Abstract
Statistical methodologies, including Plackett–Burman design and Box–Behnken design, were employed to optimize the fermentation conditions for the production of active substances against aquatic pathogen Vibrio harveyi by marine-derived Aspergillus flavipes strain HN4-13. The optimal crucial fermentation values for maximum production of active substances against V. harveyi were obtained as follows: X 1 (peptone) = 0.3%, X 2 (KCl) = 0.25%, and X 3 (inoculum size) = 4.5%. The predicted diameter of inhibitory zone against V. harveyi was 23.39 mm, and the practical value reached 23.71 ± 0.98 mm with a 62.3% increase. Bioassay-guided fractionation resulted in the acquisition of two compounds whose structures were identified as questin (1) and emodin (2). Questin exhibited the same antibacterial activity against V. harveyi as streptomycin (MIC 31.25 µg/mL). This is the first time to report questin as a potential antibacterial agent against aquatic pathogen V. harveyi.
Electronic supplementary material
The online version of this article (10.1007/s13205-017-1015-z) contains supplementary material, which is available to authorized users.
Keywords: Aspergillus flavipes, Vibrio harveyi, Fermentation optimization, Response surface methodology, Bioassay-guided fractionation, Questin
Introduction
For several decades, the aquaculture industry of China acquired the prompt development and the yield of aquatic products ranks the first in the world. Meanwhile some animal diseases continuously appeared. Infectious vibriosis caused by Vibrios has been identified as a main economic challenge to the aquaculture (Morya et al. 2014; Guo et al. 2016b). Among them, Vibrio harveyi is considered to be a major pathogen, which induced animal mortality during early larval stages, resulting in serious losses in the production and marketing of aquatic animals, especially on farmed and wild shrimp (Thompson et al. 2010; Morya et al. 2014). At present, the application of antibiotics in the administration of aquaculture was widely used. However, the long-term application or abuse of antibiotics may be adverse to the environment protection and human health, including the transfer of antibiotic resistance to human pathogens, other aquatic pathogens, and the accumulation of antibiotic residues (Harikrishnan et al. 2010; Cao et al. 2011). Hence, other than the optional control strategies, for example, improving husbandry and water quality, lowering stocking densities, nourishing better nutrition, the application of favorable microbes or their metabolites is also broadly expected to become an alternative way for the prevention and control of vibriosis (Cao et al. 2011; Xu et al. 2014).
Living in a unique environment characterized as high salt, oligotrophic, and weak alkali, marine fungi could metabolize different natural products (NPs) from their terrestrial counterparts (Zhao et al. 2013). To date, about 800 new NPs with antimicrobial, antitumor, and antivirus activities have been identified from the marine fungal metabolites (Blunt et al. 2015; Gomes et al. 2015). The antimicrobial reports on marine fungal NPs were mainly focused on human pathogens (Zheng et al. 2013; Schueffler and Anke 2014; Song et al. 2014; Sun et al. 2014); however, there are rarely reports on the research of aquatic pathogens (Barakat and Gohar 2012).
To discover the potential use of marine fungal NPs in the prevention and treatment of aquatic pathogens, a fungal strain HN4-13 identified as Aspergillus flavipes was purified from Lianyungang coastal sediment. The metabolites of A. flavipes strain HN4-13 showed multiple antimicrobial activities against V. harveyi, V. anguillarum, V. cholerae, and V. Parahemolyticus, especially for V. harveyi. Thus, we used the inhibition zone against V. harveyi as an indicator to optimize the fermentation conditions by Plackett–Burman design, Box–Behnken design and response surface methodology and to identify the antibacterial products. The results will be report in this paper.
Materials and methods
Microorganism
Strain HN4-13 (CCTCC AF 2015022) was isolated from marine sediment collected in Lianyungang coast and identified as a member of Aspergillus flavipes based on the morphological characters and internal transcribed spacer sequence (GenBank No. JX287370) (Guo et al. 2013). V. harveyi, V. anguillarum, V. cholerae, and V. Parahemolyticus were provided by Dr. Zhenxia Su. All strains were kept at the Marine Microbial Natural Products Chemistry Laboratory of Huaihai Institute of Technology (Lianyungang, Jiangsu, China).
Shake-flask culture
The seed culture was prepared by adding mycelia on a sabouraud’s dextrose agar medium (peptone 1%, glucose 4%, agar 1.5%, dissolved in natural seawater) into a 500-mL conical bottle with 200 mL of liquid sabouraud’s dextrose medium (peptone 1%, glucose 4%, dissolved in natural seawater). Cultures were incubated in a rotary shaker with the rotating speed (160 rpm/min) at 28 °C for 1 day. The optimization process were conducted by adding the harvested seed culture into 500-mL conical bottle including 200 mL of liquid fermentation medium with shaking at 160 rpm/min as follows.
Statistical fermentation optimization
To obtain the maximal antibacterial activity of A. flavipes strain HN4-13, the optimization procedures of fermentation conditions were divided into the following three parts.
Two-level Plackett–Burman design
The objective of this optimization procedure was to determine which fermentation factors have significant effects on the antibacterial activity of active extracts. The key variables of fermentation conditions were investigated using a two-level Plackett–Burman design, which permits for screening of nine variables in just twelve experiments. Factors were applied at two levels coded as − 1 and + 1. The center points (0) of all the nine variables were determined according to the results of single-factor experiments. The software JMP 7 (SAS, USA) was used to design the experiments (Table 1).
Table 1.
Experimental results of Plackett–Burman design of Aspergillus flavipes strain HN4-13
| No. | Glucose (%) | Peptone (%) | KCl (%) | NaH2PO4 (%) | NaCl (%) | PH | Inoculum size (%) | Temperature (°C) | Fermentation time (d) | Inhibition zone (mm) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | 1.5 | 0.15 | 0.15 | 4 | 8 | 3 | 36 | 10 | 16.38 |
| 2 | 2 | 1.5 | 0.05 | 0.15 | 4 | 8 | 1 | 28 | 6 | 10.63 |
| 3 | 2 | 0.5 | 0.15 | 0.05 | 4 | 8 | 3 | 28 | 6 | 23.78 |
| 4 | 4 | 0.5 | 0.05 | 0.15 | 2 | 8 | 3 | 36 | 6 | 21.31 |
| 5 | 2 | 1.5 | 0.05 | 0.05 | 4 | 6 | 3 | 36 | 10 | 9.07 |
| 6 | 2 | 0.5 | 0.15 | 0.05 | 2 | 8 | 1 | 36 | 10 | 22.12 |
| 7 | 2 | 0.5 | 0.05 | 0.15 | 2 | 6 | 3 | 28 | 10 | 19.71 |
| 8 | 4 | 0.5 | 0.05 | 0.05 | 4 | 6 | 1 | 36 | 6 | 14.77 |
| 9 | 4 | 1.5 | 0.05 | 0.05 | 2 | 8 | 1 | 28 | 10 | 12.52 |
| 10 | 4 | 1.5 | 0.15 | 0.05 | 2 | 6 | 3 | 28 | 6 | 19.45 |
| 11 | 2 | 1.5 | 0.15 | 0.15 | 2 | 6 | 1 | 36 | 6 | 11.39 |
| 12 | 4 | 0.5 | 0.15 | 0.15 | 4 | 6 | 1 | 28 | 10 | 17.93 |
Path of steepest ascent (descent)
The path of steepest ascent (descent) was applied to further optimize the variables screened by Plackett–Burman design to guide the triers nearly approach the optimum of the surface. The path initiated from the center of Plackett–Burman design, to run away from the center of Plackett–Burman design along the way of steepest ascent (descent) (Zhao et al. 2016). This routine should be ceased in favor of a more precise experiment once the way of steepest ascent (descent) no more led to increase (Zhang and Sang 2012). We moved − 0.2, 0.05, and 1% in peptone, KCl, and inoculum size, respectively.
Box–Behnken design
The optimization of the values of significant factors was conducted by response surface methodology. Data analysis and model building were carried out by Design Expert 7.0.0 (Stat-Ease, Minneapolis, USA) (Guo et al. 2016a). Box–Behnken design consists of five central points and twelve factorial points; one of the response surface methodologies was employed to ascertain the response mode and establish the model. The dependent variable (Y, %) was the diameters of inhibition zone against V. harveyi, while peptone (X 1), KCl (X 2), and inoculum size (X 3) were selected for independent factors. The range and values of three independent factors are shown in Table 4. By this software, the optimization objective of fermentation condition was to achieve the maximum diameter of inhibition zone. The pattern of the system was evaluated by the following second-order polynomial equation:
| 1 |
where Y represents the predicted response, β 0, β i, β ii and β ij are constant coefficients, while X i and X j are the independent variables.
Table 4.
Box–Behnken design and experimental results of strain HN4-13
| No. | Coded levels | Y (%) | ||
|---|---|---|---|---|
| X 1: Peptone (%) | X 2: KCl (%) | X 3: Inoculum size (%) | Inhibition zone (mm) | |
| 1 | − 1 (0.2) | − 1 (0.2) | 0 (5) | 22.58 |
| 2 | + 1 (0.6) | − 1 (0.2) | 0 (5) | 18.88 |
| 3 | − 1 (0.2) | + 1 (0.3) | 0 (5) | 21.28 |
| 4 | + 1 (0.6) | + 1 (0.3) | 0 (5) | 20.14 |
| 5 | − 1 (0.2) | 0 (0.25) | − 1 (4) | 23.26 |
| 6 | + 1 (0.6) | 0 (0.25) | − 1 (4) | 20.81 |
| 7 | − 1 (0.2) | 0 (0.25) | + 1 (6) | 20.37 |
| 8 | + 1 (0.6) | 0 (0.25) | + 1 (6) | 17.71 |
| 9 | 0 (0.4) | − 1 (0.2) | − 1 (4) | 21.12 |
| 10 | 0 (0.4) | + 1 (0.3) | − 1 (4) | 22.11 |
| 11 | 0 (0.4) | − 1 (0.2) | + 1 (6) | 20.83 |
| 12 | 0 (0.4) | + 1 (0.3) | + 1 (6) | 19.22 |
| 13 | 0 (0.4) | 0 (0.25) | 0 (5) | 22.36 |
| 14 | 0 (0.4) | 0 (0.25) | 0 (5) | 21.45 |
| 15 | 0 (0.4) | 0 (0.25) | 0 (5) | 23.17 |
| 16 | 0 (0.4) | 0 (0.25) | 0 (5) | 23.45 |
| 17 | 0 (0.4) | 0 (0.25) | 0 (5) | 23.38 |
Preparation of the active extracts
Harvested fermentation broths were filtered through cheesecloth to isolate broth from mycelia. The filtrate was extracted with an equivalent volume of ethyl acetate for three times to give an ethyl acetate solution. Ethyl acetate solution was further enriched under reduced pressure to produce active extracts, which was dissolved in methanol (10 mg/mL).
Bioactivity-guided fractionation of active compounds
The fungus A. flavipes strain HN4-13 was cultivated in 500-mL conical bottles including 200 mL optimal fermentation medium that was composed of peptone 0.3%, glucose 3%, KCl 0.25%, NaH2PO4 0.1%, NaCl 3%, inoculum size 4.5% and distilled water (pH 7.0) and was grown at 32 °C for 8 days with shaking at 160 rpm/min. The fermented whole broths (20 L) were filtered through cheesecloth to separate mycelia from broth. Then the filtrates were extracted by an equivalent volume of ethyl acetate three times, and concentrated under reduced pressure to give ethyl acetate extract (7.1 g).
The ethyl acetate extract was subjected to a vacuum column chromatography over silica gel, eluting with a gradually gradient of petroleum ether–CH2Cl2 (1:1 and 0:1) and then of CH2Cl2–CH3OH (100–0%) to achieve nine primary fractions (Fr.1–Fr.9). Fr.1–Fr.9 were screened for antibacterial activity against V. harveyi by the Oxford cup method. The Fr.5 (0.45 g) eluted with CH2Cl2-CH3OH (25:1) showed the antibacterial activity against V. harveyi, which was further purified into three sub-fractions (Fr.5-1 to Fr.5-3) by Sephadex LH-20 after elution with CH3OH. Then Fr.5-2 (0.1 g) was purified by preparative high-performance liquid chromatography (PHPLC) eluting with 80% MeOH-H2O to give compounds 1 (4.0 mg, t R 7.3 min) and 2 (8.5 mg, t R13.5 min).
Evaluation of antibacterial activity
The antibacterial activity against V. harveyi of the active extracts was evaluated by the Oxford cup method. The medium was beef extract peptone medium (BP) composed of beef extract 0.3%, peptone 1%, NaCl 0.5%, agar 1.5%, and the pH was adjusted to 7.0 with NaOH. These cups were placed on plates previously inoculated with the pathogens. 100 µL of each extract (10 mg/mL) was added in Oxford cups and incubation at 37 °C for 12 h. The experiments were conducted in triplicate, and the average inhibition zone (mm in diameter) was recorded as the antibacterial activity of active extracts.
Compounds were dissolved in methanol, diluted into different concentrations with sterile distilled water by continuous twofold dilution. These solutions were directly used to determine their antibacterial activities against aquatic V. harveyi, V. anguillarum, V. cholerae, and V. parahemolyticus using above method. The minimum inhibitory concentration (MIC) was defined as the lowest concentration of compounds that produced the inhibition zone against the tested pathogens (Sun et al. 2014). The experiments were performed in duplicate with full agreement between both results.
Results
Screening the significant factors using Plackett–Burman design
On the basis of single-factor experimental results, two-level Plackett–Burman design was employed to choose the most key fermentation factors. The experimental design and responses of different experiments are shown in Table 1. Table 2 shows regression coefficients of the variables on the responses, the associated t values, and significant level. The fit quality of first-order model was evaluated by the determination coefficient R 2, which was determined as 0.983. The results indicated that the model could explain 98.3% of the variability in the response (Li et al. 2008). The analysis of variance showed the model was significant. In Table 2, P value of the three variables such as peptone, KCl and inoculum size was less than 0.10, which was identified as significant, while others were not significant. It also can be seen from Table 2, the increase of KCl and inoculum size had the positive effects on inhibition zone, whereas peptone had a negative effect. Therefore, peptone, KCl and inoculum size were chosen to make further optimization.
Table 2.
Analysis of the two-level Plackett–Burman design
| Variable | Regression coefficient | t value | Sig. | Ranks |
|---|---|---|---|---|
| Glucose | 0.472 | 1.09 | 0.3887 | 7 |
| Peptone | − 3.348 | − 7.76 | 0.0162 | 1 |
| KCl | 1.92 | 4.45 | 0.0470 | 2 |
| NaH2PO4 | − 0.363 | − 0.84 | 0.4886 | 8 |
| NaCl | − 1.162 | − 2.69 | 0.1148 | 5 |
| pH | 1.202 | 2.78 | 0.1085 | 4 |
| Inoculum size | 1.695 | 3.93 | 0.0592 | 3 |
| Temperature | − 0.748 | − 1.73 | 0.2252 | 6 |
| Fermentation time | − 0.3 | − 0.69 | 0.5590 | 9 |
Approximating the optimum combination using path of steepest ascent (descent)
The path of ascent (descent) was decided to find the appropriate direction of changing variables, and then the concentration was increased or decreased according to the sign of the main effect on increasing the diameter of inhibition zone. The path of steepest ascent (descent) began from the zero level of Plackett–Burman design and shifted along the path in which KCl and inoculum size increased, while peptone decreased. Other variables were set at their zero level. The design and data obtained from the experiment are shown in Table 3. The biggest inhibition zone was 23.28 mm when the concentrations of peptone, KCl and inoculum size were 0.4, 0.25, and 5%, respectively. It offered that the point was near the region of maximum inhibition zone response. Further optimization with Box–Behnken design and response surface methodology was around this point.
Table 3.
Experimental levels and results of the path of steepest ascent (descent)
| No. | Peptone (%) | KCl (%) | Inoculum size (%) | Inhibition zone (mm) |
|---|---|---|---|---|
| 1 | 1.0 | 0.10 | 2 | 22.19 |
| 2 | 0.8 | 0.15 | 3 | 20.08 |
| 3 | 0.6 | 0.20 | 4 | 22.88 |
| 4 | 0.4 | 0.25 | 5 | 23.28 |
| 5 | 0.2 | 0.30 | 6 | 22.30 |
Identifying the optimal fermentation conditions using Box–Behnken design
Based on the results of Plackett–Burman design and path of ascent (descent) trial, peptone (X 1), KCl (X 2), and inoculum size (X 3) were chosen for further optimization by the trial of tri-factor, tri-level Box–Behnken design. Based on the parameter estimates, the application of response surface methodology can offer an empirical relationship between the response factors and the test variables. The matrices of Box–Behnken design are shown in Table 4. By applying multiple regression analysis on the experimental data, the equation for the diameter of inhibition zone was established as:
| 2 |
The value of determination R 2 (0.9113) for Eq. (2) is reasonably close to 1, which indicates the regression models defined well the true behavior of the system (Guo et al. 2014). The corresponding analysis of variance is shown in Table 5. The values of “P > F = 0.0056” indicated that the fitness of the model was significant. In addition, the lack of fit value of the model showed 0.7156, without significant difference (Guo et al. 2016a). The results also indicated that the linear effects of peptone and inoculum size are very significant (P < 0.01). The quadratic effect of peptone, KCl, and inoculum size (P < 0.05) is also significant.
Table 5.
Analysis of variance for the quadratic model of the Box–Behnken design experiments
| Source | Sum of squares | DF | Mean square | F value | P value | Sig. |
|---|---|---|---|---|---|---|
| Model | 41.56 | 9 | 4.62 | 8.20 | 0.0056 | ** |
| X 1 | 12.38 | 1 | 12.38 | 21.97 | 0.0022 | ** |
| X 2 | 0.05 | 1 | 0.05 | 0.10 | 0.7649 | |
| X 3 | 10.51 | 1 | 10.51 | 18.66 | 0.0035 | ** |
| X 1 X 2 | 1.64 | 1 | 1.64 | 2.91 | 0.1319 | |
| X 1 X 3 | 0.01 | 1 | 0.01 | 0.02 | 0.8927 | |
| X 2 X 3 | 1.69 | 1 | 1.69 | 3.00 | 0.1269 | |
| X 21 | 5.69 | 1 | 5.69 | 10.10 | 0.0155 | * |
| X 22 | 3.26 | 1 | 3.26 | 5.79 | 0.0471 | * |
| X 23 | 4.75 | 1 | 4.75 | 8.43 | 0.0228 | * |
| Residual | 3.94 | 7 | 0.56 | |||
| Lack of fit | 1.04 | 3 | 0.35 | 0.48 | 0.7156 |
* P < 0.05 and ** P < 0.01
The interactions between three variables (peptone, KCl and inoculum size) and the inhibition zone were exhibited by the response surface and contour plots (Fig. 1). The effects of peptone interaction with each of the two other factors on the diameter of inhibition zone are shown in Fig. 1a, b. The diameter of inhibition zone increased to a high value with increasing peptone from 0.2 to 0.3, but then, with increasing the concentration of peptone, the inhibition zone could decrease, which indicated that peptone has significant effect on the diameter of inhibition zone. A similar phenomenon is observed in Fig. 1b, c with inoculum size and the other two factors. The increase in inoculum size enhanced the diameter of inhibition zone, but after that, with further increasing the inoculum size over 4.5%, the inhibition zone would be decreased. The effects of KCl interaction with each of the two other factors on the diameter of inhibition zone are shown in Fig. 1a, c, which imply that KCl is less significant than the other two variables.
Fig. 1.
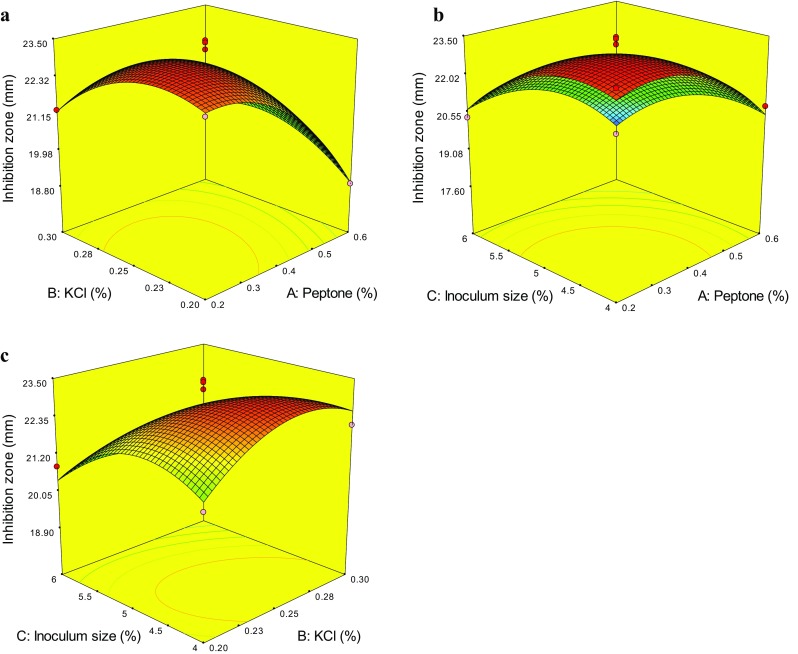
Response surface plots showing effects of pairwise factors on the inhibition zone and their interaction
The predicted optimal levels of peptone (X 1), KCl (X 2), and inoculum size (X 3) were obtained by applying the regression analysis to Eq. (2): X 1 = 0.3%, X 2 = 0.25%, and X 3 = 4.49%. To confirm the result, the rechecking experiment was performed under the optimum fermentation conditions as follows: 0.3% peptone, 3% glucose, 0.25% KCl, 0.1% NaH2PO4, 3% NaCl, pH7.0, 4.5% inoculum size, 32 °C, 160 rpm/min for 8 days. The practical value of inhibition zone reached (23.71 ± 0.98) mm, which was not significant difference to the predicted value (23.39 mm), indicating that the model was accurate and adequate. Compared with that under the original liquid SD fermentation conditions (1% peptone, 4% glucose, natural seawater, 1% inoculum size, 28 °C, 160 rpm/min for 7 days), the diameter of inhibition zone increased 62.3%.
Identification of questin and emodin
The EtOAc extract of strain HN4-13 was subjected to bioassay-guided fractionation over silica gel column, Sephadex LH-20 column and C18 column to give compounds 1 and 2 (Fig. 2). The structures 1 and 2 were identified as 1,6-dihydroxy-8-methoxy-3-methylanthracene-9,10-dione (questin) and 1,3,8-trihydroxy-6-methylanthracene-9,10-dione (emodin), respectively, by UV, 1H- and 13C-NMR, DEPT and ESI–MS data (Fig. S1–S6).
Fig. 2.
Chemical structure of questin (1) and emodin (2)
1,6-dihydroxy-8-methoxy-3-methylanthracene-9,10-dione (questin, 1): orange powder. 1H-NMR (600 MHz, DMSO-d 6) δ13.23 (br s, 1H, HO-1), 7.18 (br s, 1H, H-2), 7.41 (br s, 1H, H-4), 7.10 (br s, 1H, H-5), 6.82 (br s, 1H, H-7), 3.88 (s, 3H, H3CO-8), 2.37 (s, 3H, H3-11).13C-NMR (150 MHz, DMSO-d 6) δ163.4 (C, C-1), 119.1 (CH, C-2), 146.6 (C, C-3), 124.2 (CH, C-4), 132.0 (C, C-4a), 107.0 (CH, C-5), 161.7 (C, C-6), 105.0 (CH, C-7), 164.6 (C, C-8), 112.5 (C, C-8a), 186.3 (C, C-9), 114.4 (C, C-9a), 182.3 (C, C-10), 136.8 (C, C-10a), 56.3 (CH3, CH3O-8), 21.4 (CH3, C-11). ESI–MS m/z 285.1 [M + H]+. These data are consistent with literature report (Park and Kim 2011).
1,3,8-trihydroxy-6-methylanthracene-9,10-dione (emodin, 2): orange powder. 1H-NMR (600 MHz, DMSO-d 6) δ12.02 (s, 1H, HO-1), 7.16 (br s, 1H, H-2), 7.49 (br s, 1H, H-4), 7.11 (br s, 1H, H-5), 6.59 (d, 1H, J = 2.1 Hz, H-7), 12.08 (1H, s, HO-8), 2.41 (s, 3H, H-11). 13C-NMR (150 MHz, DMSO-d 6) δ164.5 (C, C-1), 108.0 (CH, C-2), 165.7 (C, C-3), 108.9 (CH, C-4), 135.1 (C, C-4a), 120.5 (CH, C-5), 148.3 (C, C-6), 124.2 (CH, C-7), 161.4(C, C-8), 113.4 (C, C-8a), 189.6 (C, C-9), 108.8 (C, C-9a), 181.4 (C, C-10), 132.8 (C, C-10a), 21.5 (CH3, C-11). ESI–MS m/z 271.3 [M + H]+. These data are consistent with literature report (Lv et al. 2011).
Antibacterial activities of emodin and questin against aquatic pathogens
The antibacterial activities of emodin and questin were determined by the Oxford cup assay using the aquatic pathogens V. harveyi, V. anguillarum, V. cholerae and V. parahemolyticus. Questin showed the same activity to the positive control streptomycin against V. harveyi (MIC 31.25 µg/mL). Questin also exhibited antibacterial activity against V. anguillarum, V. cholerae and V. parahemolyticus with the MIC values of 62.5, 62.5 and 125 µg/mL (Table 6), while emodin was not active against the tested aquatic pathogens.
Table 6.
Antibacterial activities against aquatic pathogens of questin
| Compound | MIC (µg/mL) | |||
|---|---|---|---|---|
| V. harveyi | V. anguillarum | V. cholerae | V. parahemolyticus | |
| Streptomycin | 31.25 | 31.25 | 31.25 | 31.25 |
| Questin | 31.25 | 62.50 | 62.50 | 125.0 |
| Emodin | – | – | – | – |
“–” Shows no activity
Discussion
The fungus A. flavipes has been reported to produce pectinase (Martínez-Trujillo et al. 2011), L-methioninase (El-Sayed 2011), alpha-galactosidase (Ozsoy and Berkkan 2003), butyrolactones (Nagia et al. 2012), cerebrosides (Jiang et al. 2004), isobenzofuran (Kwon et al. 2012), alkaloids such as spiroquinazoline (Barrow and Sun 1994) and aspochalasin (Rochfort et al. 2005). This is the first report of questin which can be secreted by the fungus A. flavipes.
Questin (emodin 8-methyl ether) can be isolated from plant such as Uvaria kurzii (Lv et al. 2011) and Cassia tora (Park and Kim 2011), and be secreted by marine-derived fungus Aspergillus sp. B-F-2 (Liu et al. 2006), marine mangrove plant-derived endophytic Eurotium rubrum (Li et al. 2009), Trewia nudiflora commensal fungus Aspergillus sp. F1 (Lin et al. 2009) and the antarctic sponge-derived Pseudogymnoascus sp. fungus (Figueroa et al. 2015). It has previously been shown to exhibit moderate or weak cytotoxicity, DPPH radical scavenging activity and antimicrobial activity against phytopathogenic Fusarium oxysporium. Besides, it is very interesting that the analogue of questin, physcion (parietin, emodin 3-methyl ether, emodin 6-methyl ether) has been used widely in the agriculture of China as a highly active botanical pesticide against phytopathogens. However, to the best of our knowledge, this is the first report of questin possessing antimicrobial activity against aquatic pathogenic Vibrios.
We report here the optimized production of antibacterial substances against aquatic pathogenic V. harveyi from A. flavipes strain HN4-13 with potential for aquatic applications. Statistical methods, Plackett–Burman design, Box–Behnken design and response surface methodology were combined and employed to identify the optimum fermentation conditions for the production of antibacterial active compounds as follows: 0.3% peptone, 3% glucose, 0.25% KCl, 0.1% NaH2PO4, 3% NaCl, pH7.0, 4.5% inoculum size, 32 °C, 160 rpm/min for 8 days. Such optimization showed a 62.3% increase in antibacterial activity. Then, two compounds, emodin and questin, were separated from the ethyl acetate extract of fermented broth of A. flavipes HN4-13 by activity-guided fractionation. Compared to streptomycin, questin exhibited significant antimicrobial activity against aquatic pathogen V. harveyi with a MIC value of 31.25 μg/mL. Work on pharmacological effect and action mechanism of questin against aquatic pathogen V. harveyi in vitro and in vivo would provide the favorable prospect in aquatic application.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study was funded by Natural Science Foundation of Jiangsu Province (No. BK20151283), Natural Science Foundation of Lianyungang (No. CG1612), 521 Talented Project of Lianyungang, Priority Academic Program Development of Jiangsu Higher Education Institutions (No. 5511201401X) and Open-end Funds of Jiangsu Key Laboratory of Marine Pharmaceutical Compound Screening (No. 2015HYB10).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13205-017-1015-z) contains supplementary material, which is available to authorized users.
References
- Barakat KM, Gohar YM. Antimicrobial agents produced by marine Aspergillus terreus var. africanus against some virulent fish pathogens. Indian J Microbiol. 2012;52:66–372. doi: 10.1007/s12088-012-0255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow CJ, Sun HH. Spiroquinazoline, a novel substance P inhibitor with a new carbon skeleton, isolated from Aspergillus flavipes. J Nat Prod. 1994;57:471–476. doi: 10.1021/np50106a005. [DOI] [PubMed] [Google Scholar]
- Blunt JW, Copp BR, Keyzers RA, Munro MH, Prinsep MR. Marine natural products. Nat Prod Rep. 2015;32:116–211. doi: 10.1039/C4NP00144C. [DOI] [PubMed] [Google Scholar]
- Cao H, He S, Wei R, Diong M, Lu L. Bacillus amyloliquefaciens G1: a potential antagonistic bacterium against eel-pathogenic Aeromonas hydrophila. Evid Based Complement Altern Med. 2011;2011:824104. doi: 10.1155/2011/824104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayed AS. Purification and characterization of a new L-methioninase from solid cultures of Aspergillus flavipes. J Microbiol. 2011;49:130–140. doi: 10.1007/s12275-011-0259-2. [DOI] [PubMed] [Google Scholar]
- Figueroa L, Jiménez C, Rodríguez J, Areche C, Chávez R, Henríquez M, de la Cruz M, Díaz C, Segade Y, Vaca I. 3-Nitroasterric acid derivatives from an Antarctic sponge-derived Pseudogymnoascus sp. fungus. J Nat Prod. 2015;78:919–923. doi: 10.1021/np500906k. [DOI] [PubMed] [Google Scholar]
- Gomes NG, Lefranc F, Kijjoa A, Kiss R. Can some marine-derived fungal metabolites become actual anticancer agents? Mar Drugs. 2015;13:3950–3991. doi: 10.3390/md13063950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Zhu WC, Liu WW, Ding GW, Zhang YQ, Zhang SL, Qiu Y, Xie Y. Identification and fermentation optimization of marine fungus HN4-13 with antibacterial activity. Microbiol China. 2013;40:951–958. [Google Scholar]
- Guo L, Zhu W, Xu F, Liu M, Xie Y, Zhang J. Optimized ultrasonic-assisted extraction of polysaccharides from Cyclina sinensis and evaluation of antioxidant activities in vitro. CyTA J Food. 2014;12:32–39. doi: 10.1080/19476337.2013.785982. [DOI] [Google Scholar]
- Guo L, Guo J, Zhu W, Jiang X. Optimized synchronous extraction process of tea polyphenols and polysaccharides from Huaguoshan Yunwu tea and their antioxidant activities. Food Bioprod Process. 2016;100:303–310. doi: 10.1016/j.fbp.2016.08.001. [DOI] [Google Scholar]
- Guo L, Wang C, Zhu W, Xu F. Bioassay-guided fractionation and identification of active substances from the fungus Aspergillus tubingensis against Vibrio anguillarum. Biotechnol Biotec Equip. 2016;30:602–606. doi: 10.1080/13102818.2016.1146635. [DOI] [Google Scholar]
- Harikrishnan R, Balasundaram C, Heo MS. Effect of probiotics enriched diet on Paralichthys olivaceus infected with lymphocystis disease virus (LCDV) Fish Shellfish Immunol. 2010;29:868–874. doi: 10.1016/j.fsi.2010.07.031. [DOI] [PubMed] [Google Scholar]
- Jiang T, Li T, Li J, Fu HZ, Pei YH, Lin WH. Cerebroside analogues from marine-derived fungus Aspergillus flavipes. J Asian Nat Prod Res. 2004;6:249–257. doi: 10.1080/1028602031000147384. [DOI] [PubMed] [Google Scholar]
- Kwon YJ, Sohn MJ, Kim CJ, Koshino H, Kim WG. Flavimycins A and B, dimeric 1,3-dihydroisobenzofurans with peptide deformylase inhibitory activity from Aspergillus flavipes. J Nat Prod. 2012;75:271–274. doi: 10.1021/np200720v. [DOI] [PubMed] [Google Scholar]
- Li X, Xu T, Ma X, Guo K, Kai L, Zhao Y, Jia X, Ma Y. Optimization of culture conditions for production of cis-epoxysuccinic acid hydrolase using response surface methodology. Bioresour Technol. 2008;99:5391–5396. doi: 10.1016/j.biortech.2007.11.017. [DOI] [PubMed] [Google Scholar]
- Li DL, Li XM, Wang BG. Natural anthraquinone derivatives from a marine mangrove plant-derived endophytic fungus Eurotium rubrum: structural elucidation and DPPH radical scavenging activity. J Microbiol Biotechnol. 2009;19:675–680. [PubMed] [Google Scholar]
- Lin T, Lu C, Shen Y. Secondary metabolites of Aspergillus sp. F1, a commensal fungal strain of Trewia nudiflora. Nat Prod Res. 2009;23:77–85. doi: 10.1080/14786410701852826. [DOI] [PubMed] [Google Scholar]
- Liu R, Zhu W, Zhang Y, Zhu T, Liu H, Fang Y, Gu Q. A new diphenyl ether from marine-derived fungus Aspergillus sp. B-F-2. J Antibiot. 2006;59:362–365. doi: 10.1038/ja.2006.52. [DOI] [PubMed] [Google Scholar]
- Lv Z, Zhang Q, Chen R, Yu D. Alkaloids and anthraquinones from branches and leaves of Uvaria kurzii. China J Chin Mat Med. 2011;36:1190–1192. [PubMed] [Google Scholar]
- Martínez-Trujillo A, Arreguín-Rangel L, García-Rivero M, Aguilar-Osorio G. Use of fruit residues for pectinase production by Aspergillus flavipes FP-500 and Aspergillus terreus FP-370. Lett Appl Microbiol. 2011;53:202–209. doi: 10.1111/j.1472-765X.2011.03096.x. [DOI] [PubMed] [Google Scholar]
- Morya VK, Choi W, Kim EK. Isolation and characterization of Pseudoalteromonas sp. from fermented Korean food, as an antagonist to Vibrio harveyi. Appl Microbiol Biotechnol. 2014;198:1389–1395. doi: 10.1007/s00253-013-4937-3. [DOI] [PubMed] [Google Scholar]
- Nagia MM, El-Metwally MM, Shaaban M. Four butyrolactones and diverse bioactive secondary metabolites from terrestrial Aspergillus flavipes MM2: isolation and structure determination. Org Med Chem Lett. 2012;2:9–16. doi: 10.1186/2191-2858-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozsoy N, Berkkan H. Production and characterization of alpha-galactosidase from Aspergillus flavipes. Cell Biochem Funct. 2003;21:387–389. doi: 10.1002/cbf.1041. [DOI] [PubMed] [Google Scholar]
- Park YB, Kim SB. Isolation and identification of antitumor promoters from the seeds of Cassia tora. J Microbiol Biotechnol. 2011;21:1043–1048. doi: 10.4014/jmb.1103.03040. [DOI] [PubMed] [Google Scholar]
- Rochfort S, Ford J, Ovenden S, Wan SS, George S, Wildman H, Tait RM, Meurer-Grimes B, Cox S, Coates J, Rhodes D. A novel aspochalasin with HIV-1 integrase inhibitory activity from Aspergillus flavipes. J Antibiot. 2005;58:279–283. doi: 10.1038/ja.2005.34. [DOI] [PubMed] [Google Scholar]
- Schueffler A, Anke T. Fungal natural products in research and development. Nat Prod Rep. 2014;31:1425–1448. doi: 10.1039/C4NP00060A. [DOI] [PubMed] [Google Scholar]
- Song F, Ren B, Chen C, Yu K, Liu X, Zhang Y, Yang N, He H, Liu X, Dai H, Zhang L. Three new sterigmatocystin analogues from marine-derived fungus Aspergillus versicolor MF359. Appl Microbiol Biotechnol. 2014;98:3753–3758. doi: 10.1007/s00253-013-5409-5. [DOI] [PubMed] [Google Scholar]
- Sun K, Li Y, Guo L, Wang Y, Liu P, Zhu W. Indole diterpenoids and isocoumarin from the fungus, Aspergillus flavus, isolated from the prawn, Penaeusvannamei. Mar Drugs. 2014;12:3970–3981. doi: 10.3390/md12073970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J, Gregory S, Plummer S, Shields RJ, Rowley AF. An in vitro and in vivo assessment of the potential of Vibrio spp. as probiotics for the Pacific white shrimp Litopenaeusvannamei. J Appl Microbiol. 2010;109:1177–1187. doi: 10.1111/j.1365-2672.2010.04743.x. [DOI] [PubMed] [Google Scholar]
- Xu HM, Rong YJ, Zhao MX, Song B, Chi ZM. Antibacterial activity of the lipopetides produced by Bacillus amyloliquefaciens M1 against multidrug-resistant Vibrio spp. isolated from diseased marine animals. Appl Microbiol Biotechnol. 2014;98:127–136. doi: 10.1007/s00253-013-5291-1. [DOI] [PubMed] [Google Scholar]
- Zhang H, Sang Q. Statistical optimization of cellulases production by Penicillium chrysogenum QML-2 under solid-state fermentation and primary application to chitosan hydrolysis. World J Microbiol Biotechnol. 2012;28:1163–1174. doi: 10.1007/s11274-011-0919-8. [DOI] [PubMed] [Google Scholar]
- Zhao C, Zhu T, Zhu W. New marine natural products of microbial origin from 2010 to 2013. Chin J Org Chem. 2013;33:1195–1234. doi: 10.6023/cjoc201304039. [DOI] [Google Scholar]
- Zhao C, Guo L, Wang L, Zhu G, Zhu W. Improving the yield of (+)-terrein from the salt-tolerant Aspergillus terreus PT06-2. World J Microbiol Biotechnol. 2016;32:77. doi: 10.1007/s11274-016-2029-0. [DOI] [PubMed] [Google Scholar]
- Zheng J, WangY Wang J, Liu P, Li J, Zhu W. Antimicrobial ergosteroids and pyrrole derivatives from halotolerant Aspergillus flocculosus PT05-1 cultured in a hypersaline medium. Extremophiles. 2013;17:963–971. doi: 10.1007/s00792-013-0578-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



