Abstract
Parkinson's disease is a progressive neurological disorder, marked by the loss of dopaminergic neurons in the nigrostriatal pathway that leads to abnormal gait, rigidity, slowness of movement, and tremor. The ability to recapitulate and measure the neurological sequelae in rodent models of Parkinson's disease is important for studying and evaluating potential therapeutics. Individual variability in lesion severity and injury progression are key factors in the 6-hydroxydopamine model that require normalization when evaluating therapeutic effects. The gait parameters that were found to be affected by 6-hydroxydopamine lesioning of the nigrostriatal pathway in rats may be used to study novel transgenic models of Parkinson's disease as well as to test novel therapeutics. Previously, studies have used a video-based system to analyze gait abnormalities in the 6-hydroxydopamine model of Parkinson's disease, but these studies did not account for individual variability on reported gait parameters. By analyzing the ratio of parameters from the injured to uninjured sides and correcting for speed in related parameters, hindpaw step cycle parameters, hindpaw print area, and step sequence are significantly altered in different ways for each type of lesion, when compared to saline-injected controls. These findings enable new metrics for evaluating therapeutic efficacy of drug-, gene-, or cell-based therapies in rat models of Parkinson's disease.
Keywords: CatWalk, Parkinson's disease (PD), Gait analysis, Step sequence, 6-Hydroxydopamine (6-OHDA)
Introduction
Parkinson's disease (PD) is a progressive neurodegenerative disease characterized by a loss of the nigrostriatal dopamine neurons that control movement, which leads to diminished locomotor ability. Behavioral assessments of locomotor ability are used as a principle method for tracking the progression and treatment of PD. In animal models of the disorder, nigrostriatal dopamine neurons are degraded by exposure to toxins or through genetic manipulation. These methods yield similar phenotypic characteristics as those seen in the human disorder, including a reduction in tyrosine hydroxylase (TH) immunoreactivity and diminished locomotor behavior. Unilateral lesioning of the nigrostriatal dopamine neurons is useful for PD models because it allows for the contralateral hemisphere of the brain to serve as an internal reference for an intact dopamine pathway. Injection of 6-hydroxydopamine (6-OHDA) in the right hemisphere, for example, creates a dopamine imbalance that can be indirectly measured through pharmacologically induced rotational behavior (e.g., with amphetamine or apomorphine)1. Amphetamine causes the release of dopamine in the striatum, which is imbalanced as a result of the loss of dopamine-producing neurons in the lesioned hemisphere. The injured animal will rotate ipsilaterally relative to the 6-OHDA-induced lesion2. In contrast, apomorphine, a dopamine receptor agonist, causes contralateral rotation due to an increased expression and response of dopamine receptors in the lesioned hemisphere3. Although a robust and simple test to administer, this is an involuntary behavior that does not reflect any clinical PD symptoms. One of the hallmark symptoms of PD is a festinating or “parkinsonian” gait characterized by a generally slow pace with small, shuffling steps. Typically, there is a reduction in the length of each stride and an increased cadence rate. In addition, the typical parkinsonian gait includes increased time spent with both legs supporting the body4.
Gait analysis can be conducted on voluntary movement and allows for the longitudinal monitoring of motor phenotypes that mimic PD symptoms. Commercially available video-tracking systems, such as the CatWalk XT system, are designed for gait analysis in rodents. The CatWalk XT system consists of a glass platform illuminated by green light that causes any contact with the glass plate to be highlighted and recorded for analysis. The CatWalk XT is increasingly common in PD research5 and has been used to evaluate the therapeutic potential of transplanted embryonic stem cell-derived dopaminergic cells in a rat model of PD6. Here we use CatWalk XT for gait analysis of two types of 6-OHDA lesion models: partial and complete. Injections of 6-OHDA in the medial forebrain bundle (MFB) and the striatum (intra-STR) are both used as models of PD7. When assessed by reduced TH immunoreactivity and behavioral deficits, the MFB lesion produces a complete loss of functional connectivity on one side, while the intra-STR lesion produces a partial loss.
To date, the speed of locomotion has not been considered in the analysis of PD models using the CatWalk XT system. The impairment of motor function expected from degradation of nigrostriatal dopamine neurons should include a reduction in speed as observed in the human PD. In a rapidly progressing model such as 6-OHDA (1-2 weeks), an easy-to-use, rapidly performed assay is critical for tracking locomotor degradation throughout the progressive development of the disease state.
The use of a unilateral lesion allows for a better behavioral assay for rodent models of PD by allowing for the normalization of gait parameters measured contralateral to the lesion against the ipsilateral side. Here we identify step sequence as a useful metric of gait impairment in the unilateral lesion model for PD.
Materials and Methods
Animals
All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committees (IACUCs) of the National Institutes of Health (NIH). Young adult (5-6 weeks old) male Long Evans rats weighing approximately 250-350 g at the time of surgery were housed separately with ad libitum access to food and water during a 12-h normal light/dark cycle. Forty rats received intracranial 6-OHDA or saline injections in the MFB or intra-STR region after training on the CatWalk XT system (Noldus, Leesburg, VA, USA) (Fig. 1A). Rats were grouped into one of three experimental groups [MFB lesion (n = 14), intra-STR lesion (n = 14), sham injection (n = 12)] based on their average prelesion speed measured from a single session of three runs. Group assignment for each individual was confirmed by immunohistochemistry at the end of the experiment.
Figure 1.
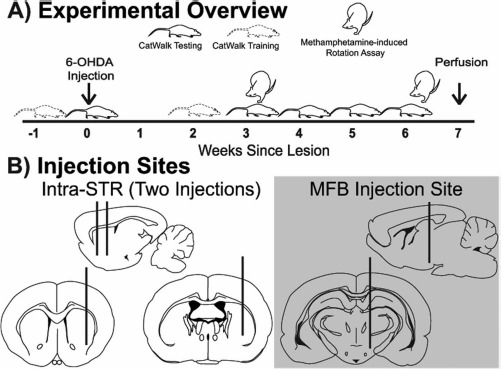
Experimental overview. (A) Timeline of behavioral assays (CatWalk and methamphetamine-induced rotations) in relation to induction of lesion. (B) Schematic of targeted sites for unilateral 6-hydroxydopamine (6-OHDA) lesion to create the intrastriatal (intra-STR; two striatal injections) and medial forebrain bundle (MFB; single injection) model of Parkinson's disease.
Chemicals and Reagents
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise noted. Ketamine, xylazine, and methamphetamine were obtained from the NIDA Pharmacy (Baltimore, MD, USA).
Surgery
Animals were anesthetized with isoflurane followed by 80 mg/kg of ketamine and 8 mg/kg of xylazine and then placed into a stereotaxic frame where they received an injection of either 6-OHDA (lesion) or 0.9% saline (sham). A 10-μl Nanofil syringe with a 33-gauge needle mounted on a microprocessor control syringe pump [UMP4; World Precision Instruments (WPI), Sarasota, FL, USA] was used to infuse 6-OHDA at a rate of 1 μl/min. The 6-OHDA was prepared in deoxygenated saline to 10 mM (+0.01% ascorbic acid). The unilateral MFB lesions were made by injecting 4 μl into the left MFB at the coordinates: anterior-posterior (AP) = −4.4 mm (bregma), medial-lateral (ML) = +1.3 mm, dorsal-ventral (DV) = −8.0 mm (Fig. 1B). The unilateral intra-STR lesion consists of two striatal injections on the left hemisphere at the coordinates: AP = −1.0 mm, ML = +3.5 mm, DV = −6.0 mm and AP = +1.0, ML = +3.5 mm, DV = −6.0 mm. Each striatal site received 2 μl of the 10 mM 6-OHDA solution (Fig. 1B).
Immunohistochemistry and Density Analysis
Upon completion of the behavioral study, the brains were fixed by transcardial perfusion with 0.9% saline followed by 4% paraformaldehyde (PFA), removed from the skull, postfixed for 2 h in 4% PFA, and then cryopreserved using a sucrose gradient of 18% sucrose followed by 30% sucrose. Coronal cryosections (30 μm) of the striatum and midbrain were blocked with phosphate buffer solution containing 4% goat serum and 0.3% Triton X-100 for 1 h and then incubated in anti-TH primary antibody (1:2,000; MAB318; Merck Millipore, Billerica, MA, USA) at 4°C overnight. The following day, sections were incubated in IR-800 secondary antibody (1:2,000; Rockland Immunochemicals Inc., Pottstown, PA, USA) for 1 h at room temperature, followed by a 15-min TO-PRO-3 incubation (1:1,000; Molecular Probes, Eugene, OR, USA). For each animal, three sections within the striatum and three sections within the MFB on the ipsilateral and contralateral sides were mounted and imaged using Odyssey Infrared Imager 9120 (LI-COR Biosciences, Lincoln, NE, USA). The three sections were selected to be from the rostral, medial, and caudal portions of the region of interest, respectively. ImageJ software (version 1.48; NIH, Bethesda, MD, USA) was used to compare the optical density of TH immunofluorescence between the two hemispheres of each striatal and midbrain sections. TH optical density in the striatum was normalized to TO-PRO-3 fluorescence to account for any differences in section thickness between brain hemispheres. TO-PRO-3 normalization was not performed on midbrain sections because of loss of TO-PRO-3 cell labeling that occurred as a result of the 6-OHDA lesion.
Gait Analysis
All gait analysis recording and paw print identification were done using the CatWalk XT hardware and software. The apparatus consists of a 130-cm tunnel, raised 152 cm from the ground. The raised tunnel has a glass floor with an internally reflected green light and a ceiling that contains a red light to provide contrast to the internally reflected green light. A high-speed video camera records the green light reflected from each point of contact with the animal at 100 frames per second from underneath the glass-bottom floor of the walkway. The pattern of green light is interpreted by custom software and translated into a quantitative assessment of the rodent paw prints (Table 1).
Table 1.
CatWalk XT Parameter Definitions
| Parameter | Units | Definition |
|---|---|---|
| Print area | cm2 | Surface area of complete print |
| Step sequence | % | Order in which paws were placed on glass plate |
| Types of sequences: alternate (A, B), cruciate (A, B), and rotate (A, B) | ||
| Stand | s | Duration of contact of paw with glass |
| Swing | s | Duration in seconds of no contact of paw with glass |
| Step cycle | s | Time between two consecutive initial contacts of the same paw |
| Step cycle = stand + swing |
All rats were trained on traversing the CatWalk for 5 consecutive days in the 2 weeks before surgeries. Training involved allowing each rat to cross the runway until the rat completed three uninterrupted movements across the platform. Any rat that turned around or stopped for longer than 30 s on the glass platform was immediately removed from the CatWalk and allowed to begin a new crossing attempt. Each rat was given a maximum of 10 consecutive attempts to make three successful crossings per training day. After each successful crossing, rats were prompted to enter the goal box (their home cage) and given a Froot Loop (Kellogg's, Battle Creek, MI, USA) reward. Retraining took place 2 weeks after lesioning over 3 consecutive days.
A trial (or run) was considered successful if the duration was between 0.8 and 5 s and performed at a steady speed (<70% variation). This percentage of variation was determined to be the maximum variation allowable without rats fully stopping or turning on the CatWalk runway.
Methamphetamine-Induced Rotational Behavior
Methamphetamine-induced rotational behavior was measured at 3 and 6 weeks after lesioning. Rats were placed in round chambers with cameras recording from above using EthoVision XT 8.5 (Noldus) for 1 h. Following this habituation period, rats were subcutaneously injected with 2.5 mg/kg methamphetamine8 and placed back into the same chamber. Clockwise and counterclockwise rotations were manually counted for 1 h after injection using the recorded videos by a technician blind to the experimental groups. One rat in the sham group had unusually high rotational bias toward the ipsilateral side and therefore was excluded from the study, and another rat was excluded from data analysis due to early death. The final numbers of animals in the methamphetamine-induced rotation experiments were 10 shams, 14 intra-STR, and 12 MFB.
Data Analysis
Statistical analyses were done using SPSS 22.0 software (IBM, Armonk, NY, USA). For each animal, an average score of the successful runs (one to three per animal) was calculated for each metric and presented as mean ± standard error. All lateralized parameters were normalized by dividing the ipsilateral side by the contralateral side prior to averaging. There were no differences observed between the MFB sham and intra-STR sham animals, so these are analyzed as a single sham group. The significance of speed on data analysis was determined by a multivariate analysis of covariance (MANCOVA) test of between-subjects effects with average speed as covariate. Tukey post hoc tests were performed to determine significance between groups, except in cases where comparisons were needed to control for prelesion variability that required a post hoc comparison with a Dunnett's t-test. All graphs were rendered with Prism, version 6.0 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Characterizing Lesions
As expected5, the group that received MFB 6-OHDA lesions had the greatest decrease in TH immunoreactivity relative to the contralateral side, with almost complete ablation of TH+ neurons in the nigrostriatal pathway. Saline-injected controls exhibited no significant loss of TH immunostaining in either striatum or midbrain (Fig. 2A). The intra-STR group exhibited a partial lesion phenotype, with TH terminal loss observed only in a portion of the striatum and minimal loss in the midbrain (Fig. 2B and C). Striatum: analysis of variance (ANOVA), F = 178.5, p < 0.0001. Midbrain: ANOVA, F = 117.3, p < 0.0001.
Figure 2.
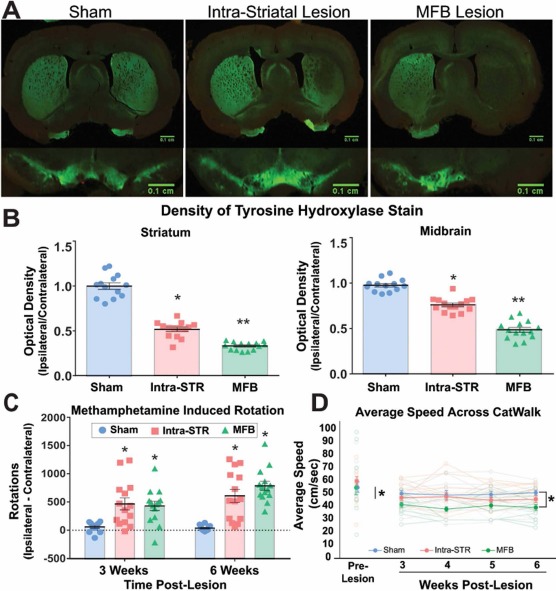
Loss of dopamine neurons impairs locomotion. (A) Representative images of tyrosine hydroxylase (TH) loss in the striatum (above) and substantia nigra (below) of each lesion group compared to sham. (B) Semiquantification optical density measurements of TH immunoreactivity relative to contralateral hemisphere in the striatum and midbrain. (C) Net ipsilateral turns (ipsilateral minus contralateral turns) following methamphetamine administration. (D) Average speeds of rats tracked throughout the study. Intra-STR, group with injections of 6-OHDA in the striatum; MFB, medial forebrain bundle/group with injections of 6-OHDA in the medial forebrain bundle.
Methamphetamine-Induced Rotations
The partial lesion (intra-STR) group showed the most variation in both the histological and rotational phenotype. Most notably, the intra-STR group had a significantly higher average rotational behavior when compared to the MFB group in the first testing session, which may be a result of variable nigrostriatal loss from the striatal lesions. However, the MFB 6-OHDA rats performed more ipsilateral rotations at the latter time point (repeated-measures ANOVA, p < 0.0001).
Gait Parameters
The average speed to complete the locomotor task was significantly slower (ANOVA, F = 2.868, p < 0.01) (Fig. 2D) for the MFB group (41.784 cm/s, standard error: ± 0.546) compared to either the intra-STR (47.865 cm/s, standard error: ± 1.865; Tukey post hoc, p < 0.01) or sham-injured group (50.254 cm/s, standard error: ± 1.300; Tukey post hoc, p < 0.01).
The print area of the paw measures the amount of skin in physical contact with the floor. The average of the hind paw print areas, quantified as lesioned/nonlesioned limb, for lesioned animals was significantly larger than the print area of sham-injected animals (0.969 ± 0.335; ANOVA, F = 2.259, p < 0.01). In addition, the MFB-lesioned animals had larger print areas (1.290 ± 0.051) compared to the intra-STR lesion group (1.154 ± 0.042; Tukey post hoc, p = 0.046) (Fig. 3A). This could be indicative of changes in limb loading observed in PD, even though the physics of limb loading in quadrupeds is fundamentally different from bipeds.
Figure 3.
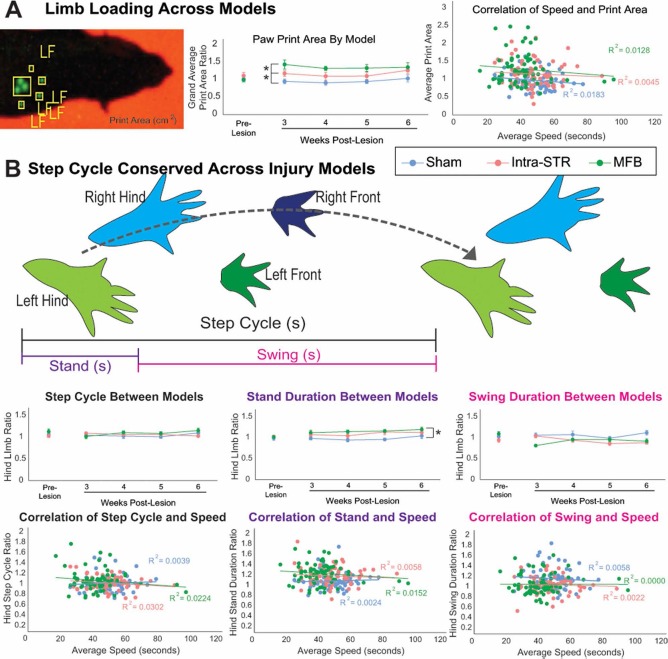
The 6-OHDA lesion alters proportion of step cycle spent in stand phase. (A) Left: Image of left front paw print taken from the CatWalk software. Middle: Ratio of hindpaw (left or lesioned side/right or unlesioned side) print area is different between types of lesion, indicating a change in limb loading. Right: Print area does not correlate with speed of crossing the apparatus. (B) Schematic of step cycle made up of the stand (paw contact with glass) and swing (paw not in contact with glass) phases. Step cycle as a whole did not change in both the lesion and sham groups. 6-OHDA, 6-hydroxydopamine; Intra-STR, group with injections of 6-OHDA in the striatum; MFB, medial forebrain bundle/group with injections of 6-OHDA in the medial forebrain bundle; LF, left front paw.
The step cycle is defined by the duration of a paw's full contact with the glass (stand), through the forward movement of the paw in the air (swing), until its next contact (Fig. 3B). The ratio of hindpaw step cycles did not significantly change in the 6-OHDA or sham groups over the four testing days (ANOVA, F = 0.963, p = 0.539) (Fig. 3B), even when corrected for speed (MANCOVA, F = 0.957, p = 0.550). Of the subcomponents of the step cycle, the ratio of hindlimb stand duration significantly changed after lesion in both groups (ANOVA, F = 1.514, p = 0.038; with Tukey post hoc, p < 0.01 for sham to lesion groups), while the swing duration was not significantly different as defined by our cutoff value of p < 0.01 (ANOVA, F = 1.423, p = 0.066). Factoring in speed did not alter these relationships (stand duration MANCOVA, F = 1.494, p = 0.042; swing duration MANCOVA, F = 1.381, p = 0.063).
The gait parameter most significantly affected by the 6-OHDA lesion is the step sequence. There are three principal sequences: cruciate, alternate, and rotate, which are each subdivided into two forms (Fig. 4A). From the sham group and prelesion data, it appears that healthy rats tend to preferentially traverse the CatWalk using alternate step patterns, with a secondary inclination for cruciate-type sequences (Fig. 4A). In the testing weeks following lesion by 6-OHDA, the preference toward alternate step patterns diminishes and is replaced with cruciate step patterns (Fig. 4B). The most drastic change was observed in the proportion of cruciate A step sequence (Fig. 4C), which increased in frequency in lesioned groups (ANOVA, F = 3.352, p < 0.01), even after considering variability from the prelesion trials (Dunnett's t-test post hoc, p < 0.01) or accounting for speed (MANCOVA, F = 3.515, p < 0.01). We also observed negative and positive correlations with TH immunostaining and alternate step sequences and cruciate step sequences, respectively (Fig. 5).
Figure 4.
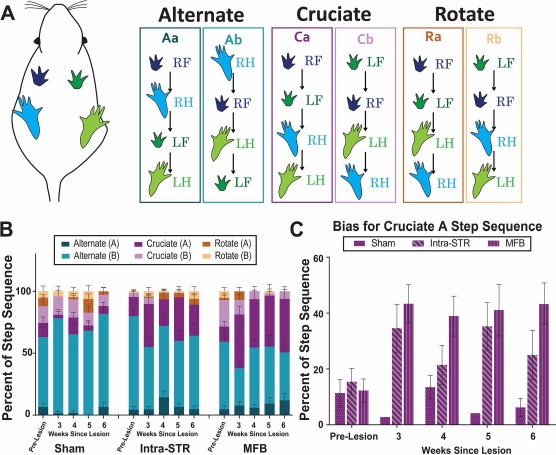
The 6-OHDA lesions alter step sequence preference. (A) Schematic demonstrates the order of each paw in the six-step sequence pattern classifications. (B) Overall step sequence: the proportions of each step sequence type out of total step patterns identified. (C) The cruciate A step sequence is more common after lesion, permitting quantification of parkinsonian gait in an animal model. LF, left front paw; LH, left hind paw; RF, right front paw; RH, right hind paw; 6-OHDA, 6-hydroxydopamine; Intra-STR, group with injections of 6-OHDA in the striatum; MFB, medial forebrain bundle/group with injections of 6-OHDA in the medial forebrain bundle.
Figure 5.
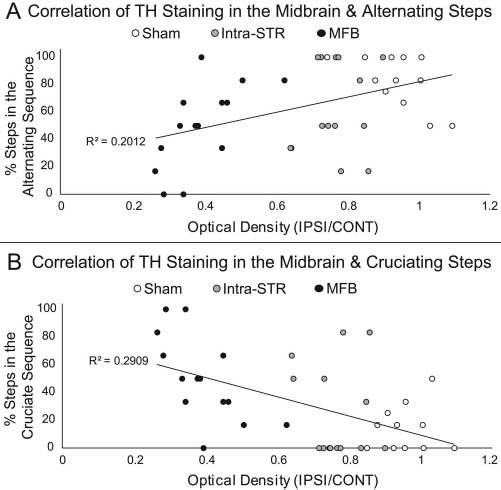
Correlation of step sequence and TH immunoreactivity in the midbrain. To examine the relationship between TH staining and changes in gait, optical density of TH staining in the midbrain was correlated to (A) the percentage of steps taken in the alternate sequences and (B) in the cruciate sequences on week 6. Although negative and positive correlations are shown with TH staining/alternate step sequences and TH staining/cruciate step sequences, respectively, a floor effect (0% of alternate sequence in MFB lesion) and a ceiling effect (100% steps taken as alternate in sham lesion) negate statistical analysis. TH, tyrosine hydroxylase; 6-OHDA, 6-hydroxydopamine; Intra-STR, group with injections of 6-OHDA in the striatum; MFB, medial forebrain bundle/group with injections of 6-OHDA in the medial forebrain bundle.
Discussion
Unilateral 6-OHDA lesions of the MFB and STR in rats can be used to model specific changes in locomotor gait in PD. By using the contralateral side to control for individual differences and analyzing the step sequence with the CatWalk XT system, it is possible to assess disease progression as a function of changes in gait. Although MFB and STR lesion rats are significantly slower than sham rats, speed did not account for the significant changes we observed in hindpaw step cycle parameters, hindpaw print area, and step sequence as a result of lesioning.
As previously observed7, we found that rats that received intra-STR injections exhibited a less severe loss of dopaminergic neurons in the nigrostriatal pathway [including in the ventral tegmental area (VTA)] when compared to rats that received MFB injections (Fig. 2B). Three weeks following 6-OHDA injections, the intra-STR group exhibited higher net ipsilateral rotational behavior in the methamphetamine-induced rotational behavior paradigm than did the MFB group. However, 6 weeks following 6-OHDA lesion, the MFB group showed higher net ipsilateral rotational behavior than the intra-STR group. It should also be noted that the intra-STR group showed more variable rotational count than the MFB group. As expected from the severity of the lesion, the MFB group showed greater deviation in parameters reported from the sham group compared to the intra-STR group. In fact, most of the reported parameters changed in a manner which corresponded to the severity of the lesion. In some parameters reported, including print area, the intra-STR group did not reach statistical significance, although a trend can be seen.
The use of a unilateral lesion model for PD allows for the comparison of the lesioned side to the nonlesioned side. Comparing the lesioned side (left in this study) to the nonlesioned side (right) using a left/right ratio allows for the objective analysis of step cycle gait parameters in a speed-independent manner that corrects for individual differences in gait. Prelesion gait can be measured to account for preexisting bias that could skew the data. We did not observe any animals showing a strong bias in the key parameters described in this study.
For a unilateral lesion, the overall speed of the locomotion confounds many of the gait parameters measured by the CatWalk XT and should be a factor in the analysis9. Following surgery, the MFB-lesioned rats were significantly slower than sham controls, but the intra-STR-lesioned animals demonstrated an insignificant change in average speed (Fig. 2D). The speed of successful CatWalk trials did not correlate strongly with the overall step cycle or the subcomponents of the step cycle (Fig. 3B). The slower pace of locomotion with the more severe lesion (MFB) is consistent with clinical observations of impaired movement in PD patients.
Deficits in interlimb coordination have previously been observed in patients with PD10. We observed an alteration in limb coordination in unilaterally 6-OHDA-lesioned rats as measured by a shift in preference toward the cruciate A step sequence (Fig. 4C). The alternate step sequences require the animal to shift from front to hind-paws (or hind to front) on the same side of the body. In contrast, the cruciate A and B sequences involve shifting from the front (or hind) paw on one side of the body to the front (or hind) paw on the contralateral side. We speculate that this shift in step sequence preference is due to a loss of interlimb coordination in unilaterally 6-OHDA-lesioned animals, thereby making it difficult for the lesioned rats to step consecutively on the same side of the body. In a previous study using CatWalk to examine gait in mice lesioned with 1-methyl-4-phenyl-1, 2,3, 6-tetrahydropyridine (MPTP), no changes to step patterns were observed11. In MPTP lesioning, the loss of dopaminergic neurons occurs bilaterally, and changes to the step sequence may only manifest as an adaptation to a unilateral lesion of dopaminergic neurons. This notion is supported by previous studies using unilateral or bilateral 6-OHDA lesioning of MFB in rats where no significant difference in regularity index, a measurement of step sequence, was observed6,12. Our data support that there is a shift in the pattern of walking (step sequence), but the regularity of the lesion-related pattern is comparable to the regularity of the prelesion. In our study, we see a significant shift from alternate step sequence to cruciate step sequence with no significant difference in the regularity index. We also observed negative and positive correlations with TH immunostaining and alternate step sequences and cruciate step sequences, respectively (Fig. 5). Although a floor effect (0% of alternate sequence in MFB lesion) and a ceiling effect (100% steps taken as alternate in SHAM lesion) were observed, the expanded analyses of the step sequence serve as a robust metric of lateralized gait defects resulting from nigrostriatal degeneration induced by the unilateral injections of 6-OHDA.
By analyzing the changes in the step sequence after induction of the 6-OHDA lesion, the gait abnormalities were evident at 3, 4, 5, and 6 weeks after the injury. Using the model presented herein, therapeutic strategies based on neurotransplantation or neuroregeneration could be tested as early as 3 weeks following lesion (MFB or STR) for ability to reverse the change in step sequence. Additional studies can also be performed to determine the effectiveness of earlier interventions, such as trophic factor delivery or L-dopa, to counter the loss of dopamine production and associated change in gait. Such studies may aid in determining if normal gait can be recovered after the induction of parkinsonian gait or if the induction of parkinsonian gait is irreversible.
Using a video-based gait analysis system, we were able to identify changes in the step sequence of moderate (intra-STR) and severe (MFB) unilateral lesions to the rat nigrostriatal pathway that are common models of PD. After controlling for variability in speed and individual differences, the effects of the lesions on the duration of the average step cycle were minimal even though the overall speed was diminished as expected. Lesioned animals also demonstrated changes in limb loading consistent with observations of parkinsonian gait. Critically, 6-OHDA lesions led to an alteration in the step sequence that can be used to model changes in gait observed in the human disease associated with a dysfunctional nigrostriatal pathway.
Acknowledgment
The authors declare no conflicts of interest.
References
- 1.Schober A. Classic toxin-induced animal models of Parkinson's disease: 6-OHDA and MPTP. Cell Tissue Res. 2004; 318(1): 215–24. [DOI] [PubMed] [Google Scholar]
- 2.Ungerstedt U., Arbuthnott G.W. Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Res. 1970; 24(3): 485–93. [DOI] [PubMed] [Google Scholar]
- 3.Ungerstedt U. Striatal dopamine release after amphetamine or nerve degeneration revealed by rotational behaviour. Acta Physiol Scand Suppl. 1971; 367: 49–68. [DOI] [PubMed] [Google Scholar]
- 4.Goetz C.G., Tilley B.C., Shaftman S.R., Stebbins G.T., Fahn S., Martinez-Martin P., Poewe W., Sampaio C., Stern M.B., Dodel R., Dubois B., Holloway R., Jankovic J., Kulisevsky J., Lang A.E., Lees A., Leurgans S., LeWitt P.A., Nyenhuis D., Olanow C.W., Rascol O., Schrag A., Teresi J.A., van Hilten J.J., LaPelle N. Movement Disorder Society UPDRS Revision Task Force. Movement disorder society-sponsored revision of the unified Parkinson's disease rating scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov Disord. 2008; 23(15): 2129–70. [DOI] [PubMed] [Google Scholar]
- 5.Zhou M., Zhang W., Chang J., Wang J., Zheng W., Yang Y., Wen P., Li M., Xiao H. Gait analysis in three different 6-hydroxydopamine rat models of Parkinson's disease. Neu rosci Lett. 2015; 584: 184–9. [DOI] [PubMed] [Google Scholar]
- 6.Chuang C.S., Su H.L., Cheng F.C., Hsu S.H., Chuang C.F., Liu C.S. Quantitative evaluation of motor function before and after engraftment of dopaminergic neurons in a rat model of Parkinson's disease. J Biomed Sci. 2010; 17: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deumens R., Blokland A., Prickaerts J. Modeling Parkinson's disease in rats: An evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol. 2002; 175(2): 303–17. [DOI] [PubMed] [Google Scholar]
- 8.Colcher A., Simuni T. Clinical manifestations of Parkinson's disease. Med Clin North Am. 1999; 83(2): 327–47. [DOI] [PubMed] [Google Scholar]
- 9.Batka R.J., Brown T.J., Mcmillan K.P., Meadows R.M., Jones K.J., Haulcomb M.M. The need for speed in rodent locomotion analyses. Anat Rec. (Hoboken) 2014; 297(10): 1839–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roemmich R.T., Field A.M., Elrod J.M., Stegemoller E.L., Okun M.S., Hass C.J. Interlimb coordination is impaired during walking in persons with Parkinson's disease. Clin Biomech. (Bristol, Avon) 2013; 28(1): 93–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X.H., Lu G., Hu X., Tsang K.S., Kwong W.H., Wu F.X., Meng H.W., Jiang S., Liu S.W., Ng H.K., Poon W.S. Quantitative assessment of gait and neurochemical correlation in a classical murine model of Parkinson's disease. BMC Neurosci. 2012; 13: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westin J.E., Janssen M.L., Sager T.N., Temel Y. Automated gait analysis in bilateral parkinsonian rats and the role of L-DOPA therapy. Behav Brain Res. 2012; 226(2): 519–28. [DOI] [PubMed] [Google Scholar]


