Abstract
Syphilitic alopecia (SA) is considered an uncommon manifestation of secondary syphilis. SA can present in a diffuse form, resembling telogen effluvium, or in a moth-eaten form that mimics a variety of conditions (i.e., alopecia areata, trichotillomania, lichen planus pilaris or tinea capitis). When the two forms coexist, we observe a mixed pattern. Essential SA manifests without evidence of mucocutaneous syphilis manifestations and its diagnosis is often delayed. To date, trichoscopic description of SA forms are based on very few cases (i.e., five patients with moth-eaten SA and one with diffuse SA). This is the first report of a mixed pattern of essential SA: some new trichoscopic features—such as tapered bended hairs, erythematous background, diffuse scaling and perifollicular hyperkeratosis—are described in a 32-year-old man. In the absence of secondary syphilis manifestations, dermoscopy can be a useful tool that helps suspect and differentiate SA from its common mimickers.
Keywords: secondary syphilis, moth-eaten and diffuse syphilitic alopecia, trichoscopy
Introduction
Syphilis is well known as “the great imitator” as it can present with a wide range of clinical manifestations mimicking other diseases. In addition, atypical manifestations are common and different stages can overlap. Among the signs of secondary syphilis, syphilitic alopecia (SA) is traditionally considered infrequent, with a prevalence ranging from 2.9 to 22.2% [1–3]. However, its prevalence could be underestimated due to its possible subtle presentation and difficult diagnosis. SA is defined essential when alopecia is the only manifestation and symptomatic when mucocutaneous signs are [2–6]. According to its clinical appearance, SA is further classified into three forms: 1) moth-eaten, or patchy alopecia characterized by small alopecic patches irregularly distributed over the scalp; 2) diffuse alopecia, characterized by a diffuse hair loss; and 3) mixed form (i.e., combination of diffuse hair loss and alopecic moth-eaten patches) [2–7]. The trichoscopic findings of SA reported to date are based on the observation of six cases only: five cases of moth-eaten SA [4,5] and one case of diffuse SA [5]. We here report a case of a mixed SA form, highlighting the presence of some new trichoscopic features that have not been previously observed.
Clinical Case
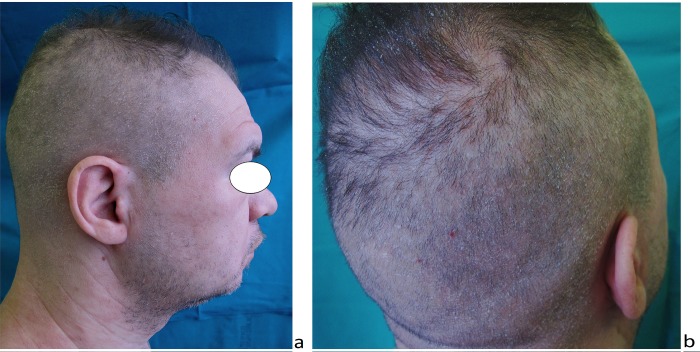
A 34-year-old man presented for alopecia of the scalp and eyebrows for one month. He was under treatment with neuroleptic drugs for bipolar disease, anxiety and obsessive traits. He described a mild erythematous rash involving the trunk and headache and malaise for some weeks. Clinical history was otherwise unremarkable. He denied having any genital lesions before hair loss started. On clinical examination, scattered small alopecic patches of 0.8 to 1.5 cm in diameter were present on the parietal-temporal-occipital areas of the scalp (Figure 1) with a moth-eaten appearance. Diffuse hair loss of the entire scalp was also evident. The eyebrows showed mild diffuse reduction in hairs rather than clear alopecic patches (Figure 1a). The scalp appeared diffusely erythematous and scaling. Neither trichodynia nor itching was present. Gentle pull test was negative. Trichoscopic examination of the moth-eaten alopecic patches revealed a nonscarring alopecia (Figure 2). Yellow dots were detected in the center of the patches, along with few black dots. Diffuse whitish scaling, erythematous background, and focal follicular hyperkeratosis were also observed. Vellus hairs were detected at the periphery of the alopecic patches. Interestingly, we could observe some variously bent tapering hairs; in some cases a double-bending was visible, resulting in a serpiginous appearance.
Figure 1.
Clinical aspect. Diffuse alopecia involving the scalp and the eyebrows, with moth-eaten patches on the temporal, parietal and occipital regions. Diffuse fine scaling is present on the scalp. [Copyright: ©2017 Tognetti et al.]
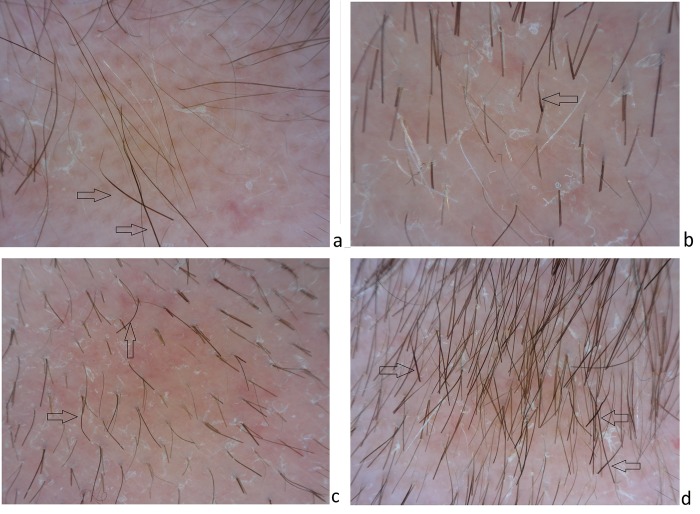
Figure 2.
Dermoscopy of the scalp. Empty ostia and yellow dots are visible in the center of the alopecic patches over an erythematous background. Tapered bent hairs (arrows) are present at the periphery of the alopecic patches. Vellus hair are visible at the periphery (a,b,d). Scales appear to be thin and withish; perifollicular hyperkeratosis is focally visible (b,c,d). [Copyright: ©2017 Tognetti et al.]
Clinical history along with physical and trichoscopic findings suggested a diagnosis of secondary syphilis with a SA of mixed pattern. Serological screening was performed, revealing a positive Venereal Disease Research Laboratory (VDRL) at a titer of 1:256 and a reactive Treponema pallidum particle hemoagglutination assay. Routine blood analysis revealed leukocytosis, raised erythrocyte sedimentation rate, and C-reactive protein. Serological tests for HIV and hepatitis B and C virus were negative. The patient received benzathine penicillin G 2.4 million units intramuscularly. Hair regrowth occurred after two months. VDRL titer was 1:64 after four months and 1:16 after eight months.
Discussion
Alopecia could be an important sign for orienting the clinical diagnosis towards secondary syphilis, as in our case. The tropism of Treponema pallidum for the hair bulge epithelium and peribulbar capillaries was demonstrated by scalp biopsies detecting spirochetes in the peribulbar region and penetrating into the follicle matrix [8]. The current hypothesis supporting the pathogenesis of SA is a vasculitis of peribulbar capillaries causing a perifollicular lymphocytic infiltration with scattered plasma cells that stops the hair cell cycle [6,7].
SA may clinically mimic a wide range of hair disorders, including alopecia areata (AA) [7], trichotillomania, lichen planus pilaris, tinea capitis, telogen effluvium, and androgenetic alopecia [2–10]. Thus, the diagnosis may be delayed, especially when SA is the unique manifestation of secondary syphilis and primary syphilis signs are absent or not reported (i.e., essential SA).
Scalp dermoscopy can help in diagnosing SA: the trichoscopic findings of moth-eaten SA were recently described, based on the observation of five patients [4,5]. In particular, Ye et al. observed black dots, focal atrichia, hypopigmentation of hair shaft and yellow dots in the center of the alopecic patches along with few black dots at the periphery of the patches [4]. Piraccini et al. described reduction in the number of terminal hairs and the presence of empty hair follicles, vellus hairs, red-brown background and irregularly dilated capillaries with small blood extravasation in four patients [5]. Diffuse SA involves the whole scalp as telogen effluvium, but the alopecic areas are more evident.
The trichoscopic observation of one patient did not show any significant alterations [5]. Our patient presented a clinical aspect of mixed pattern SA, about which the dermoscopic appearance has never been described. We identified some of the features previously observed in moth-eaten SA cases (i.e., reduction of terminal hairs, yellow dots, black dots, erythematous background, dilated capillaries and vellus hair) [4,5]. Moreover, we observed additional features, including tapering hairs, diffuse scaling and focal follicular hyperkeratosis. The tapering hairs were detected at the periphery of the moth-eaten patches and were single or double bending; thus we defined them as “tapered bended hairs.” Tapered bended hairs were of normal length and gradually narrowed from the proximal to the distal part. When detected in AA patients, tapering hairs without bending were considered the result of a sudden cessation of hair follicle production by the matrix due to the hair follicle inflammation [9,10]. In our case, tapered bended hairs are likely to be the expression of the chronic peribulbar sparse lymphocytic infiltration elicited by T. pallidum [6–8]. The diffuse fine scaling and the erythematous background that we observed in our case was in favor of a large involvement of the scalp (diffuse type of SA) and can be regarded as part of the exanthema of the secondary syphilitic infection. Interestingly, hyperkeratosis was found both around the proximal part of some hair shafts and within some empty follicular ostia.
Clinically, moth-eaten SA is considered the main simulator of AA [2,3,7] (Figure 3), and a scalp biopsy of moth-eaten SA forms may be required to exclude AA in doubtful cases [6,7]. Trichoscopy helps to differentiate between these two conditions; exclamation point hairs (3–5 mm short, wide at the top and very thin as they enter the scalp) are considered the hallmark of AA, along with numerous yellow and black dots and vellus hairs [9,10]. In addition, vellus hairs are usually observed in the center of the AA patch, whereas in SA they appear at the periphery [9.10]. Unbended tapering hairs are seldom observed in AA and mainly in the subacute phase [10]. Trichotillomania can clinically simulate both SA and AA, presenting with irregularly shaped alopecic patches distributed mainly on the occipital, parietal and vertex region, over an erythematous background. On dermoscopy, trichotillomania patches show broken hairs of varying length and occasionally a few black dots [2–5,9]. In some cases, SA could also simulate scalp manifestations of lichen planus pilaris, especially when scarring is not clinically evident [3,9]. However, in lichen planus pilaris, scarring areas are present and hyperkeratosis is more severe and usually limited to hair follicles (Figure 4). Finally, tinea capitis can also clinically mimic SA manifestations [2–5,9]. However, dermoscopic examination of tinea capitis reveals black dots and commas, corkscrew, zigzag and Morse code hairs irregularly distributed within the alopecic patches.
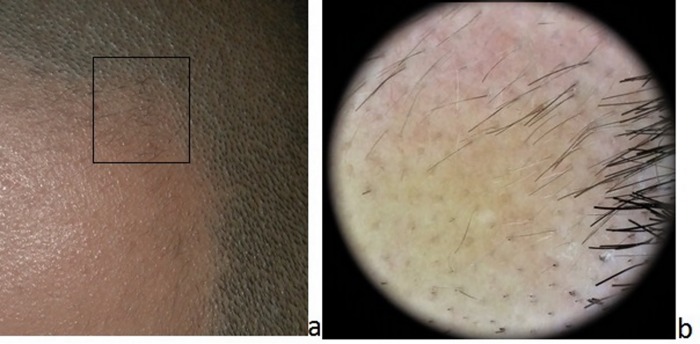
Figure 3.
Clinical (a) and dermoscopic (b) appearance of alopecia areata. Multiple black and yellow dots and vellus hairs are observable at the periphery of the alopecic patch (a,b) along with some exclamation mark hairs (b). [Copyright: ©2017 Tognetti et al.]
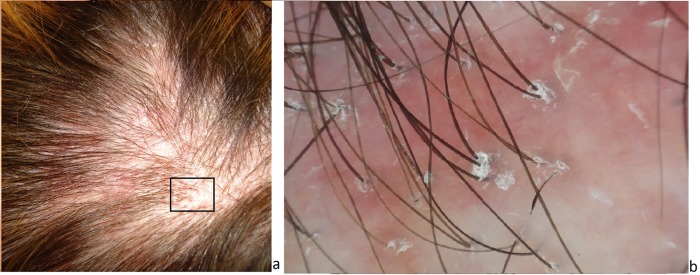
Figure 4.
Clinical (a) and dermoscopic (b) aspect of lichen planus pilaris alopecia. Confluent small alopecic patches of the parietal area are present on an erythematous scalp (a). Dermoscopy shows dilated capillaries and severe follicular hyperkeratosis at the periphery of the patch and absence of follicular ostia in the center (b). [Copyright: ©2017 Tognetti et al.]
Physicians should be aware of the possibility of secondary syphilis with SA in the absence of clear primary syphilis manifestations, a condition that is increasing nowadays, likely due to an improper concomitant antibiotic treatments [1–6]. Dermoscopy represents a useful, noninvasive, rapid, and inexpensive tool that helps the physician to suspect SA [10]. Further descriptions are necessary to confirm these trichoscopic findings of the mixed pattern of SA, a rare condition where moth-eaten and diffuse alopecia coexist.
Footnotes
Funding: None.
Competing interests: The authors have no conflicts of interest to disclose.
All authors have contributed significantly to this publication.
References
- 1.Vafaie J, Weinberg JM, Smith B, Mizuguchi RS. Alopecia in association with sexually transmitted disease: a review. Cutis. 2005;76:361–366. [PubMed] [Google Scholar]
- 2.Hernandez-Bel P, Unamuno B, Sanchez-Carazo JL, et al. Syphilitic alopecia: a report of 5 cases and a review of the literature. Actas Dermosifiliogr. 2013;104:512–517. doi: 10.1016/j.ad.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Bi MY, Cohen PR, Robinson FW, Gray JM. Alopecia syphilitica—report of a patient with secondary syphilis presenting as moth-eaten alopecia and a review of its common mimickers. Dermatol Online J. 2009;15:6. [PubMed] [Google Scholar]
- 4.Ye Y, Zhang X, Zhao Y, et al. The clinical and trichoscopic features of syphilitic alopecia. J Dermatol Case Rep. 2014;3:78–80. doi: 10.3315/jdcr.2014.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piraccini BM, Broccoli A, Starace M, et al. Hair and scalp manifestations in secondary syphilis: epidemiology, clinical features and trichoscopy. Dermatology. 2015;231(2):171–176. doi: 10.1159/000431314. [DOI] [PubMed] [Google Scholar]
- 6.Jordaan HF, Louw M. The moth-eaten alopecia of secondary syphilis. A histopathological study of 12 patients. Am J Dermatopathol. 1995;17:158. doi: 10.1097/00000372-199504000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Lee JY, Hsu ML. Alopecia syphilitica, a simulator of alopecia areata: histopathology and differential diagnosis. J Cutan Pathol. 1991;10:87. doi: 10.1111/j.1600-0560.1991.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 8.Nam-Cha Sh. Alopecia syphilitica with detection of Treponema Pallidum in the hair follicle. J Cutan Pathol. 2007;34(1):37–40. doi: 10.1111/j.1600-0560.2006.00726.x. [DOI] [PubMed] [Google Scholar]
- 9.Miteva M, Tosti A. Hair and scalp dermatoscopy. J Am Acad Dermatol. 2012;67(5):1040–1048. doi: 10.1016/j.jaad.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Inui S, Nakajima T, Nakagawa K, Itami S. Clinical significance of dermoscopy in alopecia areata: Analysis of 300 cases. Int J Dermatol. 2008;47:688–693. doi: 10.1111/j.1365-4632.2008.03692.x. [DOI] [PubMed] [Google Scholar]