi: 10.1128/IAI.00718-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Obendorf J, et al. Increased expression of CD25, CD83, and CD86, and secretion of IL-12, IL-23, and IL-10 by human dendritic cells incubated in the presence of Toll-like receptor 2 ligands and Giardia duodenalis. Parasit Vectors. 2013;6:317. doi: 10.1186/1756-3305-6-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heyworth MF, et al. Clearance of Giardia muris infection requires helper/inducer T lymphocytes. J Exp Med. 1987;165:1743–1748. doi: 10.1084/jem.165.6.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Singer SM, Nash TE. T-cell-dependent control of acute Giardia lamblia infections in mice. Infection and immunity. 2000;68:170–175. doi: 10.1128/iai.68.1.170-175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scott KG, et al. Role of CD8+ and CD4+ T lymphocytes in jejunal mucosal injury during murine giardiasis. Infection and immunity. 2004;72:3536–3542. doi: 10.1128/IAI.72.6.3536-3542.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bayraktar MR, et al. Serum cytokine changes in Turkish children infected with Giardia lamblia with and without allergy: Effect of metronidazole treatment. Acta Trop. 2005;95:116–122. doi: 10.1016/j.actatropica.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 86.Singer SM. Control of Giardiasis by Interleukin-17 in Humans and Mice-Are the Questions All Answered? Clin Vaccine Immunol. 2015;23:2–5. doi: 10.1128/CVI.00648-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dann SM, et al. IL-17A promotes protective IgA responses and expression of other potential effectors against the lumen-dwelling enteric parasite Giardia. Exp Parasitol. 2015;156:68–78. doi: 10.1016/j.exppara.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li E, et al. Complement Activation by Giardia Parasites through the Lectin Pathway Contributes to Mast Cell Responses and Parasite Control. Infection and immunity. 2016;84:1092–1099. doi: 10.1128/IAI.00074-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saghaug CS, et al. Human memory CD4+ T cell immune responses against Giardia lamblia. Clin Vaccine Immunol. 2015;23:11–18. doi: 10.1128/CVI.00419-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dreesen L, et al. Giardia muris infection in mice is associated with a protective interleukin 17A response and induction of peroxisome proliferator-activated receptor alpha. Infection and immunity. 2014;82:3333–3340. doi: 10.1128/IAI.01536-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grit GH, et al. Evaluation of cellular and humoral systemic immune response against Giardia duodenalis infection in cattle. Vet Parasitol. 2014;202:145–155. doi: 10.1016/j.vetpar.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 92.Snider DP, et al. Chronic Giardia muris infection in anti-IgM-treated mice. I. Analysis of immunoglobulin and parasite-specific antibody in normal and immunoglobulin-deficient animals. Journal of immunology. 1985;134:4153–4162. [PubMed] [Google Scholar]
- 93.Snider DP, et al. Chronic giardiasis in B-cell-deficient mice expressing the xid gene. Infection and immunity. 1988;56:2838–2842. doi: 10.1128/iai.56.11.2838-2842.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zinneman HH, Kaplan AP. The association of giardiasis with reduced intestinal secretory immunoglobulin A. Am J Dig Dis. 1972;17:793–797. doi: 10.1007/BF02231148. [DOI] [PubMed] [Google Scholar]
- 95.Cerutti A, Rescigno M. The biology of intestinal immunoglobulin A responses. Immunity. 2008;28:740–750. doi: 10.1016/j.immuni.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Strugnell RA, Wijburg OL. The role of secretory antibodies in infection immunity. Nat Rev Microbiol. 2010;8:656–667. doi: 10.1038/nrmicro2384. [DOI] [PubMed] [Google Scholar]
- 97.Langford TD, et al. Central importance of immunoglobulin A in host defense against Giardia spp. Infection and immunity. 2002;70:11–18. doi: 10.1128/IAI.70.1.11-18.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Davids BJ, et al. Polymeric immunoglobulin receptor in intestinal immune defense against the lumen-dwelling protozoan parasite Giardia. Journal of immunology. 2006;177:6281–6290. doi: 10.4049/jimmunol.177.9.6281. [DOI] [PubMed] [Google Scholar]
- 99.Li E, et al. Mast Cell Mediated Changes in Smooth Muscle Contractility During Mouse Giardiasis. Infection and immunity. 2007;75:4514–4518. doi: 10.1128/IAI.00596-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hsu LT, et al. Gut-derived cholecystokinin contributes to visceral hypersensitivity via nerve growth factor-dependent neurite outgrowth. J Gastroenterol Hepatol. 2016;31:1594–1603. doi: 10.1111/jgh.13296. [DOI] [PubMed] [Google Scholar]
- 101.Ito K, et al. Recognition of the product of a novel MHC TL region gene (27b) by a mouse gamma delta T cell receptor. Cell. 1990;62:549–561. doi: 10.1016/0092-8674(90)90019-b. [DOI] [PubMed] [Google Scholar]
- 102.Leslie FC, et al. Plasma cholecystokinin concentrations are elevated in acute upper gastrointestinal infections. Qjm. 2003;96:870–871. doi: 10.1093/qjmed/hcg140. [DOI] [PubMed] [Google Scholar]
- 103.Buret A, et al. Intestinal protozoa and epithelial cell kinetics, structure and function. Parasitol Today. 1990;6:375–380. doi: 10.1016/0169-4758(90)90145-t. [DOI] [PubMed] [Google Scholar]
- 104.Scott KG, et al. Jejunal brush border microvillous alterations in Giardia muris-infected mice: role of T lymphocytes and interleukin-6. Infection and immunity. 2000;68:3412–3418. doi: 10.1128/iai.68.6.3412-3418.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Buret A, et al. Effects of murine giardiasis on growth, intestinal morphology, and disaccharidase activity. J Parasitol. 1990;76:403–409. [PubMed] [Google Scholar]
- 106.Buret A, et al. Pathophysiology of small intestinal malabsorption in gerbils infected with Giardia lamblia. Gastroenterology. 1992;103:506–513. doi: 10.1016/0016-5085(92)90840-u. [DOI] [PubMed] [Google Scholar]
- 107.Goto R, et al. Poor intestinal permeability in mildly stunted Nepali children: associations with weaning practices and Giardia lamblia infection. Br J Nutr. 2002;88:141–149. doi: 10.1079/bjnbjn2002599. [DOI] [PubMed] [Google Scholar]
- 108.Astiazaran-Garcia H, et al. Crosstalk between Zinc Status and Giardia Infection: A New Approach. Nutrients. 2015;7:4438–4452. doi: 10.3390/nu7064438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lima AA, et al. Effects of vitamin A supplementation on intestinal barrier function, growth, total parasitic, and specific Giardia spp infections in Brazilian children: a prospective randomized, double-blind, placebo-controlled trial. J Pediatr Gastroenterol Nutr. 2010;50:309–315. doi: 10.1097/MPG.0b013e3181a96489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Centeno-Lima S, et al. [Giardia duodenalis and chronic malnutrition in children under five from a rural area of Guinea-Bissau] Acta Med Port. 2013;26:721–724. [PubMed] [Google Scholar]
- 111.Hollm-Delgado MG, et al. Lack of an adverse effect of Giardia intestinalis infection on the health of Peruvian children. Am J Epidemiol. 2008;168:647–655. doi: 10.1093/aje/kwn177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Farthing MJ, et al. Natural history of Giardia infection of infants and children in rural Guatemala and its impact on physical growth. Am J Clin Nutr. 1986;43:395–405. doi: 10.1093/ajcn/43.3.395. [DOI] [PubMed] [Google Scholar]
- 113.Prado MS, et al. Risk factors for infection with Giardia duodenalis in preschool children in the city of Salvador, Brazil. Epidemiol Infect. 2003;131:899–906. doi: 10.1017/s0950268803001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Boeke CE, et al. Intestinal protozoan infections in relation to nutritional status and gastrointestinal morbidity in Colombian school children. J Trop Pediatr. 2010;56:299–306. doi: 10.1093/tropej/fmp136. [DOI] [PubMed] [Google Scholar]
- 115.Donowitz JR, et al. A Prospective Longitudinal Cohort to Investigate the Effects of Early Life Giardiasis on Growth and All Cause Diarrhea. Clin Infect Dis. 2016;63:792–797. doi: 10.1093/cid/ciw391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Backhed F, et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe. 2015;17:690–703. doi: 10.1016/j.chom.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 117.Rogawski ET, et al. Early Antibiotic Exposure in Low-Resource Settings is Associated with Increased Weight in The First Two Years of Life. J Pediatr Gastroenterol Nutr. 2017 doi: 10.1097/MPG.0000000000001640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ivanov II, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Atarashi K, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Smith PM, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Furusawa Y, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 122.Ivanov I, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]