Abstract
Background
The recognition of nodular melanoma is clinically challenging, and the diagnostic accuracy of dermoscopy and confocal microscopy is lower than for superficial spreading melanoma.
Objectives
To test a management strategy consisting in the excision of any nodular lesion that cannot be confidently and precisely classified as a benign tumor after clinical and dermoscopic examination.
Methods
Clinical and dermoscopic images of excised nodular lesions were retrospectively collected and evaluated. The evaluators were asked to record the level of diagnostic confidence for each lesion, by declaring if they were confident or doubtful about the given diagnosis. The NNE (number needed to excise) value was used to evaluate the efficacy of the proposed method.
Results
A total of 1,319 excised nodular lesions formed the study set. The NNE for any malignancy was 3.9 (634/164), while the NNE for melanoma was 13.2 (634/48). NNE for hypo and amelanotic melanoma was 27.3 (327/12).
Conclusions
Excising doubtful nodular lesions seems to be an effective management strategy not to miss nodular melanoma, resulting in an acceptable rate of unnecessary excision of benign lesions.
Keywords: nodular melanoma, number needed to excise, skin cancer, dermatoscopy
Introduction
Nodular melanoma (NM) is a very aggressive subtype of melanoma lacking a significant superficial spreading phase and, consequently, being thicker at diagnosis as compared with other melanoma subtypes [1]. Although NM accounts only for 9 to 15% of melanomas, it is responsible for 43% of melanoma deaths, according to a population-based study in Australia [2]. Because of its peculiar clinical characteristics, the ABCD clinical criteria are usually of no help for NM recognition [3–5]. The tumor typically develops as a rapidly growing, firm papule or nodule. These clinical characteristics have been summarized by the EGF rule (Elevation, Growth, Firmness), which has been proposed to facilitate the recognition of NM by patients and clinicians [6,7]. The introduction of noninvasive diagnostic tools in dermatology, such as dermoscopy and confocal microscopy, has significantly improved melanoma diagnostic accuracy [5,8–18]. Despite these advances, the recognition of NM remains a challenge, because its clinical and dermoscopic features may be similar to benign nodular lesions, such as hemangioma, pyogenic granuloma, seborrheic keratosis, dermatofibroma, and dermal nevi. Several studies have investigated the accuracy of dermoscopic criteria in the diagnosis of NM, reporting sensitivity, specificity, and predictive values lower than those for superficial spreading melanoma (SSM) [8]. Reflectance confocal microscopy (RCM), a recently introduced diagnostic tool allowing skin evaluation at a quasi histologic level, has been tested for the diagnosis of nodular lesions in a retrospective study [17]. However, the use of RCM is limited by its cost and availability. Moreover, the presence of hyperkeratosis and ulceration may prevent the deeper evaluation of the tumor structures and render RCM of limited value in NM [17].
When taking into consideration the limitations of clinical, dermoscopic and confocal diagnosis, a practical management strategy has been suggested so as not to miss NM [8,19]. According to this approach, any nodular lesion that cannot be confidently and precisely classified as a benign tumor after clinical and dermoscopic examination should be histopathologically diagnosed [19]. However, one might argue that applying this strategy would result in a high number of excisions of benign lesions. The aim of this study was to evaluate the effectiveness of this strategy, by evaluating the number needed to excise (NNE) when applying the rule of excising any doubtful nodular lesion.
Material and Methods
Clinical and dermoscopic images of excised nodular lesions were retrospectively collected from the databases of two pigmented lesion clinics in Naples and Reggio Emilia, Italy. As a general rule, excision for cosmetic concern or patient will are not routinely performed at our referral centers, but all excisions are performed to rule out malignancy. The inclusion criteria for the current study were the availability of clinical and dermoscopic images of a nodular lesion that was excised and histopathologically diagnosed. Nodularity was evaluated on clinical images, in which a ruler was available. Pure nodular and flat palpable lesions with a nodular component were included. We did not select lesions based on the histopathologic diagnosis; thus, both benign and malignant lesions were included for evaluation. The study period was 2008 to 2014. Clinical images were acquired using a high-resolution digital camera. Dermoscopic images were captured using non-polarized dermoscopes (DermLite Foto, 3Gen LLC, Dana Point, CA).
All images were evaluated by two residents in dermatology with an average expertise in dermoscopy. The evaluators were blinded to the final histopathologic diagnosis. The presence of pigmentation was assessed on the dermoscopic images, and a lesion was classified as pigmented when pigmentation was involving 50% or more of the lesion surface, hypopigmented when pigmentation was present in less than 50% of the lesion surface, and amelanotic when no pigmentation was detected at all. The evaluators were asked to provide a diagnosis based on the clinical and dermoscopic aspects of the lesion. To serve the main aim of our study, the evaluators were also asked to record the level of diagnostic confidence for each lesion, by declaring if they were confident or doubtful about the given diagnosis. The NNE (number needed to excise) value was used to evaluate the efficacy of the proposed method [20–22]. This was obtained by dividing the total number of doubtful lesions excised to the number of those histopathologically proven to be malignant. NNE values were calculated for all malignancies (NNE total) and for melanoma diagnosis in particular.
Results
In all, 1,319 excised nodular lesions formed the study set. Of these, 747 (56.6%) were malignant and 572 (43.4%) were benign. The study sample included: hemangioma (27; 2.0%), angiocheratoma (3; 0.2%), angioleiomioma (1; 0.1%), clear cell acanthoma (5; 0.4%), angiosarcoma (2; 0.2%), basal cell carcinoma (283; 21.4%), squamous cell carcinoma (205; 15.5%); Merkel cell carcinoma (3; 0.2%), cylindroma (2; 0.2%), epidermal cyst (20; 1.5%), dermatofibroma (60; 4.5%), pyogenic granuloma (24; 1.8%), Kaposi sarcoma (12; 0.9%), melanoma (199; 15.0%), metastasis from internal tumors (2; 0.2%), melanocytic nevi (342; 25.9%), other benign lesions (88; 6.7%), and other malignant lesions (41; 3.1%).
On clinical-dermoscopic evaluation, 685 of 1,319 lesions (51.9%) were assessed as surely malignant and 634 (48.1%) as doubtful. Of 685 lesions clinically/dermoscopically judged as surely malignant, 583 were indeed malignant histopathologically (85.1%), and 102 (14.9%) were histopathologically benign. Of 634 clinically/dermoscopically doubtful lesions, 164 (25.9%) were histopathologically malignant, including 48 melanomas. Thus, the NNE for any malignancy was 3.9 (634/164), while the NNE for melanoma was 13.2 (634/48).
Two hundred eighty-three BCC were included in the study set, and of these 238 were judged “sure malignant,” and 45 clinically doubtful. Two hundred five SCC were included in the study set, and 154 were judged clinically “sure malignant,” and 34 clinically doubtful. Of the 199 melanomas, 151 were clinically “sure malignant,” and 48 were clinically doubtful.
Regarding pigmentation, 755 lesions were hypo or amelanotic and, of these, 509 were malignant. Three hundred twenty-seven lesions were hypo or amelanotic and clinically/dermoscopically doubtful. Of these, 116 lesions were judged doubtful and histopathologically malignant. In the subgroup of hypo- or amelanotic lesions, the NNE for any malignancy was 2.8 (327/116). A subset of 33 melanomas consisted of hypo- or amelanotic lesions. Of these, 12 lesions were judged doubtful; thus, the NNE for hypo and amelanotic melanoma was 27.3 (327/12).
The majority of histopathologically benign lesions that were judged to be doubtful under clinical and dermoscopic examination were melanocytic nevi (279 lesions, 44.0%), including Spitz nevus (102; 16.1%), compound nevus (86; 13.6%), dermal nevus (24; 3.8%), blue nevus (16; 2.5%), Clark nevus (15; 2.4%), congenital nevus (14; 2.2%), and 22 (3.5%) other nevi, not specified. Dermatofibroma accounted for 50 (7.9%) of the benign doubtful lesions, whereas 32 (5.0%) were angiomas, 22 (3.5%) pyogenic granulomas, and 15 (2.4%) epidermal cysts.
Discussion
When facing nodular lesions, clinicians are asked to provide one of two mutually exclusive management recommendations, namely, excision or “no action” [19]. Follow-up of nodular lesions is not an option, because of the possibility of missing a nodular melanoma, which may result in worsened prognosis given the high growth rate of the tumor [1,19]. To address this problem, a practical management strategy has been suggested, namely to recommend surgical excision of all nodular lesions for which a confident and specific diagnosis of a benign tumor is not feasible after clinical and dermoscopic examination [19]. However, one could argue that applying this rule in daily practice could result in a high number of unnecessary excisions of benign lesions.
Our results suggest that the aforementioned strategy is effective, resulting in an NNE of 13.2 for the diagnosis of melanoma. The number needed to excise is a value indicating the accuracy of a screening strategy, measuring how many benign lesions are excised to find one malignancy [20–22]. The NNE depends on both the expertise of the clinician and the prevalence of the disease. Usually, NNE values for melanoma diagnosis vary between 4 and 30, with lower values achieved in specialized or referral centers [23]. Our results are in line with previous studies, confirming that diagnosing nodular melanoma can be challenging.
When considering malignant lesions overall, including BCC, SCC, and others, an NNE value of 3.9 was achieved. In simple words, when applying the rule of excising doubtful nodular lesions, 1 out of 4 will be proven to be malignant. This result highlights that applying this approach results in an acceptable number of excisions of benign lesions.
Within the group of clinically doubtful benign lesions, the great majority were melanocytic nevi (44%), with Spitz, compound and dermal nevi accounting for 16.1%, 13.6 and 3.8% of excisions, respectively. Apart from Spitz nevi that are well-known simulators of melanoma, compound, and dermal nevi are usually easy to recognize both clinically and dermoscopically. However, sometimes their morphologic characteristics might not allow a confident diagnosis. As shown by our results, such morphologically atypical nevi should be removed when applying the rule of excising doubtful nodular lesions. (Figures 1 and 2) However, given that the NNE remains absolutely acceptable, sacrificing a small number of nevi to avoid missing nodular melanoma seems a reasonable compromise.
Figure 1. Clinical presentation of 4 nodular lesions excised to rule out melanoma.
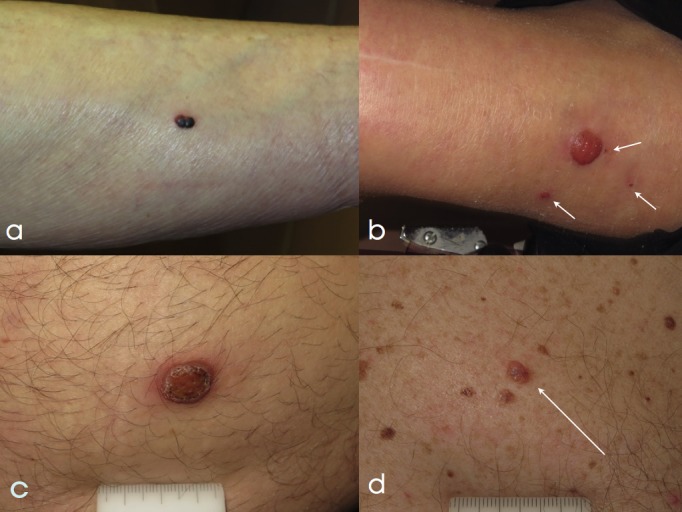
(a) A red-purple-to-black nodule, arising on the leg of a 72-year-old woman. The patient reported recent onset and sudden change in color. The lesion was excised and histopathologically diagnosed as angioma. (b) A large amelanotic nodule, arising on the leg of 80-year-old woman. Three smaller papules were visible in the surrounding skin (white arrows). The patient was unaware of the time duration of the lesions. A biopsy was taken, and a final diagnosis of clear cell acanthoma with satellites was given. (c) An 8 mm diameter amelanotic, ulcerated nodule arising on the abdomen of a 40-year-old man. The lesion was noticed three months before and was rapidly growing in size. Histopathologic examination revealed a melanoma 2.5 mm Breslow thickness. (d) A 6 mm nodule (white arrow) arising on the chest of a 64-year-old man with a prior history of multiple primary melanomas. Color variegation and presence of central bluish hue prompted excision. Histopathology revealed a dermal nevus. [Copyright: ©2017 Moscarella et al.]
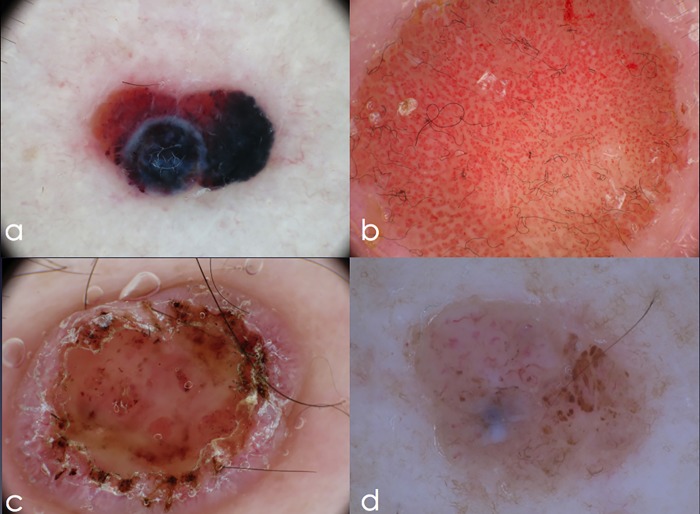
Figure 2.
Corresponding dermoscopy image of the four excised nodular lesions (thrombosed hemangioma, clear cell acanthoma, nodular melanoma and dermal nevus). (a) Asymmetric lesion, with red-to-purple background, purple globules and two areas of structureless blue pigmentation. (b) A symmetric amelanotic lesion, with multiple dotted vessels in a chain-like distribution, the so-called “string of pearls.” (c) An amelanotic nodule displaying central ulceration, scaling, and peripheral short linear vessels over a pink background. (d) Asymmetric nodular lesion, with brown globules and short linear vessels. A central area of structureless white-blue pigmentation is visible. [Copyright: ©2017 Moscarella et al.]
With a value of 2.8 lesions to excise to find one malignancy, our results highlight that the rule of excising doubtful nodular lesions is particularly effective for hypo- or non-pigmented tumors. Among nonmelanocytic lesions, the most problematic in our series were dermatofibroma, hemangioma, and pyogenic granuloma, accounting for 16.4% of doubtful lesions. This confirms that benign nonmelanocytic lesions represent a consistent proportion of the lesions to be considered in the differential diagnosis of nodular melanoma.
One limitation of our study was the retrospective design, which did not allow us to evaluate prospectively the factors prompting excision of a given lesion. However, as a general rule, at our referral centers, all excisions are performed to rule out malignancy.
In conclusion, excising doubtful nodular lesions seems to be an effective management strategy not to miss nodular melanoma, resulting in an acceptable rate of unnecessary excision of benign lesions.
Footnotes
Funding: None.
Competing interests: The authors have no conflicts of interest to disclose.
All authors have contributed significantly to this publication.
References
- 1.Liu W, Dowling JP, Murray WK, et al. Rate of growth in melanomas: characteristics and associations of rapidly growing melanomas. Arch Dermatol. 2006;142(12):1551–1558. doi: 10.1001/archderm.142.12.1551. [DOI] [PubMed] [Google Scholar]
- 2.Mar V, Roberts H, Wolfe R, English DR, Kelly JW. Nodular melanoma: a distinct clinical entity and the largest contributor to melanoma deaths in Victoria, Australia. J Am Acad Dermatol. 2013;68(4):568–575. doi: 10.1016/j.jaad.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 3.Rigel DS, Friedman RJ, Kopf AW, Polsky D. ABCDE: an evolving concept in the early detection of melanoma. Arch Dermatol. 2005;141(8):1032–1034. doi: 10.1001/archderm.141.8.1032. [DOI] [PubMed] [Google Scholar]
- 4.Lin MJ, Mar V, McLean C, Wolfe R, Kelly JW. Diagnostic accuracy of malignant melanoma according to subtype. Australas J Dermatol. 2014;55(1):35–42. doi: 10.1111/ajd.12121. [DOI] [PubMed] [Google Scholar]
- 5.Rigel DS, Russak J, Friedman R. The evolution of melanoma diagnosis: 25 years beyond the ABCDs. CA Cancer J Clin. 2010;60(5):301–316. doi: 10.3322/caac.20074. [DOI] [PubMed] [Google Scholar]
- 6.Kelly JW, Chamberlain AJ, Staples MP, McAvoy B. Nodular melanoma: no longer as simple as ABC. Aust Fam Physician. 2003;32(9):706–709. [PubMed] [Google Scholar]
- 7.Kelly JW. Nodular melanoma: how current approaches to early detection are failing. J Drugs Dermatol. 2005;4(6):790–793. [PubMed] [Google Scholar]
- 8.Menzies SW, Moloney FJ, Byth K, et al. Dermoscopic evaluation of nodular melanoma. JAMA Dermatol. 2013;149(6):699–709. doi: 10.1001/jamadermatol.2013.2466. [DOI] [PubMed] [Google Scholar]
- 9.Argenziano G, Longo C, Cameron A, et al. Blue-black rule: a simple dermoscopic clue to recognize pigmented nodular melanoma. Br J Dermatol. 2011;165(6):1251–1255. doi: 10.1111/j.1365-2133.2011.10621.x. [DOI] [PubMed] [Google Scholar]
- 10.Kalkhoran S, Milne O, Zalaudek I, et al. Historical, clinical, and dermoscopic characteristics of thin nodular melanoma. Arch Dermatol. 2010;146(3):311–318. doi: 10.1001/archdermatol.2009.369. [DOI] [PubMed] [Google Scholar]
- 11.Menzies SW, Kreusch J, Byth K, et al. Dermoscopic evaluation of amelanotic and hypomelanotic melanoma. Arch Dermatol. 2008;144(9):1120–1127. doi: 10.1001/archderm.144.9.1120. [DOI] [PubMed] [Google Scholar]
- 12.Cavicchini S, Tourlaki A, Bottini S. Dermoscopic vascular patterns in nodular “pure” amelanotic melanoma. Arch Dermatol. 2007;143(4):556. doi: 10.1001/archderm.143.4.556. [DOI] [PubMed] [Google Scholar]
- 13.Menzies SW, Ingvar C, Crotty KA, McCarthy WH. Frequency and morphologic characteristics of invasive melanomas lacking specific surface microscopic features. Arch Dermatol. 1996;132(10):1178–1182. [PubMed] [Google Scholar]
- 14.Pizzichetta MA, Kittler H, Stanganelli I, et al. Italian Melanoma Intergroup. Pigmented nodular melanoma: the predictive value of dermoscopic features using multivariate analysis. Br J Dermatol. 2015 Jul;173(1):106–114. doi: 10.1111/bjd.13861. [DOI] [PubMed] [Google Scholar]
- 15.Carli P, De Giorgi V, Palli D, et al. Patterns of detection of superficial spreading and nodular-type melanoma: a multicenter Italian study. Dermatol Surg. 2004;30(11):1371–1375. doi: 10.1111/j.1524-4725.2004.30434.x. [DOI] [PubMed] [Google Scholar]
- 16.Segura S, Pellacani G, Puig S, et al. In vivo microscopic features of nodular melanomas: dermoscopy, confocal microscopy, and histopathologic correlates. Arch Dermatol. 2008;144(10):1311–1320. doi: 10.1001/archderm.144.10.1311. [DOI] [PubMed] [Google Scholar]
- 17.Longo C, Farnetani F, Ciardo S, et al. Is confocal microscopy a valuable tool in diagnosing nodular lesions? A study of 140 cases. Br J Dermatol. 2013;169(1):58–67. doi: 10.1111/bjd.12259. [DOI] [PubMed] [Google Scholar]
- 18.Longo C, Farnetani F, Moscarella E, et al. Can noninvasive imaging tools potentially predict the risk of ulceration in invasive melanomas showing blue and black colors? Melanoma Res. 2013;23(2):125–131. doi: 10.1097/CMR.0b013e32835d90b8. [DOI] [PubMed] [Google Scholar]
- 19.Lallas A, Zalaudek I, Apalla Z, et al. Management rules to detect melanoma. Dermatology. 2013;226(1):52–60. doi: 10.1159/000346645. [DOI] [PubMed] [Google Scholar]
- 20.English DR, Del Mar C, Burton RC. Factors influencing the number needed to excise: excision rates of pigmented lesions by general practitioners. Med J Aust. 2004;80:16–19. doi: 10.5694/j.1326-5377.2004.tb05766.x. [DOI] [PubMed] [Google Scholar]
- 21.Hansen C, Wilkinson D, Hansen M, Argenziano G. How good are skin cancer clinics at melanoma detection? Number needed to treat variability across a national clinic group in Australia. J Am Acad Dermatol. 2009;61:599–604. doi: 10.1016/j.jaad.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 22.Ahnlide I, Nielsen K, Bjellerup M. Diagnosis of pigmented skin tumours in a dermatological setting: different aspects of the number needed to excise as a measure of efficiency. Acta Derm Venereol. 2014;94(6):683–686. [Google Scholar]
- 23.Argenziano G, Cerroni L, Zalaudek I, et al. Accuracy in melanoma detection: a 10-year multicenter survey. J Am Acad Dermatol. 2012;67(1):54–59. doi: 10.1016/j.jaad.2011.07.019. [DOI] [PubMed] [Google Scholar]