Abstract
Tumors consist of a mixture of heterogeneous cell types. Cancer stem cells (CSCs) are a minor sub-population within the bulk cancer fraction which has been found to reconstitute and propagate the disease and to be frequently resistant to chemotherapy, irradiation, cytotoxic drugs and probably also against immune attack. CSCs are considered as the seeds of tumor recurrence, driving force of tumorigenesis and metastases. This underlines the urgent need for innovative methods to identify and target CSCs. However, the role and existence of CSCs in therapy resistance and cancer recurrence remains a topic of intense debate. The underlying biological properties of the tumor stem cells are extremely dependent on numerous signals, and the targeted inhibition of these stem cell signaling pathways is one of the promising approaches of the new antitumor therapy approaches. This perspective review article summarizes the novel methods of tracing CSCs and discusses the hallmarks of CSC identification influenced by the microenvironment or by having imperfect detection markers. In addition, explains the known molecular mechanisms of therapy resistance in CSCs as reliable and clinically predictive markers that could enable the use of new targeted antitumor therapy in the sense of personalized medicine.
Keywords: Cancer stem cells, Cancer recurrence, Cancer therapy, Combination therapy, Chemotherapy, Radiation therapy, Immunotherapy
Core tip: Cancer stem cells (CSCs) are small subpopulation of the tumor that can survive from conventional treatment, scape from the immune system and can cause recurrence of cancer disease. Therefore, any attempt in detection and selective therapeutic targeting of CSCs will ultimately lead to better cancer treatments and can play an important role in reducing the cancer related mortalities. This review highlights the trends and approaches in CSC tracing, isolating, characterizing and targeting, which are key strategies for a novel personalized molecular cancer therapy.
INTRODUCTION
Cancer originates from deregulation of growth and resistance to apoptosis of transformed cells that acquire proliferative and metastatic capacity. While only a few genetic and epigenetic alterations can initiate the malignant transformation of healthy cells, clinically visible tumors are extraordinarily complex structures with cancer cells displaying a large number of mutations and altered gene expression[1]. The hierarchical model of tumor organization represents a similar, albeit distorted, arrangement of the tumor cells, as are their tissues of origin. The stem cell population is positioned at the top of the cell hierarchy and has the ability to self-renew and multilineage differentiate to progenitors or differentiated cell types whose proliferation capacity is restricted[2,3].
The theory of the cancer stem cell (CSC) was postulated in the 1970s and was confirmed experimentally by the isolation of tumor-initiating cells using cellular/molecular biomarkers that allowed the isolation of CSCs in acute myeloid leukemia[4]. Further, CSC has been demonstrated in a variety of solid tumors such as tumors in brain, colorectal, head and neck, liver, lung, mammary glands, pancreatic prostate carcinomas, melanoma and hematopoietic malignancies (e.g., myeloid or lymphoid leukemia)[5-7]. Cell lines derived from these tumors also contain CSCs and tumor precursor cells, which represent a promising model for cancer stem cell research[1]. The functional characterization of CSCs revealed that these cells represent a small subpopulation of the tumor that can survive from conventional treatment, scape from the immune system and therefore can cause recurrence of cancer disease. Therefore, CSCs are driving force of tumorigenesis and metastases (Figure 1). According to the concept of a stem cell, it is assumed that even a few surviving CSCs after tumor therapy, is sufficient to form a new tumor[8].
Figure 1.
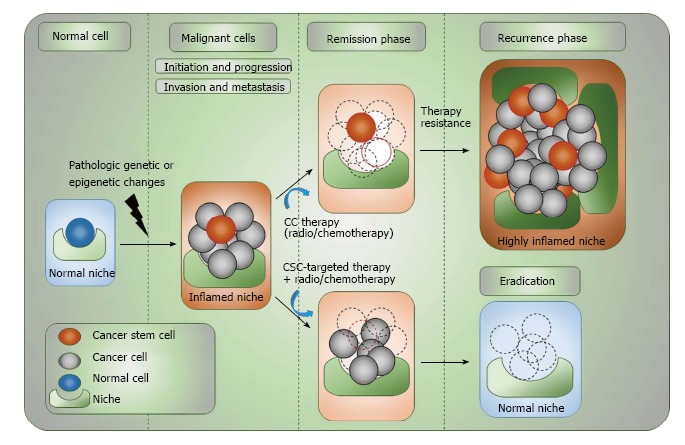
Complex organization of cancer initiation, progress, remission and relapse. CSCs are capable of undergoing extensive cell proliferation after acquiring different pathologic genetic/epigenetic changes while retaining their stemness and giving rise to differentiated progenies. Acquiring further genetic/epigenetic changes during different stages of tumor progression will evolve CSCs, but this may also be advanced through having dynamic interplay with the stem cell-niche. Both CSCs and non-CSCs can be found at the invasive front of primary tumors, which is linked to the process of EMT. However, only CSCs are capable of surviving from immune-surveillance or conventional tumor therapies and are able to give rise to distance metastasis or cause cancer recurrence. The potential eradication of tumor cells and CSCs can be resulted only upon combination targeted therapeutic approaches. Tumor stem cell-targeting drugs should be able to prolong the efficacy of cytotoxic tumor therapy and reduce the recurrence risk. CSC: Cancer stem cell; CC: Cancer cell; NC: Normal cell; EMT: Epithelial-mesenchymal transition.
In each cancer cell clone, which is characterized by harboring different combinations of mutations or genetic alterations, the processes of self-renewal, and differentiation occur differently based on the type of genetic lesions[9]. Nevertheless, significant similarities between normal and tumorigenic, experimentally identified stem cells could be expected. Both stem cell types (normal or cancerous) are rarely active, dependent on a specific microenvironment (so-called “stem cell-niche”) and have a number of self-protection mechanisms[2]. This niche enables a dynamic interaction between stem cells and surrounding cells including immune cells (“immune-niche”), cytokines and chemokines that regulates maintenance, quiescence, self-renewal and differentiation of stem cells to provide an optimal stem cell-supporting setting. What contributes to formation of the niche for tumor stem cells is the subject of intensive research[10]. Normal stem cells are more microenvironment dependent in order to get dynamic input to balance between activation and differentiation or self-renewal and quiescence “extrinsic factors”[11,12]. Although CSCs can represent more autonomous regulatory characterization “intrinsic factors”, similar concept of stem cell niche support could also hold for them[13]. The majority of studies using the isolated CSCs, shows the dominant effect of intrinsic factors on CSC regulation. While, other studies propose a role for the CSC niche[12]. This model suggests that less malignant tumors may have more demand on the stem cell-niche but upon cancer progress this dynamic interplay might be weaken or even diminished[14].
An inductor of the stem cell phenotype is hypoxia[15-17]. The self-protection mechanisms are due to the expression of numerous proteins, which reduce the effects of genotoxic xenobiotics. These include the members of efflux pumps, such as ABCB1-MDR1, ABCC1-MRP1 and ABCG2-BCRP, other specific detoxification systems, such as aldehyde dehydrogenase and increased DNA repair capacity. The symmetric cell division and asymmetric distribution of the DNA can also be regarded as part of stem cell self-protection mechanisms[9]. For the tumor stem cells, the existence of the same mechanisms is a crucial cause of their therapeutic resistance.
In addition to hypoxia as a triggering factor, growth factors play an important role, leading to epithelial-mesenchymal transition (EMT) in cells. It is shown a high-level regulation of stem cell markers after the induction of EMT in normal epithelial cells of the breast gland tissue and in mammary carcinoma cells[18]. One of the EMT effects can be the induction of the stem cell phenotype[18].
Numerous findings could show that routine tumor therapy approaches (classical chemotherapy or radiation therapy) and even the majority of currently used targeted antitumor drugs, so-called biological therapy, have little effect on the tumor stem cells even in chemo- or radio-sensitive tumors[19]. While, the stationary tumor stem cells largely retain their epithelial character and are therefore responsible for the primary tumor growth or recurrence, the migrating tumor stem cells exhibit ability for invasion and distance metastasis. This highlights the above-mentioned plasticity of the tumor stem cells (Figure 1).
THERAPY RESISTANCE IN CSCs
A small number of immortal cells within the bulk tumor with a character of CSC causes the chemo/radiotherapy resistance. Such cells with stem cell characteristics, seem to grow aggressively and metastasize easily. It is not yet clear how CSCs are formed, whether they develop from tissue stem cells or are formed from differentiated cells by recovering embryonic properties. Chemotherapeutic agents and radiotherapy mainly destroy dividing cells[20]. Since CSCs are particularly dormant, in one hand they are not detected by the routine screening measures, and in the other hand, they are positively selected upon the routine therapy approaches.
MOLECULAR MECHANISMS OF THE THERAPY RESISTANCE OF CSCs
Central regulators of the cellular response to DNA damage are checkpoint kinases 1 and 2 (Chk1/2), which are activated after genotoxic stress and stop cell proliferation to allow DNA repair. Activation of Chk1 as a response to DNA damage by ionizing radiation or chemotherapy agents can be detected preferentially in CD133+ glioblastoma precursor cells[21]. By pharmacological inhibition of Chk1, it was possible to increase the sensitivity of CD133+ glioblastoma precursor cells against therapy[21].
An efficient inactivation of reactive oxygen species (ROS) is another feature of CSCs. The excessive production of ROS under chemo/radiotherapy leads to a cell damage because of its interaction with DNA and proteins and triggering the cell death. In some tumors, including mammary carcinoma and gastrointestinal carcinoma, fewer amounts of ROS were detected in CSCs with a simultaneously increased amount of free-radical scavenger compared to the cell populations without CSC phenotype[22]. In addition, the expression of stem cell marker CD44 in tumor cells was associated with an increased expression of the glutathione as a free-radical scavenger[23,24]. Pharmacologically induced reduction in the concentration of free-radical scavenger in tumor cells can significantly increase their sensitivity to the chemo/radiotherapy[25]. It remains unclear whether the increased CD44 expression as a biomarker is suitable for the detection of ROS-resistant CSCs and thus can identify patients who can benefit from therapy with inhibitors of free-radical scavengers in combination with the chemo/radiotherapy.
Another factor contributing to the chemo/radiotherapy resistance of CSCs is hypoxia. Among other factors, hypoxia is the most common cause of therapy-resistance CSCs, which activates the hypoxia inducible factor signaling pathway and triggers cellular processes that can lead to a better survival and expansion of CSCs[26]. The presence of hypoxia in the tumor tissue or its decrease by reoxygenation in the course of chemo/radiotherapy could be correlated with an accelerated repopulation of CSCs with therapy-resistance phenotype[27].
There are several critical proliferation-promoting and survival-inducing pathways triggering the maintenance and survival of CSCs. The canonical Wnt pathway, which is central signaling pathway for stem cell maintenance and development, is constitutively active in breast cancer, colorectal cancer, myeloid leukemia, lung cancer and skin cancer[28,29]. Hedgehog Signaling (HH), which has three different homologues desert Hedgehog, Indian Hedgehog and Sonic Hedgehog is essential in a variety of molecular and cellular processes during tissue homeostasis, development or embryogenesis. Aberrant HH activation which regulates the CSC’s maintenance and potential proliferation, is reported in different cancers including acute myeloid leukemia (AML), breast cancer, chronic myeloid leukemia (CML), glioblastoma, lung carcinoma, myeloma, pancreatic adenocarcinoma[7,30,31]. Canonical Notch signaling is the other conserved signaling pathway in tissue homeostasis and development. Activation of Notch signaling upon binding of the extracellular ligands, regulates the expression of target genes involving in CSC self-renewal such as Myc, Nanog, Oct-4, and Sox2[32]. Abnormal Notch activation plays a critical role in breast cancer, myeloid leukemia (AML and CML), glioblastoma, lung cancer and pancreatic cancer[7,32,33]. Phosphoinositide-3-kinase/protein kinase B, canonical and non-canonical nuclear factor-κB (NF-κB), stromal-derived factor-1α/CXCR4, ErbB signaling and hedgehog/glioma-associated oncogene are other critical pathways that regulates CSC-related maintenance and proliferation[34-38]. The majority of cancer and CSC-related pathways do not act as isolated units but rather often interact with other pathways as a linked biological network. The predicted crosstalk among Wnt signaling, Notch pathway, Hedgehog signaling and other pathways like EGF/VEGF signaling is illustrated in the Figure 2.
Figure 2.
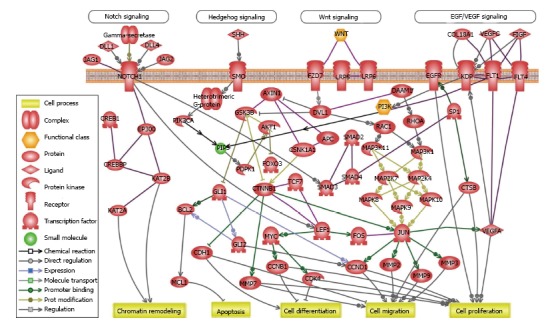
Crosstalk between cancer and cancer stem cell-related pathways. Predicted crosstalk among Wnt signaling, Notch pathway, Hedgehog signaling and other cancer-related pathways like EGF/VEGF signaling in CSCs and cancer. Gene networks and canonical pathways were assessed using the Ariadne Genomics Pathway Studio® program and database (Elsevier). EGF: Epidermal growth factor; VEGF: Vascular endothelial growth factor; WNT: Wnt signaling pathways; PI3K: Phosphoinositide 3-kinase; PIP3: Phosphatidylinositol 3,4,5 trisphosphate; CSC: Cancer stem cell; SHH: Sonic Hedgehog.
Therefore, therapies that target CSCs could be more effective than therapies targeting a general reduction in tumor mass. Thus, it can be postulated that the efficacy of the chemo/radiotherapy to eradicate CSCs, can be enhanced by a combination therapy with drugs specifically targeting CSCs (Figure 1).
METHODS FOR SCREENING OF CSCs
Over the past decade, different CSC markers were identified in a wide range of hematopoietic malignancies and solid tumors[39,40]. A widely used method for characterizing CSC-related markers is multiparameter flow cytometry. This method, which is originally developed for the analysis of blood cells and hematopoietic stem cells, offers the possibility to detect CSCs by means of specific surface markers that are stained with fluorescence-coupled antibodies. Frequently, the expression of CD133 or CD44 alone or in combination with further markers such as CD20, CD24, CD90 or α2-β1-integrin is used as a CSC-specific marker (Figure 3). Functional detection of CSC is also possible and is based on the increased expression of detoxification enzyme aldehyde dehydrogenase 1 (ALDH1) or the high activity of multidrug resistance transport proteins. These CSC-specific staining methods allow the isolation of single CSCs for further molecular characterization using single cell based molecular approaches (Figure 3). However, identified markers are not always reliable and none of the reported markers solely identify CSCs, therefore need to be used with caution (Table 1).
Figure 3.
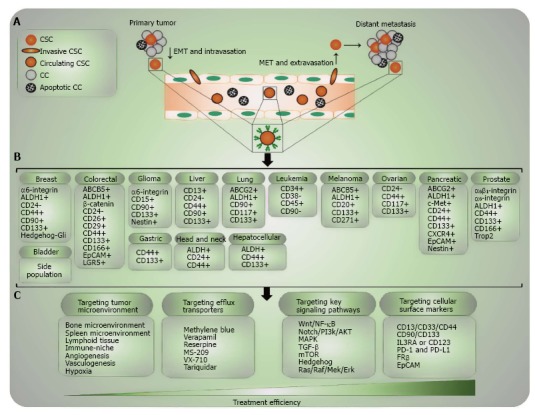
Tracing and targeting cancer stem cells. A: The complex process of distant metastasis including invasion of the tumor microenvironment, EMT, shedding of CSCs into the blood stream (intravasation), MET and invasion of circulating CTCs to the other tissues (extravasation). Only circulating CSCs are able to survive in the circulating blood, escape from immune-surveillance and home to secondary organs; B: The list of known compilation CSC-related molecular markers for different solid tumors and hematopoietic malignancies. The level of specificity of these markers differs per each type of tumor. Markers are ordered alphabetically and not according to their sensitivity or specificity; C: Four important approaches of CSC-targeted therapy. CSC: Cancer stem cell; CC: Cancer cell; NC: Normal cell; EMT: Epithelial-mesenchymal transition; MET: Mesenchymal-epithelial transition; PI3K: Phosphoinositide 3-kinase; MAPK: Mitogen-activated protein kinase; TGF: Transforming growth factor; mTOR: Mechanistic target of rapamycin; RAS: Ras-activated signaling; PD-1: Programmed death 1; PD-L1: Programmed death-ligand 1; EpCAM: Epithelial cell adhesion molecule.
Table 1.
Hallmarks of using cancer stem cell-related markers
| Problems | Potential solutions |
| CSC-related markers may not be specific by their own for a certain type of tumor | Combined used of different markers may be the solution |
| Some of CSC-related markers may be down-regulated or suppressed in a given tumor due to different genetic or epigenetic regulatory mechanisms | Using of distinct markers or a combination them |
| Splice variants of some CSC-related markers may render detection difficult | The exact splice variant should be considered for the detection |
| Markers can be detected using one method (e.g., FACS), but not with other methods (e.g., immunohistochemistry) | Stringent selection of related markers might be required |
| Different tumors have clonal variation and heterogeneous cell population. Less malignant clones may harbor CSCs that express different markers. Therefore, CSC-related markers may be differentially regulated within different clone or be completely missed | Using more specific and sensitive methods, isolate more enriched CSC populations |
| Many of reported CSC-related markers are not validated, since they derived from cell-line or mouse model studies | Markers should be validated in xenotransplants or primary human materials |
CSC: Cancer stem cell; FACS: Fluorescence-activated cell sorting.
For example, inter- or intra-tumor heterogeneity may completely render CSC markers inapt. Such tumor heterogeneity can be the result of different genetically distinct clones within the tumor due to having various genetic lesions or dysregulation of markers via pathologic epigenetic regulations[16,37-43]. For example, CD133 marker is frequently inactivated due to the DNA methylation and therefore often inadequate[44]. Inactivation of specific markers due to any scape mechanism in a particular clone may render these CSCs undetectable in the absence of other distinct markers.
While high-throughput genetic screening studies provide essential information about genes which are associated with a particular phenotype, molecular pharmacology can play an important role in development of a specific molecular therapy. Low molecular weight substances (“small molecules”) show a higher penetrance in cell-based screening methods. Therefore, small molecules are one of the most frequently used therapeutic agents. The screening of large substance banks has identified many valuable compounds that can be used to modulate biological systems in cancer cells[45]. In order to systematically identify the genes that regulate the death and differentiation of CSCs, high-throughput screenings of RNA interference (RNAi) or chemical substance libraries are carried out using different approaches. The readout of such screen approaches can be survival analysis, reporter assays, luminescence or fluorescence-based analyzes of particular genes or pathways and imaging methods, in which several cellular properties can be examined on a single cell level.
Since CSCs only make up a small fraction in the entire tumor cell pool (Figure 1), appropriate enrichment methods must be applied. Gupta et al[46] enriched CD44hi/CD24lo cells within the CSC population of mammary carcinoma cell lines by inducing the EMT. After treatment with inhibitors, the survival of the enriched and the non-selected cell population was investigated using a luminescence-based reporter assay. This study was able to identify salinomycin as a selective inhibitor of the CSC population in breast carcinoma[46].
Recent advances in computer-based image analysis have enabled rapid achievements in the development of image-based high-throughput analysis approaches. The direct visualization of cellular features and biological processes allows a more comprehensive measurement of responses to interferences. Xia et al[47] have developed a novel fluorescence imaging method to identify cancer cells with CSC properties through their increased ability to deliver fluorescent dyes via dedicated molecular transporters. Based on this method, a library of active substances was examined for their effect in CSCs. It was possible to identify substances that selectively inhibit the molecular transporters[47].
A further high-throughput method has recently been developed to characterize the biochemical and biophysical environmental conditions of CSCs. Microarray glass slides with over 2000 test chambers can be used to cultivate stem cells in different cell densities in a hydrogel of polyethylene glycol, to which different biological molecules have been coupled by robot technology[48]. Using the microscopic imaging, cell proliferation, morphology and differentiation can be monitored at a single cell level. This method as a platform for the investigation of individual stem cells in a microfluid culture system with simultaneous live-cell microscopy, represents an important step towards the miniaturization of the cellular processes as a high-throughput screening approach[49].
TARGETING CSCs
Targeting tumor microenvironment
The heterogeneous tumor microenvironment or cancer cell-niche, provides different self-protection mechanisms which enables a dynamic interaction with surrounding cells including immune cells, cytokines and chemokines to regulate proliferation, maintenance and self-renewal of CSCs. CSCs can represent more autonomous regulatory characterization in an independent manner[13]. Less malignant tumors may have more demand on the stem cell-niche but upon cancer progress this dynamic interplay might be weaken or even diminished[14]. It is known that dormant cancer cells via reducing their immunogenicity, can escape the immune surveillance[50]. Therefore, targeting CSC microenvironment may stimulate the host antitumor responses[51]. Strategies to hit the tumor-promoting inflammation are under investigation. Production of prostaglandin E2 (PGE2) by tumor cells in breast cancer, colorectal cancer and melanoma has a key role in the escape phase as it suppresses immunity and induces inflammation[52]. Therefore, the use of antagonists of PGE2 receptor (PTGER4) has proven successful in blocking immuno-suppression and preventing cancer metastases[53].
Targeting efflux transporters
Membrane efflux transporters, which are mainly located in blood-brain barrier, hepatocytes, intestinal cells or kidney proximal tubules, play important roles in drug metabolism, availability, and toxicity of drugs in human body[54]. Several studies indicate that transporter-mediated drug disposition plays an important role in mediating chemo-sensitivity and -resistance of cancer cells and CSCs[55]. The interaction between efflux transporters and chemotherapeutic drugs on cancer cells is significantly linked to the efficacy of cancer therapy. Two major superfamilies of efflux transporters are the ATP-binding cassette (ABC) transporters [ABCB1 (MDR1), ABCC1 (MRP1), ABCC2 (MRP2) and ABCG2 (BCRP)] and the solute carrier (SLC) transporters [SLC19A1 (RFC1) and SLCO1B1 (SLC21A6)]. Therefore, targeting efflux transporters within cancer therapy combined with routine therapies could significantly increase the eradication rate of resistant cancer cells[56].
Targeting key signaling pathways
The CSC phenotype depends on various cellular signals, which are triggered by the underlying genetic lesions and by the support of the stem cell niche. Some of these signals have already been identified; the most disease cussed signaling pathways are the classic Wnt-β-catenin, Notch and Sonic Hedgehog signaling[57-59]. For these three pathways, pharmacological inhibitors have been developed which are now undergoing clinical trials in many independent studies[60]. However, the clinical effect is largely depending on the tumor type and not all three pathways are equally important in all types of tumors. It has been shown that, although some signaling pathways are highly tumor-promoting in a certain type of cancers (which makes it a suitable therapeutic target), they might react as tumor suppressive in another tumor type; therefore, their inhibition may become dangerous (e.g., Notch-1 has been identified as a tumor suppressor in urinary bladder carcinoma)[61]. High Wnt pathway activity marks colon or leukemia CSCs and is required for stemness signature as a prognostic marker[6,7,62]. In addition, Wnt activity is associated with the CSC markers CD133, CD44 and LGR5 in colon cancer[63] whereas Hedgehog activity is linked to ABC transporter expression in esophageal and prostate cancer[17,64] TGF-β signaling via the family members Nodal and Activin is attributed to pancreatic CSCs[65]. The effect of Hedgehog inhibitors is actually the most evident in the basal cell carcinoma. In addition, inhibition of Hedgehog pathway blocks stemness in breast CSCs, whereas its activation enhances self-renewal[66]. It is also necessary to distinguish whether those signaling pathway has been activated within CSCs only because of harbored genetic lesions[67]. If only the CSCs are targeted, it is hardly possible to expect a dramatic tumor shrinkage as in classical successful chemo/radiotherapy; rather, this would be a disease stabilization and a slowing of the progression (Figure 1).
Disulfiram was developed as an inhibitor of aldehyde dehydrogenase for the treatment of alcoholism. This inhibition leads to the accumulation of acetaldehyde after alcohol consumption, resulting in a marked nausea that should reduce the probability of further alcohol consumption[68]. The same enzymatic activity - aldehyde dehydrogenase - is, however, a component of the self-protection of the CSCs, and thus disulfiram was used for the elimination of CSCs. Thioridazine is an inhibitor of dopamine receptors, and is a standard medication for mental disorders such as schizophrenia. Its rational use in tumor therapy is based on the finding that CSCs in several types of tumors (e.g., AML, breast carcinoma, glioblastoma), in contrast to the corresponding normal tissue stem cells, upregulate the expression of dopamine receptors[69].
Niclosamide was identified that specifically targeted Wnt-β-catenin signaling pathways[70]. Interestingly, niclosamide is known as an antiparasitic and inhibitor of oxidative phosphorylation, which has been used in human medicine for almost 50 years[71]. However, what has emerged is that these two antiparasitics are inhibitors of numerous other signaling pathways. Niclosamide inhibits not only the Wnt-β-catenin signaling pathway, but also the Notch, PI3’K-Akt - mTOR, STAT-3 and NFκB signaling pathways, which are essential for tumor stem cells[72]. Salinomycin was similarly described as an inhibitor of ABC efflux pumps and the Wnt-β-catenin signaling pathway[73]. Not enough, analogous effects have been discovered for disulfiram and thioridazine. Disulfiram has not only proved to be an effective inhibitor of aldehyde dehydrogenase, but also polo-like kinase 1 and O6-methylguanine methyltransferase as well as NFκB[74,75].
The advantages of identifying such new indications for old drugs are obvious. These drugs have long been out of patent protection and their use should therefore be much cheaper than for newly developed drugs, which is an important aspect in the current discussion on costs of tumor treatment. In addition, they have already undergone clinical trials, their potential toxicity, side effects, pharmacokinetics, contraindications, and possible drug-drug interactions are known. Therefore, their use in tumor therapy should be relatively easy. Perhaps the best opportunity to see how the effects of tumor stem cell-targeted therapy can be demonstrated is in the area of combination therapy (Figure 1). Tumor stem cell-directed drugs should be able to prolong the efficacy of cytotoxic therapy and reduce recurrence risk[76,77]. On the other hand, combined administration has significantly greater chances of total elimination of all tumor cells. Taken together, there are many possibilities for therapeutic treatment for the elimination of tumor stem cells, both from the group of newly developed inhibitors of some stem cell-specific signaling pathways as well as for some old drugs that can find a new application in tumor therapy.
Targeting cellular surface markers (tumor immunotherapy and cancer vaccination)
Many types of normal cells like immune cells infiltrate tumors. Over the last years, immune infiltration has become a central focus in cancer research[50]. It is increasingly recognized that cancer cells and CSCs need to escape immune recognition. IL-6/JAK/STAT3 signaling an important pathway in many solid tumors. Anti-IL-6 mAb siltux-imab was tested on various cancer types, which was not able to provide promising outcome to improve overall survival of patients with multiple myeloma according to a recent Phase II clinical trial on patients[78]. While, checkpoint blockade antibodies such as cytotoxic T-lymphocyte antigen 4 (CTLA-4) or programmed death-ligand 1 (PD-1/PD-L1) like ipilimumab or nivolumab could provide marked clinical benefits for lung adenocarcinoma, melanoma or Hodgkin lymphomas[79,80]. These agents can boost the immune system and display clinical benefits for a fraction of patients[50].
Many tumors cells including CSCs, alter the expression of their genes or down-modulate of antigen processing and presentation to build an immuno-suppressive microenvironment that creates physical or chemical barriers against immune cells[81]. Indeed, CSCs by low express of MHC-I, and over expressing of IL-4 are escaping from cytotoxic T lymphocytes[82]. Boosting T-cell response can be a promising approach to eradicate CSCs. This can be achieved by boosting neo-antigens within CSCs, considered as tumor vaccination. Adoptive transfer of CSC-specific T-cells into tumor-bearing mice could show a success[83]. In addition, genetically modified T cells to express chimeric antigen receptors (CAR T-cells) upon adaptive transfer could provide remarkable benefit for patients suffering from different solid tumors or leukemia[84]. Therefore, the major goal of immunotherapy is to thwart these barriers in order to enhance pre-existing or elicit a new immune response against cancer.
CONCLUSION
Because of the CSCs’ ability to therapy resistance and initiate a recurrence after therapy, cancer stem cell is an important therapeutic target. Future research is essential to elucidate how CSCs dictate metastasis, therapy-resistance or immune-scape signature. However, without having reliable markers it will be a challenging pursuit. An exact molecular characterization of this small subpopulation in the tumor tissue requires the development of specific CSC markers and suitable enrichment methods. Particularly from innovative high-throughput screening technologies, we can expect valuable insights regarding suitable CSC-associated biomarkers and new therapeutic approaches to target CSCs. This could be an important step towards individualized cancer therapy.
Footnotes
Conflict-of-interest statement: There is no conflict of interest.
Manuscript source: Unsolicited manuscript
Specialty type: Cell and tissue engineering
Country of origin: Switzerland
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: July 14, 2017
First decision: August 7, 2017
Article in press: September 4, 2017
P- Reviewer: Cao T, Kiselev SL, Qin JM, Sipos F, Wang LS, Xiao Y S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
References
- 1.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hatina J. The dynamics of cancer stem cells. Neoplasma. 2012;59:700–707. doi: 10.4149/neo_2012_092. [DOI] [PubMed] [Google Scholar]
- 3.Vermeulen L, de Sousa e Melo F, Richel DJ, Medema JP. The developing cancer stem-cell model: clinical challenges and opportunities. Lancet Oncol. 2012;13:e83–e89. doi: 10.1016/S1470-2045(11)70257-1. [DOI] [PubMed] [Google Scholar]
- 4.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 5.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 6.Riether C, Schürch CM, Flury C, Hinterbrandner M, Drück L, Huguenin AL, Baerlocher GM, Radpour R, Ochsenbein AF. Tyrosine kinase inhibitor-induced CD70 expression mediates drug resistance in leukemia stem cells by activating Wnt signaling. Sci Transl Med. 2015;7:298ra119. doi: 10.1126/scitranslmed.aab1740. [DOI] [PubMed] [Google Scholar]
- 7.Riether C, Schürch CM, Bührer ED, Hinterbrandner M, Huguenin AL, Hoepner S, Zlobec I, Pabst T, Radpour R, Ochsenbein AF. CD70/CD27 signaling promotes blast stemness and is a viable therapeutic target in acute myeloid leukemia. J Exp Med. 2017;214:359–380. doi: 10.1084/jem.20152008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baumann M, Krause M, Hill R. Exploring the role of cancer stem cells in radioresistance. Nat Rev Cancer. 2008;8:545–554. doi: 10.1038/nrc2419. [DOI] [PubMed] [Google Scholar]
- 9.Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328–337. doi: 10.1038/nature12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plaks V, Kong N, Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16:225–238. doi: 10.1016/j.stem.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medema JP, Vermeulen L. Microenvironmental regulation of stem cells in intestinal homeostasis and cancer. Nature. 2011;474:318–326. doi: 10.1038/nature10212. [DOI] [PubMed] [Google Scholar]
- 12.Shestopalov IA, Zon LI. Stem cells: The right neighbour. Nature. 2012;481:453–455. doi: 10.1038/481453a. [DOI] [PubMed] [Google Scholar]
- 13.Borovski T, De Sousa E Melo F, Vermeulen L, Medema JP. Cancer stem cell niche: the place to be. Cancer Res. 2011;71:634–639. doi: 10.1158/0008-5472.CAN-10-3220. [DOI] [PubMed] [Google Scholar]
- 14.Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Barekati Z, Radpour R, Kohler C, Zhang B, Toniolo P, Lenner P, Lv Q, Zheng H, Zhong XY. Methylation profile of TP53 regulatory pathway and mtDNA alterations in breast cancer patients lacking TP53 mutations. Hum Mol Genet. 2010;19:2936–2946. doi: 10.1093/hmg/ddq199. [DOI] [PubMed] [Google Scholar]
- 17.Medema JP. Cancer stem cells: the challenges ahead. Nat Cell Biol. 2013;15:338–344. doi: 10.1038/ncb2717. [DOI] [PubMed] [Google Scholar]
- 18.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdullah LN, Chow EK. Mechanisms of chemoresistance in cancer stem cells. Clin Transl Med. 2013;2:3. doi: 10.1186/2001-1326-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wicha MS, Hayes DF. Circulating tumor cells: not all detected cells are bad and not all bad cells are detected. J Clin Oncol. 2011;29:1508–1511. doi: 10.1200/JCO.2010.34.0026. [DOI] [PubMed] [Google Scholar]
- 21.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 22.Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baumann M, Krause M. CD44: a cancer stem cell-related biomarker with predictive potential for radiotherapy. Clin Cancer Res. 2010;16:5091–5093. doi: 10.1158/1078-0432.CCR-10-2244. [DOI] [PubMed] [Google Scholar]
- 24.Kokko LL, Hurme S, Maula SM, Alanen K, Grénman R, Kinnunen I, Ventelä S. Significance of site-specific prognosis of cancer stem cell marker CD44 in head and neck squamous-cell carcinoma. Oral Oncol. 2011;47:510–516. doi: 10.1016/j.oraloncology.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 25.Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell. 2011;19:387–400. doi: 10.1016/j.ccr.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 26.Morrison R, Schleicher SM, Sun Y, Niermann KJ, Kim S, Spratt DE, Chung CH, Lu B. Targeting the mechanisms of resistance to chemotherapy and radiotherapy with the cancer stem cell hypothesis. J Oncol. 2011;2011:941876. doi: 10.1155/2011/941876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JJ, Tannock IF. Repopulation of cancer cells during therapy: an important cause of treatment failure. Nat Rev Cancer. 2005;5:516–525. doi: 10.1038/nrc1650. [DOI] [PubMed] [Google Scholar]
- 28.Heidel FH, Bullinger L, Feng Z, Wang Z, Neff TA, Stein L, Kalaitzidis D, Lane SW, Armstrong SA. Genetic and pharmacologic inhibition of β-catenin targets imatinib-resistant leukemia stem cells in CML. Cell Stem Cell. 2012;10:412–424. doi: 10.1016/j.stem.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malanchi I, Peinado H, Kassen D, Hussenet T, Metzger D, Chambon P, Huber M, Hohl D, Cano A, Birchmeier W, et al. Cutaneous cancer stem cell maintenance is dependent on beta-catenin signalling. Nature. 2008;452:650–653. doi: 10.1038/nature06835. [DOI] [PubMed] [Google Scholar]
- 30.Merchant AA, Matsui W. Targeting Hedgehog--a cancer stem cell pathway. Clin Cancer Res. 2010;16:3130–3140. doi: 10.1158/1078-0432.CCR-09-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dierks C, Beigi R, Guo GR, Zirlik K, Stegert MR, Manley P, Trussell C, Schmitt-Graeff A, Landwerlin K, Veelken H, et al. Expansion of Bcr-Abl-positive leukemic stem cells is dependent on Hedgehog pathway activation. Cancer Cell. 2008;14:238–249. doi: 10.1016/j.ccr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Miele L. Notch signaling. Clin Cancer Res. 2006;12:1074–1079. doi: 10.1158/1078-0432.CCR-05-2570. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Li D, Liu H, Xu H, Zheng H, Qian F, Li W, Zhao C, Wang Z, Wang X. Notch-1 signaling facilitates survivin expression in human non-small cell lung cancer cells. Cancer Biol Ther. 2011;11:14–21. doi: 10.4161/cbt.11.1.13730. [DOI] [PubMed] [Google Scholar]
- 34.Hambardzumyan D, Becher OJ, Holland EC. Cancer stem cells and survival pathways. Cell Cycle. 2008;7:1371–1378. doi: 10.4161/cc.7.10.5954. [DOI] [PubMed] [Google Scholar]
- 35.Dubrovska A, Kim S, Salamone RJ, Walker JR, Maira SM, García-Echeverría C, Schultz PG, Reddy VA. The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc Natl Acad Sci USA. 2009;106:268–273. doi: 10.1073/pnas.0810956106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dubrovska A, Elliott J, Salamone RJ, Telegeev GD, Stakhovsky AE, Schepotin IB, Yan F, Wang Y, Bouchez LC, Kularatne SA, et al. CXCR4 expression in prostate cancer progenitor cells. PLoS One. 2012;7:e31226. doi: 10.1371/journal.pone.0031226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radpour R, Barekati Z, Kohler C, Lv Q, Bürki N, Diesch C, Bitzer J, Zheng H, Schmid S, Zhong XY. Hypermethylation of tumor suppressor genes involved in critical regulatory pathways for developing a blood-based test in breast cancer. PLoS One. 2011;6:e16080. doi: 10.1371/journal.pone.0016080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Radpour R, Kohler C, Haghighi MM, Fan AX, Holzgreve W, Zhong XY. Methylation profiles of 22 candidate genes in breast cancer using high-throughput MALDI-TOF mass array. Oncogene. 2009;28:2969–2978. doi: 10.1038/onc.2009.149. [DOI] [PubMed] [Google Scholar]
- 39.Radpour R, Barekati Z, Kohler C, Holzgreve W, Zhong XY. New trends in molecular biomarker discovery for breast cancer. Genet Test Mol Biomarkers. 2009;13:565–571. doi: 10.1089/gtmb.2009.0060. [DOI] [PubMed] [Google Scholar]
- 40.Aghagolzadeh P, Radpour R. New trends in molecular and cellular biomarker discovery for colorectal cancer. World J Gastroenterol. 2016;22:5678–5693. doi: 10.3748/wjg.v22.i25.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Radpour R, Haghighi MM, Fan AX, Torbati PM, Hahn S, Holzgreve W, Zhong XY. High-throughput hacking of the methylation patterns in breast cancer by in vitro transcription and thymidine-specific cleavage mass array on MALDI-TOF silico-chip. Mol Cancer Res. 2008;6:1702–1709. doi: 10.1158/1541-7786.MCR-08-0262. [DOI] [PubMed] [Google Scholar]
- 42.Radpour R, Barekati Z, Kohler C, Schumacher MM, Grussenmeyer T, Jenoe P, Hartmann N, Moes S, Letzkus M, Bitzer J, et al. Integrated epigenetics of human breast cancer: synoptic investigation of targeted genes, microRNAs and proteins upon demethylation treatment. PLoS One. 2011;6:e27355. doi: 10.1371/journal.pone.0027355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barekati Z, Radpour R, Lu Q, Bitzer J, Zheng H, Toniolo P, Lenner P, Zhong XY. Methylation signature of lymph node metastases in breast cancer patients. BMC Cancer. 2012;12:244. doi: 10.1186/1471-2407-12-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yi JM, Tsai HC, Glöckner SC, Lin S, Ohm JE, Easwaran H, James CD, Costello JF, Riggins G, Eberhart CG, et al. Abnormal DNA methylation of CD133 in colorectal and glioblastoma tumors. Cancer Res. 2008;68:8094–8103. doi: 10.1158/0008-5472.CAN-07-6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winquist RJ, Furey BF, Boucher DM. Cancer stem cells as the relevant biomass for drug discovery. Curr Opin Pharmacol. 2010;10:385–390. doi: 10.1016/j.coph.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 46.Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xia X, Yang J, Li F, Li Y, Zhou X, Dai Y, Wong ST. Image-based chemical screening identifies drug efflux inhibitors in lung cancer cells. Cancer Res. 2010;70:7723–7733. doi: 10.1158/0008-5472.CAN-09-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gobaa S, Hoehnel S, Roccio M, Negro A, Kobel S, Lutolf MP. Artificial niche microarrays for probing single stem cell fate in high throughput. Nat Methods. 2011;8:949–955. doi: 10.1038/nmeth.1732. [DOI] [PubMed] [Google Scholar]
- 49.Lecault V, Vaninsberghe M, Sekulovic S, Knapp DJ, Wohrer S, Bowden W, Viel F, McLaughlin T, Jarandehei A, Miller M, et al. High-throughput analysis of single hematopoietic stem cell proliferation in microfluidic cell culture arrays. Nat Methods. 2011;8:581–586. doi: 10.1038/nmeth.1614. [DOI] [PubMed] [Google Scholar]
- 50.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 51.Pitt JM, Marabelle A, Eggermont A, Soria JC, Kroemer G, Zitvogel L. Targeting the tumor microenvironment: removing obstruction to anticancer immune responses and immunotherapy. Ann Oncol. 2016;27:1482–1492. doi: 10.1093/annonc/mdw168. [DOI] [PubMed] [Google Scholar]
- 52.Zelenay S, van der Veen AG, Böttcher JP, Snelgrove KJ, Rogers N, Acton SE, Chakravarty P, Girotti MR, Marais R, Quezada SA, et al. Cyclooxygenase-Dependent Tumor Growth through Evasion of Immunity. Cell. 2015;162:1257–1270. doi: 10.1016/j.cell.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma X, Holt D, Kundu N, Reader J, Goloubeva O, Take Y, Fulton AM. A prostaglandin E (PGE) receptor EP4 antagonist protects natural killer cells from PGE2-mediated immunosuppression and inhibits breast cancer metastasis. Oncoimmunology. 2013;2:e22647. doi: 10.4161/onci.22647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bodó A, Bakos E, Szeri F, Váradi A, Sarkadi B. The role of multidrug transporters in drug availability, metabolism and toxicity. Toxicol Lett. 2003;140-141:133–143. doi: 10.1016/s0378-4274(02)00497-6. [DOI] [PubMed] [Google Scholar]
- 55.Huang Y, Sadée W. Membrane transporters and channels in chemoresistance and -sensitivity of tumor cells. Cancer Lett. 2006;239:168–182. doi: 10.1016/j.canlet.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 56.Huang Y. Pharmacogenetics/genomics of membrane transporters in cancer chemotherapy. Cancer Metastasis Rev. 2007;26:183–201. doi: 10.1007/s10555-007-9050-6. [DOI] [PubMed] [Google Scholar]
- 57.Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Staal FJ, Famili F, Garcia Perez L, Pike-Overzet K. Aberrant Wnt Signaling in Leukemia. Cancers (Basel) 2016;8:pii: E78. doi: 10.3390/cancers8090078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tohda S. NOTCH signaling roles in acute myeloid leukemia cell growth and interaction with other stemness-related signals. Anticancer Res. 2014;34:6259–6264. [PubMed] [Google Scholar]
- 60.Takebe N, Miele L, Harris PJ, Jeong W, Bando H, Kahn M, Yang SX, Ivy SP. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol. 2015;12:445–464. doi: 10.1038/nrclinonc.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rampias T, Vgenopoulou P, Avgeris M, Polyzos A, Stravodimos K, Valavanis C, Scorilas A, Klinakis A. A new tumor suppressor role for the Notch pathway in bladder cancer. Nat Med. 2014;20:1199–1205. doi: 10.1038/nm.3678. [DOI] [PubMed] [Google Scholar]
- 62.Vermeulen L, De Sousa E Melo F, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 63.Van der Flier LG, Sabates-Bellver J, Oving I, Haegebarth A, De Palo M, Anti M, Van Gijn ME, Suijkerbuijk S, Van de Wetering M, Marra G, et al. The Intestinal Wnt/TCF Signature. Gastroenterology. 2007;132:628–632. doi: 10.1053/j.gastro.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 64.Sims-Mourtada J, Izzo JG, Ajani J, Chao KS. Sonic Hedgehog promotes multiple drug resistance by regulation of drug transport. Oncogene. 2007;26:5674–5679. doi: 10.1038/sj.onc.1210356. [DOI] [PubMed] [Google Scholar]
- 65.Lonardo E, Hermann PC, Mueller MT, Huber S, Balic A, Miranda-Lorenzo I, Zagorac S, Alcala S, Rodriguez-Arabaolaza I, Ramirez JC, et al. Nodal/Activin signaling drives self-renewal and tumorigenicity of pancreatic cancer stem cells and provides a target for combined drug therapy. Cell Stem Cell. 2011;9:433–446. doi: 10.1016/j.stem.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 66.Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, Suri P, Wicha MS. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Amakye D, Jagani Z, Dorsch M. Unraveling the therapeutic potential of the Hedgehog pathway in cancer. Nat Med. 2013;19:1410–1422. doi: 10.1038/nm.3389. [DOI] [PubMed] [Google Scholar]
- 68.Mutschler J, Kiefer F. [Mechanism of action of disulfiram and treatment optimization in prevention of recurrent alcoholism] Praxis (Bern 1994) 2013;102:139–146. doi: 10.1024/1661-8157/a001178. [DOI] [PubMed] [Google Scholar]
- 69.Sachlos E, Risueño RM, Laronde S, Shapovalova Z, Lee JH, Russell J, Malig M, McNicol JD, Fiebig-Comyn A, Graham M, et al. Identification of drugs including a dopamine receptor antagonist that selectively target cancer stem cells. Cell. 2012;149:1284–1297. doi: 10.1016/j.cell.2012.03.049. [DOI] [PubMed] [Google Scholar]
- 70.Chen M, Wang J, Lu J, Bond MC, Ren XR, Lyerly HK, Barak LS, Chen W. The anti-helminthic niclosamide inhibits Wnt/Frizzled1 signaling. Biochemistry. 2009;48:10267–10274. doi: 10.1021/bi9009677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Al-Hadiya BM. Niclosamide: comprehensive profile. Profiles Drug Subst Excip Relat Methodol. 2005;32:67–96. doi: 10.1016/S0099-5428(05)32002-8. [DOI] [PubMed] [Google Scholar]
- 72.Li Y, Li PK, Roberts MJ, Arend RC, Samant RS, Buchsbaum DJ. Multi-targeted therapy of cancer by niclosamide: A new application for an old drug. Cancer Lett. 2014;349:8–14. doi: 10.1016/j.canlet.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boesch M, Zeimet AG, Rumpold H, Gastl G, Sopper S, Wolf D. Drug Transporter-Mediated Protection of Cancer Stem Cells From Ionophore Antibiotics. Stem Cells Transl Med. 2015;4:1028–1032. doi: 10.5966/sctm.2015-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Triscott J, Lee C, Hu K, Fotovati A, Berns R, Pambid M, Luk M, Kast RE, Kong E, Toyota E, et al. Disulfiram, a drug widely used to control alcoholism, suppresses the self-renewal of glioblastoma and over-rides resistance to temozolomide. Oncotarget. 2012;3:1112–1123. doi: 10.18632/oncotarget.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang Y, Li W, Patel SS, Cong J, Zhang N, Sabbatino F, Liu X, Qi Y, Huang P, Lee H, et al. Blocking the formation of radiation-induced breast cancer stem cells. Oncotarget. 2014;5:3743–3755. doi: 10.18632/oncotarget.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ke XY, Lin Ng VW, Gao SJ, Tong YW, Hedrick JL, Yang YY. Co-delivery of thioridazine and doxorubicin using polymeric micelles for targeting both cancer cells and cancer stem cells. Biomaterials. 2014;35:1096–1108. doi: 10.1016/j.biomaterials.2013.10.049. [DOI] [PubMed] [Google Scholar]
- 77.Murone M, Radpour R, Attinger A, Chessex AV, Huguenin AL, Schürch CM, Banz Y, Sengupta S, Aguet M, Rigotti S, et al. The Multi-kinase Inhibitor Debio 0617B Reduces Maintenance and Self-renewal of Primary Human AML CD34+ Stem/Progenitor Cells. Mol Cancer Ther. 2017;16:1497–1510. doi: 10.1158/1535-7163.MCT-16-0889. [DOI] [PubMed] [Google Scholar]
- 78.San-Miguel J, Bladé J, Shpilberg O, Grosicki S, Maloisel F, Min CK, Polo Zarzuela M, Robak T, Prasad SV, Tee Goh Y, et al. Phase 2 randomized study of bortezomib-melphalan-prednisone with or without siltuximab (anti-IL-6) in multiple myeloma. Blood. 2014;123:4136–4142. doi: 10.1182/blood-2013-12-546374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schatton T, Schütte U, Frank NY, Zhan Q, Hoerning A, Robles SC, Zhou J, Hodi FS, Spagnoli GC, Murphy GF, et al. Modulation of T-cell activation by malignant melanoma initiating cells. Cancer Res. 2010;70:697–708. doi: 10.1158/0008-5472.CAN-09-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lanzardo S, Conti L, Rooke R, Ruiu R, Accart N, Bolli E, Arigoni M, Macagno M, Barrera G, Pizzimenti S, et al. Immunotargeting of Antigen xCT Attenuates Stem-like Cell Behavior and Metastatic Progression in Breast Cancer. Cancer Res. 2016;76:62–72. doi: 10.1158/0008-5472.CAN-15-1208. [DOI] [PubMed] [Google Scholar]
- 83.Joosse SA, Pantel K. Tumor-Educated Platelets as Liquid Biopsy in Cancer Patients. Cancer Cell. 2015;28:552–554. doi: 10.1016/j.ccell.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 84.Yong CSM, Dardalhon V, Devaud C, Taylor N, Darcy PK, Kershaw MH. CAR T-cell therapy of solid tumors. Immunol Cell Biol. 2017;95:356–363. doi: 10.1038/icb.2016.128. [DOI] [PubMed] [Google Scholar]


