Abstract
AIM
To review the incidence of graft loss and acute rejection among renal transplant recipients with early reduction of immunosuppression for BK viremia.
METHODS
We performed a retrospective analysis of consecutive de-novo kidney-only transplants from January 2009 to December 2012 to evaluate the incidence of Polyoma-virus associated nephropathy (PyVAN). Recipient plasma was screened for BKV DNA via quantitative polymerase chain reaction (PCR) at months 1, 3, 6, 9 and 12 post-transplant and on worsening graft function. Immunosuppression was reduced at ≥ 3-log copies/mL. Those with viremia of ≥ 4-log copies/mL (presumptive PyVAN) underwent renal transplant biopsy. Presumptive PyVAN (PP) and definitive PyVAN (DP; biopsy-proven) were treated by immunosuppression reduction (IR) only.
RESULTS
Among 319 kidney transplant recipients, the median age was 53 years (range 19-83), 65.8% were male, and 58.9% were white. Biopsy-proven acute rejection was found in 18.5% within 0-168 wk. Death-censored graft loss occurred in 5.3% (n = 17) and graft loss attributable to PyVAN was 0.6% (n = 2). Forty-seven patients were diagnosed with PP (14.7%) and 18 (5.6%) with DP. Graft loss among participants with PyVAN (8.5%) and those without (4.8%) was not significantly different. Deceased donor kidney transplantation (OR = 2.3, 95%CI = 1.1-4.6) and AR (OR = 2.3, 95%CI = 1.2-4.7) were associated with PyVAN in the multivariate analysis. BK viremia between 3 and 4-log copies/mL occurred in 27 patients, all of whom underwent IR. Of these, 16 (59%) never developed PyVAN while 11 (41%) developed PyVAN (4 DP, 7 PP) within a range of 11-39 wk.
CONCLUSION
Instituting an early reduction of immunosuppression, in the absence of adjunctive antivirals, is effective at preventing PyVAN and may be associated with a lower incidence of graft-loss without a reciprocal increase in the incidence of acute rejection.
Keywords: BK virus, Renal transplant, Screening, PyVAN, Prevention, Graft loss
Core tip: The authors describe results of a retrospective study of 319 renal transplant recipients who underwent reduction of immunosuppression for BK viremia at a BK viral of ≥ 3-log copies/mL. Instituting early reduction of immunosuppression in the absence of adjunctive antivirals was effective in reducing the incidence of graft loss due to Polyoma-virus associated nephropathy (PyVAN) without a reciprocal increase in acute rejection. Our study also emphasizes that efforts to implement universal BK virus polymerase chain reaction assay standards recently developed by the World Health Organization are key in establishing a preventative strategy for PyVAN that is widely applicable and highly effective.
INTRODUCTION
BK virus (BKV) is a polyomavirus that causes widespread sub-clinical infection at a young age and subsequently establishes long-term latency in cells of the renal and urinary systems. In recipients of renal allograft transplants and allogeneic hematopoietic stem cell transplants, high-level BKV replication may lead to overt clinical disease. BK-polyoma virus associated nephropathy (PyVAN) is a major complication of kidney transplantation, occurring in 1%-10% of renal transplant recipients[1,2]. PyVAN is directly associated with graft failure[3,4] due to progressive interstitial nephritis and indirectly linked to allograft rejection due to immunosuppression reduction (IR), which is the cornerstone of PyVAN treatment[5]. Guidelines currently recommend prospective screening for BKV reactivation post-transplantation, by using urine cytology to detect decoy cells or testing for high-level BK viruria and/or viremia. In the event of a sustained BK viremia of ≥ 4-log copies/mL for more than 3 wk, a renal biopsy is recommended to confirm the diagnosis of PyVAN by demonstrating polyomavirus cytopathic changes with confirmation by immunohistochemical staining[6]. In addition, a prompt reduction of immunosuppression is critical in an attempt to abrogate the full-fledged manifestations of the disease. Although agents with anti-BK activity such as leflunomide[7], cidofovir[8] and quinolones[9] have been used[10], randomized controlled trials proving their efficacy are lacking.
The positive predictive value of BK viremia at a cut-off of 7 × 103 copies/mL (approximately 4 log copies/mL) has been estimated at 50% to 60% for detecting proven PyVAN within 2 to 6 wk but rises to 90% when a threshold of 6-log copies/mL is implemented[1,11,12]. A lower threshold of 3-log copies/mL may increase the sensitivity, leading to the identification of more cases, and earlier in the natural course when intervention may be more effective and graft loss more likely to be averted. In this paper, we aim to assess the incidence of PyVAN and graft loss in a single transplant center while implementing a reduction of immunosuppression at BKV loads of ≥ 3-log BKV copies/mL.
MATERIALS AND METHODS
We performed a retrospective analysis of consecutive de-novo kidney transplants at Yale-New Haven Hospital (YNHH), who underwent screening for PyVAN screening and prevention, from January 2009 to December 2012. The Yale University Institutional Review Board approved this study and all procedures conducted were in accordance with the Helsinki Declaration of 1975. Individuals included in the study were above the age of 18 years and underwent primary kidney-only transplant. Medical records were reviewed for data on demographics, comorbidities, and transplant parameters including type of transplant (deceased or living donor), CMV donor and recipient serostatus, induction and maintenance immunosuppression, presence of delayed graft function (DGF), biopsy-proven acute rejection (AR), graft loss and its etiology, last follow-up visit, deaths, BK viral load (copies/mL) and biopsy-proven PyVAN. Graft loss was censored for episodes of death with a functioning graft.
Presumptive PyVAN (PP) was defined as sustained BK viremia ≥ 4-log copies/mL while definitive PyVAN (DP) required cytopathic changes on renal biopsy that were confirmed by positive BKV immunohistochemistry[6]. Renal allograft rejection was graded in accordance with the BANFF working classification of renal allograft pathology[13]. DGF was defined as acute renal failure requiring dialysis within 7 d of transplantation. Graft failure was defined as chronic allograft nephropathy leading to the resumption of chronic renal replacement therapy. Primary outcomes included both presumptive and definitive PyVAN while the secondary outcomes were graft survival and acute rejection.
The YNHH kidney transplant program has been active since 1967 and performs approximately 100 kidney transplantations annually. The standard maintenance immunosuppressive regimen consists of tacrolimus, mycophenolate mofetil and low dose prednisone (5-10 mg daily). The target tacrolimus trough level for the first 30 d post-transplant was 8-10 ng/mL and 5-7 ng/mL thereafter. As part of the institutional protocol for PyVAN screening and prevention, transplant recipient plasma is screened for BKV DNA via quantitative PCR. For the first two years of the study, an NIH-developed, real-time BKV PCR assay targeting the viral T antigen gene was used[14]. Due to concerns about potential under-quantitation of some BKV subtypes, a multiplex real-time PCR assay developed at the University of Washington (UW) that targets both VP1 and T genes using two primer sets and three probes was implemented in December 2010[15].
Per protocol, a serum BKV DNA viral load (VL) is obtained at months 1, 3, 6, 9 and 12 post-transplant and in case of worsening graft function. A BKV DNA VL between 3 and 3.99 log copies/mL prompted a 50% dose reduction of mycophenolate mofetil, a reduced target tacrolimus trough level of 5 ng/mL and monthly plasma BKVL until negative. Additionally, mycophenolate mofetil was discontinued and a renal biopsy was with immunohistochemical staining was performed if the serum BKV VL was above ≥ 4-log copies/mL PP and DP were treated by reduction of immunosuppression, without adjunctive anti-viral treatment.
Statistical analysis
Statistical analysis was performed using SPSS software, version 16.0.0.0. In univariate analyses, χ2 and Fisher’s exact test (when appropriate) were used to evaluate categorical variables and Mann-Whitney U test was used for continuous variables. Predictors of PyVAN were identified using a multivariate logistic regression model. Only variables with a P-value < 0.10 on univariate analysis were entered into a stepwise multivariate logistic regression model to identify factors independently associated with Presumptive PyVAN. All tests were double-tailed, with an assumed type 1 error risk a equal to 5%. Kaplan-Meier survival curves were plotted using GraphPad Prism version 6.03 (GraphPad Software, San Diego, CA, United States).
RESULTS
A total of 330 primary kidney transplant recipients were identified and were followed for a median time of 42 ± 14.7 mo. BK screening data on 11 patients was unavailable and thus they were excluded, leaving 319 patients available for analysis. The median age was 53 years (range 19-83), 65.8% were male, 58.9% were white, and 27.0% had diabetes mellitus. A CMV D+/R- serostatus was present in 18.2% of transplants and 54.5% of recipients underwent a deceased-donor kidney transplantation (DDKT). Induction immunosuppressive therapy consisted of basiliximab (44.8%), anti-thymocyte globulin (37.0%) or daclizumab (17.6%). Maintenance immunosuppressive therapy included both a calcineurin inhibitor and mycophenolate mofetil in 95% of cases and 95% received steroids. Biopsy-proven rejection was found in 18.5% (n = 59) of transplant recipients within 0-168 wk. Graft loss occurred in 5.3% (n = 17) and PyVAN-associated graft los occurred in 0.6% (n = 2). Causes of graft loss included: AR (n = 7), antibody-mediated chronic rejection (n = 2), PyVAN (n = 2), CMV nephropathy (n = 1), hypoplastic kidney disease (n = 1), ureteral obstruction (n = 1), renal graft vein thrombosis (n = 1) and unknown cause (n = 2). Death ensued in 6.6% (n = 21) of the sample. A detailed list of demographics is found in Table 1.
Table 1.
Demographics and outcomes n (%)
| Variable | All sample (n = 319) | PyVAN negative (n = 272) | PyVAN positive (n = 47) |
| Age (mean, yr) | 53 | 51 | 53.1 |
| Male | 210 (65.8) | 177 (65.1) | 33 (70.2) |
| Black | 86 (27.0) | 67 (24.6) | 19 (40.4) |
| Diabetes mellitus | 86 (27.0) | 71 (26.1) | 15 (32.0) |
| CMV D+/R- | 58 (18.2) | 49 (18.0) | 9 (19.1) |
| DDKT | 174 (54.5) | 140 (51.5) | 34 (72.3) |
| Induction immunosuppression | |||
| Thymoglobulin | 118 (37.0) | 97 (35.7) | 21 (44.7) |
| Basiliximab | 143 (44.8) | 124 (45.6) | 19 (40.4) |
| Daclizumab | 56 (17.6) | 50 (18.4) | 6 (12.8) |
| Delayed graft function | 58 (18.2) | 47 (17.3) | 11 (23.4) |
| Acute rejection | 59 (18.5) | 44 (16.2) | 15 (32.0) |
| Graft loss | 17 (5.3) | 13 (4.8) | 4 (8.5) |
| Death | 21 (6.6) | 15 (5.5) | 6 (12.8) |
DDKT: Deceased-donor kidney transplantation; PyVAN: Polyoma-virus associated nephropathy.
BK viremia of ≥ 3-log copies/mL was detected in 63 (19.7%) recipients. Of these, 47 (14.7%) were subsequently diagnosed with PP at a median time of 25 wk from initial screening. A renal biopsy was performed in 34 of these recipients and 18 (5.6% of the original sample) were confirmed to have DP. Two patients with DP progressed to graft failure and 4 developed AR within 90 d after reduction of immunosuppression.
The majority of the 319 patients included in the study (85.3%) never developed PyVAN. Time to first BK viremia was 190 d in patients with PyVAN and 235 d in those without. Graft loss occurred in 8.5% of patients with PyVAN vs. 4.8% of those without. Graft survival for 1-year, 3-year and 5-years were 99%, 95% and 92% respectively. A Kaplan-Meier curve of graft survival over time for recipients with and without PP (Figure 1) showed that survival was not significantly different between groups (logrank P = 0.93).
Figure 1.
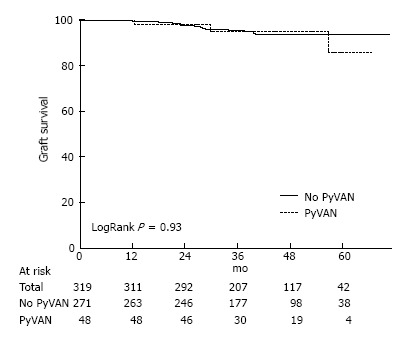
Kaplan-Meier Survival curve showing graft survival over time for recipients with and without presumptive polyoma-virus associated nephropathy. PyVAN: Polyoma-virus associated nephropathy.
In a univariate analysis of recipients diagnosed with PP (n = 47) compared to recipients without PyVAN (n = 272), black race, DDKT and AR were significantly associated with PyVAN (P < 0.10). In a subsequent multivariate analysis, only DDKT (OR = 2.24; 95%CI = 1.1-4.54) and AR (OR = 2.42; 95%CI = 1.19-4.29) were significantly associated with PyVAN (P < 0.05). In this model, PyVAN was not associated with delayed graft function, graft loss or increased mortality. A full description covariates included in the fit-model is found in Table 2.
Table 2.
Predictors of polyoma-virus associated nephropathy
| Predictor |
Univariate analysis |
Multivariate analysis |
||||
| PyVAN negative (n = 272) | PyVAN positive (n = 47) | P value | OR | CI | P value | |
| Age (mean, yr) | 51 | 53.1 | 0.343 | |||
| Male | 177 | 33 | 0.493 | |||
| Black race | 67 | 19 | 0.024a | 1.68 | 0.86-3.31 | 0.13 |
| Diabetes mellitus | 71 | 15 | 0.407 | |||
| CMV D+/R- | 49 | 9 | 0.839 | |||
| DDKT | 140 | 34 | 0.008a | 2.24 | 1.1-4.54 | 0.03a |
| Thymoglobulin | 97 | 21 | 0.237 | |||
| Basiliximab | 124 | 19 | 0.511 | |||
| Daclizumab | 50 | 6 | 0.412 | |||
| Delayed graft function | 47 | 11 | 0.315 | |||
| Acute rejection | 44 | 15 | 0.010a | 2.42 | 1.19-4.29 | 0.02a |
| Graft loss | 13 | 4 | ||||
| Death | 16 | 6 | 0.112 | |||
P < 0.05. DDKT: Deceased-donor kidney transplantation; PyVAN: Polyoma-virus associated nephropathy.
While the majority of patients with high-level viremia were found to have an initial BKVL above 4 log copies/mL, an initial BK viral load between 3 and 4-log copies/mL was reported in 27 transplant recipients, all of whom underwent reduction of immunosuppression, without administration of adjunctive anti-viral therapy. Of these, 16 (59%) never developed PyVAN while 11 (41%) developed PyVAN within a range of 11-39 wk. Among the 11 recipients with PyVAN, 4 were proven by renal biopsy and 7 were presumptive. Two of 27 recipients developed AR and none developed graft loss.
Since the BK PCR assay changed midway through the study, we compared the incidence of PyVAN when using the NIH assay (January 2009 to December 2010) to the incidence when using the UW assay (January 2011 to December 2012). In a univariate chi-square analysis of all recipients with first BK viremia, 17/33 (52%) had PyVAN before the assay change versus 30/59 (51%) after the change (P = 0.95).
In addition, we reviewed BKVL data of patients with PP to evaluate for adherence or deviation from the set protocol for post-transplant viral load screening. Among 47 patients with PP, 16 patients underwent screening beyond the recommended interval during the study period and were found to have high-level viremia on belated screening. For 3 patients, screening was done within 10 d of recommended time point. A summary of patients in whom protocol deviation occurred is found in Table 3.
Table 3.
Protocol deviation among patients with presumptive polyoma-virus associated nephropathy1
| Patient | Time point of protocol deviation post-transplant | Target days between serial screening | Actual days between serial screening | BKVL change (copies/mL) |
| 1 | Month 1 to month 3 | 60 d | 100 d | ND to 1065190 |
| 2 | Month 1 to month 3 | 60 d | 138 d | ND to 17478 |
| 3 | Month 9 to month 12 | 90 d | 214 d | < 1000 to 1076120 |
| 4 | Month 6 to month 9 | 90 d | 393 d | < 1000 to 1269650 |
| 5 | Month 6 to month 9 | 90 d | 113 d | ND to 57982 |
| 6 | Month 1 to month 3 | 60 d | 134 d | ND to 392527 |
| 7 | Month 3 to month 6 | 90 d | 133 d | ND to 627218 |
| 8 | Month 3 to month 6 | 90 d | 108 d | ND to 82354 |
| 9 | DOT to month 1 | 30 d | 121 d | 157939 at month 1 |
| 10 | Month 3 to month 6 | 90 d | 137 d | ND to 74389 |
| 11 | Month 1 to month 3 | 60 d | 179 d | ND to 28592 |
| 12 | Month 1 to month 3 | 60 d | 94 d | ND to 39000 |
| 13 | DOT to month 1 | 30 d | 58 d | 17558 at month 1 |
Three patients with < 10 d of deviation from protocol were not included. ND: Not detectable.
DISCUSSION
Though established guidelines recommend a reduction of immunosuppression at a sustained BK viremia of ≥ 4-log copies/mL, studies vary significantly with regards to the implemented threshold. Cutoffs of any viremia[16,17], of ≥ 3-log copies/mL[18] and of ≥ 4-log copies/mL[19] have been used with varying rates of PyVAN, graft loss and acute rejection. The incidence of PP in this study, using a lower threshold (≥ 3-log copies/mL) of BK viremia for reduction of immunosuppression, was consistent with previously reported rates. In contrast to several investigations conducted in the last decade, which reported rates of graft loss of 15%-60% within 5 years of transplant, our incidence of graft loss, was 5.3%[5,20-23]. The incidence of PyVAN-associated graft loss in this study (0.6%) was commensurate with more recently published data from 2009-2013, in which BK associated graft loss ranged from 0%-0.85%[1,19,24-26]. Early reduction of immunosuppression in the setting of BK viremia, though potentially associated with decreased rates of graft loss due to BK nephropathy, carries the potential for increased rates of acute rejection. However, in this study, the incidence of acute rejection (18.5%) was also in keeping with previously reported rates[19,25], suggesting that early reduction of immunosuppression may not necessarily increase the risk of acute rejection.
In the past decade, there has been a steady trend towards decreased rates of graft loss. This is thought to be the result of improved diagnostic tools including immunostaining and PCR, which better differentiate virus-induced nephropathy from acute rejection, as well as targeted interventions to promptly identify BK viremia and reduce immunosuppression earlier[27]. A multitude of factors including the potency of induction and maintenance immunosuppressive regimens, demographic differences such as in age and race, frequency of BK viral load monitoring and use of adjunctive anti-virals may account for observed differences. The heterogeneity of these studies is further compounded by variation in the sensitivity, lower limit of detection of the BK virus PCR assay and most importantly a lack of equivalence of quantitation between different assays. Complicating matters is the presence of multiple viral subtypes, some of which (serotypes 3 and 4) are particularly prone to under-quantitation. In this study, we found no statistical difference in the number of patients with any viremia when sequentially comparing two different molecular assays but results from laboratories using different assays have been shown to vary significantly, even when performed on the same sample[15]. Since specific BK VL cutoffs are used to trigger interventions, assay variability is a critical issue and may indeed explain the variability in thresholds used across different transplant centers. A BK PCR standard that can be applied across laboratories is paramount in implementing a uniform BK viremia threshold for reduction of immunosuppression. In 2015, the World Health Organization (WHO) took steps to establish an international standard for BKV DNA nucleic acid amplification technique-based assays, using purified virions from BKV infected cell cultures[28]. This standard, however, has not yet been widely adopted and additional in-vitro verification data and in-vivo clinical data are needed to ascertain its performance characteristics. Until then, performing serial testing on individual patients using the same assay within the same laboratory, eschewing over-interpretation of small viral load changes as biologically important and establishing center-specific viral load cutoffs to guide clinical decision making in local patient populations will facilitate the interpretation of current BK viral load testing.
Certainly, adequate implementation of screening protocols is another critical factor in optimizing preventative strategies. In our study, a substantial number of patients with presumptive PyVAN did not adhere to the scheduled BKVL screening time-points. Strict adherence to screening protocol is likely to reduce the incidence of PyVAN by identifying viremia earlier and allowing for early interventions.
Instituting an early reduction of immunosuppression at ≥ 3-log copies/mL, in the absence of adjunctive antivirals, was effective at preventing PyVAN in our center and may be associated with a lower incidence of graft-loss without an increased rate of acute rejection compared to published data. However, efforts to implement the WHO BK standard will be key in establishing a universal preventive strategy for PyVAN that is both highly effective and widely applicable.
COMMENTS
Background
Polyoma virus associated nephropathy (PyVAN) caused by BK virus (BKV) is a major complication occurring in 1%-10% of renal transplant recipients that is directly associated with graft loss and indirectly associated with graft rejection.
Research frontiers
Guidelines currently recommend prospective screening for BKV reactivation post-transplantation, with reduction of immunosuppression at > 4-log copies/mL of BK virus. Additional research is needed to determine the best screening strategy.
Innovations and breakthroughs
The present study describes results of early reduction of immunosuppression (at ≥ 3-log copies/mL) in the absence of antivirals. This strategy effective at preventing PyVAN and was associated with a lower incidence of graft-loss without a reciprocal increase in the incidence of acute rejection.
Applications
Early reduction of immunosuppression should be considered as a strategy for prospective screening for BKV reactivation post-transplantation.
Terminology
PyVAN is a disease of the kidney that results from reactivation of BK virus in the setting of immune suppression, leading to cytopathic effect on renal tubular cells and secondary inflammation.
Peer-review
The information provided by the authors adds to the existing knowledge on the subject and will prove useful to the transplant community.
Footnotes
Institutional review board statement: The Yale University Institutional Review Board approved this study and all procedures conducted were in accordance with the Helsinki Declaration of 1975.
Conflict-of-interest statement: The authors have no conflicts of interest.
Data sharing statement: No additional data are available.
Manuscript source: Unsolicited manuscript
Specialty type: Transplantation
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: February 15, 2017
First decision: April 17, 2017
Article in press: August 2, 2017
P- Reviewer: Shrestha BM S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
Contributor Information
Marwan M Azar, Department of Pathology, Section of Microbiology, Massachusetts General Hospital, Boston, MA 02145, United States; Section of Infectious Diseases, Yale School of Medicine, New Haven, CT 06510, United States. mmazar@mgh.harvard.edu.
Roland Assi, Department of Surgery, Yale University School of Medicine, New Haven, CT 06510, United States.
Aziz K Valika, Adventist Health Partners, Chicago, IL 60521, United States.
David B Banach, Division of Infectious Diseases, University of Connecticut School of Medicine, Farmington, CT 06032, United States.
Isaac E Hall, Division of Hypertension and Nephrology, University of Utah School of Medicine, Dalt Lake City, UT 84132, United States.
Marie-Louise Landry, Department of Laboratory Medicine, Yale University School of Medicine, New Haven, CT 06510, United States.
Maricar F Malinis, Section of Infectious Diseases, Yale School of Medicine, New Haven, CT 06510, United States; Department of Surgery, Yale University School of Medicine, New Haven, CT 06510, United States.
References
- 1.Hirsch HH, Knowles W, Dickenmann M, Passweg J, Klimkait T, Mihatsch MJ, Steiger J. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med. 2002;347:488–496. doi: 10.1056/NEJMoa020439. [DOI] [PubMed] [Google Scholar]
- 2.Ramos E, Drachenberg CB, Portocarrero M, Wali R, Klassen DK, Fink JC, Farney A, Hirsch H, Papadimitriou JC, Cangro CB, et al. BK virus nephropathy diagnosis and treatment: experience at the University of Maryland Renal Transplant Program. Clin Transpl. 2002:143–153. [PubMed] [Google Scholar]
- 3.Hirsch HH. Polyomavirus BK nephropathy: a (re-)emerging complication in renal transplantation. Am J Transplant. 2002;2:25–30. doi: 10.1034/j.1600-6143.2002.020106.x. [DOI] [PubMed] [Google Scholar]
- 4.Randhawa PS, Demetris AJ. Nephropathy due to polyomavirus type BK. N Engl J Med. 2000;342:1361–1363. doi: 10.1056/NEJM200005043421809. [DOI] [PubMed] [Google Scholar]
- 5.Ramos E, Drachenberg CB, Papadimitriou JC, Hamze O, Fink JC, Klassen DK, Drachenberg RC, Wiland A, Wali R, Cangro CB, et al. Clinical course of polyoma virus nephropathy in 67 renal transplant patients. J Am Soc Nephrol. 2002;13:2145–2151. doi: 10.1097/01.asn.0000023435.07320.81. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch HH, Randhawa P; AST Infectious Diseases Community of Practice. BK polyomavirus in solid organ transplantation. Am J Transplant. 2013;13 Suppl 4:179–188. doi: 10.1111/ajt.12110. [DOI] [PubMed] [Google Scholar]
- 7.Josephson MA, Gillen D, Javaid B, Kadambi P, Meehan S, Foster P, Harland R, Thistlethwaite RJ, Garfinkel M, Atwood W, et al. Treatment of renal allograft polyoma BK virus infection with leflunomide. Transplantation. 2006;81:704–710. doi: 10.1097/01.tp.0000181149.76113.50. [DOI] [PubMed] [Google Scholar]
- 8.Wu SW, Chang HR, Lian JD. The effect of low-dose cidofovir on the long-term outcome of polyomavirus-associated nephropathy in renal transplant recipients. Nephrol Dial Transplant. 2009;24:1034–1038. doi: 10.1093/ndt/gfn675. [DOI] [PubMed] [Google Scholar]
- 9.Sharma BN, Li R, Bernhoff E, Gutteberg TJ, Rinaldo CH. Fluoroquinolones inhibit human polyomavirus BK (BKV) replication in primary human kidney cells. Antiviral Res. 2011;92:115–123. doi: 10.1016/j.antiviral.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Wright AJ, Gill JS. Strategies to prevent BK virus infection in kidney transplant recipients. Curr Opin Infect Dis. 2016;29:353–358. doi: 10.1097/QCO.0000000000000278. [DOI] [PubMed] [Google Scholar]
- 11.Bressollette-Bodin C, Coste-Burel M, Hourmant M, Sebille V, Andre-Garnier E, Imbert-Marcille BM. A prospective longitudinal study of BK virus infection in 104 renal transplant recipients. Am J Transplant. 2005;5:1926–1933. doi: 10.1111/j.1600-6143.2005.00934.x. [DOI] [PubMed] [Google Scholar]
- 12.Dall A, Hariharan S. BK virus nephritis after renal transplantation. Clin J Am Soc Nephrol. 2008;3 Suppl 2:S68–S75. doi: 10.2215/CJN.02770707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solez K, Axelsen RA, Benediktsson H, Burdick JF, Cohen AH, Colvin RB, Croker BP, Droz D, Dunnill MS, Halloran PF. International standardization of criteria for the histologic diagnosis of renal allograft rejection: the Banff working classification of kidney transplant pathology. Kidney Int. 1993;44:411–422. doi: 10.1038/ki.1993.259. [DOI] [PubMed] [Google Scholar]
- 14.Ryschkewitsch C, Jensen P, Hou J, Fahle G, Fischer S, Major EO. Comparison of PCR-southern hybridization and quantitative real-time PCR for the detection of JC and BK viral nucleotide sequences in urine and cerebrospinal fluid. J Virol Methods. 2004;121:217–221. doi: 10.1016/j.jviromet.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman NG, Cook L, Atienza EE, Limaye AP, Jerome KR. Marked variability of BK virus load measurement using quantitative real-time PCR among commonly used assays. J Clin Microbiol. 2008;46:2671–2680. doi: 10.1128/JCM.00258-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knight RJ, Gaber LW, Patel SJ, DeVos JM, Moore LW, Gaber AO. Screening for BK viremia reduces but does not eliminate the risk of BK nephropathy: a single-center retrospective analysis. Transplantation. 2013;95:949–954. doi: 10.1097/TP.0b013e31828423cd. [DOI] [PubMed] [Google Scholar]
- 17.Hardinger KL, Koch MJ, Bohl DJ, Storch GA, Brennan DC. BK-virus and the impact of pre-emptive immunosuppression reduction: 5-year results. Am J Transplant. 2010;10:407–415. doi: 10.1111/j.1600-6143.2009.02952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaub S, Hirsch HH, Dickenmann M, Steiger J, Mihatsch MJ, Hopfer H, Mayr M. Reducing immunosuppression preserves allograft function in presumptive and definitive polyomavirus-associated nephropathy. Am J Transplant. 2010;10:2615–2623. doi: 10.1111/j.1600-6143.2010.03310.x. [DOI] [PubMed] [Google Scholar]
- 19.Sood P, Senanayake S, Sujeet K, Medipalli R, Zhu YR, Johnson CP, Hariharan S. Management and outcome of BK viremia in renal transplant recipients: a prospective single-center study. Transplantation. 2012;94:814–821. doi: 10.1097/TP.0b013e31826690c6. [DOI] [PubMed] [Google Scholar]
- 20.Brennan DC, Agha I, Bohl DL, Schnitzler MA, Hardinger KL, Lockwood M, Torrence S, Schuessler R, Roby T, Gaudreault-Keener M, et al. Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am J Transplant. 2005;5:582–594. doi: 10.1111/j.1600-6143.2005.00742.x. [DOI] [PubMed] [Google Scholar]
- 21.Vasudev B, Hariharan S, Hussain SA, Zhu YR, Bresnahan BA, Cohen EP. BK virus nephritis: risk factors, timing, and outcome in renal transplant recipients. Kidney Int. 2005;68:1834–1839. doi: 10.1111/j.1523-1755.2005.00602.x. [DOI] [PubMed] [Google Scholar]
- 22.Wadei HM, Rule AD, Lewin M, Mahale AS, Khamash HA, Schwab TR, Gloor JM, Textor SC, Fidler ME, Lager DJ, et al. Kidney transplant function and histological clearance of virus following diagnosis of polyomavirus-associated nephropathy (PVAN) Am J Transplant. 2006;6:1025–1032. doi: 10.1111/j.1600-6143.2006.01296.x. [DOI] [PubMed] [Google Scholar]
- 23.Drachenberg CB, Papadimitriou JC, Wali R, Nogueira J, Mendley S, Hirsch HH, Cangro CB, Klassen DK, Weir MR, Bartlett ST, et al. Improved outcome of polyoma virus allograft nephropathy with early biopsy. Transplant Proc. 2004;36:758–759. doi: 10.1016/j.transproceed.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 24.Koukoulaki M, Grispou E, Pistolas D, Balaska K, Apostolou T, Anagnostopoulou M, Tseleni-Kotsovili A, Hadjiconstantinou V, Paniara O, Saroglou G, et al. Prospective monitoring of BK virus replication in renal transplant recipients. Transpl Infect Dis. 2009;11:1–10. doi: 10.1111/j.1399-3062.2008.00342.x. [DOI] [PubMed] [Google Scholar]
- 25.Alméras C, Vetromile F, Garrigue V, Szwarc I, Foulongne V, Mourad G. Monthly screening for BK viremia is an effective strategy to prevent BK virus nephropathy in renal transplant recipients. Transpl Infect Dis. 2011;13:101–108. doi: 10.1111/j.1399-3062.2011.00619.x. [DOI] [PubMed] [Google Scholar]
- 26.Thakur R, Arora S, Nada R, Minz M, Joshi K. Prospective monitoring of BK virus reactivation in renal transplant recipients in North India. Transpl Infect Dis. 2011;13:575–583. doi: 10.1111/j.1399-3062.2011.00634.x. [DOI] [PubMed] [Google Scholar]
- 27.Hariharan S. BK virus nephritis after renal transplantation. Kidney Int. 2006;69:655–662. doi: 10.1038/sj.ki.5000040. [DOI] [PubMed] [Google Scholar]
- 28.Sheila Govind JH, Clare Morris, Collaborative Study Group. Collaborative Study to establish the 1st WHO International Standard for BKV DNA for nucleic acid amplification technique (NAT)-based assays. Biologicals. 2016;44:423–433. doi: 10.1016/j.biologicals.2016.04.010. [DOI] [PubMed] [Google Scholar]


