Abstract
Alpha2u-globulin is an adult male rat-specific protein that accumulates spontaneously or inductively in the renal proximal tubular epithelium and forms microscopically observable deposits, which are generally referred to as “hyaline droplets,” whereas a specific type of deposits is referred to as “eosinophilic bodies” by Japanese toxicologic pathologists. We compared hyaline droplets and eosinophilic bodies using special stains including immunostaining for α2u-globulin and lysosome-associated membrane protein in spontaneously occurring and d-limonene-induced cases. Eosinophilic bodies appeared simultaneously and increased in parallel with the hyaline droplets in the induced case. In both of the spontaneous and induced cases, hyaline droplets and eosinophilic bodies were associated with α2u-globulin and lysosomes, although there were differences in the forms and staining properties that probably reflected the purity or density of α2u-globulin. According to the results, it is not necessary for eosinophilic bodies to be strictly distinguished from hyaline droplets, and it is reasonable to identify eosinophilic bodies as hyaline droplets in α2u-globulin nephropathy in routine toxicity studies, as they have been recognized to be a sequence of changes associated with accumulation of α2u-globulin.
Keywords: alpha2u-globulin, male rat, kidney, eosinophilic body, hyaline droplet
Introduction
Alpha2u-globulin is an adult male rat-specific low-molecular-weight (18 to 20 kDa) protein. The blood α2u-globulin produced in the liver is filtered freely through the glomerulus, and about 60% of the filtered protein is reabsorbed by tubules1. Certain chemicals bind noncovalently to α2u-globulin, and the resultant complex is even more poorly digested than α2u-globulin, causing accumulation of the complex in the proximal tubular epithelium of the kidney2. When the level of the accumulation intensifies, it becomes detectable as eosinophilic and spherical to polyangular deposits, generally referred to as “hyaline droplets.”
In Japanese toxicologic pathology, the term “eosinophilic body” has also been used for a certain types of deposits as different entities from hyaline droplets since proposal of the term3 following its first description4, although eosinophilic bodies are associated with α2u-globulin accumulation, which is similar to hyaline droplets. Eosinophilic bodies are described as pale eosinophilic, homogenous/amorphous, and larger than hyaline droplets (usually larger than nuclei) in Japanese textbooks5,6,7. Moreover, they are round to irregular in shape (but not angular), present singly in a cell in general and frequently with a halo, and are believed to be large lysosomes. To the best of our knowledge, however, there are no reports describing the detailed morphology of eosinophilic bodies yet, resulting in a little misunderstanding about this type of deposit8, and the term is not referred to in the guide published by INHAND for the urinary system9. Meanwhile, eosinophilic bodies have been referred to in toxicity study reports and reported to increase simultaneously with hyaline droplets in male rats after administration of several industrial chemicals10,11,12. Moreover, eosinophilic body is described as a synonym of hyaline droplet as well as eosinophilic droplet in the latest Japanese toxicologic pathology textbook13.
The aim of this study was to demonstrate the detailed comparative morphology of hyaline droplets and eosinophilic bodies using the d-limonene-induced and spontaneously occurring cases and to elucidate the nature of eosinophilic bodies.
Materials and Methods
Experimental animals and treatment
A total of 18 male Crl(CD):SD rats (Charles River Laboratories Japan Inc., Kanagawa, Japan) were obtained at 10 weeks of age and used at 11 weeks. d-Limonene (Nacalai Tesque Inc., Kyoto, Japan), a well-known α2u-globulin nephropathy inducer, was administered to groups of rats consisting of 3 males each at 300 mg/kg/day by gavage using corn oil (Nacalai Tesque Inc.) as a vehicle for 1, 5, 10, or 20 days. Control animals consisting of 3 males each were dosed with the vehicle for 1 or 20 days (Supplementary Table 1: online only). The rats were housed individually in stainless steel wire cages in an animal room maintained at 24 ± 2°C and 55 ± 10% humidity with a 12-hr light/dark cycle (lighting from 7:00 to 19:00) and were allowed free access to food (CRF-1, Oriental Yeast Co., Ltd., Tokyo, Japan) and water. This study was conducted in accordance with the principles outlined in the Guide for the Care and Use of Laboratory Animals of the Japanese Association for Laboratory Animal Science and those of our institution.
Tissue sampling
The rats were anesthetized the day after the last administration with 30 mg/kg of sodium pentobarbital by intraperitoneal injection and perfused with lactated Ringer’s solution (Lactec, Otsuka Pharmaceutical Factory Inc., Tokushima, Japan) added with sodium nitrite and heparin sodium through the sinus aortae to eliminate immunoreactions to α2u-globulin in the blood vessels on immunohistochemistry. The right kidney was removed following ligation of the renal artery and vein with a suture and immersed in 10% neutral buffered formalin solution as the non-perfused immersion sample. Thereafter, animals were perfused with Karnovsky-type fixative (2% paraformaldehyde, 1.25% glutaraldehyde, and 2.5% sucrose in 0.2 M phosphate buffer, pH 7.4), and the left kidney was removed to be used as the perfusion sample. Then the left kidney samples for histopathology were postfixed by immersion in 10% neutral buffered formalin solution. After that, both the right and left kidney samples were trimmed and embedded in paraffin.
For comparative samples with spontaneously occurring renal lesions including eosinophilic bodies, the right kidneys from three untreated animals, which were necropsied at the age of 13 weeks, from one of our in-house pharmacological studies were used; the kidneys were fixed and preserved in 10% neutral buffered formalin solution.
Hematoxylin-eosin (HE) stain, special stain, and immunohistochemistry
Serial paraffin sections were prepared and deparaffinized before staining with each stain. Sections of the left kidney to be used as perfused samples were stained with HE. Sections of the right kidney to be used as formalin-fixed (non-perfused) samples including the comparative samples were subjected to HE staining, Azan-Mallory (AM) staining, the periodic acid-Schiff (PAS) reaction, and immunostaining for α2u-globulin. Furthermore, serial sections from selected animals treated with d-limonene for 20 days and the comparative samples were stained with chromotrope-aniline blue (CAB, de Rijk, et al.14) and immunostained with anti-lysosome-associated membrane protein (LAMP) antibody as in Supplementary Table 1. As for immunostaining, the sections were deparaffinized, hydrated, and quenched for endogenous peroxidase with hydrogen peroxide, and nonspecific reactions were blocked with a normal serum. Epitope retrieval was performed using pronase treatment for 20 minutes (for all animals) or for 5 or 10 minutes (for selected animals) at 37 to 40°C for anti-α2u-globulin antibody (our in-house antibody15) and was performed in a microwave for 5 minutes for anti-LAMP (LAMP1, Abcam plc, Cambridge, UK). Anti-α2u-globulin antibody was incubated overnight at 4°C at a 1:80,000 dilution, and anti-LAMP antibody was incubated for 60 minutes at room temperature at a 1:400 dilution. Simple Stain MAX-PO (MULTI) (Nichirei Biosciences, Inc., Tokyo, Japan) was used as the secondary antibody. Specific stains for proteins were visualized using 3,3′-diaminobenzidine (DAB) as a chromogen, and sections were lightly counterstained with methyl green.
All HE-stained sections from both immersion and perfusion samples were examined histopathologically with special regard to hyaline droplets and eosinophilic bodies. All the findings were evaluated with five grades (0, none; 1, minimal; 2, mild; 3, moderate; 4, marked) based on their severity and distribution. In addition, the staining properties of these deposits were confirmed using the serial sections stained with CAB and immunostained with anti-α2u-globulin and anti-LAMP antibodies for the representative animals.
Results
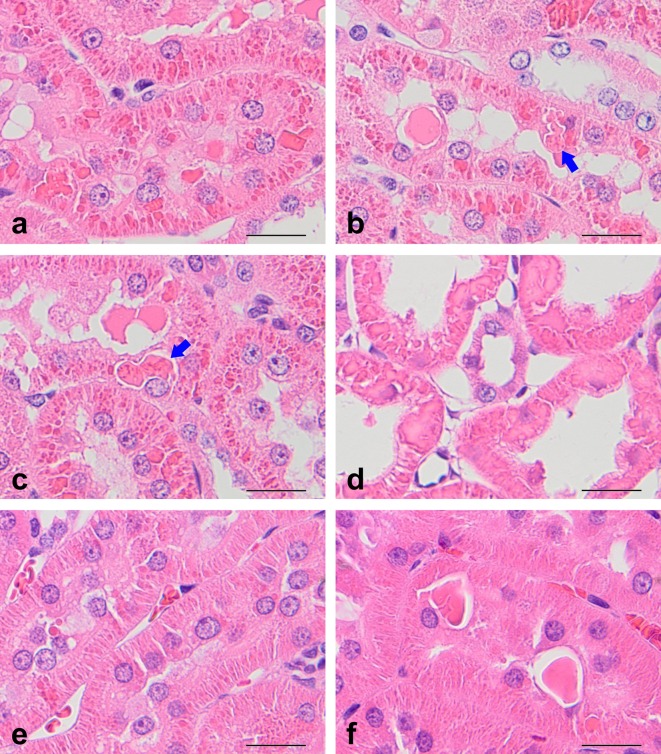
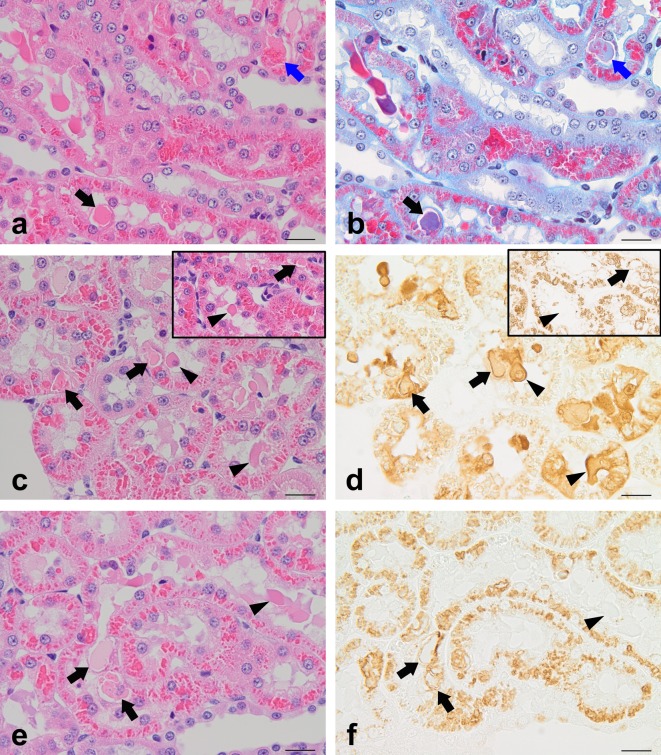
Histological findings for HE-stained sections are shown in Table 1, and the staining properties of hyaline droplets and eosinophilic bodies subjected to staining with special stains and immunostaining are summarized in Table 2.
Table 1. Histological Findings in the Kidney of Rats Treated with d-Limonene.
Table 2. Staining Properties of Hyaline Droplets and Eosinophilic Bodies.
Histological appearances of d-limonene-induced cases
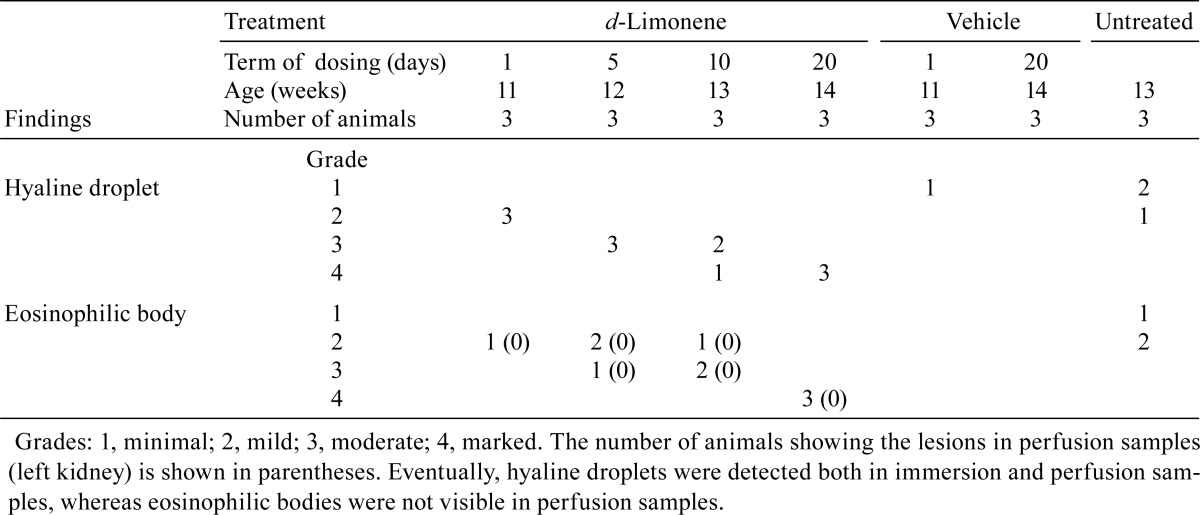
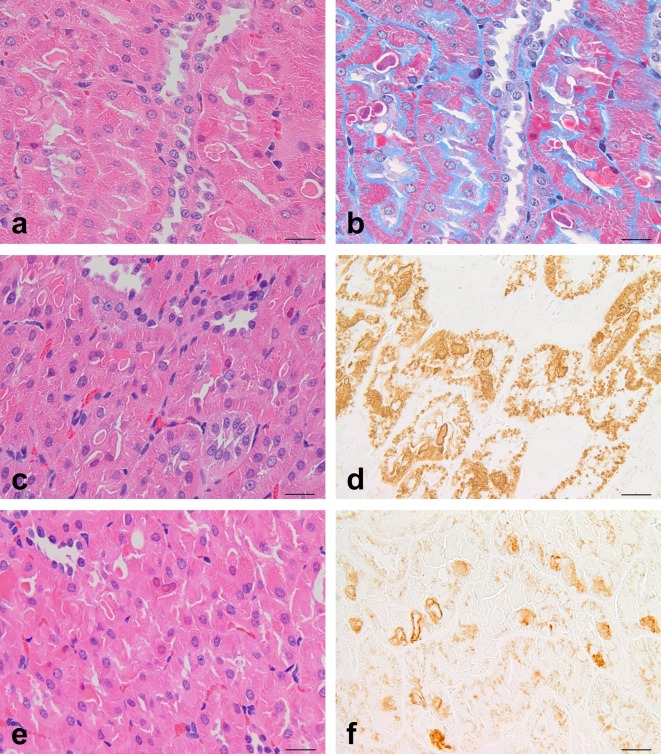
In the HE-stained sections from the immersion samples, hyaline droplets were observed in the proximal tubules of all animals receiving 1 to 20 doses of d-limonene (Table 1), and the amount, size, and angularity of the hyaline droplets increased in proportion to the number of doses (Fig. 1). Hyaline droplets were brilliant red, small to large, and round to angular/crystalloid in shape (Fig. 2a). In the animals receiving 10 to 20 doses, hyaline droplets larger than the nuclei of epithelial cells were frequently present, and epithelial cells filled with hyaline droplets were occasionally visible. Eosinophilic bodies were also observed in the proximal tubules of most of the animals that received 1 to 20 doses of d-limonene (Table 1). They were generally larger and lighter than hyaline droplets and roughly divided into two types. The first type was pale to pink, apparent in the cytoplasm, and frequently encircled with a halo resembling spontaneously occurring eosinophilic bodies (Fig. 2b). Another type was pink to dark red without a halo, and this type existed freely in the tubular lumens. However, the latter type was identified by its staining properties to be the cytoplasm of one kind of characteristic cells. These cells have homogenous pink to dark red cytoplasm with a pyknotic and/or red-tinged nucleus and are referred to as “dense eosinophilic cells” in this study. Dense eosinophilic cells were located in the proximal tubular epithelium or present freely in the tubular lumen, and they tended to appear near the cells containing eosinophilic bodies. The cytoplasm of dense eosinophilic cells occasionally contained eosinophilic bodies but no or few hyaline droplets. Eosinophilic bodies and dense eosinophilic cells increased proportionally with the increase in hyaline droplets (Fig. 1). Moreover, hyaline droplets were occasionally detectable within eosinophilic bodies (Fig. 2b and c). A few granular casts were detected in the corticomedullary junction in animals receiving 10 or 20 doses of d-limonene, and mild regeneration of the proximal tubular epithelium was observed in animals receiving 20 doses of d-limonene.
Fig. 1.
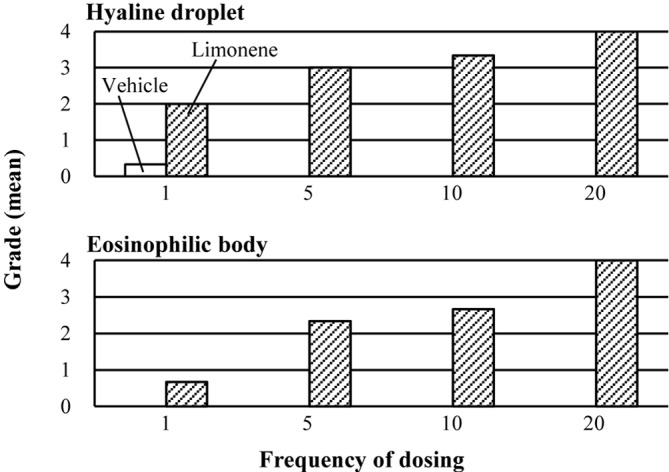
Results of histopathology in the kidney from male rats treated with d-limonene. Each bar shows mean grade of three animals. (Grades: 0, none; 1, minimal; 2, mild; 3, moderate; 4, marked)
Fig. 2.
Histopathological features in the kidneys treated with d-limonene at 300 mg/kg/day for 20 days (a–d) or untreated (e, f). The photographs show (a) hyaline droplets, (b, c) eosinophilic bodies in immersion samples, (d) hyaline droplets in perfusion samples, (e) hyaline droplets, and (f) eosinophilic bodies. Eosinophilic bodies (b, c, and f) are clearly discriminated from hyaline droplets (a, e) in both the induced and spontaneous cases. Eosinophilic bodies occasionally contain hyaline droplets (blue arrows in b and c). There were no eosinophilic bodies in perfusion samples (d). HE stain. Bar = 20 μm.
In the HE-stained sections from the perfusion samples that were well fixed, hyaline droplets were observed that were similar to those from the immersion samples mentioned above except that they were more closely packed in the cytoplasm (Fig. 2d). However, there were no figures corresponding to eosinophilic bodies and dense eosinophilic cells. Granular casts and regenerations were detected even in the perfusion samples as well as in the immersion samples.
Histological appearances of spontaneously occurring cases
Minimal or mild hyaline droplets were detected in the proximal tubules of all three animals (Table 1). They were small and stained brilliant red but showed lesser angularity than those in the induced cases (Fig. 2e). Minimal or mild eosinophilic bodies were also detected in all three animals. They were homogenous/amorphous and round to irregular-shaped deposits, and they were larger than hyaline droplets (Fig. 2f). They were certainly eosinophilic but stained somewhat deeper than those in the induced cases. Eosinophilic bodies were present singly in a cell in general and frequently with a halo. Dense eosinophilic cells were detected that were similar to those in the induced cases.
Staining properties of d-limonene-induced cases with special stains and immunostainings
In all of the d-limonene-dosed animals, hyaline droplets were negative for the PAS reaction and stained orange with AM stain (Table 2). Hyaline droplets in the sections pretreated with pronase for 20 minutes showed clearly positive immunostaining for α2u-globulin (Fig. 3d inset). Eosinophilic bodies were also negative for the PAS reaction in all cases but showed a variety of colors with AM stain. In the sections pretreated with pronase for 20 minutes, eosinophilic bodies failed to show immunoreactivity for α2u-globulin because the bodies were digested by pronase (Fig. 3d inset).
Fig. 3.
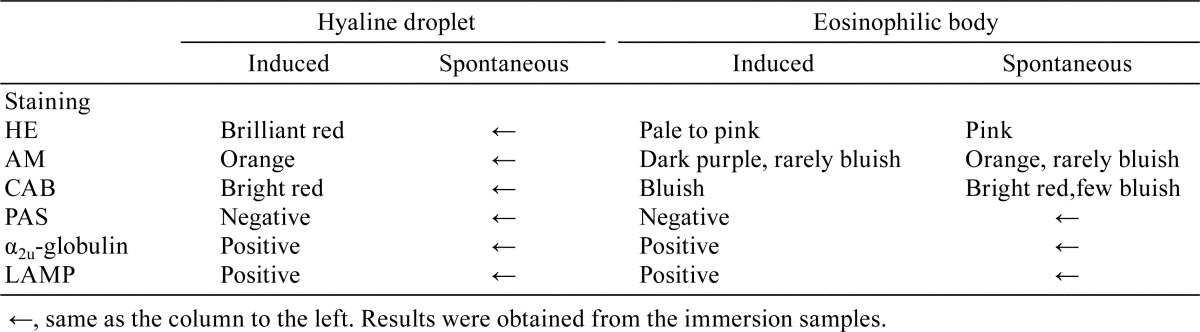
d-Limonene-induced hyaline droplets and eosinophilic bodies. (a, c, and e) HE stain, (b) CAB stain, (d) α2u-globulin immunostaining (pronase treatment: 5 min and 20 min (inset)), and (f) LAMP immunostaining. Hyaline droplets show bright red staining with CAB and are positive for α2u-globulin and LAMP. Hyaline droplets in eosinophilic bodies also show bright red staining with CAB (blue arrows). Eosinophilic bodies show bluish staining with CAB and are positive for α2u-globulin and LAMP (black arrows). The cytoplasm of dense eosinophilic cells is frequently positive for α2u-globulin but negative for LAMP (arrowheads). The insets in photographs c and d show that a prolonged pronase treatment causes eosinophilic bodies and dense eosinophilic cells to disappear (arrows and arrowheads) due to complete digestion and makes the reaction of hyaline droplets stand out. Bar = 20 μm.
In the sections of representative animals, hyaline droplets were stained bright red with CAB stain (Fig. 3b), and their rims reacted clearly positive for LAMP immunostaining (Fig. 3f). Eosinophilic bodies were stained bluish with CAB stain (Fig. 3b). In the sections pretreated with pronase for 5 or 10 minutes, eosinophilic bodies showed positive immunostaining for α2u-globulin because pronase did not digest the eosinophilic bodies entirely (Fig. 3d). These results of α2u-globulin immunostaining demonstrate that a prolonged pronase treatment causes eosinophilic bodies to disappear due to a complete digestion and makes the reaction of only hyaline droplets stand out. The cytoplasm of dense eosinophilic cells showed a variety of reactions with α2u-globulin immunostaining (Fig. 3d). Most eosinophilic bodies showed positive reactions in their rims with LAMP immunostaining, while the cytoplasm of dense eosinophilic cells showed a negative reaction (Fig. 3f).
Staining properties of spontaneously occurring cases with special stains and immunostainings
The staining properties of spontaneously occurring hyaline droplets were similar to those of induced ones (Table 2). Eosinophilic bodies in spontaneous cases were negative for the PAS reaction, which was similar to the eosinophilic bodies in the induced cases. However, spontaneous eosinophilic bodies were stained orange with AM stain, and most of them were stained bright red with CAB stain (Fig. 4b). These results showed that the staining properties of spontaneous eosinophilic bodies with AM and CAB stain were rather close to those of induced hyaline droplets compared with induced eosinophilic bodies. Immunostaining for α2u-globulin revealed a moderate to intense positive reaction for eosinophilic bodies in proportion to treatment time (5 to 20 minutes) with pronase (Fig. 4d). With immunostaining for LAMP, most of the spontaneous eosinophilic bodies showed positive reactions similar to the induced cases (Fig. 4f). Dense eosinophilic cells in the spontaneous cases showed similar staining properties to the induced cases.
Fig. 4.
Spontaneously occurring hyaline droplets and eosinophilic bodies. (a, c, and e) HE stain, (b) CAB stain, (d) α2u-globulin immunostaining (pronase treatment: 20 min), and (f) LAMP immunostaining. Hyaline droplets show bright red staining with CAB and are positive for α2u-globulin and LAMP. Eosinophilic bodies show bright red to bluish staining with CAB and are positive for α2u-globulin and LAMP. Bar = 20 μm.
Discussion
Male rat-specific hyaline droplets are associated with α2u-globulin and easily increase by administration of certain chemicals2. Eosinophilic bodies are also acknowledged to be close but not identical to hyaline droplets despite both probably being associated with α2u-globulin, and they are therefore distinguished from hyaline droplets3,4,5,6,7. We frequently encounter simultaneous occurrence and increase in these two types of deposits in actual toxicity studies10,11,12. However, eosinophilic bodies or the like have rarely been reported outside of Japan16, though it is unlikely that they occur only in studies conducted in Japan. The most probable reason for this is that this type of deposit is recognized as a type of hyaline droplet, neglected as a physiologically normal structure due to its occasional occurrence in normal healthy male rats, or recognized as a certain artifact due to its similarity to intratubular inclusions/vesicles/debris, which are recognized as agonal/artificial changes occurring within seconds after an interruption in blood flow17, 18.
Both the induced and spontaneously occurring hyaline droplets showed similar steady staining properties that included a PAS-negative reaction, staining orange with AM, and staining bright red with CAB, although the hyaline droplets of the induced cases tended to become large and highly polygonal, i.e., crystalloid. Immunostaining for α2u-globulin and LAMP showed a positive reaction for hyaline droplets, clearly demonstrating that hyaline droplets are lysosomes that contained α2u-globulin.
Eosinophilic bodies were PAS negative both in induced and spontaneous cases, which was similar to hyaline droplets. Spontaneously occurring eosinophilic bodies showed similar staining properties to hyaline droplets with AM and CAB stains and with immunostaining for α2u-globulin and LAMP. These results demonstrated that spontaneously occurring eosinophilic bodies are certainly associated with α2u-globulin accumulation in lysosomes having a similar nature to hyaline droplets.
Induced eosinophilic bodies were positive for both the anti-α2u-globulin and anti-LAMP antibodies, which was similar to hyaline droplets and spontaneously occurring eosinophilic bodies. These results demonstrate that induced eosinophilic bodies are also associated with α2u-globulin in lysosomes, though there is a probable difference in the condition, purity, or density of α2u-globulin between these two types of deposits considering their staining properties. In addition, the induced cases occasionally contained a few hyaline droplets, which were stained similarly to the abovementioned hyaline droplets with all the special stains and immunostaining for α2u-globulin but not anti-LAMP antibody. This interesting feature has not yet been reported to date and suggests a close relationship between hyaline droplets and eosinophilic bodies.
We were not able to detect eosinophilic bodies in the kidneys fixed by perfusion irrespective of the occurrence of eosinophilic bodies in the contralateral kidneys fixed by immersion in formalin in this study. This discrepancy suggests that the fixation procedure, fixative, or postmortem changes may influence the occurrence of eosinophilic bodies. Similarly, dense eosinophilic cells were observed in the immersion samples but not in the perfusion samples, suggesting that these cells were also associated with the abovementioned causes rather than single cell necrosis as reported previously19.
This report aimed to demonstrate the detailed morphology of eosinophilic bodies and the like as compared with hyaline droplets. Our study revealed simultaneous occurrence and close correlation of hyaline droplets and eosinophilic bodies induced by d-limonene administration and an association of both to α2u-globulin irrespective of some differences. Although the occurrence mechanism and pathological meaning of eosinophilic bodies have not been explained that well at present, it is not considered necessary to strictly distinguish eosinophilic bodies from hyaline droplets, and it is reasonable to identify eosinophilic bodies as hyaline droplets in α2u-globulin nephropathy9 in routine toxicity studies, as they are regarded as a sequence of changes associated with α2u-globulin.
Supplementary Table 1.
Acknowledgments
We would like to thank Ms. Tamami Murata, Hiromi Yamaguchi, and Miwako Ishii for excellent technical assistance and Mr. Stephen Kean Filiatrault and Ms. Kanae Tamatsukuri for language editing of the manuscript.
Footnotes
Disclosure of Potential Conflicts of Interest: The authors declare that they have no conflicts of interest.
References
- 1.Neuhaus OW, Flory W, Biswas N, and Hollerman CE. Urinary excretion of α2u-globulin and albumin by adult male rats following treatment with nephrotoxic agents. Nephron. 28: 133–140. 1981. [DOI] [PubMed] [Google Scholar]
- 2.U.S. EPA Alpha2u-globulin: Association with chemically induced renal toxicity and neoplasia in the male rat. U.S. Environmental Protection Agency, Washington, D.C. 1991. [Google Scholar]
- 3.Kawai K. [Report from Working Group on Long-Term Holding of Experimental Animals]. Exp Anim. 29: 181–231. 1980; (in Japanese). [Google Scholar]
- 4.Niki R. Jikken Dobutsu Kenkyukai (Laboratory Animal Research Committee). Meeting in Shizuoka in Japan. 1974. [Google Scholar]
- 5.Enomoto M, and Akazaki K. [Eosinophilic body appearing in the epithelium of the proximal convoluted tubules]. In: Color Atlas of Toxicological Pathology: Fundamental of Morphological Observation. M Enomoto, and K Akazaki (eds). Soft Science Publications, Tokyo. 44–45. 1987. (in Japanese). [Google Scholar]
- 6.Watanabe M. [Non-neoplastic Lesions, Chapter 5 Urinary System]. In: Dokusei Siken Koza No. 5 Toxicologic Pathology. A Maekawa, and Y Hayashi (eds). Chijin-Syokan, Tokyo. 267–300. 1991. (in Japanese). [Google Scholar]
- 7.Watanabe M, and Nishikawa A. [Kidney]. In: Toxicologic Histopathology. The Japanese Society of Toxicologic Pathology, Nagoya. 247–266. 2000. (in Japanese). [Google Scholar]
- 8.Hard GC. Some aids to histological recognition of hyaline droplet nephropathy in ninety-day toxicity studies. Toxicol Pathol. 36: 1014–1017. 2008. [DOI] [PubMed] [Google Scholar]
- 9.Frazier KS, Seely JC, Hard GC, Betton G, Burnett R, Nakatsuji S, Nishikawa A, Durchfeld-Meyer B, and Bube A. Proliferative and nonproliferative lesions of the rat and mouse urinary system. Toxicol Pathol. 40(Suppl): 14S–86S. 2012. [DOI] [PubMed] [Google Scholar]
- 10.Hoshino N, Aoyama R, Yamagishi Y, Toyota N, Takano K, and Suzuki Y. [Combined repeat dose and reproductive/developmental toxicity screening test of 1-chloro-2-(chloromethyl)benzene by oral administration in rats]. In: Toxicity Testing Reports of Environmental Chemicals (Supervised by Office of Environmental Chemicals Safety, Environmental Health Bureau, Ministry of Health & Welfare, Japan), Chemicals Investigation Promoting Council, Tokyo. Vol. 7. 492–502. 1999. (in Japanese). [Google Scholar]
- 11.Yoshimura H, Shigeno H, Nagaya J, Furukawa M, Kawamura K, Takeda M, and Hikichi N. [Twenty-eight-day repeat dose oral toxicity test of 5-ethylidene-2-norbornene in rats]. In: Toxicity Testing Reports of Environmental Chemicals (Supervised by Office of Environmental Chemicals Safety, Environmental Health Bureau, Ministry of Health & Welfare, Japan), Chemicals Investigation Promoting Council, Tokyo. Vol. 8. 1089–1100. 2001. (in Japanese). [Google Scholar]
- 12.Sunaga M, Kiguchi M, Hirata T, Kasahara M, Makino H, Hirata M, and Furukawa M. [Combined repeat dose and reproductive/developmental toxicity screening test of 4-methyl-1-pentene by oral administration in rats]. In: Toxicity Testing Reports of Environmental Chemicals (Supervised by Office of Environmental Chemicals Safety, Environmental Health Bureau, Ministry of Health & Welfare, Japan), Chemicals Investigation Promoting Council, Tokyo. Vol. 13. 119–137. 2006. (in Japanese). [Google Scholar]
- 13.Nishikawa A, Yamate J, Hayashi S, and Miyata K. [Kidney]. In: Toxicologic Pathology. The Japanese Society of Toxicologic Pathology (ed), Nishimura Co., Tokyo. 299–334. 2017. (in Japanese). [Google Scholar]
- 14.de Rijk EPCT, Ravesloot WTM, Wijnands Y, and van Esch E. A fast histochemical staining method to identify hyaline droplets in the rat kidney. Toxicol Pathol. 31: 462–464. 2003. [DOI] [PubMed] [Google Scholar]
- 15.Hamamura M, Hirose A, Kamata E, Katoku K, Kuwasaki E, Oshikata T, Nakahara Y, Ema M, and Hasegawa R. Semi-quantitative immunohistochemical analysis of male rat-specific α2u-globulin accumulation for chemical toxicity evaluation. J Toxicol Sci. 31: 35–47. 2006. [DOI] [PubMed] [Google Scholar]
- 16.Peter CP, Burek JD, and van Zwieten MJ. Spontaneous nephropathies in rats. Toxicol Pathol. 14: 91–100. 1986. [DOI] [PubMed] [Google Scholar]
- 17.Griffith LD, Bulger RE, and Trump BF. The ultrastructure of the functioning kidney. Lab Invest. 16: 220–246. 1967. [PubMed] [Google Scholar]
- 18.Longley JB, and Burstone MS. Intraluminal nuclei and other inclusions as agonal artifacts of the renal proximal tubules. Am J Pathol. 42: 643–655. 1963. [PMC free article] [PubMed] [Google Scholar]
- 19.Hard GC, Rodgers IS, Baetcke KP, Richards WL, McGaughy RE, and Valcovic LR. Hazard evaluation of chemicals that cause accumulation of α2u-globulin, hyaline droplet nephropathy, and tubule neoplasia in the kidneys of male rats. Environ Health Perspect. 99: 313–349. 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.