Abstract
Exposure to zinc oxide (ZnO) has been linked to adverse health effects, but the renal effects of ZnO nanoparticles (ZnONPs) remain unclear. The objective of this study was to determine the renal toxicity of inhaled ZnONPs. Sprague Dawley (SD) rats were exposed to occupationally relevant levels of 1.1 (low dose) and 4.9 mg/m3 (high dose) ZnONPs or high-efficiency particulate arresting-filtered air (HEPA-FA) via inhalation for 2 weeks. Histopathological examinations of rat kidneys were performed at 24 hours, 7 days, and 1 month after exposure. A significant increase in microscopic inflammatory foci with pronounced periglomerular inflammation and interstitial lymphocytic infiltration was found in rats exposed to low and high doses of ZnONPs compared with rats exposed to HEPA-FA at the three time points following 2 weeks of exposure. Tubulitis featuring lymphocytic infiltrate within the tubular epithelium was found after 24 hours but had disappeared at 7 and 30 days in both the low- and high-dose exposure groups. Our findings demonstrate that inhaled ZnONPs cause sustained renal periglomerular and interstitial inflammation through lymphocytic infiltration. These findings provide histopathological evidence regarding sustained renal inflammation of nanoparticle exposure in rats and may provide some insight into the occupational health effects of ZnONPs on exposed workers.
Keywords: zinc oxide, nanoparticles, inflammation, histopathology, kidney, rat
Introduction
Nanotechnology is defined as the development and application of materials and structures with nanoscale dimensions, usually ranging 1–100 nm1. Zinc oxide nanoparticles (ZnONPs) are typical metal oxide nanoparticles (NPs) widely used in many commercial products, and the potential for human exposure to them is increasing2. Compared with ordinary Zinc oxide (ZnO) powder, ZnONPs are highly functional fine inorganic materials with increased chemical activity, strong oxidation and corrosion resistance, and a uniquely stronger absorption3.
While the pulmonary toxicity of ZnONPs has been reported4, 5, whether or not inhaled ZnONPs exhibit renal toxicity remains unclear. One previous study showed that a single gastrointestinally administered high dose of ZnONPs (5,000 mg/kg body weight) can cause proteinaceous casts in the renal tubules of mice6. Another study further demonstrated that ZnONPs (at a dose of 1,000 mg/kg body weight, intragastrically administered daily for 14 continuous days) resulted in significant increases in serum creatinine and blood urea nitrogen and overt tubular epithelial cell necrosis in rats7. These findings indicate that high doses of ZnONPs can cause nephrotoxicity. These toxicological studies based on oral administration of high doses of ZnONPs, however, do not reflect the real effect of ZnONPs inhaled under common workplace conditions. Thus, we conducted a pulmonary exposure study in which Sprague Dawley (SD) rats were exposed to occupationally relevant ZnONPs (at the levels of 1.1 and 4.9 mg/m3 of ZnONP inhalation for 2 weeks). While the cardiopulmonary toxicity results of this exposure have already been reported5, the purpose of this study was to report the effect of this exposure on the kidney as assessed by histopathology.
Materials and Methods
Animals
All of the animal experiments were approved by the Laboratory Animal Centre of National Taiwan University. Male Sprague Dawley (SD) rats (7 weeks old, 250–300 g) were obtained from BioLASCO (Taiwan) for this study.
Experimental design
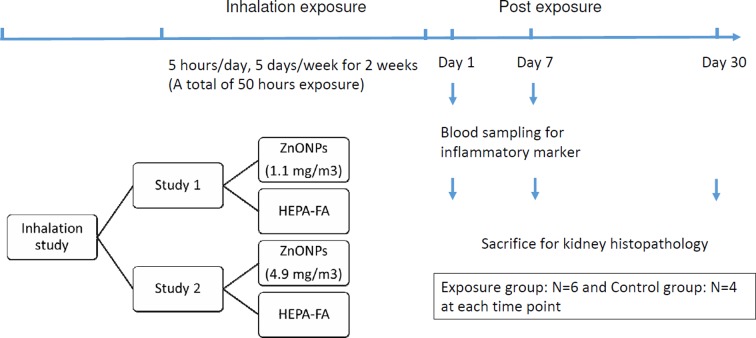
This study was performed to investigate the renal effects of inhalation of occupationally relevant doses of ZnONPs. To do this, we randomly divided 60 rats into two exposure groups, a low-dose group and a high-dose group. Each group contained 30 rats and was then further divided into two subgroups, those exposed to either ZnONPs (n=18; exposure) and those exposed to high-efficiency particulate arresting (HEPA)-filtered air (HEPA-FA) (n=12; control) for two weeks (5 h/day and 5 days/week, simulation of workers with common workplace exposure). The rats were sacrificed (6 rats from the ZnONPs exposure group and 4 rats from the HEPA-FA control group) at 1 day, 7 days, or 30 days after the two weeks of exposure (Fig. 1)5.
Fig. 1.
Experimental design. Rats were divided into low- (1.1 mg/m3) and high-dose (4.9 mg/m3) exposure groups; further divided into two subgroups, those exposed to either ZnONPs or HEPA-FA; and sacrificed at 1 day, 7 days, or 30 days after exposure for two weeks. ZnONPs: zinc oxide nanoparticles. HEPA-FA: high-efficiency particulate arresting (HEPA)-filtered air.
ZnONPs exposure
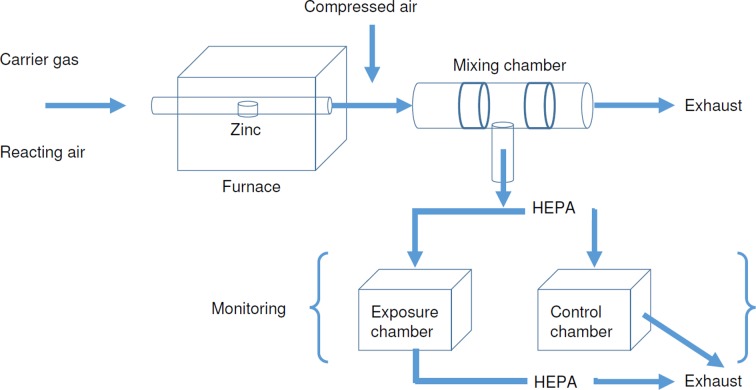
An evaporation-condensation ZnONPs generation system was used to generate ZnONPs for inhalation (Fig. 2)5. The system produces two different levels of ZnONPs with similar size fractions but with different numbers, masses, and surface areas of the ZnONPs for inhalation studies. Model 1 (low dose) of the ZnONP generation system resulted in 3.9 × 105 ± 1.4 × 105 ZnONPs/cm3 with a mass of 1.1 ± 0.3 mg/m3, a surface area of 8.1 × 105 ± 1.4 × 106 mm2/m3, and a geometric mean diameter (GMD) of 48 nm (1.8 geometric standard deviation, GSD). Model 2 (high dose) yielded 2.7 × 106 ± 1.1 × 106 ZnONPs/cm3 with a mass of 4.9 ± 1.6 mg/m3, surface area of 4.2 × 107 ± 1.3 × 108 mm2/m3, and a GMD of 51 nm (1.8 GSD).
Fig. 2.
ZnONPs inhalation exposure system.
Histopathology
The rat’s kidneys were excised and washed with ice-cold phosphate-buffered saline (PBS). Each organ was fixed with 10% neutral buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E) and periodic acid-Schiff (PAS) reagent. A coronal section from the mid-portion of the kidney was removed from each sacrificed rat. The histological samples were examined under light microscopy by two pathologists (CC and JS) who were ignorant of the classification of the samples (control or exposure, low dose or high dose group). The whole field of the coronal section was examined, and the inflammatory activity was semiquantitatively measured as the number of inflammatory foci/2 cm2 (50 LPF, 1 low power field (LPF) = 100× total magnification). An inflammatory focus was defined as an aggregate of two or more lymphocytes and/or plasma cells. The patterns of inflammatory distribution were also recorded. These included periglomerular, perivascular, and interstitial inflammation with or without tubulitis (defined as lymphocytes or plasma cells infiltrate within the renal tubular epithelium).
Statistical analyses
Results are expressed as the mean ( ± standard deviation) unless otherwise indicated. Differences between two groups were compared with the Mann-Whitney U test and Fisher’s exact test. All p values were 2-tailed, and p values less than 0.05 were considered significant. All statistical operations were performed using the IBM SPSS Statistics Version 20.0 (IBM Corp., Armonk, NY, USA) statistical software.
Results
We compared the lesions observed in treated rats and the controls at each time point (24 hours, 7 days, and 30 days). Compared with the controls at 7 days and 30 days, the average numbers of inflammatory foci/50 LPF were significantly higher at 7 days and 30 days after exposure in the low-dose group (2.17 vs. 0 foci/50 LPF, p=0.038, and 2.5 vs. 0.75 foci/50 LPF, p=0.038, respectively). There were no significant differences in the remaining pairwise comparisons unless all the low-dose and/or high-dose exposure rats were grouped together, rendering increased numbers of rats, which resulted in some significant differences between the controls and the low- and/or high-dose groups (see below).
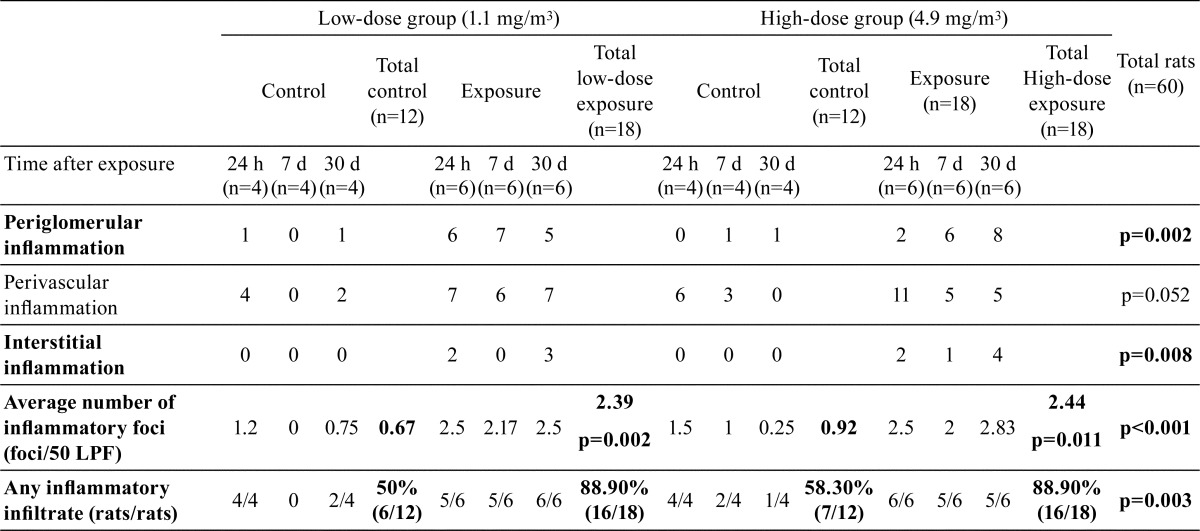
Therefore, we grouped all the low-dose exposure rats as the total low-dose exposure group (n=18), all the high-dose exposure rats as the total high-dose exposure group (n=18), and all the low- and high-dose exposure rats as the total exposure group (n=36). In the low-dose exposure group, we observed inflammatory cells infiltrate in the cortical periglomerular and perivascular spaces with predominantly lymphocytes and few plasma cells in 16 (88.9%) of the 18 ZnONP-exposed rats and 6 (50%) of the 12 control rats. We observed a significant increase in the light microscopic inflammatory foci in the total low-dose exposure group (average: 2.39 foci/50 LPF) compared with the control (average: 0.67 focus/50 LPF (p=0.002) (Table 1). Intertubular interstitial lymphocytic infiltration was a prominent morphological feature in the ZnONP-exposed rats. None of the controls were found to have interstitial inflammation. Representative photomicrographs of the histopathological studies at 24 hours, 7 days, and 30 days after exposure are shown in Fig. 3 (periglomerular, perivascular, and interstitial inflammation).
Table 1. Semiquantitative Measurement of the Inflammatory Foci/2 cm2 (50 LPF, Low Power Field = 100× Total Magnification).
Fig. 3.
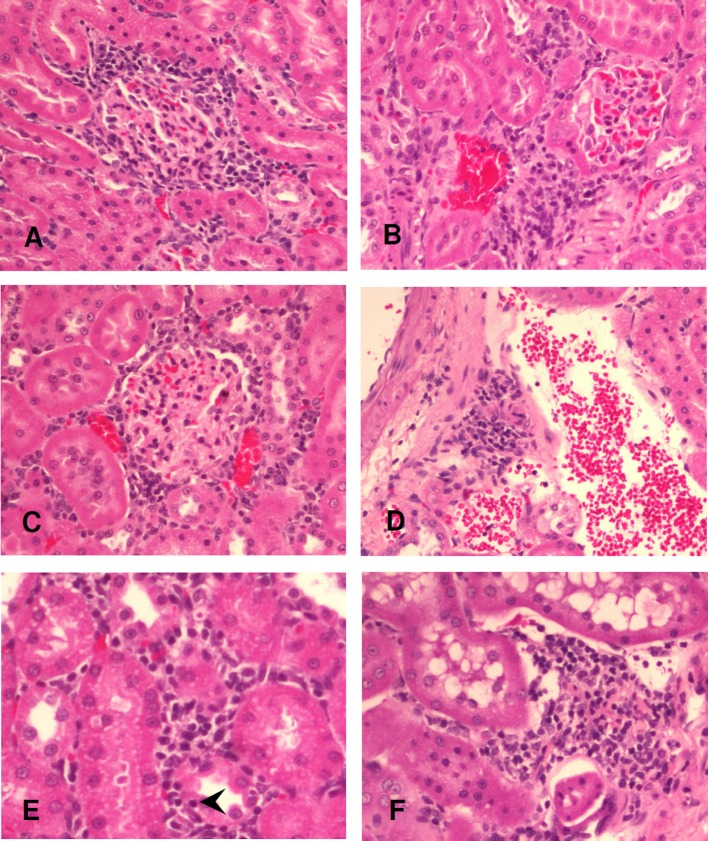
Histopathology of the low-dose (1.1 mg/m3) exposure group. Periglomerular inflammation at 24 hours, 7 days, and 30 days after exposure (A–C). Perivascular inflammation at 7 days after exposure (D). Interstitial inflammation with tubulitis featuring lymphocytic infiltrate within the tubular epithelium (arrowhead) at 24 hours after exposure (E). Interstitial inflammation at 30 days after exposure (F). Hematoxylin and eosin stain, original magnification = 200×.
The number of rats affected and the inflammation scores were similar between the total low-dose exposure and total high-dose exposure groups (Table 1). In the total high-dose exposure group, lymphocytic infiltration was observed in 16 (88.9%) of the 18 ZnONP-exposed rats and 7 (58.3%) of the 12 control rats. We also observed a significant increase in the inflammatory foci in the total high-dose exposure group (average: 2.44 foci/50 LPF) compared with the controls (average: 0.92 foci/50 LPF) (p=0.011) (Table 1). None of the control rats were found to have interstitial inflammation. Representative photomicrographs recorded during the histopathological studies at 24 hours, 7 days, and 30 days after exposure are shown in Fig. 4 (periglomerular, perivascular, and interstitial inflammation).
Fig. 4.
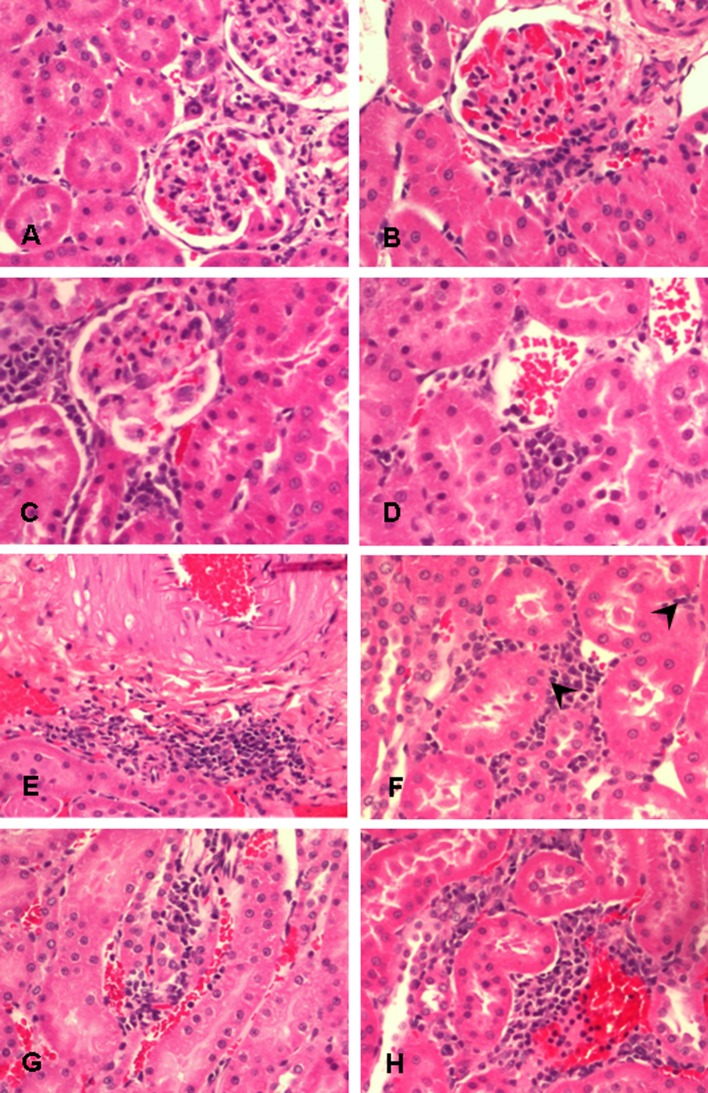
Histopathology of the high-dose (4.9 mg/m3) exposure group. Periglomerular inflammation at 24 hours, 7 days, and 30 days after exposure (A–C). Perivascular inflammation at 24 hours and 7 days after exposure (D, E). Interstitial inflammation with tubulitis featuring lymphocytic infiltrate within the tubular epithelium (arrowhead) at 24 hours after exposure (F). Interstitial inflammation at 7 days and 30 days after exposure (G, H). Hematoxylin and eosin stain, original magnification = 200×.
In contrast to the control group, the total exposure group had significant and consistent interstitial lymphocytic infiltration (p=0.008). Slightly increased perivascular lymphocytic infiltration was also noted in the total exposure group, but it lacked significance (p=0.052). Periglomerular inflammation was significantly more pronounced in the total exposure group (p=0.002). There was no neutrophilic infiltration or interstitial fibrosis in the exposed rats. Tubular epithelial cell degeneration or necrosis was not observed. Tubulitis was found after 24 hours but had disappeared at 7 and 30 days in both the low- and high-dose exposure groups (Fig. 3E and 4F). The results of semiquantitative measurement of the inflammatory foci/50 LPF are shown in Table 1. Compared with the controls, both the total low-dose exposure group and the total high-dose exposure group showed a significant increase in the average number of inflammatory foci (2.39 foci/50 LPF, p=0.002, and 2.44 foci/50 LPF, p=0.011, respectively). A significant difference was also observed when the total exposure group was compared with the controls (p<0.001). Any types of inflammatory infiltrate, regardless of whether they are periglomerular, perivascular, or interstitial inflammation, were significantly more pronounced in the ZnONP-exposed rats compared with the controls (p=0.003) (Table 1).
Discussion
This study is the first to report histopathological evidence of sustained renal inflammation following 2 weeks of inhalation of ZnONPs in SD rats. High dissolution of the ZnONPs in the lung tissue, translocation of Zn into the blood circulation, and the concentration, mode, and duration of the exposure may play important roles in the toxicity of the ZnONPs2. This work is a continuation of the authors’ previous studies on the adverse health effects of ZnONPs on the cardiopulmonary systems4, 5. We exposed rats to occupationally relevant mass concentrations of ZnONPs by inhalation for 2 weeks to investigate the cardiopulmonary effects as determined by oxidative-inflammatory responses and histopathology. The results showed neutrophilic infiltration in the alveolar space and septum of the lung. The degree of inflammatory cell infiltration was decreased after 30 days5.
This current study further demonstrates that occupationally relevant mass concentrations of ZnONPs cause a significant, sustained increase in the lymphocytic inflammatory foci in the kidney of rats at 24 hours, 7 days, and 30 days after low-dose (1.1 mg/m3) and high-dose (4.9 mg/m3) exposure. Such sustained renal lymphocytic inflammation in the kidney differs from acute neutrophilic infiltration in the lung5. We suspect that a minor neutrophilic infiltrate in the kidney might have been present but not sampled, because the neutrophilic infiltrate can be quite evanescent and give way within days to lymphocytes and plasma cells8. Our results also showed that the periglomerular and interstitial lymphocytic inflammation was specific to the exposure group, observed at 24 hours, and still present at 30 days after subchronic ZnONP inhalation.
Previous nanoparticle studies have demonstrated that intragastric administration of titanium dioxide nanoparticles (TiO2 NPs) and ZnONPs may cause nephric inflammation, cell necrosis and dysfunction, and overt renal tubular epithelial cell necrosis with alteration of inflammatory cytokine expression7, 9. Mice exposed to ZnONPs through oral gavage have also been shown to have glomerular segmentation, hydropic degeneration and necrosis of tubular epithelial cells, and swelling of the proximal tubular epithelial cells10. However, the doses of the particles in that study were relatively high, and the exposure route was not pulmonary inhalation. Our study is unique in demonstrating that subchronic pulmonary exposure of ZnONPs at occupationally relevant mass concentrations causes sustained renal inflammation. Our experimental conditions reflect common inhalation conditions of the nanoparticles in the workplace, and our results may provide insight into the occupational health hazard effects on workers exposed to ZnONPs.
Previously, it has been shown that intratracheal exposure to cadmium-containing silica nanoparticles (Cd-SiNPs) in SD rats results in altered expression of the renal genes which have been linked to immune function, oxidative stress, and inflammation processes at day 7. Gene categories implicated in cell regulation and apoptosis are also modified by the nanoparticles at day 30 after exposure11, 12. These findings indicate that the kidney may be a susceptible target of the nanoparticles when locally instilled into the lung and that the subtle long-lasting alterations may be produced via the inflammatory processes. One recent report has also suggested that, through the translocation process across biological barriers, nanoparticles can reach and be deposited in secondary target organs where they may induce adverse biological reactions. This suggests that evaluation of renal nanotoxicity is urgently needed, as renal excretion is an expected and possible elimination route for nanoparticles13.
In this study, a pathologic criteria for acute renal allograft rejection is used for the diagnosis of tubulitis, that is, tubular inflammation in which one or more mononuclear cells have penetrated into tubules and located between tubular epithelial cells or between tubular cells and their basement membranes14. Tubulitis is the hallmark of tubulointerstitial nephritis but may also occur in other renal diseases15. Knowledge of the clinical implications, pathogenesis, and consequences of tubulitis is limited due to the complex structural–functional relationships between the tubular epithelium, the peritubular interstitium, capillaries, and the complicated segmental structure of the tubules. It seems likely that tubulitis results from a T-cell-mediated reaction in most cases16.
A previous study found renal effects, including tubular epithelial cell damage, when rats with diabetes mellitus were exposed to particulate matter (PM)17. Tubular epithelial cell damage, such as thinning, detachment, vacuolization, individual cell necrosis, nuclear enlargement, cytoplasmic basophilia, or mitotic figure, was inconspicuous in our study. However, tubulitis was observed at 24 hours but had disappeared at 7 days and 30 days post exposure in both the low- and high-dose exposure groups. A previous study showed that persistence of cycling T cells within intact tubules can be identified after acute or active episodes of injury under the anti-apoptotic microenvironment provided by the IL-15-rich tubular epithelium18. This finding may indicate that tubular epithelial cell damage caused by inhaled ZnONPs is acute and reversible, with some residual persistence of cycling T cells within intact tubules, and foci of interstitial lymphocytic infiltrate. It should be noted that although acute phase inflammation includes predominantly neutrophil and macrophage infiltrate in other organs, the characteristic feature of acute interstitial nephritis in the kidney is diffuse or patchy interstitial inflammatory infiltrate composed dominantly of T lymphocytes, monocytes, and occasionally plasma cells, with tubulitis, and the pathology of chronic interstitial nephritis is dominated by tubular atrophy and interstitial fibrosis19.
The limitation of this study is that the number of exposed rats was restricted by the experimental design. We compared the lesions observed in the exposed rats and the controls at each time point (24 hours, 7 days, and 30 days). Compared with the controls, the average numbers of inflammatory foci/50 LPF were significantly higher at 7 days and 30 days after exposure in the low-dose group, but there was no significant difference in the remaining pairwise comparisons. Grouping the entire low-dose and/or high-dose exposure rats, rendering increase numbers of rats, resulted in significant differences between the controls and both the low- and high-dose groups. The average number of the inflammatory foci/50 LPF, periglomerular inflammation, and interstitial inflammation, were significantly more pronounced in the ZnONP-exposed rats compared with the controls.
This study found that pulmonary exposure to the occupationally relevant mass concentrations of ZnONPs may result in sustained inflammation of the kidney. The inflammatory infiltrate in the kidney may result from direct toxicity of the nanoparticles after translocation from the lung tissue into the blood circulation and into the kidney and/or may result from lung-kidney crosstalk via systemic oxidative stress-inflammatory cascades or chemical mediators20. The ZnONP accumulation, excretion, and deposition process may play an important role. The concentration of ZnO in the form of nanoparticles or ions in the rat model may actually be playing a part. The inflammation reported in this study was probably associated with Zn ions that induce cellular and systemic metal ion homeostasis. Data concerning ZnONP deposition in the kidney was lacking in the current study. Further histopathological and mechanistic studies are needed to investigate how much of the ZnONPs leached out Zn ions, the concentration levels in the rat system, phenomenon of ion homeostasis and the efflux system, and the accumulation of Zn ions in the organs or the rat system4, 21.
The presence of persistent interstitial lymphocytic infiltration in the ZnONP-exposed rats raises concerns about disease progression to renal fibrosis and chronic kidney disease22. Although there was no tubular atrophy or interstitial fibrosis in our study, further long-term and larger number studies are needed to elucidate the renal effects of the inflammatory response resulting from the inhalation of nanoparticles. The findings of this study may serve as an important histopathological basis for further studies regarding the potential nephrotoxicity of ZnONPs under occupational conditions.
Footnotes
Disclosure of Potential Conflicts of Interest: None declared.
References
- 1.Lee JW, Kim JE, Shin YJ, Ryu JS, Eom IC, Lee JS, Kim Y, Kim PJ, Choi KH, and Lee BC. Serum and ultrastructure responses of common carp (Cyprinus carpio L.) during long-term exposure to zinc oxide nanoparticles. Ecotoxicol Environ Saf. 104: 9–17. 2014. [DOI] [PubMed] [Google Scholar]
- 2.Adamcakova-Dodd A, Stebounova LV, Kim JS, Vorrink SU, Ault AP, O’Shaughnessy PT, Grassian VH, and Thorne PS. Toxicity assessment of zinc oxide nanoparticles using sub-acute and sub-chronic murine inhalation models. Part Fibre Toxicol. 11: 15 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan R, Kang T, Lu F, Zhang Z, Shen H, and Liu M. Cytotoxicity, oxidative stress, and genotoxicity in human hepatocyte and embryonic kidney cells exposed to ZnO nanoparticles. Nanoscale Res Lett. 7: 602 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho M, Wu KY, Chein HM, Chen LC, and Cheng TJ. Pulmonary toxicity of inhaled nanoscale and fine zinc oxide particles: mass and surface area as an exposure metric. Inhal Toxicol. 23: 947–956. 2011. [DOI] [PubMed] [Google Scholar]
- 5.Chuang HC, Juan HT, Chang CN, Yan YH, Yuan TH, Wang JS, Chen HC, Hwang YH, Lee CH, and Cheng TJ. Cardiopulmonary toxicity of pulmonary exposure to occupationally relevant zinc oxide nanoparticles. Nanotoxicology. 8: 593–604. 2014. [DOI] [PubMed] [Google Scholar]
- 6.Wang B, Feng WY, Wang TC, Jia G, Wang M, Shi JW, Zhang F, Zhao YL, and Chai ZF. Acute toxicity of nano- and micro-scale zinc powder in healthy adult mice. Toxicol Lett. 161: 115–123. 2006. [DOI] [PubMed] [Google Scholar]
- 7.Yan G, Huang Y, Bu Q, Lv L, Deng P, Zhou J, Wang Y, Yang Y, Liu Q, Cen X, and Zhao Y. Zinc oxide nanoparticles cause nephrotoxicity and kidney metabolism alterations in rats. J Environ Sci Health A Tox Hazard Subst Environ Eng. 47: 577–588. 2012. [DOI] [PubMed] [Google Scholar]
- 8.Heptinstall RH. Urinary tract infection, pyelonephritis, reflux nephropathy. In: Heptinstall’s pathology of the kidney, 5th ed. Jennette JC, Olson JL, Schwartz MM, and Silva FG (eds). Lippincott-Raven, Philadelphia. 725–783. 1998. [Google Scholar]
- 9.Gui S, Zhang Z, Zheng L, Cui Y, Liu X, Li N, Sang X, Sun Q, Gao G, Cheng Z, Cheng J, Wang L, Tang M, and Hong F. Molecular mechanism of kidney injury of mice caused by exposure to titanium dioxide nanoparticles. J Hazard Mater. 195: 365–370. 2011. [DOI] [PubMed] [Google Scholar]
- 10.Esmaeillou M, Moharamnejad M, Hsankhani R, Tehrani AA, and Maadi H. Toxicity of ZnO nanoparticles in healthy adult mice. Environ Toxicol Pharmacol. 35: 67–71. 2013. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell LA, Lauer FT, Burchiel SW, and McDonald JD. Mechanisms for how inhaled multiwalled carbon nanotubes suppress systemic immune function in mice. Nat Nanotechnol. 4: 451–456. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coccini T, Roda E, Fabbri M, Sacco MG, Gribaldo L, and Manzo L. Gene expression profiling in rat kidney after intratracheal exposure to cadmium-doped nanoparticles. J Nanopart Res. 14: 925 2012. [Google Scholar]
- 13.Iavicoli I, Fontana L, and Nordberg G. The effects of nanoparticles on the renal system. Crit Rev Toxicol. 46: 490–560. 2016. [DOI] [PubMed] [Google Scholar]
- 14.Colvin RB, Cohen AH, Saiontz C, Bonsib S, Buick M, Burke B, Carter S, Cavallo T, Haas M, Lindblad A, Manivel JC, Nast CC, Salomon D, Weaver C, and Weiss M. Evaluation of pathologic criteria for acute renal allograft rejection: reproducibility, sensitivity, and clinical correlation. J Am Soc Nephrol. 8: 1930–1941. 1997. [DOI] [PubMed] [Google Scholar]
- 15.Iványi B, and Olsen S. Tubulitis in Renal Disease. In: Tubulointerstitial and Cystic Disease of the Kidney. Springer Berlin Heidelberg, Heidelberg. 117–143. 1995. [Google Scholar]
- 16.Wong WK, Robertson H, Carroll HP, Ali S, and Kirby JA. Tubulitis in renal allograft rejection: role of transforming growth factor-beta and interleukin-15 in development and maintenance of CD103+ intraepithelial T cells. Transplantation. 75: 505–514. 2003. [DOI] [PubMed] [Google Scholar]
- 17.Yan YH, C-K Chou C, Wang JS, Tung CL, Li YR, Lo K, and Cheng TJ. Subchronic effects of inhaled ambient particulate matter on glucose homeostasis and target organ damage in a type 1 diabetic rat model. Toxicol Appl Pharmacol. 281: 211–220. 2014. [DOI] [PubMed] [Google Scholar]
- 18.Robertson H, and Kirby JA. Post-transplant renal tubulitis: the recruitment, differentiation and persistence of intra-epithelial T cells. Am J Transplant. 3: 3–10. 2003. [DOI] [PubMed] [Google Scholar]
- 19.Michel DM, and Kelly CJ. Acute interstitial nephritis. J Am Soc Nephrol. 9: 506–515. 1998. [DOI] [PubMed] [Google Scholar]
- 20.Domenech P, Perez T, Saldarini A, Uad P, and Musso CG. Kidney-lung pathophysiological crosstalk: its characteristics and importance. Int Urol Nephrol. 49: 1211–1215. 2017. [DOI] [PubMed] [Google Scholar]
- 21.Kao YY, Chen YC, Cheng TJ, Chiung YM, and Liu PS. Zinc oxide nanoparticles interfere with zinc ion homeostasis to cause cytotoxicity. Toxicol Sci. 125: 462–472. 2012. [DOI] [PubMed] [Google Scholar]
- 22.Zeisberg M, and Kalluri R. Physiology of the renal interstitium. Clin J Am Soc Nephrol. 10: 1831–1840. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]