Abstract
Background:
Postmenopausal osteoporosis is the most common bone metabolic disease associated with low bone mineral density (BMD) and osteopathic fragility fractures, which can lead to significant morbidity. The objective of this study was to investigate the relationship between serum follicle-stimulating hormone (FSH), luteinizing hormone (LH), and estradiol (E2) levels and bone mineral density (BMD) across the stages of menopause in Mongolian women.
Methods:
Two hundred sixty participants aged 50.1±4.4 years were enrolled in the study. Blood samples were obtained from each participant and analyzed using ELISA. Data were first stratified and analyzed by bone mineral density status (osteoporotic, osteopenic, and normal) and then by menopause status. Between group differences were analyzed using t-tests, and correlations were assessed using the Spearman rank order test, with Bonferonni correction. The data were analyzed using Statistical Package Statistical Software version 20.0 (SPSS Inc., Chicago, IL). Significance was set at p<0.05.
Results:
The mean menopausal age was 48.4±3.4, which is comparable to the Mongolian population mean menopausal age. The mean serum estradiol level in the normal BMD group was 18.3±13.1 pg/ml and 15.8±10.7 pg/ml in the osteoporotic group. The mean serum FSH in the normal BMD group was 54.5±44.1 pg/ml and 81.3±34.2 pg/ml in the osteoporotic group. The mean serum LH level in the normal BMD group was 53.1±41.2 and 75.1±26.1 pg/ml in the osteoporotic group. The mean T and Z score were lower in the osteoporotic group. FSH and LH levels significantly differed across menopause stages in that those who were post-menopausal had higher levels compared to those who were pre- or peri-menopausal. Both hormones, FSH and LH, showed weak negative correlations with BMD level, but not E2. There were significant negative correlations between FSH and Speed of Sound (SOS) (r=−0.16; p<0.01), and between osteoporosis with age (r=−0.30, p<0.05) and number of childbirths (r=−0.14 p<0.05).
Discussion:
Osteoporosis is a significant problem with associations to hormone levels in post-menopausal women. In our study, mean serum estradiol levels decreased with age, and the mean FSH and LH levels were higher in women of later menopausal stage. Further study is warranted to investigate the bone related studies to establish better statistical references among Mongolian women.
Keywords: osteoporosis, post-menopausal women, reproductive hormone, menopause, Mongolia
Postmenopausal osteoporosis is the most common bone metabolic disease associated with low bone mineral density (BMD) and osteopathic fragility fractures, which has been associated with significant disability and mortality.1 The majority of women spend one third of their lifetime in the postmenopausal period typically accompanied by an estrogen deficit state.2 The World Health Organization (WHO) defined osteoporosis as a reduction of 2.5 standard deviations in T-scores below the normal mean for young females at the age of peak bone mass.3 A T score between 1 to 2.5 SDs below average indicates osteopenia, which is a pre-osteoporotic state.3 The diagnostic measurement of Z-score indicates the bone density relative to patients’ age and sex.4 A Z-score below −2.0 is classified as below the expected range for that age, while above −2.0 is considered to be within the expected range for a given age.4,5 Worldwide, there are nearly 9 million osteoporotic fractures each year, generating a massive burden both to individuals and to health services.6 About 13–18% of women over the age of 50 years have osteoporosis and 37–50% have osteopenia in the USA.7 Unfortunately, no epidemiologic data have been published about the rate of osteoporosis in Mongolia. Anecdotal evidence suggests that rates in Mongolia are similar to those reported in the USA.
Low estradiol levels are a risk factor for osteoporosis and influences the quality of life for older women.8 Estrogen deficiency can lead to excessive bone re-absorption after menopause.9 To the best of our knowledge, there are no published studies on the relationship between serum reproductive hormonal level and BMD in postmenopausal women in Mongolia. Therefore, the purpose of this study is to evaluate the correlation between reproductive hormone levels such as follicle- stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), and osteoporosis among postmenopausal women in Mongolia.
Osteoporosis research is inadequate in Mongolia due to the lack of reliable diagnostic instruments and poor research infrastructure. Conducting such research is valuable to gerontology, endocrinology, and gynecology fields in Mongolia. Hence, the secondary purpose of this study is to establish a research database for future research in related fields.
Methods
Participants
Two hundred sixty women with a mean age of 50.1±4.4 years were included in the study. Participants were recruited from four districts from Ulaanbaatar City of Mongolia. All participants signed informed consents prior to study participation. The study was approved by the ethical committee of Health Sciences University of Mongolia.
Participants were interviewed using a questionnaire developed by the investigators, which included demographics, body mass index (BMI), years since menopause, menarche age, menopause age, and health history. The participants were divided into three different groups based on their BMD value: osteoporosis, osteopenia, and normal. The mean age of the osteoporosis (T score: −2.5), osteopenia (T score: −2.5 to −1), and normal (T score: >−1) group was 54.0±3.9, 51.5±4.3, and 48.5±3.8 years, respectively. The participants were further classified into four menopausal stages: premenopausal, perimenopausal, postmenopausal early and postmenopausal late using the Staging Reproductive Aging in Women (STRAW).10 Exclusion criteria included: history of bone disease, metabolic and/or endocrine disorders including hyperthyroidism, hyperparathyroidism, diabetes mellitus, liver disease, renal disease, and medications known to affect bone metabolism (e.g., corticosteroids, anticonvulsants, and heparin sodium). None of the participants had a history of medications for the treatment of osteoporosis, such as active vitamin D11, bisphosphonates, selective estrogen receptor modulators (SERM), or calcium.
Laboratory measurements
Five milliliters of fasting peripheral blood were drawn, centrifuged and kept frozen in the −20° C freezer until assayed. Serum samples were evaluated for levels of E2, FSH, LH levels, calcium, phosphorus, and vitamin D by ELISA (Thermo Fisher Scientific, USA) method.
Bone mineral density measurements
The bone ultrasound Mini-Omni (Sunlight, Beammed, Israel) was used to assess BMD. The parameter of speed of sound (SOS) m/s is used for the analysis.12 The SOS refers to the division of transmission time of the sound waves by the length of the body part studied. The forearm and tibia bone scans were performed with the patient on the imaging table using the protocols recommended by the manufacturer. Osteoporosis was defined according to the conventional World Health Organization (WHO) definition.3,13
Data analysis
The comparisons of scores between groups were performed using t-tests, and correlations were assessed with the Spearman rank order test, with Bonferonni correction. The data were analyzed using Statistical Package Statistical Software version 20.0 (SPSS Inc., Chicago, IL). Significance was set at p<0.05 and was two-sided.
Results
Participant Characteristics
Two hundred sixty (n=260) participants aged 50.1±4.4 years were enrolled in the study. The average BMI was 27.3±5.2 kg/m2 and mean age of menopause was 48.4±3.4 years.
Factors associated with bone density status
The age of menopause (p=0.002), breastfeeding time (p=0.041), and serum vitamin D (p=0.025) were significantly associated with osteoporotic status. However, there were no statistically significant differences of BMI, menarche age, serum calcium, serum phosphorus, and parathyroid hormone levels noted between the three groups. The result of hormone analysis showed serum estradiol levels were lower in the osteoporotic group than in the normal BMD group (normal: 18.3±13.1 pg/ml, osteoporotic: 15.8±10.7 pg/ml). The FSH and LH levels were higher in the osteoporosis group than in the normal BMD group (FSH normal: 54.5±44.1 pg/ml, FSH osteoporosis: 81.3±34.2 pg/ml, LH normal: 53.1±41.2, LH osteoporosis: 75.1±26.1 pg/ml). Clinical and laboratory characteristics of the participants are listed in Table 1.
Table 1.
Demographic and laboratory characteristics of participants
| Characteristics | Osteoporosis N=30 | Osteopenia N=90 | Normal N=140 | p-value |
|---|---|---|---|---|
| Mean age | 54.0±3.9 | 51.5±4.3 | 48.5±3.8 | 0.433 |
| BMI (kg/m2) | 28.98±4.0 | 27.97±5.3 | 26.55±5.2 | 0.460 |
| Age at Menopause (years) | 48.6±3.6 | 48.2 ±3.5 | 48.5±3.2 | 0.002 |
| Age at menarche (years) | 14.72±1.8 | 14.47±1.7 | 14.98±1.9 | 0.464 |
| Number of birth (n) | 3.3±1.5 | 3.2 ±1.5 | 2.6±1.2 | 0.167 |
| Serum calcium (mmol/l) | 2.17±0.3 | 2.18 ±0.2 | 2.14±0.2 | 0.077 |
| Serum phosphorus (mmol/l) | 3.63±0.9 | 3.76 ±1.0 | 3.55±0.9 | 0.261 |
| Serum PTH (pg/ml) | 7.91±4.5 | 7.62 ±4.2 | 7.61±5.6 | 0.565 |
| Serum VitD (mmol/l) | 13.28±6.1 | 12.52 ±4.9 | 12.58±6.1 | 0.025 |
| Breastfeeding time (yrs) | 2.06±1.2 | 2.43±1.6 | 2.38±1.6 | 0.041 |
Significant findings are indicated in bold.
Correlation of reproductive hormones with menopausal status
Serum estradiol levels were not significantly different across menopause stages. The FSH and LH levels were higher in the postmenopausal late stage than in the premenopausal stage (Table 2). The study found a weak statistical correlation between FSH and SOS from the ultrasound (r=−0.16, p<0.01). There was a significant negative correlation between SOS and BMI (r=−0.306, p<0.01). The SOS was significantly different in all four groups (p<0.05).
Table 2.
Levels of reproductive hormones (by STRAW) stratified by menopausal status
| Stage of menopause | Premenopausal N=63 | Perimenopausal N=77 | Postmenopausal early N=79 | Postmenopausal late N=41 |
|---|---|---|---|---|
| Estradiol (pg/ml) | 29.9±3.1 | 30.7±4.7 | 34.0±1.7 | 26.3±7.7 |
| FSH (pg/ml) | 26.7±4.7 | 36.9±4.2 | 64.6±4.9 | 61.0±6.3 |
| LH (pg/ml) | 20.5±3.6 | 34.4±3.5 | 62.3±4.7 | 59.7±6.9 |
Analysis of bone mineral density
The average T score was −0.18±0.6 in the normal BMD group, 1.67±0.4 in the osteopenia group, and −3.18±0.6 in the osteoporosis group. The Z score was 0.29±1.2 in normal patients, −1.64±1.3 in the osteopenia group, and −2.17±1.1 in osteoporosis patient group.
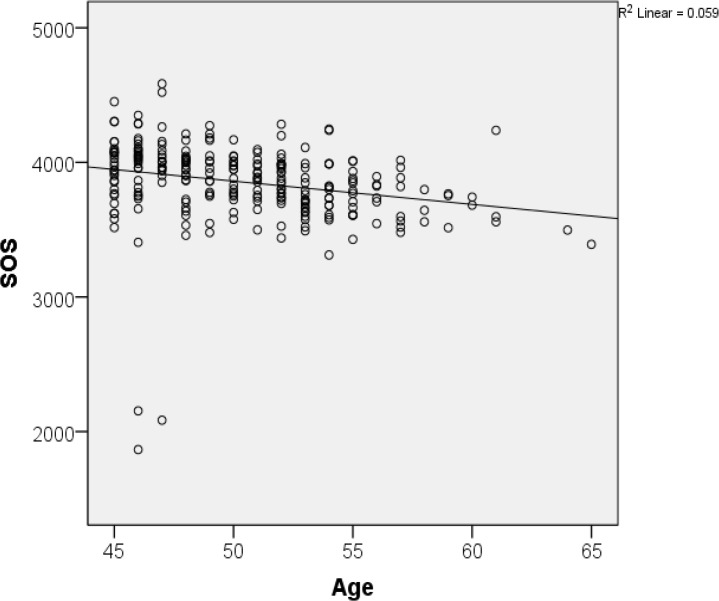
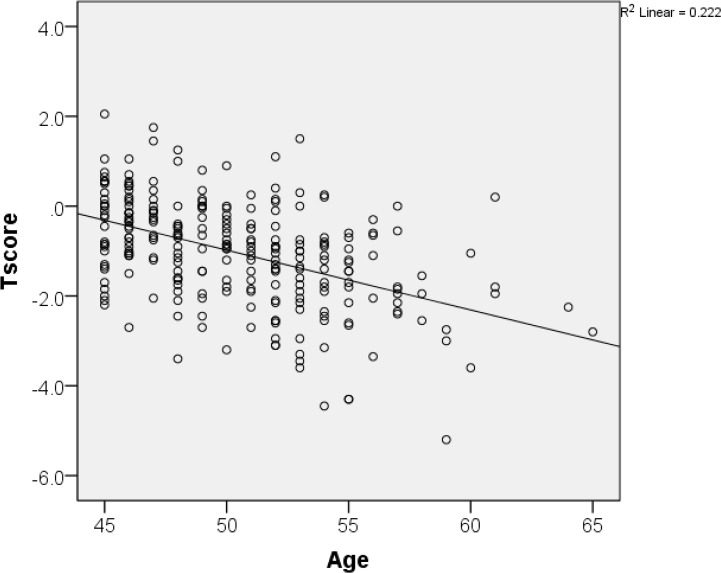
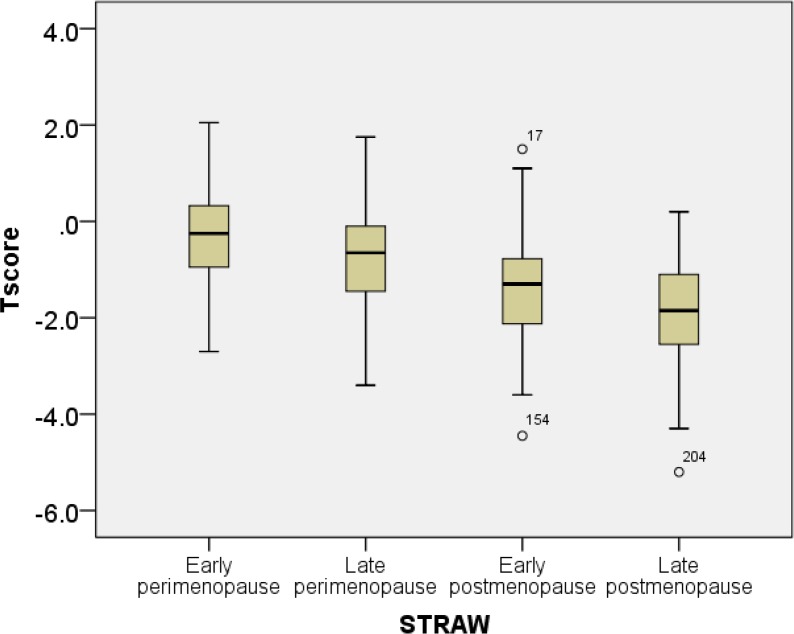
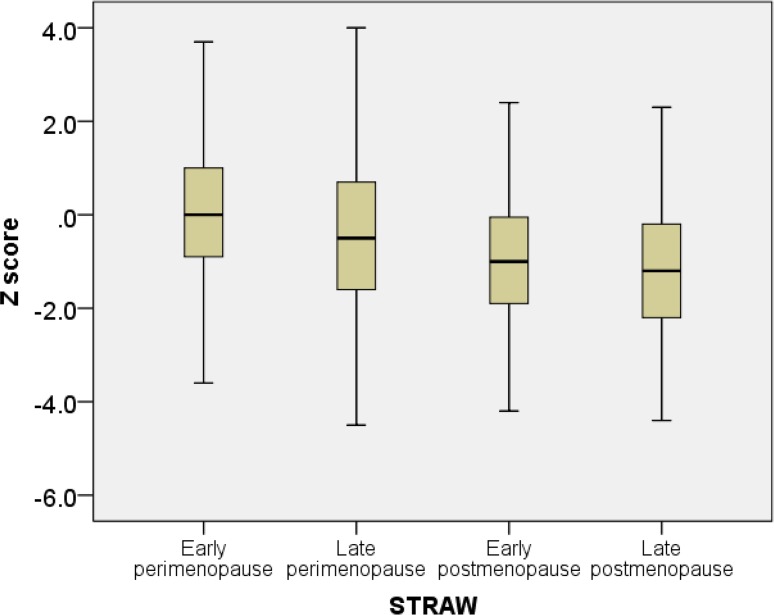
T-score and speed of sound progressively decreased with with age (Figures 1 and 2). The box plots of T and Z scores of the participants stratified by menopausal status are shown in Figures 3 and 4.
Figure 1.
Correlation between age and bone mineral density
Figure 2.
Correlation between age and speed of sound
Figure 3.
Box plots of T scores stratified by menopausal status
Figure 4.
Box plots of Z scores stratified by menopausal status
Discussion
The menopausal period is a normal physiological process that is accompanied by various symptoms.8 The researchers identified that the average age of onset of the menopause is 48–52, and it is similar in women across various countries.14 Recently published study demonstrated that the average age of menopause onset among Mongolian women was 49.3±3.1, 48.6±1.7 and 48.2±2.9 years in 2008, 2010, and 2011, respectively.15 Similarly, in our study, the mean age of menopause for our research participants was 48.4±3.4 years.
The current study showed that the rate of osteoporosis was 11.2% and osteopenia was 34.6%, rates that were similar to results of other Asian women from reports of WHO in 2008.16 Osteoporosis incidence was higher in women with advanced stages of menopause. In our study, FSH and LH levels significantly differed across menopause stages in that those who were post-menopausal had higher levels compared to those who were pre- or peri-menopausal. Both hormones, FSH and LH, showed weak negative correlations with BMD level, but not E2. The result was significant in FSH level and SOS (r=−0.16; p<0.01), which was similar to previous studies.17,18 This could indicate that low BMD in later stages of menopause is associated with serum FSH rather than E2. The weak correlation may be due to our small sample size. Other limitations of the current study include: participants were only selected from city areas and rural areas were not sampled, and the BMD was only tested in forearm and tibia. Ideally, the most common fracture sites, such as hips and spine, should be tested in future studies.
This is one of the first studies in Mongolia to determine the correlation between the hormonal levels and menopausal status. We concluded that women from older age groups had lower levels of serum estradiol, which were associated with loss of BMD. However, there was significant correlation between FSH and BMD in more advanced stages of menopause. Further study is needed to investigate the bone metabolism related studies to establish better epidemiological data sources among Mongolian population. Authors would like to encourage similar studies in Mongolia and Central Asia, consistent with the need of Cental Asian Region for better-developed chronic disease research.19
Acknowledgments
We wish to thank you all volunteers and the consultant gynecologist and the staff of the First Maternity Hospital.
Footnotes
Conflict of Interest
The author declares that they have no competing interests.
This journal is published by the University Library System of the University of Pittsburgh as part of its D-Scribe Digital Publishing Program and is cosponsored by the University of Pittsburgh Press.
References
- 1.Darba J, Kaskens L, Perez-Alvarez N, Palacios S, Neyro JL, Rejas J. Disability-adjusted-life-years losses in postmenopausal women with osteoporosis: a burden of illness study. BMC Public Health. 2015;15:324. doi: 10.1186/s12889-015-1684-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster JY. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2013;24(1):23–57. doi: 10.1007/s00198-012-2074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO Assessment of osteoporosis at primary health care level. Summary Meeting Report. 2004 http://www.who.int/chp/topics/Osteoporosis.pdf. [Google Scholar]
- 4.McKiernan FE, Berg RL, Linneman JG. The utility of BMD Z-score diagnostic thresholds for secondary causes of osteoporosis. Osteoporos Int. 2011;22(4):1069–1077. doi: 10.1007/s00198-010-1307-1. [DOI] [PubMed] [Google Scholar]
- 5.Kanis JA, Melton LJ, 3rd, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9(8):1137–1141. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 6.Moon R, Cooper C, Harvey NC. The Duration and Safety of Osteoporosis Treatment. Springer International Publishing; 2016. Osteoporosis: Pathophysiology and Epidemiology; pp. 1–16. [Google Scholar]
- 7.Keating NL, Cleary PD, Rossi AS, Zaslavsky AM, Ayanian JZ. Use of hormone replacement therapy by postmenopausal women in the United States. Ann Intern Med. 1999;130(7):545–553. doi: 10.7326/0003-4819-130-7-199904060-00002. [DOI] [PubMed] [Google Scholar]
- 8.Cauley JA, Seeley DG, Ensrud K, Ettinger B, Black D, Cummings SR. Estrogen replacement therapy and fractures in older women. Study of Osteoporotic Fractures Research Group. Ann Intern Med. 1995;122(1):9–16. doi: 10.7326/0003-4819-122-1-199501010-00002. [DOI] [PubMed] [Google Scholar]
- 9.Boroňová IBJ, Kľoc J, Tomková Z, Petrejčíková E, Mačeková S, Blaščáková MM. Analysis of OPG Gene Polymorphism T245G (rs3134069) in Slovak Postmenopausal Women. Analysis. 2014;2(12859) [Google Scholar]
- 10.Soules MR, Sherman S, Parrott E, et al. Stages of Reproductive Aging Workshop (STRAW). J Womens Health Gend Based Med. 2001;10(9):843–848. doi: 10.1089/152460901753285732. [DOI] [PubMed] [Google Scholar]
- 11.Heinemann DF. Osteoporosis. An overview of the National Osteoporosis Foundation clinical practice guide. Geriatrics. 2000;55(5):31–36. quiz 39. [PubMed] [Google Scholar]
- 12.Chin KY, Ima-Nirwana S. Calcaneal quantitative ultrasound as a determinant of bone health status: what properties of bone does it reflect? Int J Med Sci. 2013;10(12):1778–1783. doi: 10.7150/ijms.6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO . Prevention and management of osteoporosis Report of a WHO Scientific Group. Geneva: World Health Organization; 2003. [Google Scholar]
- 14.Palacios S, Henderson VW, Siseles N, Tan D, Villaseca P. Age of menopause and impact of climacteric symptoms by geographical region. Climacteric. 2010;13(5):419–428. doi: 10.3109/13697137.2010.507886. [DOI] [PubMed] [Google Scholar]
- 15.Ulziinorov G. The Relationship between Menopause Symptoms and the Quality of life of Women in Ulaabaatar City, Mongolia. Mahidol University: Institute for Population and Social Research; 2009. [Google Scholar]
- 16.Mithal A, Kaur P. Osteoporosis in Asia: a call to action. Curr Osteoporos Rep. 2012;10(4):245–247. doi: 10.1007/s11914-012-0114-3. [DOI] [PubMed] [Google Scholar]
- 17.Wu XYS, Zhang H, Xie H, Luo X, Peng Y, Liao E, et al. Early bone mineral density decrease is associated with FSH and LH, not estrogen. Clinica Chimica Acta. 2013;415:69–73. doi: 10.1016/j.cca.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Xu ZWA, Wu X, Zhang H, Sheng Z, Wu X, Liao E. Relationship of age-related concentrations of serum FSH and LH with bone mineral density, prevalence of osteoporosis in native Chinese women. Clinica Chimica Acta. 2009;400(1–2):8–13. doi: 10.1016/j.cca.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 19.Adambekov S, Kaiyrlykyzy A, Igissinov N, Linkov F. Health challenges in Kazakhstan and Central Asia. J Epidemiol Community Health. 2016;70(1):104–108. doi: 10.1136/jech-2015-206251. [DOI] [PubMed] [Google Scholar]