Abstract
For the purpose of clarifying the histopathological effects of methotrexate (MTX) on medaka testes, wild-type and homogenic p53-deficient male medaka at 4 to 6 months post-hatching were exposed to 0.25 mg/ml of MTX for 96 h with histopathological examination of testes at 24, 48, 72 and 96 h. At 72 and 96 h after the start of MTX exposure, numerous apoptotic cells were observed in the spermatogonia and spermatocytes, and the pyknotic cell rate and the TUNEL-positive and cleaved caspase-3-positive rates in the spermatogonia and spermatocytes of MTX-treated wild type medaka were higher compared with those in the control wild-type medaka. Starting at 48 h, the phospho-histone H3-positive rate in the spermatogonia and spermatocytes of was significantly lower in MTX-treated wild-type medaka than in control wild-type medaka. In homogenic p53-deficient medaka, apoptosis was not induced in the spermatogonia and spermatocytes by exposure to MTX. Starting at 48 h, the phospho-histone H3-positive rate in spermatogonia and spermatocytes of MTX-treated homogenic p53-deficient medaka was lower than in control homogenic p53-deficient medaka. Throughout the entire experimental period, there were no significant differences in phospho-histone H3-positive rates in the spermatogonia and spermatocytes between the MTX-treated homogenic p53-deficient medaka group and the MTX-treated wild-type medaka group. In the present study, spermatogonia and spermatocytes of medaka testes were sensitive to MTX at 0.25 mg/ml in the culture water, and MTX-induced apoptosis in the testes was dependent on p53 expression; however, inhibition of MTX-induced cell proliferation was independent of p53 expression.
Keywords: apoptosis, cell proliferation inhibition, p53, spermatocyte, spermatogonia
Introduction
Methotrexate (MTX), a methyl derivative of aminopterin, is a folate antagonist that inhibits dihydrofolate reductase, resulting in a block in the conversion of dihydrofolate to tetrahydrofolate1, 2. Because folates play a role in the transfer of one-carbon units, they are essential to the synthesis of pyrimidine, purine and thymidine required for DNA and RNA synthesis1. Therefore, MTX inhibits DNA and RNA synthesis and cell proliferation by preventing synthesis of pyrimidine, purine and thymidine1, 3. Additionally, MTX induces apoptosis via the upregulation of p53 and p21 expression by c-Jun N-terminal kinase (JNK) 1- and JNK2-dependent mechanisms4. MTX is used clinically in the treatment of neoplastic diseases, such as leukemias and lymphomas, and autoimmune and inflammatory diseases, including rheumatoid arthritis, systemic lupus and Crohn’s disease5, 6. However, testicular toxicity has been observed after administration of MTX, and it may cause subsequent infertility7. Administration of MTX caused a decreased sperm number, an increase in numbers of abnormally shaped sperm, sperm DNA damage, damage to testicular germ cells and atrophy of the seminiferous tubules8, 9. MTX inhibited cell proliferation and induced apoptosis in the germ cells of the testes in rodents10,11,12, and rodent testes exposed to MTX showed a significant increase in mRNA expression of p5310.
Previous studies demonstrated that MTX was detected in the effluents from a hospital or wastewater treatment plant13,14,15. Therefore there is a possibility that MTX contaminates the aquatic environment and exerts adverse effects on the testes of aquatic vertebrates. Nevertheless, there are no reports examining the histopathologic effects of MTX on the testes of aquatic vertebrates, and the pathogenesis of MTX-induced testicular disorder in aquatic vertebrates remains unclear.
The medaka (Oryzias latipes) serves as a model vertebrate organism in various fields of biology including toxicology, development, genetics and evolution16. Completion of the medaka genome sequencing project has promoted the use of medaka as a comparative and complementary material for research on other vertebrates such as zebrafish, sticklebacks, mice and humans16. Within the Organization for Economic Cooperation and Development (OECD) guidelines, the medaka is recognized as a suitable model fish for chemical toxicity tests. On the other hand, in the testes of the medaka, germ cells are distributed separately into the cell differentiation stages, such as spermatogonia, spermatocytes, spermatids and spermatozoa17. Therefore, it is easy to distinguish the germ cell differentiation stage showing high sensitivity for chemicals by histomorphological examinations.
In the present study, we examined temporal changes in histopathologic characteristics in the testes of adult male medaka following exposure to MTX. Additionally, the involvement of p53 gene expression in the pathogenesis of MTX-induced testicular disorders has not been clarified, so a histopathological analysis was also performed to clarify the significance of p53 gene expression in the pathogenesis of MTX-induced testicular disorders in medaka. The purpose of the present study was to clarify the histopathological characteristics and pathogenesis of testicular disorder induced by MTX in medaka.
Material and Methods
Animals
Adult male wild-type and homogenic p53-deficient medaka (Oryzias latipes; Cab strain) obtained from the National Institute for Basic Biology (Aichi, Japan) were used at 4 to 6 months post-hatching. The p53-deficient medaka were originally produced by TILLING. In this study, these medaka were backcrossed ten times with inbred Cab strain medaka to establish a p53-deficient strain with a Cab genomic background. The fish were maintained at 25 to 26°C with a 14:10 h (light:dark) cycle in a recirculating aquaculture system equipped with carbon filtration and biofiltration. The present experiments were performed according to the guidelines of the Animal Research Committee of Tottori University.
Experimental design
For the exposure experiments, 160 male medaka (body length: 19.11 ± 0.18 mm) were divided into four groups as follows: (1) control wild-type medaka group (n=40), (2) MTX-treated wild-type medaka group (n=40), (3) control homogenic p53-deficient (p53 (−/−)) medaka group (n=40), and (4) MTX-treated homogenic p53-deficient (p53 (−/−)) medaka group (n=40).
MTX (Pfizer Japan Inc., Tokyo, Japan) was dissolved in dechlorinated tap water to a concentration of 0.25 mg/ml, and fish were kept in the MTX-containing water for up to 96 h. The water was exchanged daily with MTX-containing water of the same concentration. Fish from all treatment groups were removed (10 fish per group per time point) and examined histopathologically at 24, 48, 72 and 96 h. A preliminary study using wild-type medaka with exposure for 96 h showed that 0.025 mg/ml and 0.0025 mg/ml induced few histopathologic changes in the testes, whereas exposure to 0.25 mg/ml caused stable pyknotic changes in the testes with no individual differences in the rate of pyknotic cells.
Histopathological examination
Fish were euthanized by prolonged immersion in 100 mg/l tricaine methanesulfonate (Tokyo Chemical Industry, Tokyo, Japan) and fixed in toto in Bouin’s fluid overnight before being fixed again in neutral buffered formalin, embedded in paraffin, cut into sagittal sections, and routinely stained with hematoxylin-eosin. Histopathological examination of whole organs was carried out.
TUNEL method
Cells with DNA fragmentation in testicular tissue were detected by terminal deoxynucleotidyl-transferase (TdT)-mediated deoxyuridine triphosphate-digoxigenin (dUTP) nick-end labeling (TUNEL) assay using a TACS® 2 TdT-DAB In Situ Apoptosis Detection Kit (Trevigen, Inc., Gaithersburg, MD, USA). The TUNEL-positive rate was calculated as the percentage of TUNEL-positive cells among the total number of the spermatogonia and spermatocytes with histomorphometric analysis software (Olympus Corporation, Tokyo, Japan).
Immunohistochemical examination
Immunohistochemical staining was carried out using a labeled polymer method with Histofine Simple Stain MAX-PO (R) (Nichirei, Tokyo, Japan). To retrieve the antigen, tissue sections for the detection of cleaved caspase-3 were immersed in citrate buffer at pH 6.0 (Dako, Glostrup, Denmark) and autoclaved for 15 min at 121°C. Tissue sections for the detection of phospho-histone H3 were immersed in citrate buffer (pH 6.0; Dako) and microwaved for 15 min. Histone H3, a protein involved in chromatin structure, is phosphorylated at serine 10 during chromatin condensation in mitosis18; therefore, phospho-histone H3 is recognized as a mitosis-specific marker19, 20. Endogenous peroxidase activity in the sections was quenched by immersing the sections in 3% hydrogen peroxide in methanol for 15 min. The sections were incubated with a cleaved caspase-3 rabbit polyclonal antibody (1:50 dilution; Cell Signaling Technology, Inc., Danvers, MA, USA) for 30 min at room temperature. The sections were incubated with a phospho-histone H3 rabbit monoclonal antibody (1:1,500 dilution; Abcam, Tokyo, Japan) for 30 min at room temperature. They were then treated with Histofine Simple Stain MAX-PO (R) (Nichirei, Tokyo, Japan) for 30 min at room temperature. After exposure to a 3,3′-diaminobenzidine solution containing hydrogen peroxide (Nichirei, Tokyo, Japan) to facilitate a peroxidase color reaction, the sections were counterstained with Mayer’s hematoxylin. The cleaved caspase-3-positive rate and the phospho-histone H3-positive rate were calculated as the percentages of cleaved caspase-3-positive cells and phospho-histone H3-positive cells among the total number of spermatogonia and spermatocytes with the above-mentioned histomorphometric analysis software.
Statistical analysis
All data were expressed as means ± SE in each group. The results in each group were compared by Tukey’s multiple comparison test with the Excel Toukei statistical software (SSRI Co., Ltd., Tokyo, Japan). Comparisons with P<0.05 or P<0.01 were considered to be statistically significant.
Results
In all four test groups (wild-type medaka groups and homogenic p53-deficient medaka groups with and without MTX exposure), all fish survived, and none showed behavior disorders or abnormal appearance throughout the entire experimental period.
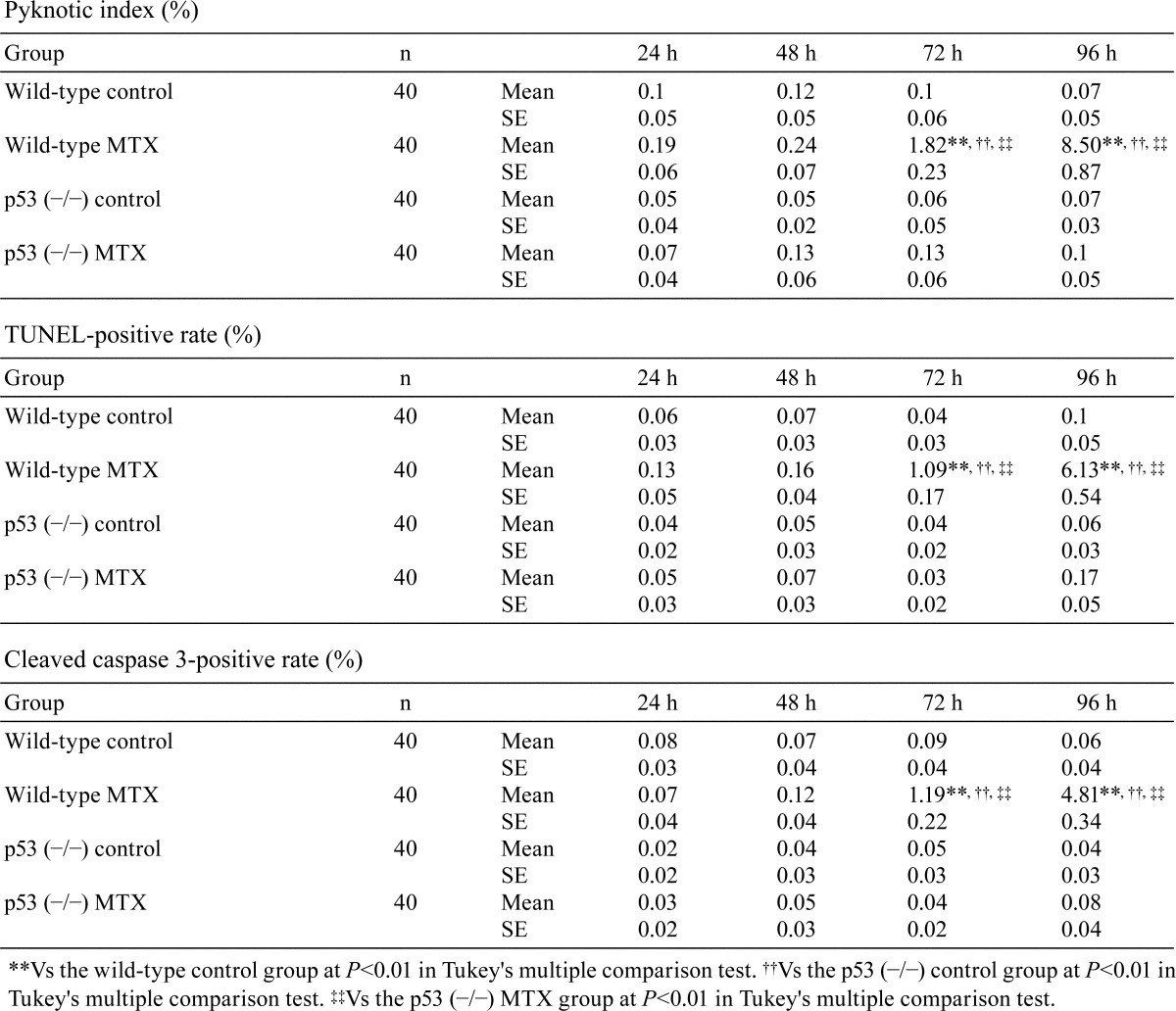
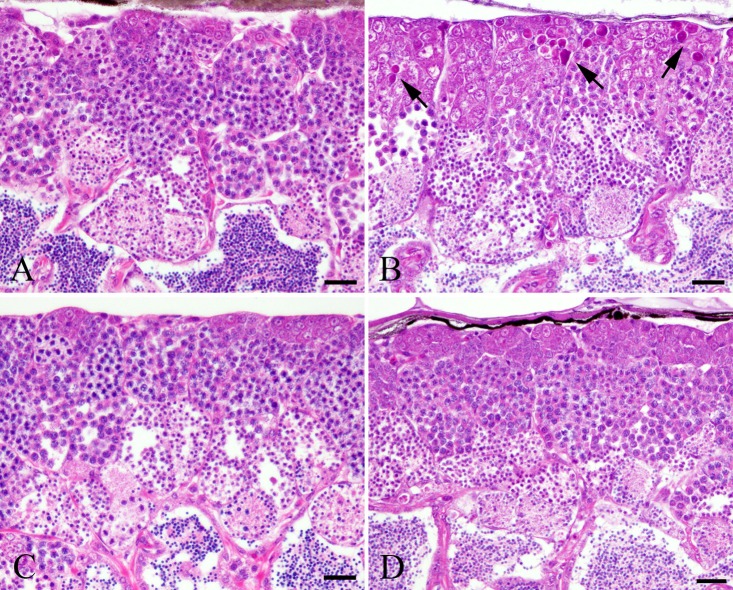
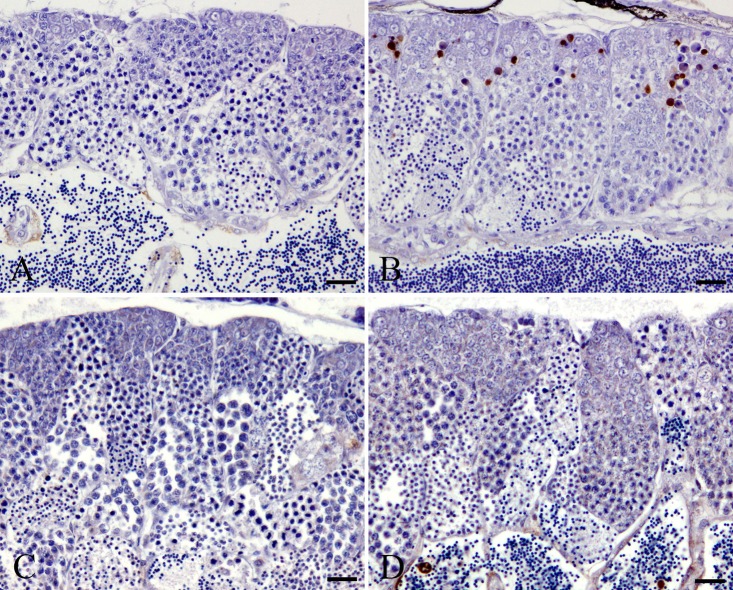
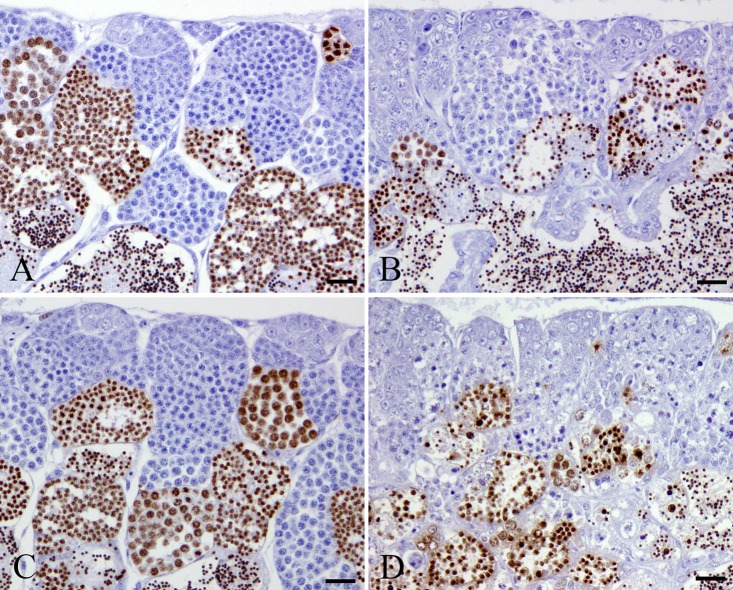
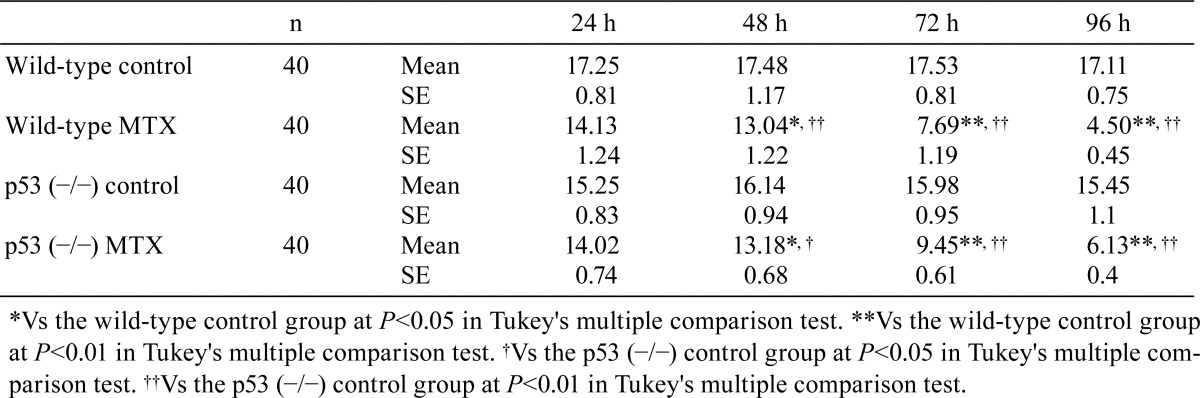
In the MTX-treated wild-type medaka group, there were few pyknotic cells in the spermatogonia and spermatocytes at 24 and 48 h after MTX treatment, which is similar to the results in the control wild-type group (Table 1). At 72 and 96 h after the start of MTX exposure, numerous pyknotic cells were observed in the spermatogonia and spermatocytes of MTX-treated wild-type medaka (Fig. 1). These pyknotic cells in MTX-treated wild-type medaka were positive for TUNEL staining and anti-cleaved caspase 3 antibody (Fig. 2 and 3). At 72 and 96 h after the start of MTX treatment, pyknotic cell rates and TUNEL-positive and cleaved caspase-3 rates in the spermatogonia and spermatocytes in the MTX-treated wild-type medaka group were significantly higher than those in the control wild-type medaka group (Table 1). Throughout the entire experimental period, the homogenic p53-deficient medaka exposed to MTX showed few pyknotic cells, TUNEL-positive cells or cleaved caspase-3-positive cells in the spermatogonia and spermatocytes, which is similar to observations in the control homogenic p53-deficient medaka group and the control wild-type medaka group (Fig. 1, 2 and 3, Table 1). Throughout the entire experimental period, a comparison of pyknotic cells rates, TUNEL-positive rates and cleaved caspase-3-positive rates in the spermatogonia and spermatocytes showed no significant differences between the MTX-treated homogenic p53-deficient medaka group, the control homogenic p53-deficient medaka group and the control wild-type medaka group (Table 1).
Table 1. Time Course Changes in Pyknotic Index, TUNEL-positive Rate, and Cleaved Caspase 3-positive Rate in Spermatogonia and Spermatocytes.
Fig. 1.
Pyknotic changes in spermatogonia and spermatocytes after 96 h MTX treatment. A. Control wild-type medaka group. B. MTX-treated wild-type medaka group. C. Control homogenic p53-deficient (p53 (−/−)) medaka group. D. MTX-treated homogenic p53-deficient (p53 (−/−)) medaka group. Arrows indicate pyknosis. Bar = 30 μm.
Fig. 2.
TUNEL-positive cells in spermatogonia and spermatocytes after 96 h MTX treatment. A. Control wild-type medaka group. B. MTX-treated wild-type medaka group. C. Control homogenic p53-deficient (p53 (−/−)) medaka group. D. MTX-treated homogenic p53-deficient (p53 (−/−)) medaka group. Bar = 30 μm.
Fig. 3.
Cleaved caspase-3-positive cells in spermatogonia and spermatocytes after 96 h MTX treatment. A. Control wild-type medaka group. B. MTX-treated wild-type medaka group. C. Control homogenic p53-deficient (p53 (−/−)) medaka group. D. MTX-treated homogenic p53-deficient (p53 (−/−)) medaka group. Bar = 30 μm.
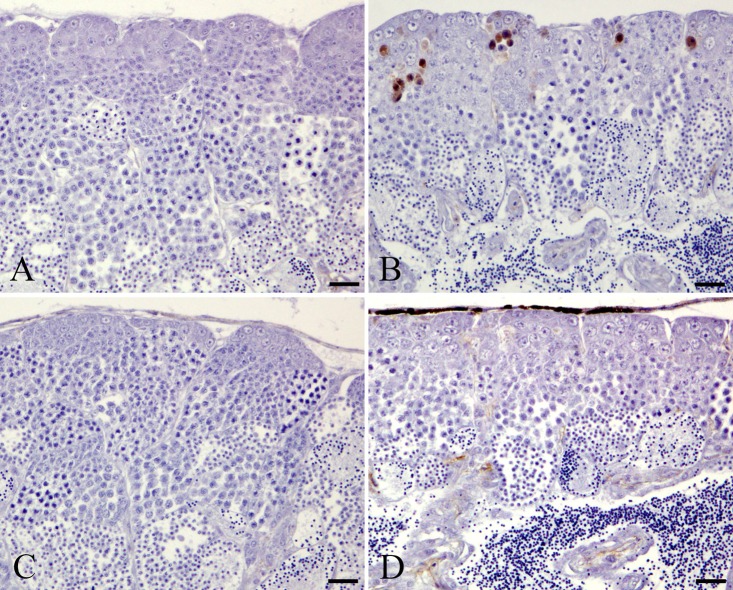
Fewer phospho-histone H3-positive cells were observed in the spermatogonia and spermatocytes in the MTX-treated wild-type and MTX-treated homogenic p53-deficient medaka groups than in the control wild-type and control homogenic p53-deficient medaka groups (Fig. 4). Starting at 48 h after MTX exposure, the phospho-histone H3-positive rates in the spermatogonia and spermatocytes in the MTX-treated wild-type and homogenic p53-deficient medaka groups were significantly lower than those in the control wild-type and homogenic p53-deficient medaka groups (Table 2). Throughout the entire experimental period, the phospho-histone H3-positive rates in the spermatogonia and spermatocytes showed no significant differences between the MTX-treated homogenic p53-deficient medaka and the MTX-treated wild-type medaka groups.
Fig. 4.
Phospho-histone H3-positive cells in spermatogonia and spermatocytes after 96 h MTX treatment. A. Control wild-type medaka group. B. MTX-treated wild-type medaka group. C. Control homogenic p53-deficient (p53 (−/−)) medaka group. D. MTX-treated homogenic p53-deficient (p53 (−/−)) medaka group. Bar = 30 μm.
Table 2. Time Course Changes in Phospho-histone H3-positive Rate (%) in Spermatogonia and Spermatocytes.
Discussion
The results of the present study revealed that spermatogonia and spermatocytes in wild-type medaka were sensitive to 96 h exposure to 0.25 mg/ml MTX, and MTX exposure induced pyknotic changes in the spermatogonia and spermatocytes of wild-type medaka testes. The pyknotic cells in spermatogonia and spermatocytes were positive for TUNEL staining and cleaved caspase-3. Cleavage of caspase-3 is associated with apoptosis and thus serves as an apoptosis marker21. These results demonstrate that the pyknotic changes induced by 0.25 mg/ml MTX in the spermatogonia and spermatocytes of the wild-type medaka testes occurred through the mechanism of apoptosis. A single intraperitoneal injection of MTX at 20 mg/kg induced apoptosis of germinal epithelial cells in rat testes10, 11. Spermatogonia and spermatocytes in rodents were sensitive to MTX in vivo and in vitro22,23,24. These findings suggest that the testicular disorders induced by MTX in medaka are similar to those in rodents. A previous study suggested that testicular germinal epithelial cells in rodents are especially susceptible to cytotoxic drugs because testicular germinal epithelial cells have high mitotic activity23. Spermatogonia and spermatocytes in medaka testes also show high cell proliferative activity25, and this finding may be related to the induction of apoptosis in spermatogonia and spermatocytes in medaka testes exposed to MTX in the present study.
The p53 protein induces apoptosis in response to DNA damage via intrinsic (mitochondrial) and extrinsic (death receptor-mediated) pathways26. MTX-induced apoptosis in mouse testes was involved in upregulation of the p53 gene10. In the present study, MTX exposure induced apoptosis in spermatogonia and spermatocytes in the testes of the wild-type medaka but not in those of the homogenic p53-deficient medaka. Consistent with the results of the previous study in mice10, the results of the present study revealed that MTX-induced apoptosis in wild-type medaka testes was dependent on p53 gene expression.
MTX inhibited cell proliferation by preventing synthesis of thymidylate and purine nucleotides required for DNA and RNA synthesis, and this action mechanism is independent of p53 expression1, 3, 27. Additionally, MTX also induced inhibition of cell proliferation via cell cycle arrest arising from upregulation of p21 expression, and this action mechanism is dependent of p53 expression4, 28. In the present study, inhibition of cell proliferation was observed in the testes of MTX-treated homogenic p53-deficient medaka, and it was similar to that in MTX-treated wild-type medaka. This result demonstrates that under the exposure conditions used in the present study (exposure concentration, 0.25 mg/ml; exposure time, 96 h), MTX mainly inhibits cell proliferation via a p53-independent mechanism, namely, preventing synthesis of thymidylate and purine nucleotides in medaka testes.
In conclusion, 96 h exposure to 0.25 mg/ml MTX induced apoptosis of spermatogonia and spermatocytes and inhibited their cell proliferation. MTX-induced apoptosis in spermatogonia and spermatocytes was dependent on p53 expression. These findings were similar to those for rodent testes exposed to MTX. To the best of our knowledge, this is the first report demonstrating histopathological findings of testicular disorders induced by MTX exposure in aquatic vertebrates.
Footnotes
Disclosure of Potential Conflicts of Interest: We have no conflicts of interest to declare.
References
- 1.Lloyd ME, Carr M, McElhatton P, Hall GM, and Hughes RA. The effects of methotrexate on pregnancy, fertility and lactation. QJM. 92: 551–563. 1999. [DOI] [PubMed] [Google Scholar]
- 2.Rajamani R, Muthuvel A, Senthilvelan M, and Sheeladevi R. Oxidative stress induced by methotrexate alone and in the presence of methanol in discrete regions of the rodent brain, retina and optic nerve. Toxicol Lett. 165: 265–273. 2006. [DOI] [PubMed] [Google Scholar]
- 3.Wessels JA, Huizinga TW, and Guchelaar HJ. Recent insights in the pharmacological actions of methotrexate in the treatment of rheumatoid arthritis. Rheumatology (Oxford). 47: 249–255. 2008. [DOI] [PubMed] [Google Scholar]
- 4.Spurlock CF, 3rd, Tossberg JT, Fuchs HA, Olsen NJ, and Aune TM. Methotrexate increases expression of cell cycle checkpoint genes via JNK activation. Arthritis Rheum. 64: 1780–1789. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genestier L, Paillot R, Quemeneur L, Izeradjene K, and Revillard JP. Mechanisms of action of methotrexate. Immunopharmacology. 47: 247–257. 2000. [DOI] [PubMed] [Google Scholar]
- 6.Kozma C, and Ramasethu J. Methotrexate and misoprostol teratogenicity: further expansion of the clinical manifestations. Am J Med Genet A. 155: 1723–1728. 2011. [DOI] [PubMed] [Google Scholar]
- 7.Daggulli M, Dede O, Utangac MM, Bodakci MN, Hatipoglu NK, Penbegul N, Sancaktutar AA, Bozkurt Y, Türkçü G, and Yüksel H. Protective effects of carvacrol against methotrexate-induced testicular toxicity in rats. Int J Clin Exp Med. 7: 5511–5516. 2014. [PMC free article] [PubMed] [Google Scholar]
- 8.Nouri HS, Azarmi Y, and Movahedin M. Effect of growth hormone on testicular dysfunction induced by methotrexate in rats. Andrologia. 41: 105–110. 2009. [DOI] [PubMed] [Google Scholar]
- 9.Padmanabhan S, Tripathi DN, Vikram A, Ramarao P, and Jena GB. Cytotoxic and genotoxic effects of methotrexate in germ cells of male Swiss mice. Mutat Res. 655: 59–67. 2008. [DOI] [PubMed] [Google Scholar]
- 10.Sheikhbahaei F, Khazaei M, Rabzia A, Mansouri K, and Ghanbari A. Protective effects of thymoquinone against methotrexate-induced germ cell apoptosis in male mice. Int J Fertil Steril. 9: 541–547. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sönmez MF, Çilenk KT, Karabulut D, Ünalmış S, Deligönül E, Öztürk İ, and Kaymak E. Protective effects of propolis on methotrexate-induced testis injury in rat. Biomed Pharmacother. 79: 44–51. 2016. [DOI] [PubMed] [Google Scholar]
- 12.Vardi N, Parlakpinar H, Ates B, Cetin A, and Otlu A. Antiapoptotic and antioxidant effects of beta-carotene against methotrexate-induced testicular injury. Fertil Steril. 92: 2028–2033. 2009. [DOI] [PubMed] [Google Scholar]
- 13.Ferrando-Climent L, Rodriguez-Mozaz S, and Barceló D. Incidence of anticancer drugs in an aquatic urban system: from hospital effluents through urban wastewater to natural environment. Environ Pollut. 193: 216–223. 2014. [DOI] [PubMed] [Google Scholar]
- 14.Isidori M, Lavorgna M, Russo C, Kundi M, Žegura B, Novak M, Filipič M, Mišík M, Knasmueller S, de Alda ML, Barceló D, Žonja B, Česen M, Ščančar J, Kosjek T, and Heath E. Chemical and toxicological characterisation of anticancer drugs in hospital and municipal wastewaters from Slovenia and Spain. Environ Pollut. 219: 275–287. 2016. [DOI] [PubMed] [Google Scholar]
- 15.Yin J, Shao B, Zhang J, and Li K. A preliminary study on the occurrence of cytostatic drugs in hospital effluents in Beijing, China. Bull Environ Contam Toxicol. 84: 39–45. 2010. [DOI] [PubMed] [Google Scholar]
- 16.Sasado T, Tanaka M, Kobayashi K, Sato T, Sakaizumi M, and Naruse K. The National BioResource Project Medaka (NBRP Medaka): an integrated bioresource for biological and biomedical sciences. Exp Anim. 59: 13–23. 2010. [DOI] [PubMed] [Google Scholar]
- 17.Dietrich DR, and Krieger HO. Male gonad anatomy and morphology. In: Histological analysis of endocrine disructive effects in small laboratory fish. John Wiley and Sons, Inc., New Jersey. 88–114. 2009. [Google Scholar]
- 18.Hendzel MJ, Wei Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, Bazett-Jones DP, and Allis CD. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 106: 348–360. 1997. [DOI] [PubMed] [Google Scholar]
- 19.Duregon E, Molinaro L, Volante M, Ventura L, Righi L, Bolla S, Terzolo M, Sapino A, and Papotti MG. Comparative diagnostic and prognostic performances of the hematoxylin-eosin and phospho-histone H3 mitotic count and Ki-67 index in adrenocortical carcinoma. Mod Pathol. 27: 1246–1254. 2014. [DOI] [PubMed] [Google Scholar]
- 20.Veras E, Malpica A, Deavers MT, and Silva EG. Mitosis-specific marker phospho-histone H3 in the assessment of mitotic index in uterine smooth muscle tumors: a pilot study. Int J Gynecol Pathol. 28: 316–321. 2009. [DOI] [PubMed] [Google Scholar]
- 21.Gown AM, and Willingham MC. Improved detection of apoptotic cells in archival paraffin sections: immunohistochemistry using antibodies to cleaved caspase 3. J Histochem Cytochem. 50: 449–454. 2002. [DOI] [PubMed] [Google Scholar]
- 22.Meistrich ML, Finch M, da Cunha MF, Hacker U, and Au WW. Damaging effects of fourteen chemotherapeutic drugs on mouse testis cells. Cancer Res. 42: 122–131. 1982. [PubMed] [Google Scholar]
- 23.Palo AK, and Choudhury RC. Modulation of methotrexate-induced cytogenotoxicity in mouse spermatogonia and its transmission in the male germline by caffeine. Environ Toxicol Pharmacol. 21: 254–259. 2006. [DOI] [PubMed] [Google Scholar]
- 24.Saxena AK, Dhungel S, Bhattacharya S, Jha CB, and Srivastava AK. Effect of chronic low dose of methotrexate on cellular proliferation during spermatogenesis in rats. Arch Androl. 50: 33–35. 2004. [DOI] [PubMed] [Google Scholar]
- 25.Iwasaki Y, Ohkawa K, Sadakata H, Kashiwadate A, Takayama-Watanabe E, Onitake K, and Watanabe A. Two states of active spermatogenesis switch between reproductive and non-reproductive seasons in the testes of the medaka, Oryzias latipes. Dev Growth Differ. 51: 521–532. 2009. [DOI] [PubMed] [Google Scholar]
- 26.Vousden KH, and Lu X. Live or let die: the cell’s response to p53. Nat Rev Cancer. 2: 594–604. 2002. [DOI] [PubMed] [Google Scholar]
- 27.Segal R, Yaron M, and Tartakovsky B. Methotrexate: mechanism of action in rheumatoid arthritis. Semin Arthritis Rheum. 20: 190–200. 1990. [DOI] [PubMed] [Google Scholar]
- 28.Georgakilas AG, Martin OA, and Bonner WM. p21: A Two-Faced Genome Guardian. Trends Mol Med. 23: 310–319. 2017. [DOI] [PubMed] [Google Scholar]