Abstract
Amyotrophic lateral sclerosis (ALS) is a progressive untreatable neurodegenerative disorder, leading to the death of the cortical and spinal motoneurons (MNs). Bone marrow-derived mesenchymal stem/stromal cells (BM-MSCs) may represent a new approach to slowing down the progression of ALS by providing neurotrophic support to host MNs and by having an anti-inflammatory effect. We have designed a prospective, nonrandomized, open-label clinical trial (phase I/IIa, EudraCT No. 2011-000362-35) to assess the safety and efficacy of autologous multipotent BM-MSCs in ALS treatment. Autologous BM-MSCs were isolated and expanded under GMP conditions. Patients received 15 ± 4.5 × 106 of BM-MSCs via lumbar puncture into the cerebrospinal fluid. Patients were monitored for 6 months before treatment and then for an 18-month follow-up period. Potential adverse reactions were assessed, and the clinical outcome was evaluated by the ALS functional rating scale (ALSFRS), forced vital capacity (FVC), and weakness scales (WSs) to assess muscle strength on the lower and upper extremities. In total, 26 patients were enrolled in the study and were assessed for safety; 23 patients were suitable for efficacy evaluation. After intrathecal BM-MSC application, about 30% of the patients experienced a mild to moderate headache, resembling the headaches after a standard lumbar puncture. No suspected serious adverse reactions (SUSAR) were observed. We found a reduction in ALSFRS decline at 3 months after application (p < 0.02) that, in some cases, persisted for 6 months (p < 0.05). In about 80% of the patients, FVC values remained stable or above 70% for a time period of 9 months. Values of WS were stable in 75% of patients at 3 months after application. Our results demonstrate that the intrathecal application of BM-MSCs in ALS patients is a safe procedure and that it can slow down progression of the disease.
Keywords: Clinical trial, Cell-based therapy, Stem cells, Amyotrophic lateral sclerosis (ALS) patients, Intrathecal application
Introduction
Amyotrophic lateral sclerosis (ALS) is a rapidly progressing degenerative disease that selectively attacks motoneurons (MNs) in the cortex, brain stem, and spinal cord, resulting in muscle weakness, muscle atrophy, fasciculations, spasticity, and paralysis, leading to death usually within 3-5 years after the onset of clinical symptoms. Ninety percent of all cases are considered to be sporadic, while the remaining 10% of patients suffer from familial ALS, where approximately 20% are caused by mutations in the gene encoding superoxide dismutase 1 (SOD1), located on chromosome 21q; the corresponding protein is known to detoxify potentially cell-damaging free radicals1. Other genetically identified causes of familial ALS affect RNA metabolism and protein aggregation2.
Effective treatment for this devastating disease has evaded researchers for many years. Recently, stem cell-based therapies, as potentially effective treatments of ALS, have emerged employing intraspinal, intrathecal, intramuscular, intracerebral, or intravenous autologous stem cell administration routes. Human undifferentiated mesenchymal stem cells (hMSCs) of different origin (bone marrow, umbilical cord blood, adipose, and Wharton's jelly derived) have been repeatedly tested in rodent models to treat diseases such as ALS, multiple sclerosis, spinal cord/brain injury, and Alzheimer's disease3-5. Their transplantation increased neuron survival and prevented astrogliosis and microglia activation6. Several preclinical studies demonstrated that the intrathecal, intraspinal, intravenous, or combined (intraspinal with intravenous) administration of hMSCs (either single or repeated application) is a safe procedure that is able to delay motor function decline, increase survival of symptomatic transgenic animals, have anti-inflammatory effects, and stimulate secretion of specific cytokines and growth factors that promote cell survival rather than cell replacement4,7-9. MSCs promote the resistance of neurons and oligodendrocytes to apoptosis through the release of trophic and antiapoptotic molecules, resulting in the induction of a neuroprotective microenvironment. Engraftment of hMSCs into symptomatic ALS rats influenced the extent of apoptosis in motor neurons, supported the survival of larger size neurons, and modified the affected extracellular matrix (ECM) and cytokine homeostasis. hMSCs have both anti-inflammatory and neuroprotective effects and, due to its ability to remodel the recipient's gene expression profile, can reactivate central nervous system (CNS) plasticity. Quantitative analyses of Wisteria floribunda agglutinin (WFA) fluorescence intensity, measured in the ventral horns of the cervical and lumbar levels of the spinal cord, revealed significantly greater numbers of perineuronal nets (PNNs) in the hMSC-treated animals when compared with the sham-treated group7.
Different types of stem cells were used in published clinical trials, some with outcomes indicating safety and efficiency of such therapy: bone marrow mononuclear cells10-13, fetal neural stem cells14,15, and bone marrow-derived mesenchymal stem cells (BM-MSCs)16-20. In a recently published clinical trial, MSCs secreting neurotrophic factor were reported to decrease the slope of ALS progression18. Considering that neuroinflammation plays an important role in the progression of neurodegenerative diseases, including ALS, the anti-inflammatory effects of MSC-based therapy could explain their beneficial effects in animal models and also in clinical trials16,19,21. Thus, application of autologous BM-MSCs appears to be an attractive strategy to treat ALS due to their neuroprotective and immunomodulatory properties, such as secretion of growth factors [brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), and insulin-like growth factor-1 (IGF-1)] and anti-inflammatory effects22-24.
To further elaborate on the above-mentioned therapeutic effects of stem cells, and consistent with the current worldwide interest in stem cell-based ALS treatment, we performed a phase I/IIa clinical trial in ALS patients to assess the safety and efficacy of intrathecal application of autologous BM-MSCs. Intrathecal application seems to be preferential to intravenous, where the cells can be trapped in different organs25. Intrathecally implanted cells quickly spread in the cerebrospinal fluid (CSF) around the brain and spinal cord without the need to cross the blood-brain barrier or blood-spinal cord barrier. When compared to previous trials, our study included the largest group of ALS patients, had a longer pre- and posttreatment assessment period, and had a relatively small dose of injected cells.
Materials and Methods
Study Design
The study was designed as a single-center, prospective, open-label study, without a placebo control group, to assess the safety and efficacy of a single intrathecal administration of ex vivo-expanded autologous BM-MSCs in patients with ALS. The study protocol and informed consent form (ICF) were approved by the State Institute for Drug Control and by the ethics committee of the University Hospital Motol in Prague, Czech Republic. The study was conducted at the Department of Neurology, University Hospital Motol.
Patient Selection and Recruitment
The study was designed for patients with a diagnosis of definite ALS who met all inclusion criteria and had no exclusion criteria. According to the expected number of eligible patients, 20 to 30 patients were planned to be enrolled in the study. All subjects entering the study provided informed consent before any procedures specified in the protocol were performed. The patients were assured that the procedures involved in the study protocol would not interfere with the standard method of care and treatment.
The following inclusion criteria were employed to establish diagnosis of definite ALS: El Escorial Revised criteria26, data available from detailed neurological observations, ALS functional rating scale (ALSFRS), Norris scale, forced vital capacity (FVC), brain and spinal cord magnetic resonance imaging (MRI) for at least 6 months prior to the study commencement to exclude pretreatment pathology such as tumor or spine stenosis, riluzole-naive or on a stable dose for at least 2 months, aged between 18 and 65 years, either male or female, and a life expectancy of more than 2 years.
Exclusion criteria were FVC less than 70%, paralysis less than 15 points on the Norris bulbar scale in case of primary bulbar, less than 15 points on the Norris spinal scale, pregnancy, breastfeeding, coagulopathy, skin infection at the site of bone marrow aspiration or administration of the cell product, gastrostomy, any significant medical condition that could compromise the safety of the patient (e.g., recent myocardial infarction, congestive heart failure, renal failure, liver failure, cancer, systemic infection, recurrent thromboembolic disease), alcohol or drug abuse, or women of childbearing potential not using effective contraception.
Patient Follow-Up
The patients were neurologically examined three times at 6, 3, and 1 month (±1 week) before BM-MSC application (prescreening period) to evaluate the rate of pretreatment disease progression. The follow-up period was 18 months, with regular intervals between examinations at 3, 6, 9, 12, and 18 months, to assess safety and efficacy of the treatment (Table 1).
Table 1.
Time Plan of Patient Foliow-Up in the Clinical Trial
| Visit | Timing | Informed Consent | Medical History | Bone Marrow Aspiration | Intrathecal Application IP | Laboratory Assessment | Physical Examinatioi | Neurological Examination | ALSFRS | Spirometry (FVC) | Brain and Spinal Cord MRI* | Concomitant Medication | Adverse Event Assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | -6 months (±4 weeks) | X | X | ||||||||||
| II | -3 months (±3 weeks) | X | |||||||||||
| III | -5 weeks (±1 week) | X | X | X | X | X | X | X | X | X | |||
| IV | -3 weeks (±1 week) | X | X | X | |||||||||
| V | Day 0 | X | X | X | X | X | |||||||
| VI | +1 day | X | X | X | X | ||||||||
| VII | +3 days | X | X | X | X | ||||||||
| VIII | +3 months (±1 week) | X | X | X | X | X | |||||||
| IX | +6 months (±1 week) | X | X | X | X | ||||||||
| X | +9 months (±1 week) | X | X | X | X | ||||||||
| XI | +12 months (±1 week) | X | X | X | X | X | X | X | X | ||||
| XII | +18 months (±4 weeks) | X | X | X | X |
MRI examination was performed at visit III, if not available from visit I or II.
Safety was the primary objective of the current trial. To assess adverse events (AEs) after intrathecal BM-MSC delivery, all patients' complaints regarding their medical conditions worsening, as well as every new neurological deficit, were registered. After treatment, patients were closely monitored within 3 days for immediate AEs/adverse reactions (ARs), both systemic (i.e., allergic reaction, fever, and sepsis) and local (pain, bleeding, local infection, urinary incontinence, paralysis, or sensory loss below the level of the injection site or other). During their stay in the hospital, a neurologic examination and vital function monitoring (respiratory and heart rate, blood pressure, and body temperature) were performed every day. At days 1 and 3, serum biochemistry and blood count were evaluated to exclude liver or renal dysfunction, mineral imbalance, or systemic infection. If no complication occurred, patients were discharged after 3 days and followed up at regular intervals according to the study protocol. During the 18-month-long posttreatment follow-up period, AEs/ARs were assessed by clinical and laboratory examination. To exclude treatment-related tumor formation, pathological contrast enhancement, or other structural pathology, the brain and spinal cord were examined by a 1.5- and/or 3-T MRI scanner at 12 months after BM-MSC administration. These images were then compared to those obtained from pretreatment MRI examination.
Assessment of Efficacy
The secondary objective of the study was to evaluate the effect of BM-MSC application on the rate of disease progression using ALSFRS, FVC15, and muscle weakness scale (WS). While the ALSFRS may be influenced by the patient's mental status (i.e., depression) or by the subjective view of the investigators, the FVC and WS examinations provide more objective data. According to the study protocol, the changes in ALSFRS after the treatment were compared to the changes observed prior to the treatment. The pretreatment ALSFRS decline was defined as a decline over 1 point per 3 months. The WS corresponds to the standard scaled neurological examination of muscle strength on the lower and upper extremities (range: 0-5, with 0 representing plegia and 5 representing normal strength). Strength of the upper extremities was tested on the shoulder, elbow, and wrist; the strength on lower extremities was tested on the hip, knee, and ankle.
Bone Marrow Harvesting and Processing
In this clinical trial (EudraCT No. 2015-000139-33), we used an investigational advanced therapy medicinal product (IP), which was a suspension of human autologous MSC 3P in 1.5 ml (Bioinova Ltd., Prague, Czech Republic), which consists of BM-MSCs in 1.5 ml of diluent (Ringer's solution) with stabilizer (human albumin).
In summary, after obtaining negative virology and bacteriology blood test results [HIV, HBV, HCV, and Treponema pallidum], 12 ml of bone marrow blood was collected by a single aspiration from the patient's iliac crest under local anesthesia at 3-4 weeks before BM-MSCs were administered to the patient. Isolation and expansion of MSCs from the bone marrow mononuclear fraction were performed by Bioinova Ltd. according to good manufacturing practice (GMP). First, bone marrow was applied on Gelofusine® (B. Braun Meslungen AG, Meslungen, Germany), and the mononuclear fraction was collected and used for cultivation. Cells were seeded on a plastic surface and allowed to adhere. Nonadherent cells were removed by cultivation medium replacement. Adherent cells were then cultured at 37°C in a humidified atmosphere, containing 5% CO2 and platelet lysate (Bioinova Ltd.) in enriched minimum essential medium-α (Alpha MEM; Lonza, Basel, Switzerland). The medium was changed twice a week. According to their spindle-shaped morphology and plastic adherence, the cells were identified as BM-MSCs. After reaching near confluence, BM-MSCs were detached by TrypLE™ (Gibco, Thermo Fisher Scientific, Waltham, MA, USA), passaged, and again seeded on a larger plastic surface. BM-MSCs were harvested at the third passage (3 to 4 weeks after the initial seeding), counted, and characterized by flow cytometry. Cells were characterized by surface markers showing high expression levels of major histocompatibility complex class I (MHC I; Exbio Ltd., Vestec, Czech Republic), CD90 (BioLegend, San Diego, CA, USA), CD73 (BioLegend), and CD105 (Exbio Ltd.), and low expression levels of CD34 (Beckman Coulter, Inc., Brea, CA, USA) and CD45 (Exbio Ltd.). All antibodies were used according to the manufacturer's instructions. Briefly, cell pellets were washed with PBS, and after spinning the cells were resuspended in PBS again. Cells were incubated in 50 μl of PBS containing specific antibody for 15 min at room temperature. Cells were washed with PBS and measured using the FACSCanto II (BD, Franklin Lakes, NJ, USA) and analyzed with FACSDiva Software (BD). Harvested BM-MSCs were diluted in Ringer's solution with minimal dose of human albumin and collected in a primary container, 2-ml Nunc CryoTube (Nunc, Roskilde, Denmark). The validation control for bacteria, fungi, and mycoplasma contamination to confirm sterility was then performed. Finally, the investigational product containing 15 ± 4.5 × 106 of autologous BM-MSCs was released and transported under controlled temperature (2°C-8°C) to the investigator's site. A single dose of the cell product was intrathecally administered by the investigators using a standard lumbar puncture at visit V (day 0).
Statistical Analysis
The clinical disease progression of ALS patients, evaluated by ALSFRS, has a linear decline up to about 20-25 points when it reaches a plateau27. In the linear phase, regression analysis was used to evaluate the efficacy of administering BM-MSCs by detecting changes in ALSFRS postimplantation slopes (0-3, 0-6, and 0-9 months) when compared with their preimplantation slope. The slopes were compared by paired t-test for correlated variables. Data are expressed as mean ± standard error of the mean (SEM); the level of statistical significance is marked with asterisks. We used GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Characterization of Patients
All ALS patients enrolled in the clinical trial (n = 26), treated with 15 ± 4.5 × 106 of autologous MSCs, were assessed for safety. Subject demography is shown in Table 2. The subgroup of 23 patients, with sufficient data for efficacy assessment, was eligible for statistical analysis (see Table 2). Three patients without long-term follow-up data (from visits VIII to XII) were excluded from the analyses. Patient No. 10 died 2 months after BM-MSC administration due to respiratory failure, Patient No. 21 refused to further participate in the study, and Patient No. 12 had to undergo surgery for severe cervical stenosis with myelopathy.
Table 2.
Clinical Characterization of All 26 ALS Patients Enrolled in the Clinical Trial
| Patient No. | Age (Years) | Sex | ALS Symptoms | Disease Duration (Months) | ALSFRS (Day 0) | Spirometry (FVC) | Safety Analysis | Efficacy Analysis |
|---|---|---|---|---|---|---|---|---|
| 1 | 61 | F | Spinal + bulbar | 23 | 15 | 76 | X* | X |
| 2 | 58 | F | Spinal | 82 | 22 | 74 | X | X |
| 3 | 64 | M | Spinal | 23 | 33 | 84 | X | X |
| 4 | 58 | M | Spinal | 52 | 22 | 86 | X | X |
| 5 | 33 | M | Spinal + bulbar | 24 | 29 | 82 | X | X |
| 6 | 40 | F | Spinal + bulbar | 29 | 27 | 90 | X | X |
| 7 | 63 | F | Spinal | 29 | 27 | 86 | X* | X |
| 8 | 59 | F | Spinal + bulbar | 50 | 34 | 99 | X | X |
| 9 | 39 | M | Spinal + bulbar | 48 | 34 | 92 | X | X |
| 10* | 61 | M | Spinal + bulbar | 35 | 29 | 74 | X* | RF with death |
| 11 | 52 | F | Spinal + bulbar | 99 | 26 | 118 | X | X |
| 12* | 45 | M | Spinal | 23 | 36 | 108 | X | Myelopathy |
| 13 | 53 | F | Spinal + bulbar | 79 | 27 | 76 | X | X |
| 14 | 36 | M | Spinal + bulbar | 33 | 26 | 100 | X | X |
| 15 | 61 | M | Spinal | 45 | 26 | 88 | X | X |
| 16 | 53 | F | Spinal | 47 | 32 | 95 | X | X |
| 17 | 57 | M | Spinal | 47 | 30 | 87 | X* | X |
| 18 | 47 | F | Spinal + bulbar | 27 | 32 | 92 | X | X |
| 19 | 42 | F | Spinal | 30 | 34 | 100 | X | X |
| 20 | 49 | M | Spinal + bulbar | 11 | 37 | 87 | X | X |
| 21* | 52 | M | Spinal + bulbar | 47 | 32 | 83 | X* | Withdrawal from study |
| 22 | 49 | M | Spinal | 13 | 31 | 86 | X | X |
| 23 | 49 | F | Spinal + bulbar | 27 | 27 | 74 | X | X |
| 24 | 47 | F | Spinal | 26 | 29 | 95 | X | X |
| 25 | 58 | M | Spinal | 21 | 34 | 107 | X | X |
| 26 | 45 | M | Spinal | 21 | 35 | 70 | X* | X |
| Mean ± SEM | 51.2 ± 1.7 | 38.1 ± 4.2 | 29.5 ± 1.0 | 88.8 ± 2.3 |
A subgroup of 23 patients was analyzed for efficacy. Abbreviations and explanatory notes: S, spinal; B, bulbar; X*, MRI 12 months after IP application not performed; Day 0, day of IP application; FVC, forced vital capacity.
Patients 10, 12, and 21 were excluded because of insufficient long-term follow-up data (for the reasons of RF with death, myelopathy, and withdrawal from study).
Safety Assessment
Table 3 summarizes AEs observed within the group of patients by classification of seriousness, severity, and BM-MSC application relationship (AE/SAE). After intrathecal application, 30% of the patients experienced mild/moderate headaches resembling the headaches after a standard lumbar puncture. No suspected or unexpected serious adverse reactions (SUSARs) were observed in the 26 patients enrolled in the clinical trial during the follow-up period. No new intradural cerebrospinal pathology was found by MRI in patients enrolled in the clinical trial efficacy analysis during the 12-month follow-up period.
Table 3.
Overview of AE/SAE With Classification of Seriousness, Severity, and IP Relationship
| Patient No. | AE/SAE Preferred Term | Serious | Relationship | Severity | Action Taken | Duration (Days) |
|---|---|---|---|---|---|---|
| 1 | PEG insertion | Yes | No | Mild | Hospitalization | 3 |
| 2 | Respiratory failure | Yes | No | Death | Hospitalization | 4 |
| 3 | No | - | - | - | - | - |
| 4 | Headache | No | Yes | Mild | No | 2 |
| 5 | Headache | No | Yes | Mild | No | 2 |
| Hyperhydrosis | No | Yes | Mild | No | 2 | |
| Leukocytosis | No | Yes | Mild | No | 2 | |
| 6 | No | - | - | - | - | - |
| 7 | Respiratory failure (due to bronchopneumonia) | Yes | No | Severe | Hospitalization | Persistent |
| 8 | Headache | No | Yes | Mild | Analgetics | 2 |
| 9 | Headache | No | Yes | Moderate | Analgetics | 7 |
| 10 | Respiratory failure | Yes | No | Death | No | 1 |
| 11 | No | - | - | - | - | - |
| 12 | Cervical spine stenosis (progression) | No | No | Severe | Surgery | - |
| 13 | Headache | No | Yes | Mild | Analgetics | 7 |
| Cystitis | No | No | Mild | Antibiotics | 7 | |
| 14 | No | - | - | - | - | - |
| 15 | No | - | - | - | - | - |
| 16 | Headache | No | Yes | Mild | Analgetics | 1 |
| 17 | Headache | No | Yes | Mild | Analgetics | 1 |
| 18 | No | - | - | - | - | - |
| 19 | No | - | - | - | - | - |
| 20 | PEG insertion | Yes | No | Mild | No | 3 |
| 21 | No | - | - | - | - | - |
| 22 | Respiratory failure | Yes | No | Severe | Hospitalization | 44 |
| 23 | No | - | - | - | - | - |
| 24 | No | - | - | - | - | - |
| 25 | No | - | - | - | - | - |
| 26 | Leukocytosis | No | Yes | Mild | No | 3 |
Efficacy Assessment
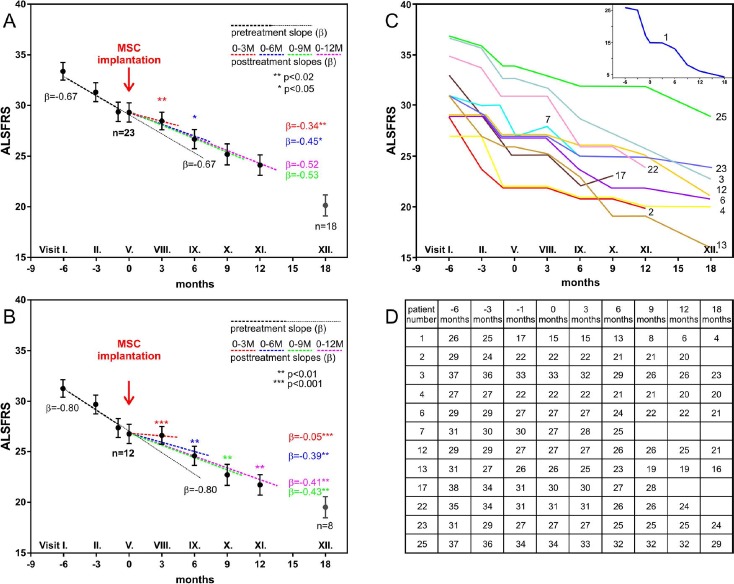
Clinical analysis was performed in 23 patients as described in the Materials and Methods section (Table 2). Table 2 shows the ALS symptoms, disease duration, and ALSFRS score before BM-MSC application and spirometry (FVC). Figure 1A shows regression analysis of ALSFRS changes in all 23 patients. Compared to the preimplantation score, we found a significant reduction/stabilization in ALSFRS decline at 3 months after BM-MSC application (p < 0.02), which was less pronounced at 6 months (p < 0.05). It should be noted that when the ALSFRS score reaches low values (<25), the clinical progression of the disease may not be linear27. For further analysis, the patients were divided into two groups according to the disease progression. We recorded disease progression during 6 months of the preimplantation period (ALSFRS decline scores in the range 2-11, n = 12) and detected a slowdown of disease progression. Figure 1B shows regression analysis of ALSFRS changes in these 12 patients. Figure 1C and D shows the time course of the disease and the patients' individual responses to the treatment (n = 12). Regression analysis revealed a significant slowdown of the disease at 3 months (p < 0.001), as well as at 6, 9, and 12 months (p < 0.01) after treatment. In patients with stable ALSFRS scores during the 6-month preimplantation period, we could not detect a slowdown of disease progression.
Figure 1.
Clinical analysis of amyotrophic lateral sclerosis (ALS) patients. (A) Regression analysis of ALS functional rating scale (ALSFRS) changes in 23 patients analyzed for efficacy compared to the preimplantation ALSFRS score. Note the significant reduction/stabilization in ALSFRS decline at 3 months after bone marrow-derived mesenchymal stem cell (BM-MSC) application (p < 0.02), which was less pronounced at 6 months (p < 0.05). The y-axis shows the ALSFRS scores, and the x-axis shows the clinical trial visits with corresponding time courses in months. (B) Regression analysis of patients with a decline of ALSFRS scores 6 months before BM-MSC application. (C) Time courses of ALSFRS scores in the individual patients. Patient No. 11 is shown in a separate inset for better visibility of data. (D) Table of the ALSFRS scores.
In about 80% of the patients, FVC values remained stable or above 70% for a time period of 9 months and remained in about 60% of patients at 12 months after application (Table 4). Values of WS remained stable in 75% of the patients at 3 months after application, which then decreased at 12 months in the follow-up period (Table 5). Table 4 shows the stable average values of the weakness score (changes < 1.0 were evaluated as stable) in the lower and upper extremities at 3 months after application.
Table 4.
Forced Vital Capacity of All 26 ALS Patients Enrolled in the Clinical Trial
| Patient No. | Visit III (-5 Weeks) | Visit VIII (+3 Months) | Visit IX (+6 Months) | Visit X (+9 Months) | Visit XI (+12 Months) | Visit XII (+18 Months) |
|---|---|---|---|---|---|---|
| 1 | 76 | - | - | - | - | - |
| 2 | 74 | 67 | 57 | 42 | 34 | - |
| 3 | 84 | 81 | 89 | 85 | 80 | 55 |
| 4 | 86 | 80 | 77 | 79 | 63 | 42 |
| 5 | 82 | 67 | 87 | 79 | 69 | 50 |
| 6 | 90 | 80 | 85 | 76 | 84 | 55 |
| 7 | 86 | 83 | 79 | - | - | - |
| 8 | 99 | 115 | 103 | 113 | 90 | 98 |
| 9 | 92 | 91 | 89 | 79 | 87 | - |
| 11 | 118 | 114 | 101 | 99 | 91 | 82 |
| 13 | 76 | 61 | 81 | 83 | 73 | - |
| 14 | 100 | 94 | 87 | 73 | 74 | 74 |
| 15 | 88 | 82 | 79 | 57 | 54 | - |
| 16 | 95 | 86 | 90 | - | 92 | 86 |
| 17 | 87 | 83 | 63 | 83 | - | - |
| 18 | 92 | 78 | 81 | 73 | 62 | - |
| 19 | 100 | 104 | 95 | 92 | 96 | 87 |
| 20 | 87 | 76 | 73 | 73 | 60 | - |
| 22 | 86 | 84 | 86 | 74 | 75 | - |
| 23 | 74 | 60 | 74 | 61 | 65 | 67 |
| 24 | 95 | 88 | 75 | 63 | 63 | - |
| 25 | 107 | 109 | 109 | 109 | 107 | 103 |
| 26 | 70 | 61 | 50 | - | - | - |
| Mean ± SEM | 89 ± 11 | 84 ± 16 | 82 ± 14 | 79 ± 17 | 75 ± 17 | 73 ± 20 |
Table 5.
Decreases of Weakness Score From Visit III
| WS Visit III (-5 Weeks) | WS Decrease Visit V (Day 0) | WS Decrease Visit VIII (+3 Months) | WS Decrease Visit XI (+12 Months) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient No. | UEL | UER | LEL | LER | UEL | UER | LEL | LER | UEL | UER | LEL | LER | UEL | UER | LEL | LER |
| 1 | 1.5 | 1.5 | 1.5 | 1.5 | 0 | 0 | 0 | 0 | -0.5 | -0.5 | -0.5 | -0.5 | -1.5 | -1.5 | -0.5 | -0.5 |
| 2 | 1.3 | 1.3 | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | -1.3 | -0.6 | -1 | -1 |
| 3 | 2.7 | 2.7 | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | -0.5 | -0.5 | -1 | -1 |
| 4 | 3.9 | 4.2 | 0.5 | 0.5 | 0 | 0 | 0 | 0 | -0.2 | -0.5 | -0.2 | -0.2 | -1.9 | -1.9 | -0.2 | -0.2 |
| 5 | 4.3 | 4.3 | 4.7 | 4.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | -1.6 | -1.6 | -1.7 | -1.7 |
| 6 | 3.8 | 4 | 4 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | -0.2 | -1 | -1 |
| 7 | 5 | 5 | 0.7 | 0.7 | 0 | 0 | 0 | 0 | 0 | 0 | -0.2 | -0.2 | - | - | - | - |
| 8 | 4.8 | 5 | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | -0.8 | -0.7 | 0 | 0 |
| 9 | 5 | 5 | 4.3 | 5 | 0 | 0 | 0 | -0.7 | -1 | -1 | -0.3 | -1 | -1 | -1 | -1.3 | -2 |
| 11 | 4 | 4 | 3.5 | 3.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | -0.5 | -0.5 |
| 13 | 3.7 | 3.7 | 4 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 14 | 3.7 | 3.7 | 4 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | -1 | -1 | -1 | -1 |
| 15 | 3 | 1 | 4 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | -1.5 | -1 | -1 | -1 |
| 16 | 3.5 | 3.5 | 3.5 | 3.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | -0.5 | -0.5 | -0.8 | -0.8 |
| 17 | 4 | 2.5 | 4 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | - |
| 18 | 4 | 3.5 | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | -1 | -1.2 | -1 | -1 |
| 19 | 4.7 | 5 | 4 | 4 | 0 | 0 | 0 | 0 | -0.4 | 0 | 0 | 0 | -1 | -1 | -1 | -1 |
| 20 | 5 | 5 | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | -0.7 | -1.3 |
| 22 | 3.3 | 3 | 4 | 4 | 0 | 0 | 0 | 0 | 0 | -0.3 | 0 | 0 | -0.3 | -0.8 | -0.2 | 0 |
| 23 | 4.7 | 4.7 | 4 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 24 | 4 | 5 | 0.7 | 0.7 | 0 | 0 | 0 | 0 | -1 | -0.3 | -0.7 | -0.7 | -3.7 | -2.7 | -0.7 | -0.7 |
| 25 | 3 | 3.1 | 5 | 5 | 0 | 0 | -1 | -1 | -1 | -0.3 | -1 | -1 | -1 | -1 | -1 | -1 |
| 26 | 5 | 5 | 4.8 | 4.8 | 0 | 0 | 0 | 0 | -0.3 | -0.3 | -0.8 | -0.8 | - | - | - | - |
WS, weakness score; UEL, upper left extremity; UER, upper right extremity; LEL, lower right extremity; LER, lower left extremity.
Our results demonstrate that the intrathecal application of BM-MSCs in ALS patients is a safe procedure and suggest that it is able, at least temporarily, to slow down the progression of the disease.
Discussion
Despite the progress made in the last decade, there is no efficient treatment for neurodegeneration in ALS. New perspectives of understanding the pathophysiology of ALS have been opened up by the discovery of disease-related genetic mutations and the creation of transgenic rodent models mimicking motor deficiency. Successful application of various stem cells in in vivo studies in transgenic animals and their promising outcomes have resulted in the emergence of several clinical trials in ALS patients testing the safety and efficacy of cell-based therapy.
Our current phase I/IIa clinical trial has been approved by the Czech State Institute for Drug Control, registered under EudraCT No. 2011-000362-35. The study involved 26 patients with sporadic ALS who received a single intrathecal (via a lumbar puncture) dose of autologous BM-MSCs. However, it has been recently shown that repeated application can enhance the effect of MSCs19, and the current trial could provide a basis for repeated application of MSCs in the future. The current trial was based on our preclinical animal studies involving SOD1-transgenic rats, with intrathecal application of human BM-MSCs, manufactured by a similar protocol to the clinical trial described here7. These cells are relatively easy to isolate and expand for autologous application, and their application has been proven to be safe in several trials using various routes of stem cell delivery10-13,16-19.
The intrathecal application of stem cells has several advantages compared to intravenous application. After the intrathecal application, a greater number of cells can reach the CNS tissue without being trapped in the lungs or other organs25. Direct application to the spinal cord parenchyma is invasive, localized to a relatively narrow locus, and may even accelerate disease progression14,15. A small percentage of intrathecally injected cells also migrate to the spinal cord parenchyma and ventricles; however, their effect is relatively temporal as most of the cells are circulating for some time in the CSF and do not home within the nervous tissue. In our earlier study, we monitored the survival of green fluorescent protein (GFP)-labeled MSCs after intrathecal application. Fourteen days after cell delivery, we could not find GFP+ cells, neither in the CNS (brain or spinal cord) nor in any other parenchymal organs (liver, spleen, or lungs)7.
The mechanisms of MSC-based therapy are very broad and have been rigorously reviewed28. In earlier studies, we studied physiological characteristics and therapeutic properties of MSCs derived from different tissues in different disease models28-30. Based on our experience and studies published by other groups, the secretion of anti-inflammatory molecules and neurotrophic factors by the grafted MSCs deserves special attention among known mechanisms31. However, delivered cell products have a short half-life in the recipient7. Given the short graft halflife, it is reasonable to assume that repeated applications of the MSCs may enhance or at least prolong the overall therapeutic effect19. Here we present a study where a total of 26 patients were enrolled. Data from 23 ALS patients were analyzed for treatment efficacy since 3 patients had no sufficient long-term follow-up data. The results of the 18-month follow-up (AEs and MRI evaluation) revealed that intrathecal application of BM-MSCs is a safe procedure. The clinical findings suggest a beneficial effect of MSCs on disease progression in some ALS patients. In a recent study, Oh et al.19 reported a similar effect in seven patients using two intrathecal injections of MSCs (1 × 106 cells/kg, 26-day interval) and much higher cell doses than those used in our study. Nevertheless, the ultimate effect in their patients was comparable to our study (i.e., the time course of disease progression was less accelerated during the 6-month follow-up period). Our data have the advantage of a longer prescreening period (6 month) and a longer follow-up period. Comparison of both phase I/IIa clinical trials suggests the need for elucidation of whether a better effect can be achieved by increasing the number of cells in a single application dose, and/or by repeated applications, or by other manufacturing processes (e.g., increased cell viability or differentiation).
The mechanism of MSC action in ALS patients is not fully understood; therefore, all effects should be accounted for as equally important. The effect of MSCs can be considered as trophic22, via production of many cytokines, angiogenetic vascular endothelial growth factor (VEGF), and the prosurvival gene Akt1. Some subtypes of MSCs produce BDNF and β nerve growth factor (βNGF)23. Trophic factors produced by MSCs (such as VEGF or BDNF) can support the survival of distant and local MNs, either by long-range diffusion and/or by local interaction with neural cells. MSCs also secrete glial cell line-derived neurotrophic factor (GDNF) and IGF-1, which play a crucial role in nourishing and protecting neurons6,32-35.
ALS affects not only neurons and astrocytes in the spinal cord but also neural elements of the brain (primary cortex, premotor and supplementary motor cortex). An ongoing compensatory process within the higher order motor-processing system of ALS patients is activated to overcome the loss of function in primary motor cortex and motor networks36. Guan et al.37 reported a significant level of potential plasticity in the adult spinal cord in response to neurodegeneration in the SOD1 model of ALS. Our experimental data suggest that, besides dysregulation of neural cells, changes in ECM molecules also contribute to ALS since delivery of human BM-MSCs protects ECM structures (PNNs), as well as modifies the expression of several host genes7. Application of stem cells supposedly leads to activation and/or stimulation of adult neural plasticity by promotion of inner neurogenesis, modification of gene and protein expression levels, and preservation of ECM structures, which could play a crucial role in stem cell therapy38. Transplanted MSCs are also able to mediate direct neuroprotection by reducing neuronal sensitivity to glutamate receptor ligands and altering gene expression, suggesting there is a link between the therapeutic effects of MSCs and the activation of cell plasticity in the damaged CNS24. By this means, MSCs can promote the proliferation and maturation of local neural precursor cells, leading to their differentiation into mature neurons and oligodendrocytes39,40.
Neuroinflammation plays an important role in neurodegenerative diseases as well as in ALS. Recently, a crosstalk between MNs, astrocytes, and immune cells such as microglia, T lymphocytes, and macrophages has been reviewed21. Since various anti-inflammatory therapeutic approaches in animal models and clinical studies of ALS failed, reduction of neuroinflammation might be better achieved by cell-based therapy41. Replacing the astrocytes and immune cells could be a proper strategy for treating ALS. Currently, MSCs with their anti-inflammatory effects are one of the best and most useful candidates.
It is necessary to mention that ALS patients are a very specific group of highly sensitive desperate patients. The placebo effect, combined with high expectations and various psychosocial circumstances affecting the psychoneuroimmunological response, can modify the outcome of any therapy in ALS patients. These factors could play a role in ALSFRS scores during the prescreening period, as well as after intrathecal application of BM-MSCs. Patients could also benefit from more individualized medical care and from more attention from physicians.
In summary, we conclude that cell-based therapy in ALS patients is promising, but it should be further investigated and confirmed in more advanced clinical trials. Animal studies often provide more promising data than the human trials because animal models sometimes give more positive effects than those observed so far in ALS patients. It also might be important to start the therapeutic intervention much earlier, similar to animal models, but this would require earlier ALS diagnosis and/or identification of early disease markers in suspected cases. There is a need for further clinical trials to elucidate the most effective cell type, the most effective methods of delivery, and proper doses in single or repeated applications.
Acknowledgments
This clinical trial was funded by Bioinova Ltd., Prague, Czech Republic, and supported by Motol Hospital (Grant Nos. 9772, GACR 15-06958S, and P304/12/G069). Publication of the results was supported by the Czech National Centre of European Infrastructure for Translational Medicine EATRIS-CZ (LM2015064). The authors thank the patients, their families, and other sponsors (e.g., students from Harvard University, Kooperativa Insurance Company) for their contribution to the Cell Therapy Foundation supporting ALS research. The patients' voluntary contribution did not affect their selection and recruitment for the clinical trial. The authors thank Michael Syka, M.D., for his valuable comments and Professor James Fawcett for his comments and critical reading of the manuscript. The authors declare no conflicts of interest.
References
- 1.Rosen D.R., Siddique T., Patterson D., Figlewicz D.A., Sapp P., Hentati A., Donaldson D., Goto J., O'Regan J.P., Deng H.X., Rahmani Z., Krizus A., McKenna-Yasek D., Cayabyab A., Gason S.M., Berger R., Tanzi R.E., Halperin J.J., Herzfeldt B., Van Den Bergh R., Hung W.Y., Bird T., Deng G., Mulder D.W., Smyth C., Laing N.G., Soriano E., Pericak-Vance M.A., Haines J., Rouleau G.A., Gusella J.S., Horvitz H.R., Brown R.H., Jr Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 1993; 362(6415): 59–62. [DOI] [PubMed] [Google Scholar]
- 2.Baumer D., Talbot K., Turner M.R. Advances in motor neurone disease. J R Soc Med. 2014; 107(1): 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehrhart J., Darlington D., Kuzmin-Nichols N., Sanberg C.D., Sawmiller D.R., Sanberg P.R., Tan J. Biodistribution of infused human umbilical cord blood cells in Alzheimer's diseaselike murine model. Cell Transplant. 2016; 25(1): 195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forostyak S., Jendelova P., Kapcalova M., Arboleda D., Sykova E. Mesenchymal stromal cells prolong the lifespan in a rat model of amyotrophic lateral sclerosis. Cytotherapy 2011; 13(9): 1036–46. [DOI] [PubMed] [Google Scholar]
- 5.Arboleda D., Forostyak S., Jendelova P., Marekova D., Amemori T., Pivonkova H., Masinova K., Sykova E. Transplantation of predifferentiated adipose-derived stromal cells for the treatment of spinal cord injury. Cell Mol Neurobiol. 2011; 31(7): 1113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vercelli A., Mereuta O.M., Garbossa D., Muraca G., Mareschi K., Rustichelli D., Ferrero I., Mazzini L., Madon E., Fagioli F. Human mesenchymal stem cell transplantation extends survival, improves motor performance and decreases neuroinflammation in mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2008; 31(3): 395–405. [DOI] [PubMed] [Google Scholar]
- 7.Forostyak S., Homola A., Turnovcova K., Svitil P., Jendelova P., Sykova E. Intrathecal delivery of mesenchymal stromal cells protects the structure of altered perineuronal nets in SOD1 rats and amends the course of ALS. Stem Cells 2014; 32(12): 3163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang C., Zhou C., Teng J.J., Zhao R.L., Song Y.Q., Zhang C. Multiple administrations of human marrow stromal cells through cerebrospinal fluid prolong survival in a transgenic mouse model of amyotrophic lateral sclerosis. Cytotherapy 2009; 11(3): 299–306. [DOI] [PubMed] [Google Scholar]
- 9.Sanberg P.R., Eve D.J., Willing A.E., Garbuzova-Davis S., Tan J., Sanberg C.D., Allickson J.G., Cruz L.E., Borlongan C.V. The treatment of neurodegenerative disorders using umbilical cord blood and menstrual blood-derived stem cells. Cell Transplant. 2011; 20(1): 85–94. [DOI] [PubMed] [Google Scholar]
- 10.Deda H., Inci M.C., Kurekci A.E., Sav A., Kayihan K., Ozgun E., Ustunsoy G.E., Kocabay S. Treatment of amyotrophic lateral sclerosis patients by autologous bone marrow-derived hematopoietic stem cell transplantation: A 1-year follow-up. Cytotherapy 2009; 11(1): 18–25. [DOI] [PubMed] [Google Scholar]
- 11.Blanquer M., Moraleda J.M., Iniesta F., Gomez-Espuch J., Meca-Lallana J., Villaverde R., Perez-Espejo M.A., Ruiz-Lopez F.J., Garcia Santos J.M., Bleda P., Izura V., Saez M., De Mingo P., Vivancos L., Carles R., Jimenez J., Hernandez J., Guardiola J., Del Rio S.T., Antunez C., De la Rosa P., Majado M.J., Sánchez-Salinas A., López J., Martínez-Lage J.F., Martínez S. Neurotrophic bone marrow cellular nests prevent spinal motoneuron degeneration in amyotrophic lateral sclerosis patients: A pilot safety study. Stem Cells 2012; 30(6): 1277–85. [DOI] [PubMed] [Google Scholar]
- 12.Martinez H.R., Molina-Lopez J.F., Gonzalez-Garza M.T., Moreno-Cuevas J.E., Caro-Osorio E., Gil-Valadez A., Gutierrez-Jimenez E., Zazueta-Fierro O.E., Meza J.A., Couret-Alcaraz P., Hernandez-Toree M. Stem cell transplantation in amyotrophic lateral sclerosis patients: Methodological approach, safety, and feasibility. Cell Transplant. 2012; 21(9): 1899–907. [DOI] [PubMed] [Google Scholar]
- 13.Sharma A.K., Sane H.M., Paranjape A.A., Gokulchandran N., Nagrajan A., D'sa M., Badhe P.B. The effect of autologous bone marrow mononuclear cell transplantation on the survival duration in amyotrophic lateral sclerosis—A retrospective controlled study. Am J Stem Cells 2015; 4(1): 50–65. [PMC free article] [PubMed] [Google Scholar]
- 14.Glass J.D., Boulis N.M., Johe K., Rutkove S.B., Federici T., Polak M., Kelly C., Feldman E.L. Lumbar intraspinal injection of neural stem cells in patients with amyotrophic lateral sclerosis: Results of a phase I trial in 12 patients. Stem Cells 2012; 30(6): 1144–51. [DOI] [PubMed] [Google Scholar]
- 15.Feldman E.L., Boulis N.M., Hur J., Johe K., Rutkove S.B., Federici T., Polak M., Bordeau J., Sakowski S.A., Glass J.D. Intraspinal neural stem cell transplantation in amyotrophic lateral sclerosis: Phase 1 trial outcomes. Ann Neurol. 2014; 75(3): 363–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karussis D., Karageorgiou C., Vaknin-Dembinsky A., Gowda-Kurkalli B., Gomori J.M., Kassis I., Bulte J.W., Petrou P., Ben-Hur T., Abramsky O., Slavin S. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010; 67(10): 1187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prabhakar S., Marwaha N., Lal V., Sharma R.R., Rajan R., Khandelwal N. Autologous bone marrow-derived stem cells in amyotrophic lateral sclerosis: A pilot study. Neurol India 2012; 60(5): 465–9. [DOI] [PubMed] [Google Scholar]
- 18.Petrou P., Gothelf Y., Argov Z., Gotkine M., Levy Y.S., Kassis I., Vaknin-Dembinsky A., Ben-Hur T., Offen D., Abramsky O., Melamed E., Karussis D. Safety and clinical effects of mesenchymal stem cells secreting neurotrophic factor transplantation in patients with amyotrophic lateral sclerosis: Results of phase 1/2 and 2a clinical trials. JAMA Neurol. 2016; 73(3): 337–44. [DOI] [PubMed] [Google Scholar]
- 19.Oh K.W., Moon C., Kim H.Y., Oh S.I., Park J., Lee J.H., Chang I.Y., Kim K.S., Kim S.H. Phase I trial of repeated intrathecal autologous bone marrow-derived mesenchymal stromal cells in amyotrophic lateral sclerosis. Stem Cells Transl Med. 2015; 4(6): 590–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nafissi S., Kazemi H., Tiraihi T., Beladi-Moghadam N., Faghihzadeh S., Faghihzadeh E., Yadegarynia D., Sadeghi M., Chamani-Tabriz L., Khanfakhraei A., Taheri T. Intraspinal delivery of bone marrow stromal cell-derived neural stem cells in patients with amyotrophic lateral sclerosis: A safety and feasibility study. J Neurol Sci. 2016; 362: 174–81. [DOI] [PubMed] [Google Scholar]
- 21.Rizzo V., Richman J., Puthanveettil S.V. Dissecting mechanisms of brain aging by studying the intrinsic excitability of neurons. Front Aging Neurosci. 2014; 6: 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caplan A.I., Dennis J.E. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006; 98(5): 1076–84. [DOI] [PubMed] [Google Scholar]
- 23.Crigler L., Robey R.C., Asawachaicharn A., Gaupp D., Phinney D.G. Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp Neurol. 2006; 198(1): 54–64. [DOI] [PubMed] [Google Scholar]
- 24.Voulgari-Kokota A., Fairless R., Karamita M., Kyrargyri V., Tseveleki V., Evangelidou M., Delorme B., Charbord P., Diem R., Probert L. Mesenchymal stem cells protect CNS neurons against glutamate excitotoxicity by inhibiting glutamate receptor expression and function. Exp Neurol. 2012; 236(1): 161–70. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi Y., Tsuji O., Kumagai G., Hara C.M., Okano H.J., Miyawaki A., Toyama Y., Okano H., Nakamura M. Comparative study of methods for administering neural stem/progenitor cells to treat spinal cord injury in mice. Cell Transplant. 2011; 20(5): 727–39. [DOI] [PubMed] [Google Scholar]
- 26.Brooks B.R., Miller R.G., Swash M., Munsat T.L. World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000; 1(5): 293–9. [DOI] [PubMed] [Google Scholar]
- 27.Proudfoot M., Jones A., Talbot K., Al-Chalabi A., Turner M.R. The ALSFRS as an outcome measure in therapeutic trials and its relationship to symptom onset. Amyotroph Lateral Scler Frontotemporal Degener. 2016; 17(5–6): 414–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forostyak S., Jendelova P., Sykova E. The role of mesenchymal stromal cells in spinal cord injury, regenerative medicine and possible clinical applications. Biochimie 2013; 95(12): 2257–70. [DOI] [PubMed] [Google Scholar]
- 29.Forostyak O., Butenko O., Anderova M., Forostyak S., Sykova E., Verkhratsky A., Dayanithi G. Specific profiles of ion channels and ionotropic receptors define adipose- and bone marrow derived stromal cells. Stem Cell Res. 2016; 16(3): 622–34. [DOI] [PubMed] [Google Scholar]
- 30.Forostyak O., Forostyak S., Kortus S., Sykova E., Verkhratsky A., Dayanithi G. Physiology of Ca(2+) signalling in stem cells of different origins and differentiation stages. Cell Calcium 2016; 59(2–3): 57–66. [DOI] [PubMed] [Google Scholar]
- 31.Kim H., Kim H.Y., Choi M.R., Hwang S., Nam K.H., Kim H.C., Han J.S., Kim K.S., Yoon H.S., Kim S.H. Dose-dependent efficacy of ALS-human mesenchymal stem cells transplantation into cisterna magna in SOD1-G93A ALS mice. Neurosci Lett. 2010; 468(3): 190–4. [DOI] [PubMed] [Google Scholar]
- 32.Li Y., Chen J., Chen X.G., Wang L., Gautam S.C., Xu Y.X., Katakowski M., Zhang L.J., Lu M., Janakiraman N., Chopp M. Human marrow stromal cell therapy for stroke in rat: Neurotrophins and functional recovery. Neurology 2002; 59(4): 514–23. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J., Li Y., Chen J., Yang M., Katakowski M., Lu M., Chopp M. Expression of insulin-like growth factor 1 and receptor in ischemic rats treated with human marrow stromal cells. Brain Res. 2004; 1030(1): 19–27. [DOI] [PubMed] [Google Scholar]
- 34.Muto T., Miyoshi K., Horiguchi T., Hagita H., Noma T. Novel genetic linkage of rat Sp6 mutation to amelogenesis imperfecta. Orphanet J Rare Dis. 2012; 7: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uccelli A., Benvenuto F., Laroni A., Giunti D. Neuroprotective features of mesenchymal stem cells. Best Pract Res Clin Haematol. 2011; 24(1): 59–64. [DOI] [PubMed] [Google Scholar]
- 36.Lule D., Diekmann V., Kassubek J., Kurt A., Birbaumer N., Ludolph A.C., Kraft E. Cortical plasticity in amyotrophic lateral sclerosis: Motor imagery and function. Neurorehabil Neural Repair 2007; 21(6): 518–26. [DOI] [PubMed] [Google Scholar]
- 37.Guan Y.J., Wang X., Wang H.Y., Kawagishi K., Ryu H., Huo C.F., Shimony E.M., Kristal B.S., Kuhn H.G., Friedlander R.M. Increased stem cell proliferation in the spinal cord of adult amyotrophic lateral sclerosis transgenic mice. J Neurochem. 2007; 102(4): 1125–38. [DOI] [PubMed] [Google Scholar]
- 38.Fawcett J.W. The extracellular matrix in plasticity and regeneration after CNS injury and neurodegenerative disease. Prog Brain Res. 2015; 218: 213–26. [DOI] [PubMed] [Google Scholar]
- 39.Tsyb A.F., Yuzhakov V.V., Roshal L.M., Sukhikh G.T., Konoplyannikov A.G., Sushkevich G.N., Yakovleva N.D., Ingel I.E., Bandurko L.N., Sevan'kaeva L.E., Mikhina L.N., Fomina N.K., Marei M.V., Semenova ZhB, Konoplyannikova O.A., Kal'sina S.S.h, Lepekhina L.A., Semenkova I.V., Agaeva E.V., Shevchuk A.S., Pavlova L.N., Tokarev O.Y., Karaseva O.V., Chernyshova T.A. Morphofunctional study of the therapeutic efficacy of human mesenchymal and neural stem cells in rats with diffuse brain injury. Bull Exp Biol Med. 2009; 147(1): 132–46. [DOI] [PubMed] [Google Scholar]
- 40.Uccelli A., Moretta L., Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008; 8(9): 726–36. [DOI] [PubMed] [Google Scholar]
- 41.Mitsumoto H., Brooks B.R., Silani V. Clinical trials in amyotrophic lateral sclerosis: Why so many negative trials and how can trials be improved? Lancet Neurol. 2014; 13(11): 1127–38. [DOI] [PubMed] [Google Scholar]