Abstract
Agmatine is a decarboxylated arginine by arginine decarboxylase. Agmatine is known to be a neuroprotective agent. It has been reported that agmatine works as a NMDA receptor blocker or a competitive nitric oxide synthase inhibitor in CNS injuries. In spinal cord injury, agmatine showed reduction of neuropathic pain, improvement of locomotor function, and neuroprotection. Macrophage is a key cellular component in neuroinflammation, a major cause of impairment after spinal cord injury. Macrophage has subtypes, M1 and M2 macrophages. M1 macrophage induces a pro-inflammatory response, but M2 inspires an anti-inflammatory response. In this study, it was clarified whether the neuroprotective effect of agmatine is related with the modulation of macrophage subdivision after spinal cord injury. Spinal cord injury was induced in rats with contusion using MASCIS. Animals received agmatine (100 mg/kg, IP) daily for 6 days beginning the day after spinal cord injury. The proportion of M1 and M2 macrophages are confirmed with immunohistochemistry and FACS. CD206+ & ED1+ cells were counted as M2 macrophages. The systemic treatment of agmatine increased M2 macrophages caudal side to epicenter 1 week after spinal cord injury in immunohistochemistry. M2 macrophage related markers, Arginase-1 and CD206 mRNA, were increased in the agmatine treatment group and M2 macrophage expressing and stimulated cytokine, IL-10 mRNA, also was significantly overexpressed by agmatine injection. Among BMPs, BMP2/4/7, agmatine significantly increased only the expression of BMP2 known to reduce M1 macrophage under inflammatory status. These results suggest that agmatine reduces impairment after spinal cord injury through modulating the macrophage phenotype.
Keywords: Agmatine, Spinal cord injury, Macrophage, M2 polarization, Neuroinflammation
INTRODUCTION
Spinal cord injury is one of the major CNS injuries in need of an optimal cure. Neuroinflammation is the main aggravator of CNS injuries, including spinal cord injury [1]. A variety of immune cell types were related to inflammation at the site of spinal cord injury [1]. Macrophage, activated microglia and infiltrated monocyte, are major players in neuroinflammation [2,3,4]. It was reported that macrophages have two major subtypes, M1 macrophage related to pro-inflammation and M2 macrophage related to anti-inflammation [3]. The portion of macrophage subtypes after spinal cord injury is one of important factors in formulating a repair strategy to overcome spinal cord injury. M1 macrophages are neurotoxic, while M2 macrophages promote axonal regeneration after CNS injuries [3,5,6]. In addition, the expression of M1 macrophages is induced quickly after injury and continued for a long time, but the expression is transient in M2 macrophages [3]. According to these reports, to increase the portion of M2 macrophages is one strategy to reduce impairment after spinal cord injury.
Agmatine is an endogenous amine formed by decarboxylation of arginine through the activation of arginine decarboxylase [7]. It is reported as a NMDA receptor blocker, competitive inhibitor of nitric oxide synthase (NOS), and neurotransmitter to α2-adrenergic and imidazoline receptors [7,8]. Exogenous administration of agmatine was reported to be neuroprotective in vitro and in vivo [9,10,11,12]. Also, it was published that agmatine reduced nitrite production from hypoxic injured BV2 microglia [13] and decreased iNOS and Iba1 double positive cells in the brain after LPS injection in vivo [14]. Based on these reports, it is hypothesized that the neuroprotective effect of agmatine be related to the modulation of M2 macrophage portion after CNS injuries. To establish this hypothesis, spinal cord injury animal model in vivo was used with or without agmatine administration in this study.
MATERIALS AND METHODS
Animals
Studies were conducted on Sprague-Dawley rats (11 weeks old, 260±15 g, Samtako, Korea). All animal experiments were performed in accordance with the Korean Food and Drug Administration (KFDA) guidelines. Protocols were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the Yonsei Laboratory Animal Research Center (YLARC, Permit #: 2014-0375). All rats were maintained in the specific pathogen-free facility of the YLARC.
Spinal cord injury and agmatine treatment
The rats were anesthetized with an intraperitoneal injection of Zoletil (tiletamine 15 mg/kg and zolazepam 15 mg/kg) and Rompun (xylazine 9.6 mg/kg). A laminectomy of thoracic vertebra (a half of Th 9 and full Th10) was performed without damaging the dura mater. The exposed spinal cord was contused via dropping a 10-g rod at 25.0-mm height using MASCIS (Rutgers, The State University of New Jersey). Saline (6 ml, SQ) and Baytril (Enrofloxacin 4.16 mg/kg, IM) were injected for 3 days after injury. Bladder was manually pressed daily. Food and water were freely accessible in their cages. Agmatine was administered for 6 days starting the day after spinal cord injury (100 mg/kg, IP). The experimental control group received normal saline in the same manner. All rats were sacrificed for immunohistochemistry, FACS and mRNA analysis 7 days after spinal cord injury.
FACS analysis
To analyze the proportion of macrophage subtypes using FACS, rats were perfused with saline for a short time and a 10-mm length of spinal cord at epicenter was examined, following the previous report [15]. In brief, the spinal cord was dissociated enzymatically with trypsin and collagenase followed by trituration. It was filtered and centrifuged to get the cell pellet, which was resuspended in 6 ml of HBSS (Welgene, Korea). The dissociated spinal cord cell solution was layered on top of OptiPrep gradient solutions (Thermo Fisher Scientific) and centrifuged with 1900 rpm for 15 minutes at room temperature (RT). The cell pellet containing inflammatory cells, glia, and red blood cells, was resuspended in 0.85% ammonium chloride solution for 5 minutes to lyse red blood cells. After washing with HBSS, cells were blocked in 10% normal rabbit or mouse serum for 30 minutes at RT. Cells were washed twice and incubated with primary antibodies, Mouse anti-ED1-FITC (1:100, AbD Serotec); Rabbit anti-CD206 labelled with PE/Cy5.5 (1:100, Abcam); Rabbit anti-iNOS labelled with PE/Cy5.5 (1:100, Chemicon) or isotype IgG solution (1:100 dilution) for 1hour at 4℃. Cells were washed twice and resuspended in 300 ul of HBSS for FACS analysis. Immunolabeled cells were sorted using FACS Verse (BD Pharmigen), and analyzed on FlowJo software (Tree Star, Inc., USA).
Immunohistochemistry, analysis and cell counting
Animals were perfused with saline and fixed with 4% paraformaldehyde solution 7 days after spinal cord injury. Spinal cords were removed and post-fixed in 4% paraformaldehyde solution at 4℃ overnight. For coronal tissue sections, spinal cords were submerged in 30% sucrose solution at 4℃ for 3 days, embedded in OCT compound, and cut to 20-um thickness. For immunofluorescence, tissue sections were blocked with 10% normal goat serum solution at RT for 2 hours and reacted with the primary antibodies against ED1 (AbD Serotec, 1:400) or CD206 (Abcam, 1:200) at 4℃ overnight. Appropriate secondary antibodies were applied to tissue sections at RT for 2 hours following washing with PBS 3 times. Hoechst33258 (SigmaAldrich, 1:2000) was used to visualize nuclei. Image analysis was performed using a LSM 700 confocal microscope with Zen imaging software (Carl Zeiss). ED1 positive and CD206 & ED1 double positive cell counting were done in 4 areas (total 0.49 mm2) containing the margin of infarct zone. The size of each area was 350 um * 350 um.
mRNA expression (RT-PCR)
To analyze the relative expression of mRNA related to inflammation, macrophage, and astrocyte, rats were perfused with saline for a short time and a 10-mm length of spinal cord at the epicenter was used to isolate total RNA with TRIzol Reagent (ThermoFisher Scientific) according to manufacturer's protocol. A total 2 ug of RNA was transcripted into cDNA with reverse transcriptase (High-Capacity RNA-to-cDNA Kit, ThermoFisher Scientific). 200 ng of cDNA was amplified with specific primer sets (Table 1) and SYBR green Master Mix (ThermoFisher Scientific) by qRT-PCR system (QuantStudio 3, Applied Biosystems). qRT-PCR duplication was performed in biological replicates (n=5 per each group).
Table 1. The specific primer sequences for RT-PCR.
| Gene | Sequence | Reference | |
|---|---|---|---|
| (PMID) | |||
| CD11b | F | 5′-TGA CGG CTC CGG TAG CAT-3′ | 24505289 |
| R | 5′-CCA TCA CAG TTG AGA CAA ATT CCT-3′ | ||
| Arg-1 | F | 5′-CCG CAG CAT TAA GGA AAG C-3′ | 25944087 |
| R | 5′-CCC GTG GTC TCT CAC ATT G-3′ | ||
| CD206 | F | 5′-AGG GGT TCA CCT GGA GTG AT-3′ | 25944087 |
| R | 5′-GCT CTC CAT AAG CCC AAT TTT-3′ | ||
| IL-1b | F | 5′-AAA TGC CTC GTG CTG TCT GAC C-3′ | 26173397 |
| R | 5′-TCC CGA CCA TTG CTG TTT CCT-3′ | ||
| IL-6 | F | 5′-TCA TTC TGT CTC GAG CCC AC-3′ | 26173397 |
| R | 5′-GAA GTA GGG AAG GCA GTG GC-3′ | ||
| IL-10 | F | 5′-AGG GCT GCC TTC AGT CAA GT-3′ | 26173397 |
| R | 5′-AGA AAT CGA TGA CAG CGT CG-3′ | ||
| BMP2 | F | 5′-CCA GGT TAG TGA CTC AGA ACA C-3′ | 19861972 |
| R | 5′-TCA TCT TGG TGC AAA GAC CTG C-3′ | ||
| BMP4 | F | 5′-TGG ACA CTT CAT CAC ACG ACT A-3′ | 19861972 |
| R | 5′-GCG ACG GCA GTT CTT ATT CTT C-3′ | ||
| BMP7 | F | 5′-AGA CGC CAA AGA ACC AAG AG-3′ | 19861972 |
| R | 5′-GCT GTC GTC GAA GTA GAG GA-3′ | ||
| GFAP | F | 5′-GGG CGA AGA AAA CCG CAT-3′ | 24505289 |
| R | 5′-TCT GGA GGT TGG AGA AAG TCT GT-3′ | ||
| b-actin | F | 5′-AGA AGA GCT ATG AGC TGC CTG ACG-3′ | 19861972 |
| R | 5′-TAC TTG CGC TCA GGA GGA GCA ATG-3′ |
Statistical analysis
All statistical analyses were performed using SPSS 18.0 (IBM). The data was presented as means±standard error (SE). Independent student t-test was used to compare the experimental groups. Differences were considered statistically significant at p<0.05.
RESULTS
There was no difference of macrophage subtypes with or without agmatine treatment in FACS analysis
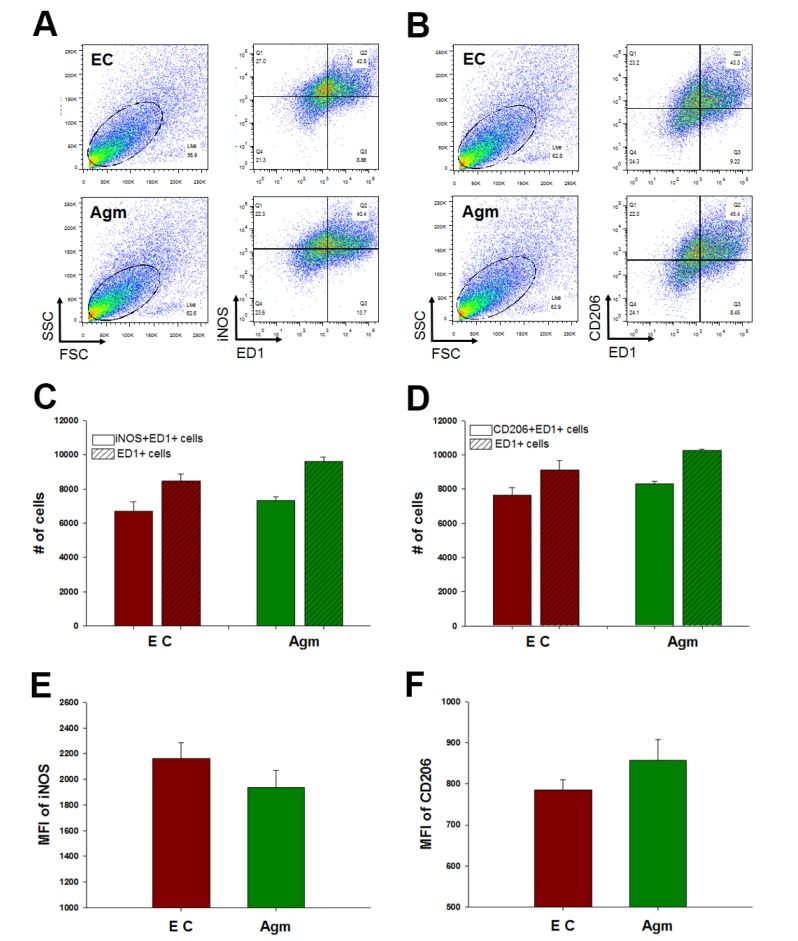
To established hypothesis, the macrophage subtypes were confirmed using FACS analysis. ED1 was used as a macrophage marker and iNOS for M1 and CD206 for M2 subtype macrophage markers were used. These markers were specific to each macrophage [16]. The portion of M1 macrophages, iNOS and ED1 double positive cells, was not reduced by agmatine treatment 1 week after spinal cord injury (Fig. 1A and C). The percent of M2 macrophages, CD206 and ED1 positive cells, was also not significantly increased in the agmatine treatment group (Fig. 1B and D). However, the median fluorescence intensity (MFI) of iNOS was decreased in the agmatine administration group and the MFI of CD206 was increased in the agmatine treatment group without significance compared to the experimental control group (Fig. 1E and F).
Fig. 1. FACS analysis of macrophages 1 week after spinal cord injury. M1 macrophages were iNOS+&ED1+ cells (A) and M2 macrophages were CD206+&ED1+ cells (B). There were no significant difference in the number of M1 and M2 macrophages between agmatine treatment group (Agm, n=3) and experimental control group (EC, n=3, C&D). Median fluorescence intensity (MFI) of iNOS was decreased with agmatine treatment (E) and MFI of CD206 was increased in agmatine treatment group (F) without significance.
M2 macrophages were increased with agmatine treatment in immunohistochemistry
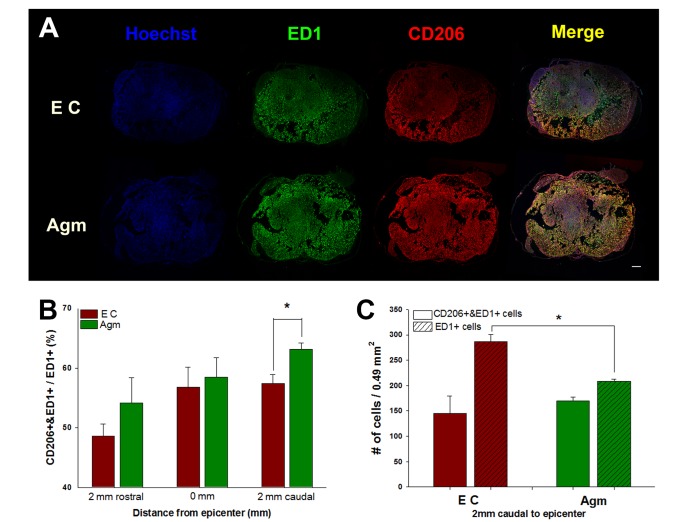
M2 macrophage expression was increased in agmatine treatment group with regional specificity (Fig. 2). Only in caudal to epicenter, M2 macrophage was significantly induced by agmatine treatment (Fig. 2A and B). The number of macrophages (ED1+ cells) was significantly reduced in agmatine treatment group but the numbers of M2 macrophages (CD206+ & ED1+ cells) were not difference between agmatine treatment group and experimental control group (Fig. 2C).
Fig. 2. Immunohistochemistry of macrophages 1 week after spinal cord injury. The representative coronal spinal cord sections 2 mm caudal to epicenter were shown in A. The portion of M2 macrophages (CD206+&ED1+/ED1+) was significantly increased in Agm only 2 mm caudal to epicenter compared to EC (B). Agmatine treatment significantly reduced the number of macrophages (ED1+ cells) 2 mm caudal to epicenter compared to EC (C). Agmatine treatment group (Agm, n=3); Experimental control group (EC, n=3). Scale bar is 200 um. *p<0.05.
The marker molecules and cytokines for M2 macrophages were increased in the agmatine treatment group
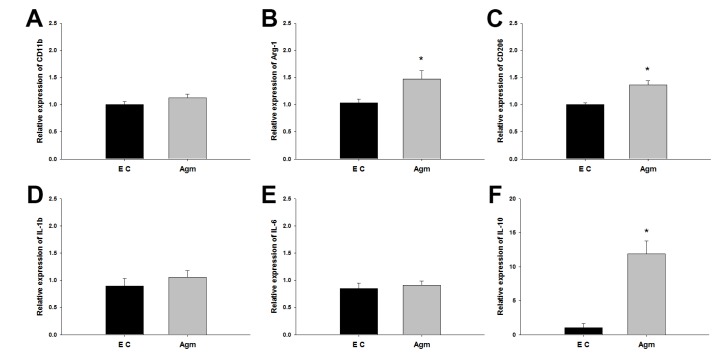
The mRNA expressions of M2 macrophage-related molecules and cytokines were confirmed 1 week after spinal cord injury using RT-PCR. In 10-mm length of spinal cord, CD11b as macrophage marker was not changed with or without agmatine administration (Fig. 3A). Arg-1 and CD206 expressed mainly in M2 macrophages [16] were significantly increased in the agmatine treatment group (Fig. 3B and C). IL-1b and IL-6 were known to be secreted in M1 macrophages [16]. Agmatine treatment made no change in IL-1b and IL-6 mRNA level (Fig. 3D and E), but IL-10 was highly overexpressed (about 11 folds) in the agmatine treatment group (Fig. 3F). IL-10 was known as a secreting factor of M2 macrophages and one of the stimuli to M2 macrophage polarization [16].
Fig. 3. The mRNA expression of immune-related molecules and cytokines 1 week after spinal cord injury. CD11b, macrophage marker, expressed similar between EC and Agm (A). Arg-1 and CD206, M2 macrophage marker, were significantly increased in Agm (B&C). Inflammatory cytokines, IL-1b and IL-6 were not changed with agmatine treatment (Agm) or without (EC, D&E) but IL-10, anti-inflammatory cytokine, was significantly increased in Agm (F). Agmatine treatment group (Agm, n=5); Experimental control group (EC, n=5). *p<0.05.
Agmatine increased BMP2 expression in mRNA level
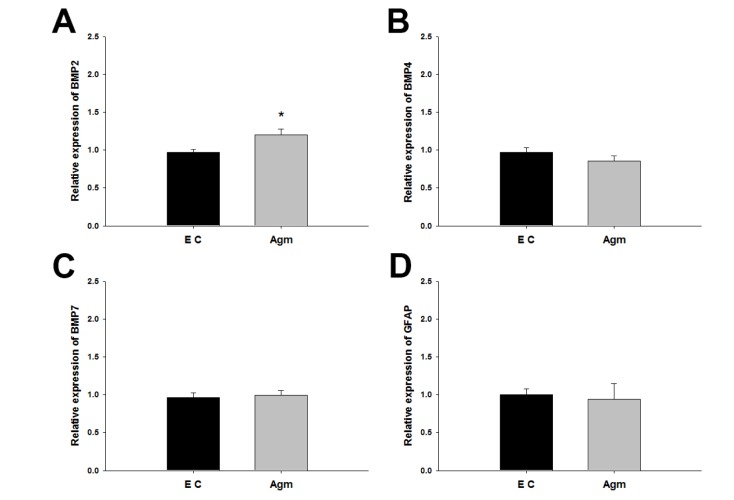
BMP family and GFAP mRNA expressions were validated 1 week after spinal cord injury. Among BMP2/4/7, only BMP2 mRNA expression was significantly increased in the agmatine treatment group (Fig. 4A). There was no change in BMP4 and BMP7 mRNA expressions between the agmatine treatment group and the experimental control group (Fig. 4B and C). The expression of GFAP mRNA, astrocyte marker, was not changed by agmatine treatment (Fig. 4D).
Fig. 4. The mRNA expression of BMPs and GFAP 1 week after spinal cord injury. BMP2 was expressed higher in Agm than EC (A) but BMP4, BMP7 and GFAP was not changed between Agm and EC (B-D). Agmatine treatment group (Agm, n=5); Experimental control group (EC, n=5). *p<0.05.
DISCUSSION
In this study, it was clarified whether agmatine modulated the M2 macrophage acute phase in spinal cord injury. Beck et al. reported that the peak expression of macrophages/microglia is 1 week after spinal cord injury in rats [17], so this study was done 1 week after spinal cord injury. In FACS results, agmatine treatment made no change in neither the number of M2 macrophages nor the number of M1 macrophages (Fig. 1A~D). Although there was no statistical significance, median fluorescence intensity (MFI) of CD206, M2 macrophage, was higher in the agmatine treatment group and MFI of iNOS, M1 macrophage, was lower in the agmatine treatment group compared to the experimental control group (Fig. 1E and F). These results suggest that agmatine could enhance the M2 macrophage property without increasing cell number. Regional expressions of M1 and M2 macrophages were confirmed by immunohistochemistry using coronal sectioned spinal cord (Fig. 2). The portion of M2 macrophage (CD206+ & ED1+/ED1+) was significantly increased only caudal to epicenter (Fig. 2B), but the number of CD206 & ED1 double positive cells was similar between the agmatine treatment and experimental control groups (Fig. 2C). In contrast, the number of ED1 positive cells (macrophages) was significantly decreased by agmatine treatment (Fig. 2C), so the portion of M2 macrophages was increased in the agmatine treatment group. This was consistent with a previous report that agmatine injection (100 mg/kg, IP) reduced the number of macrophages in cerebral ischemic injured brain and LPS-injured brain [13,14]. It was published that encapsulated human mesenchymal stromal cells (MSCs) increased M2 macrophages 8 days after spinal cord injury and increased portion of M2 macrophages was shown 2.5 mm caudal to epicenter only [18]. The reason for this was that encapsulated human MSCs were located caudal to epicenter, so the M2 macrophage population was affected only in caudal side, not in rostral side. In this study, agmatine was injected intraperitoneally which means exogenous agmatine affected the whole spinal cord, rostral and caudal to epicenter, through the blood stream. Therefore, the regional specific change of M2 macrophage expression shown in this study might be correlated with spinal cord blood flow and blood-spinal cord barrier. Spinal cord blood flow spontaneously recovered within 7 days after spinal cord injury through spontaneous angiogenesis [19,20]. Blood-spinal cord barrier permeability increased during the acute phase after spinal cord injury, but reduced and closed to sham 1 week after spinal cord injury [21]. Recently, Figley et al. reported spatial-temporal disruption of the vasculature in clip-compression spinal cord injury [21]. In this report, the number of vascular counts was significantly reduced 1mm rostral side to epicenter until 10 days after spinal cord injury. It was also reduced 1mm caudal but there was no significance. Based on this report, systemic administrated agmatine might be delivered more effectively caudal side to epicenter than rostral side, so significant increasing of M2 macrophages were shown only caudal side in the agmatine treatment group. In mRNA level, M2 macrophage marker arginase-1 and CD206 were significantly increased by agmatine treatment and M1 macrophage-expressing IL-1b and IL-6 were not changed (Fig. 3A~E). M2 macrophage-expressing IL-10 was significantly overexpressed in the agmatine treatment group (Fig. 3F) and it was reported that M1-to-M2 switch is promoted by IL-10 [5,22,23]. Therefore, overexpression of IL-10 might be one of the pathways to modulate M2 macrophages under agmatine treatment. Matsuura et al. reported that BMP2/4 inhibition enhanced axonal growth and functional recovery after spinal cord injury [24]. On the other hand, BMP2 induced dopaminergic neuronal differentiation [25,26]. BMP7 was reported to be neuroprotective in stroke and spinal cord injury [27,28] and to polarize THP-1 cells into M2 macrophages [29,30]. Agmatine was found to increase BMP2/7 expression and reduce BMP4 after spinal cord injury in mice [10,31]. GFAP expression was also reduced by agmatine after spinal cord injury in mice [10]. So far all reports for modulation of BMPs by agmatine have been done in mice, so in this study, modulation was confirmed in rat spinal cord injury. BMP7 was not changed 1 week after spinal cord injury in rats (Fig. 4C). This is different with the result found in mice [10,31]. Setoguchi et al. reported that the peak expression of BMP7 mRNA is 4 days after spinal cord injury in rats and the expression 7 days after spinal cord injury decreased about half from the peak time point [32], so the modulation of BMP7 by agmatine administration should be confirmed at an early time point, within 7 days after spinal cord injury in rats. Only one research group reported that BMP7 modulated M2 macrophage polarization [29,30], but modulation of M2 macrophages by agmatine treatment seemed to be not related to BMP7 expression, at least in this study. BMP4 and GFAP mRNA were also not changed with or without agmatine administration 1 week after spinal cord injury (Fig. 4B and D). This is also different to previous study using mice [10]. BMP2 expression was only significantly increased by agmatine 1 week after spinal cord injury in rats (Fig. 4A) and this phenomenon was also confirmed in mice spinal cord injury [10]. One paper recently reported that the supplementation of BMP-2 dramatically diminished the expression of M1 phenotypic markers including IL-1β, IL-6, and iNOS in M1 polarized macrophages and increased CD206 mRNA expression in IL-4 induced M2 macrophages [33].
Based on the results in this study and previous reports, it is suggested that agmatine treatment is one possible mechanism to modulate the expression of M2 macrophages after spinal cord injury through induced expression of IL-10 and BMP2.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (NRF-2013R1A1A2062711 and NRF-2017R1A2B2005350) and IBS-R015-D1.
References
- 1.Plemel JR, Wee Yong V, Stirling DP. Immune modulatory therapies for spinal cord injury--past, present and future. Exp Neurol. 2014;258:91–104. doi: 10.1016/j.expneurol.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 2.Hayakawa K, Okazaki R, Morioka K, Nakamura K, Tanaka S, Ogata T. Lipopolysaccharide preconditioning facilitates M2 activation of resident microglia after spinal cord injury. J Neurosci Res. 2014;92:1647–1658. doi: 10.1002/jnr.23448. [DOI] [PubMed] [Google Scholar]
- 3.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shechter R, Miller O, Yovel G, Rosenzweig N, London A, Ruckh J, Kim KW, Klein E, Kalchenko V, Bendel P, Lira SA, Jung S, Schwartz M. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity. 2013;38:555–569. doi: 10.1016/j.immuni.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma SF, Chen YJ, Zhang JX, Shen L, Wang R, Zhou JS, Hu JG, Lü HZ. Adoptive transfer of M2 macrophages promotes locomotor recovery in adult rats after spinal cord injury. Brain Behav Immun. 2015;45:157–170. doi: 10.1016/j.bbi.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Mikita J, Dubourdieu-Cassagno N, Deloire MS, Vekris A, Biran M, Raffard G, Brochet B, Canron MH, Franconi JM, Boiziau C, Petry KG. Altered M1/M2 activation patterns of monocytes in severe relapsing experimental rat model of multiple sclerosis. Amelioration of clinical status by M2 activated monocyte administration. Mult Scler. 2011;17:2–15. doi: 10.1177/1352458510379243. [DOI] [PubMed] [Google Scholar]
- 7.Li G, Regunathan S, Barrow CJ, Eshraghi J, Cooper R, Reis DJ. Agmatine: an endogenous clonidine-displacing substance in the brain. Science. 1994;263:966–969. doi: 10.1126/science.7906055. [DOI] [PubMed] [Google Scholar]
- 8.Piletz JE, Chikkala DN, Ernsberger P. Comparison of the properties of agmatine and endogenous clonidinedisplacing substance at imidazoline and alpha-2 adrenergic receptors. J Pharmacol Exp Ther. 1995;272:581–587. [PubMed] [Google Scholar]
- 9.Yu CG, Marcillo AE, Fairbanks CA, Wilcox GL, Yezierski RP. Agmatine improves locomotor function and reduces tissue d amage following s pinal c ord i njury. Neuroreport. 2000;11:3203–3207. doi: 10.1097/00001756-200009280-00031. [DOI] [PubMed] [Google Scholar]
- 10.Park YM, Lee WT, Bokara KK, Seo SK, Park SH, Kim JH, Yenari MA, Park KA, Lee JE. The multifaceted effects of agmatine on functional recovery after spinal cord injury through modulations of BMP-2/4/7 expressions in neurons and glial cells. PLoS One. 2013;8:e53911. doi: 10.1371/journal.pone.0053911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JH, Lee YW, Park KA, Lee WT, Lee JE. Agmatine attenuates brain edema through reducing the expression of aquaporin-1 after cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:943–949. doi: 10.1038/jcbfm.2009.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JH, Yenari MA, Giffard RG, Cho SW, Park KA, Lee JE. Agmatine reduces infarct area in a mouse model of transient focal cerebral ischemia and protects cultured neurons from ischemia-like injury. Exp Neurol. 2004;189:122–130. doi: 10.1016/j.expneurol.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 13.Ahn SK, Hong S, Park YM, Lee WT, Park KA, Lee JE. Effects of agmatine on hypoxic microglia and activity of nitric oxide synthase. Brain Res. 2011;1373:48–54. doi: 10.1016/j.brainres.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Ahn SK, Hong S, Park YM, Choi JY, Lee WT, Park KA, Lee JE. Protective effects of agmatine on lipopolysaccharide-injured microglia and inducible nitric oxide synthase activity. Life Sci. 2012;91:1345–1350. doi: 10.1016/j.lfs.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen HX, Beck KD, Anderson AJ. Quantitative assessment of immune cells in the injured spinal cord tissue by flow cytometry: a novel use for a cell purification method. J Vis Exp. 2011:2698. doi: 10.3791/2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gensel JC, Zhang B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015;1619:1–11. doi: 10.1016/j.brainres.2014.12.045. [DOI] [PubMed] [Google Scholar]
- 17.Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM, Anderson AJ. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain. 2010;133:433–447. doi: 10.1093/brain/awp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barminko J, Kim JH, Otsuka S, Gray A, Schloss R, Grumet M, Yarmush ML. Encapsulated mesenchymal stromal cells for in vivo transplantation. Biotechnol Bioeng. 2011;108:2747–2758. doi: 10.1002/bit.23233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loy DN, Crawford CH, Darnall JB, Burke DA, Onifer SM, Whittemore SR. Temporal progression of angiogenesis and basal lamina deposition after contusive spinal cord injury in the adult rat. J Comp Neurol. 2002;445:308–324. doi: 10.1002/cne.10168. [DOI] [PubMed] [Google Scholar]
- 20.Kang CE, Clarkson R, Tator CH, Yeung IW, Shoichet MS. Spinal cord blood flow and blood vessel permeability measured by dynamic computed tomography imaging in rats after localized delivery of fibroblast growth factor. J Neurotrauma. 2010;27:2041–2053. doi: 10.1089/neu.2010.1345. [DOI] [PubMed] [Google Scholar]
- 21.Figley SA, Khosravi R, Legasto JM, Tseng YF, Fehlings MG. Characterization of vascular disruption and blood-spinal cord barrier permeability following traumatic spinal cord injury. J Neurotrauma. 2014;31:541–552. doi: 10.1089/neu.2013.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vereyken EJ, Heijnen PD, Baron W, de Vries EH, Dijkstra CD, Teunissen CE. Classically and alternatively activated bone marrow derived macrophages differ in cytoskeletal functions and migration towards specific CNS cell types. J Neuroinflammation. 2011;8:58. doi: 10.1186/1742-2094-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weisser SB, McLarren KW, Kuroda E, Sly LM. Generation and characterization of murine alternatively activated macrophages. Methods Mol Biol. 2013;946:225–239. doi: 10.1007/978-1-62703-128-8_14. [DOI] [PubMed] [Google Scholar]
- 24.Matsuura I, Taniguchi J, Hata K, Saeki N, Yamashita T. BMP inhibition enhances axonal growth and functional recovery after spinal cord injury. J Neurochem. 2008;105:1471–1479. doi: 10.1111/j.1471-4159.2008.05251.x. [DOI] [PubMed] [Google Scholar]
- 25.Reiriz J, Espejo M, Ventura F, Ambrosio S, Alberch J. Bone morphogenetic protein-2 promotes dissociated effects on the number and differentiation of cultured ventral mesencephalic dopaminergic neurons. J Neurobiol. 1999;38:161–170. [PubMed] [Google Scholar]
- 26.Hegarty SV, Sullivan AM, O'Keeffe GW. BMP2 and GDF5 induce neuronal differentiation through a Smad dependant pathway in a model of human midbrain dopaminergic neurons. Mol Cell Neurosci. 2013;56:263–271. doi: 10.1016/j.mcn.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Chang CF, Lin SZ, Chiang YH, Morales M, Chou J, Lein P, Chen HL, Hoffer BJ, Wang Y. Intravenous administration of bone morphogenetic protein-7 after ischemia improves motor function in stroke rats. Stroke. 2003;34:558–564. doi: 10.1161/01.str.0000051507.64423.00. [DOI] [PubMed] [Google Scholar]
- 28.de Rivero Vaccari JP, Marcillo A, Nonner D, Dietrich WD, Keane RW. Neuroprotective effects of bone morphogenetic protein 7 (BMP7) treatment after spinal cord injury. Neurosci Lett. 2009;465:226–229. doi: 10.1016/j.neulet.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Rocher C, Singla DK. SMAD-PI3K-Akt-mTOR pathway mediates BMP-7 polarization of monocytes into M2 macrophages. PLoS One. 2013;8:e84009. doi: 10.1371/journal.pone.0084009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rocher C, Singla R, Singal PK, Parthasarathy S, Singla DK. Bone morphogenetic protein 7 polarizes THP-1 cells into M2 macrophages. Can J Physiol Pharmacol. 2012;90:947–951. doi: 10.1139/y2012-102. [DOI] [PubMed] [Google Scholar]
- 31.Kim JH, Lee YW, Park YM, Park KA, Park SH, Lee WT, Lee JE. Agmatine-reduced collagen scar area accompanied with surface righting reflex recovery after complete transection spinal cord injury. Spine (Phila Pa 1976) 2011;36:2130–2138. doi: 10.1097/BRS.0b013e318205e3f7. [DOI] [PubMed] [Google Scholar]
- 32.Setoguchi T, Yone K, Matsuoka E, Takenouchi H, Nakashima K, Sakou T, Komiya S, Izumo S. Traumatic injury-induced BMP7 expression in the adult rat spinal cord. Brain Res. 2001;921:219–225. doi: 10.1016/s0006-8993(01)03123-7. [DOI] [PubMed] [Google Scholar]
- 33.Wei F, Zhou Y, Wang J, Liu C, Xiao Y. The immunomodulatory role of BMP-2 on macrophages to accelerate osteogenesis. Tissue Eng Part A. 2017 doi: 10.1089/ten.TEA.2017.0232. (in press) [DOI] [PubMed] [Google Scholar]