Abstract
Unscheduled cell cycle reentry of postmitotic neurons has been described in cases of mild cognitive impairment (MCI) and Alzheimer's disease (AD) and may form a basis for selective neuronal vulnerability during disease progression. In this regard, the multifunctional protein regulator of cell cycle (RGCC) has been implicated in driving G1/S and G2/M phase transitions through its interactions with cdc/cyclin-dependent kinase 1 (cdk1) and is induced by p53, which mediates apoptosis in neurons. We tested whether RGCC levels were dysregulated in frontal cortex samples obtained postmortem from subjects who died with a clinical diagnosis of no cognitive impairment (NCI), MCI, or AD. RGCC mRNA and protein levels were upregulated by ~50%-60% in MCI and AD compared to NCI, and RGCC protein levels were associated with poorer antemortem global cognitive performance in the subjects examined. To test whether RGCC might regulate neuronal cell cycle reentry and apoptosis, we differentiated neuronotypic PC12 cultures with nerve growth factor (NGF) followed by NGF withdrawal to induce abortive cell cycle activation and cell death. Experimental reduction of RGCC levels increased cell survival and reduced levels of the cdk1 target cyclin B1. RGCC may be a candidate cell cycle target for neuroprotection during the onset of AD.
Keywords: Mild cognitive impairment (MCI), Alzheimer's disease (AD), Cell cycle, Regulator of cell cycle (RGCC), Nerve growth factor (NGF), Apoptosis
Introduction
Several lines of evidence suggest that cell cycle reactivation occurs in postmitotic neurons in Alzheimer's disease (AD) and its putative prodromal stage, mild cognitive impairment (MCI). The AD brain is characterized by neuronal expression of cell cycle regulatory proteins1,3 and DNA replication4,6, whereas we have demonstrated the presence of the cell cycle proteins proliferating cell nuclear antigen (PCNA), cyclin D1, and cyclin B1 in neurons in vulnerable brain regions in subjects with MCI7. Mechanistically, a link has been established between unscheduled cell cycle reentry and neuronal apoptosis, suggesting a pathogenic mechanism for neuronal selective vulnerability8,13. Moreover, the activation of several cell cycle-related kinases, including cdc2/cyclin-dependent kinase 1 (cdk1), cdc2-like kinase, cdk2, and cdk5, has been shown to phosphorylate tau and promote tau aggregation14,17.
The mechanisms underlying aberrant neuronal cell cycle reentry during the onset of AD have not been firmly established, but various stressors such as DNA damage and neurotrophin dysregulation have been proposed18,22. Notably, the tumor suppressor protein p53, which induces cell cycle arrest and DNA repair in damaged proliferating cells, facilitates apoptosis when the neuronal milieu is presented with toxic insults23,25. Although the link between p53, cell cycle dysregulation, and apoptosis is unclear, p53 induces the expression of the multifunctional protein regulator of cell cycle (RGCC)26,27, which is highly expressed in many cancerous tissues28. RGCC has been shown to either induce S phase entry and mitosis or promote differentiation in nonneuronal cells, which appears to be context dependent27,32. Whether RGCC dysfunction might represent a novel pathway linking aberrant cell cycle activation and apoptosis in neurons during the progression of AD remains undetermined. In the present study, we measured RGCC mRNA and protein levels in frontal cortex samples obtained postmortem from individuals who died with an antemortem diagnosis of no cognitive impairment (NCI), MCI, or AD. We also tested whether RGCC expression impacted cell survival in an in vitro experimental paradigm for neuronal cell cycle-induced apoptosis.
Materials and Methods
Subjects
Brain tissues from NCI (n = 14), MCI (n = 11), and mild/moderate AD (n = 11) cases from both genders were obtained from participants in the Rush Religious Orders Study, a longitudinal clinical pathologic study of aging and AD in elderly Catholic clergy33. Demographic, clinical, and neuropathological characteristics of the subjects are summarized in Table 1. Details of cognitive evaluations and diagnostic criteria have been extensively published33,36. Briefly, a team of investigators performed annual neuropsychological performance testing including the Mini Mental State Exam (MMSE) and 17 additional neuropsychological tests referable to five cognitive domains: orientation, attention, memory, language, and perception. A Global Cognitive Score (GCS), consisting of a composite z-score calculated from this test battery, was determined for each participant. A board-certified neurologist with expertise in the evaluation of the elderly made the clinical diagnosis based on impairments in each of the five cognitive domains and a clinical examination. The diagnosis of dementia or AD met recommendations by the joint working group of the National Institute of Neurologic and Communicative Disorders and Stroke/AD and Related Disorders Association (NINCDS/ADRDA)37. The MCI population was defined as subjects who exhibited impairment on neuropsychological testing but did not meet the criteria for AD or dementia. These criteria for MCI are consistent with those used by others in the field38.
Table 1.
Clinical, Demographic, and Neuropathological Characteristics by Diagnosis Category
| Clinical Diagnosis |
|||||
|---|---|---|---|---|---|
| Characteristics | NCI (n = 14) | MCI (n = 11) | AD (n = 11) | p Value | Pairwise |
| Comparison | |||||
| Age (years) at death | 0.1* | - | |||
| Mean ± SD | 83.9 ± 4.5 | 84.4 ± 5.2 | 86.2 ± 5.1 | ||
| Range | 76-92 | 72-91 | 78-95 | ||
| Number (%) of males | 6 (43%) | 6 (54%) | 6 (54%) | 0.5† | - |
| Years of education | 0.1* | - | |||
| Mean ± SD | 19.1 ± 2.9 | 18.9 ± 4.3 | |||
| Range | 15-22 | 8-24 | 14-21 | ||
| Number (%) with ApoE ∊4 allele | 2 (14%) | 2 (18%) | 5 (45%) | 0.01† | AD >NCI, MCI |
| MMSE | <0.0001* | NCI, MCI >AD | |||
| Mean ± SD | 28.1 ± 0.9 | 26.8 ± 2.6 | 15.1 ± 7.7 | ||
| Range | 26-29 | 22-30 | 0-27 | ||
| Global cognitive score | <0.0001* | NCI, MCI >AD | |||
| Mean ± SD | 0.0 ± 0.3 | -0.37 ± 0.4 | -1.8 ± 0.6 | ||
| Range | -0.5 to 0.4 | -1.2 to 0.3 | -2.8 to −0.7 | ||
| Postmortem interval (h) | 0.3* | - | |||
| Mean ± SD | 4.7 ± 2.9 | 6.0 ± 3.3 | 5.4 ± 3.4 | ||
| Range | 2.2-12.0 | 2.7-13.0 | 2.7-12.0 | ||
| Distribution of Braak scores | 0.1* | ||||
| 0 | 0 | 0 | 0 | ||
| I/II | 5 | 4 | 2 | ||
| III/IV | 8 | 6 | 6 | ||
| V/VI | 1 | 1 | 3 | ||
| NIA-Reagan diagnosis (likelihood of AD) | 0.2* | ||||
| No AD | 0 | 0 | 0 | ||
| Low | 6 | 6 | 5 | ||
| Intermediate | 6 | 5 | 5 | ||
| High | 2 | 0 | 1 | ||
| CERAD diagnosis | 0.2* | ||||
| No AD | 3 | 4 | 3 | ||
| Possible | 3 | 2 | 2 | ||
| Probable | 6 | 3 | 5 | ||
| Definite | 2 | 2 | 1 | ||
Kruskal-Wallis test, with Bonferroni correction for multiple comparisons.
Fisher's exact test, with Bonferroni correction for multiple comparisons.
Tissue samples were accrued as previously reported34,39,40. At autopsy, tissue from one hemisphere was immersion fixed in 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO, USA) in 0.1 M phosphate buffer (pH 7.2) for 24-72 h at 4°C. Tissue slabs from the opposite hemisphere were frozen at −80°C prior to collection of frontal cortex samples for quantitative polymerase chain reaction (qPCR) and biochemical analysis. A series of fixed tissue sections were prepared for neuropathological evaluation including visualization and quantitation of neocortical and hippocampal amyloid plaques and neurofibrillary tangles (NFTs) using antibodies directed against the Aβ peptide (Aβ; 4G8; Covance, Princeton, NJ, USA), tau (PHF1; a gift from Dr. Peter Davies)33,40, as well as thioflavine-S (Sigma-Aldrich) histochemistry and a modified Bielschowsky silver stain (components from Fisher Scientific, Pittsburgh, PA, USA). Additional sections were stained for Lewy bodies using antibodies directed against ubiquitin and α-synuclein. Exclusion criteria included argyrophilic grain disease, frontotemporal dementia, Lewy body disease, mixed dementias, Parkinson's disease, stroke, and hippocampal sclerosis. A board-certified neuropathologist blinded to the clinical diagnosis performed the neuropathological evaluation. Neuropathological criteria were based on National Institute on Aging (NIA)-Reagan, Consortium to Establish a Registry for Alzheimer's Disease (CERAD), and Braak staging41,43. Amyloid burden and apolipoprotein E (ApoE) genotype were determined for each case as described previously33,40.
qPCR
Total RNA from frozen frontal cortex (Brodmann area 10) samples was extracted using guanidine-isothiocyanate lysis (PureLink; Ambion, Waltham, MA, USA), and RNA integrity and concentration were verified using Bioanalysis (Agilent Technologies, Santa Clara, CA, USA). Samples were assayed on a real-time (RT)-PCR cycler (ABI 7500; Applied Biosystems, Foster City, CA, USA) in 96-well optical plates as described previously44,47. qPCR was performed using TaqMan hydrolysis probe primer sets (Applied Biosystems) specific for amplification of the following human transcripts: rgcc (probe set Hs00204129_m1), tumor protein p53 (tp53; Hs01034249_m1), and cdk1 (Hs00938777_m1). A primer set specific for human glyceraldehyde 3-phosphate dehydrogenase (gapdh) (Hs02758991_g1) was used as a control housekeeping transcript. For PC12 cell culture experiments (see below), total RNA was extracted and assayed as described above using primers specific for rat cyclin b1 (ccnb1; probe set Rn01494180_g1; Applied Biosystems) and rat gapdh (Rn01775763_g1). The Δ-Δ Ct (ddCT) method was employed to determine relative levels of each amplicon44-46,48. Variance component analyses revealed relatively low levels of within-case variability, and the average value of the triplicate qPCR products from each case was used in subsequent analyses. Alterations in PCR product synthesis were analyzed by one-way analysis of variance (ANOVA) with Bonferroni correction for post hoc comparison. The level of statistical significance was set at α = 0.05 (two sided).
Western Blotting
Frozen frontal cortex tissue samples from the same cases used for qPCR were sonicated in ice-cold homogenization buffer [20 mM Tris, 1 mM ethylene glycolbis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 1 mM ethylenediaminetetraacetic acid (EDTA), 10% sucrose, pH 7.4] containing protease inhibitors (2 mg/ml leupeptin, 0.01 U/ml aprotinin, 1 mg/ml pepstatin A, 1 mg/ml antipain, 2.5 mg/ml chymostatin, 10 mM benzamidine, 0.1 mM PMSF, 0.4 mg/ml TPCK, 0.4 mg/ml TLCK, 0.4 mg/ml soybean trypsin inhibitor, 0.1 mM sodium fluoride, and 0.1 mM sodium orthovanadate). All chemicals were purchased from Sigma-Aldrich. Samples were prepared by centrifugation at 100 × g for 10 min at 4°C. The protein concentration of the resulting S1 supernatant was determined by the Bradford method (Bio-Rad, Hercules, CA, USA), which uses bovine serum albumin (BSA) as the protein standard. Sample proteins from the S1 fraction were denatured in sodium dodecyl sulfate (SDS; Fisher Scientific) loading buffer to a final concentration of 5 mg/ml. Proteins (25 μg/sample) were separated by SDS polyacrylamide gel electrophoresis (10%; Lonza, Basel, Switzerland), transferred to Immobilon-FL membranes (Millipore, Billerica, MA, USA), blocked in Tris-buffered saline (pH 7.4) containing 0.1% Tween 20 (Fisher Scientific) and 2% nonfat milk, and then incubated overnight at 4°C with rabbit polyclonal antiserum to RGCC (1:500; Novus Biologicals, Littleton, CO, USA). Blots were then incubated for 1 h with near-infrared-labeled goat anti-rabbit immunoglobulin G (IgG) secondary antiserum (IRDye 680LT; 1:10,000; Licor, Lincoln, NE, USA) and analyzed on an Odyssey imaging system (Licor). Following imaging, the membranes were stripped and reprobed with a mouse monoclonal β-actin antibody (1:20,000; Millipore) overnight followed by a 1-h incubation with near-infrared-labeled goat anti-mouse IgG secondary antiserum (IRDye 680LT; 1:10,000; Licor) and Odyssey imaging. Signals for RCGG were normalized to β-actin for quantitative analysis34,47,49.
PC12 Cell Culture
PC12 cultures (a gift of Dr. Richard Burry, Ohio State University, Columbus, OH, USA) were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% horse serum (Gibco, Grand Island, NY, USA), 5% FetalClone I bovine serum (Hyclone, Logan, UT, USA), and 1% penicillin/streptomycin (Gibco). Cultures were plated at 10 K/cm2 onto Matrigel-coated dishes (1%; Collaborative Biomedical; Becton Dickinson, Frankin Lakes, NJ, USA) in DMEM with 1.5% serum. PC12 cultures were grown for 1 week in the presence of 400 pm (~50 ng/ml) mouse 7S nerve growth factor (NGF; Alomone Labs, Jerusalem, Israel). Media were replaced on days 3 and 5 in vitro. On day 7, PC12 cells were rinsed and incubated with 50 nM RGCC or scrambled siRNA (Origene, Rockville, MD, USA)/1% Lipofectamine RNAiMAX (Life Technologies, Carlsbad, CA, USA) in OptiMEM (Gibco)/400 pm NGF for 18 h48, then rinsed and exchanged into DMEM/1.5% serum without NGF for 48 h. Cultures were assayed for cell viability using the LIVE/DEAD assay (Thermo Fisher Scientific, Waltham, MA, USA). Sister cultures were analyzed for cyclin B1 (ccnb1) expression, as described above.
Statistical Analysis
Demographic variables (Table 1) were compared among clinical diagnostic groups by Kruskal-Wallis or Fisher's exact tests with Bonferroni correction for pairwise comparisons. Transcript levels (qPCR), protein levels (Western blotting), and cell viability measures were compared among groups by one-way ANOVA with Bonferroni post hoc testing. The level of statistical significance was set at p < 0.05. RGCC protein levels across diagnostic groups were tested for associations with clinical and pathological variables using Spearman rank correlations. The level of statistical significance was set at p < 0.01.
Results
Subject Demographics
The clinical diagnostic groups did not differ by age, gender, years of education, or postmortem interval (Table 1). There were significantly more subjects with an ApoE 4 allele in the AD (45%) group than in the NCI (14%) or MCI (18%) group. AD cases had significantly lower MMSE scores compared to both NCI and MCI (p < 0.001), whereas the latter two groups did not differ statistically (Table 1). GCS z-scores were significantly lower in the AD compared to the NCI and MCI cases (p < 0.0001). Subjects in the different clinical diagnostic groups displayed considerable overlap with respect to pathological diagnostic criteria. Pathological examination revealed that 64% of NCI, 64% of MCI, and 82% of AD cases were classified as Braak stages III-VI. Using the NIA-Reagan criteria, 57% of NCI, 45% of MCI, and 55% of AD cases were classified as intermediate to high likelihood of AD (Table 1). For CERAD diagnosis, 57% of NCI, 45% of MCI, and 55% of AD cases received a diagnosis of probable/definite AD. Statistical analysis did not reveal any differences in pathology among the NCI, MCI, and AD groups.
RCGG Expression Levels in MCI and AD
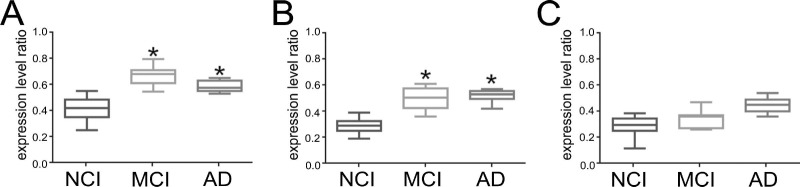
qPCR analysis was performed to quantify RGCC (rgcc), p53 (tp53), and CDK1 (cdk1) gene expression levels in frozen frontal cortex tissue samples accrued from NCI, MCI, and AD subjects (Fig. 1). A significant ~55%-60% increase in rgcc transcript levels was measured in MCI compared to NCI cases (p < 0.05), whereas rgcc levels were upregulated by ~50% in AD compared to NCI (p < 0.05) (Fig. 1A). By contrast, tp53 expression levels were significantly increased by ~55%-60% in MCI and AD compared to NCI (p < 0.05) (Fig. 1B), whereas there were no differences in cdk1 expression across the diagnostic groups (Fig. 1C).
Figure 1.
p53 (tp53) and protein regulator of cell cycle (rgcc) gene expression levels are increased in mild cognitive impairment (MCI) and Alzheimer's disease (AD). Box plots show relative expression levels of (A) tp53, (B) rgcc, and (C) cdc/cyclin-dependent kinase 1 (cdk1) normalized to glyceraldehyde 3-phosphate dehydrogenase (gapdh) levels (mean ± max/min; arbitrary units) in total RNA derived from no cognitive impairment (NCI), MCI, and AD cases. *p < 0.05 versus NCI, via one-way analysis of variance (ANOVA) with Bonferroni post hoc comparisons.
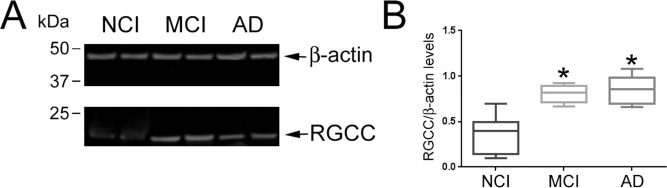
To test whether RCGG protein levels were also upregulated in the MCI and AD cases, quantitative Western blotting was performed on tissue extracts from the same cases (Fig. 2). RGCC immunoreactivity (~15-kDa band) was higher in the MCI and AD frontal cortex compared to NCI (Fig. 2A). Quantitative analysis of the Western blots showed that normalized RGCC protein levels were significantly increased by ~50%-55% in MCI and AD (p < 0.05). Spearman rank correlations showed no association between RGCC protein levels and age, gender, PMI, or ApoE status (data not shown). By contrast, increased RGCC protein levels were associated with poorer cognitive performance as measured by the MMSE (r = 0.39, p = 0.002) and GCS (r = 0.43, p = 0.005), but not with Braak, NIA-Reagan, or CERAD neuropathological criteria.
Figure 2.
Regulator of cell cycle (rgcc) protein levels are increased in mild cognitive impairment (MCI) and Alzheimer's disease (AD). (A) Representative Western blot shows greater RCGG immunoreactivity (~15 kDa) in tissue extracts derived from MCI and AD cases compared to no cognitive impairment (NCI) cases; levels of β-actin were equivalent across samples. (B) Box plots show relative quantitative measurements of RGCC immunoreactivity normalized to β-actin signals (mean+max/min; arbitrary units) in the three diagnostic groups. *p < 0.05 versus NCI, via one-way analysis of variance (ANOVA) with Bonferroni post hoc comparisons.
Inhibition of RGCC Protects PC12 Cells From Nerve Growth Factor Withdrawal
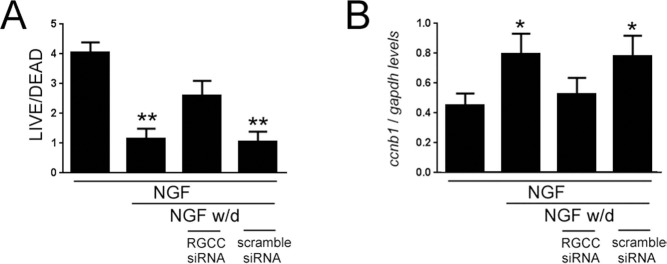
Neuronotypic differentiation of rat PC12 cells with NGF, followed by NGF deprivation in low/no serum, results in aberrant cell cycle entry and apoptosis8,50-53. In order to assess whether RGCC might play a role in neuronal apoptosis related to cell cycle reentry, we differentiated PC12 cells and then treated the cultures with rgcc-specific siRNA or scrambled control siRNA prior to NGF withdrawal (Fig. 3). There was an overall ~75% decrease in the cell survival of PC12 cultures subjected to NGF withdrawal compared to cultures maintained on NGF (p < 0.01). By contrast, rgcc downregulation rescued the PC12 cultures from NGF deprivation, resulting in an ~25% decrease in cell survival compared to NGF-maintained cultures (Fig. 3A). To assess the extent of cell cycle activation in the cultures, we used qPCR to measure expression levels of cyclin b1 (ccnb1), a CDK1 binding partner that is downregulated during NGF-induced differentiation and upregulated during NGF withdrawal and apoptosis of PC12 cells54,55. There was an overall ~80% increase in cyclin B1 levels in PC12 cultures subjected to NGF withdrawal compared to cultures maintained on NGF (p < 0.05). By contrast, rgcc downregulation prevented cyclin B1 upregulation during NGF deprivation (Fig. 3B).
Figure 3.
Regulator of cell cycle (rgcc) inhibition rescues PC12 cells from cell death induced by nerve growth factor (NGF) withdrawal. (A) Bar graph shows relative levels of cell survival as measured by the LIVE/DEAD assay [mean ± standard deviation (SD); arbitrary units] for PC12 cultures maintained on NGF, deprived of NGF for 48 h, deprived of NGF for 48 h in the presence of rgcc siRNA, or deprived of NGF for 48 h in the presence of scrambled control siRNA. (B) Bar graph shows relative levels of cyclin b1 (ccnb1) transcript levels as measured by quantitative polymerase chain reaction (qPCR) (mean ± SD; arbitrary units) for PC12 cultures maintained on NGF, deprived of NGF for 48 h, deprived of NGF for 48 h in the presence of rgcc siRNA, or deprived of NGF for 48 h in the presence of scrambled control siRNA. *p < 0.05; *p < 0.01 versus NGF, via one-way analysis of variance (ANOVA) with Bonferroni post hoc comparisons.
Discussion
For over two decades, the concept of “abortive mitosis” has been noted as a cellular mechanism of apoptosis during development and neuronal cell death in neurodegenerative disease3,56,57. With respect to AD, it has been proposed that deleterious events such as the loss of neurotrophic support needed to maintain terminal differentiation, or neuronal DNA damage from oxidative stress, result in the transition from a quiescent G0 cell cycle stage into an unscheduled attempt at DNA replication and mitosis4,7,18-20. The consequent loss of genomic and cellular homeostasis ultimately triggers programmed cell death3,58,59. Moreover, the activation of several cell cycle kinases, normally under tight regulatory control in postmitotic neurons, can lead to tau hyperphosphorylation and aggregation into NFTs14,17. Hence, the cell cycle continues to represent a viable target for disease-modifying therapies for AD21,58,59. Here we provide evidence that the cell cycle regulatory protein RGCC is upregulated in MCI and AD, correlates with global cognitive decline, and may be involved in facilitating aberrant cell cycle reentry induced by neurotrophin loss in differentiated neurons, suggesting that RGCC may be a candidate cell cycle target for neuroprotection during the onset of AD. This report may also add another provocative link to the potential mechanistic interrelationship between cell transformation in cancer and selective vulnerability in neurodegenerative disease. These diseases share many molecular pathogenic processes, including oxidative and inflammatory stress, proteostatic stress, and metabolic dysregulation21,60-64, and it has been postulated that these pathways lead to either clonal expansion in proliferating cells or clonal elimination in terminally differentiated cells such as neurons65.
The functional and mechanistic repertoire of RGCC activity has not been fully elucidated. It was originally discovered as the RGC-32 response gene during complement activation of rat oligodendrocytes26. RGCC physically associates with and activates CDK1, a key kinase involved in the G1/S and G2/M phase transitions26,29,30. However, RGCC has also been implicated in diverse functions such as cellular differentiation, inflammation, vascular remodeling, and insulin resistance32,66-70. Interestingly, RGCC was identified as a transcriptional target and mediator of p53 tumor suppression in glioma cells27. In neurons, the p53 protein possesses multifactorial properties regulating DNA damage, cell cycle control, and apoptosis71,72. Given the evidence that p53 protein is upregulated and possibly dysregulated due to structural modifications in MCI and AD73,75, we investigated whether RCGG was also upregulated in these disease stages and whether it could potentially play a role in neuronal cell cycle dysfunction and/or apoptosis. In this regard, we validated p53 upregulation74 but also found that RGCC was upregulated in the frontal cortex in MCI and AD. By contrast, transcripts encoding the RGCC-regulated cell cycle protein CDK1 were stable during disease progression despite a trend (p = 0.07) for upregulation, consistent with the notion that RGCC regulates CDK1 activity rather than expression30.
The functional consequences of RGCC upregulation in MCI and AD subjects are unclear, but its role in cell cycle activation led us to test whether this upregulation could reflect a deleterious event promoting “abortive mitosis” and neuronal vulnerability. To this end, we used the PC12 cell culture model as a well-established system for NGF-mediated neuronotypic differentiation and NGF withdrawal-mediated cell cycle reactivation and apoptosis8,50-53. Using rgcc and scrambled sequence control siRNA, we found that rgcc knockdown protected PC12 cells from NGF withdrawal and prevented the upregulation of the CDK1 binding partner cyclin B154,55, suggesting that RCGG participates in cell cycle reactivation and cell death within the context of deficient neurotrophin signaling.
A central concept underlying the selective vulnerability of neurons in AD is that they are dependent on neurotrophins such as NGF and brain-derived neurotrophic factor (BDNF) for survival76,78. NGF and BDNF are derived from proNGF and proBDNF precursor proteins, and these mature peptides interact with their cognate high-affinity receptors TrkA and TrkB, respectively, for prosurvival functions77,79,80. By contrast, proNGF and proBDNF have higher affinity for the pan neurotrophin receptor p75NTR and elicit prodeath signals81. Notably, we found that cortical TrkA protein levels were selectively reduced in mild AD compared to p75NTR 49, whereas cortical proNGF levels were elevated in MCI and AD compared to NCI82. Hence, increased cortical proNGF in combination with reduced cortical TrkA expression may result in enhanced binding between proNGF and p75NTR, potentially shifting away from prosurvival NGF signaling to apoptotic signaling. Likewise, levels of BDNF and TrkB are decreased in vulnerable brain regions in MCI and AD39,83. This observation, combined with the presence of cell cycle proteins within vulnerable brain regions in MCI and mild AD4,7, suggests that neurotrophin receptor imbalance promotes a loss of neurotrophic support and unscheduled cell cycle reentry and apoptosis during the prodromal stages of AD. In this regard, whereas cell cycle abnormalities have been linked to in vitro and in vivo amyloid and tau pathology84,86, we did not find a significant association between RGCC levels and neuropathological diagnostic criteria. This may be due to the lack of significant differences in Braak, NIA-Reagan, or CERAD scores among the diagnostic groups (Table 1). On the other hand, they may suggest that neurotrophic imbalances affect RGCC and other cell cycle events independent of plaque or tangle burden. The extent to which increased RGCC levels denote its involvement in neurotrophin- mediated mitotic cell death cascades in the MCI and AD brain is a question for future study. Furthermore, given the involvement of p53 in neuronal apoptosis following NGF withdrawal87, it will be interesting to explore whether a p53-RGCC-CDK1/cyclin B cascade mediates aberrant cell cycle activation in postmitotic neurons. If so, this pathway may present a novel therapeutic target for disease modification during the progression of AD.
Acknowledgments
We are grateful for the altruism of the Religious Orders Study participants. This study was supported by the National Institutes of Health (NIH) grants PO1AG14449, RO1AG043375, R21AG026032, and R21AG042146; the Saint Mary's Foundation; Miles for Memories of Battle Creek, MI; and Barrow Neurological Institute Barrow and Beyond. The authors declare no conflicts of interest.
References
- 1.Busser J., Geldmacher D.S., Herrup K. Ectopic cell cycle proteins predict the sites of neuronal cell death in Alzheimer's disease brain. J Neurosci. 1998; 18(8): 2801–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vincent I., Jicha G., Rosado M., Dickson D.W. Aberrant expression of mitotic cdc2/cyclin B1 kinase in degenerating neurons of Alzheimer's disease brain. J Neurosci. 1997; 17(10): 3588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vincent I., Rosado M., Davies P. Mitotic mechanisms in Alzheimer's disease? J Cell Biol. 1996; 132(3): 413–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y., Geldmacher D.S., Herrup K. DNA replication precedes neuronal cell death in Alzheimer's disease. J Neurosci. 2001; 21(8): 2661–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonda D.J., Evans T.A., Santocanale C., Llosa J.C., Vina J., Bajic V., Castellani R.J., Siedlak S.L., Perry G., Smith M.A., Lee H.G. Evidence for the progression through S-phase in the ectopic cell cycle re-entry of neurons in Alzheimer disease. Aging (Albany NY) 2009; 1(4): 382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mosch B., Morawski M., Mittag A., Lenz D., Tarnok A., Arendt T. Aneuploidy and DNA replication in the normal human brain and Alzheimer's disease. J Neurosci. 2007; 27(26): 6859–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Y., Mufson E.J., Herrup K. Neuronal cell death is preceded by cell cycle events at all stages of Alzheimer's disease. J Neurosci. 2003; 23(7): 2557–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farinelli S.E., Greene L.A. Cell cycle blockers mimosine, ciclopirox, and deferoxamine prevent the death of PC12 cells and postmitotic sympathetic neurons after removal of trophic support. J Neurosci. 1996; 16(3): 1150–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freeman R.S., Estus S., Johnson E.M., Jr Analysis of cell cycle-related gene expression in postmitotic neurons: Selective induction of cyclin D1 during programmed cell death. Neuron 1994; 12(2): 343–55. [DOI] [PubMed] [Google Scholar]
- 10.Herrup K., Busser J.C. The induction of multiple cell cycle events precedes target-related neuronal death. Development 1995; 121(8): 2385–95. [DOI] [PubMed] [Google Scholar]
- 11.Park D.S., Levine B., Ferrari G., Greene L.A. Cyclin dependent kinase inhibitors and dominant negative cyclin dependent kinase 4 and 6 promote survival of NGF-deprived sympathetic neurons. J Neurosci. 1997; 17(23): 8975–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frade J.M. Unscheduled re-entry into the cell cycle induced by NGF precedes cell death in nascent retinal neurones. J Cell Sci. 2000; 113(Pt 7): 1139–48. [DOI] [PubMed] [Google Scholar]
- 13.Malik B., Currais A., Soriano S. Cell cycle-driven neuronal apoptosis specifically linked to amyloid peptide Abeta1–42 exposure is not exacerbated in a mouse model of presenilin-1 familial Alzheimer's disease. J Neurochem. 2008; 106(2): 912–6. [DOI] [PubMed] [Google Scholar]
- 14.Noble W., Olm V., Takata K., Casey E., Mary O., Meyerson J., Gaynor K., LaFrancois J., Wang L., Kondo T., Davies P., Burns M., Veeranna, Nixon R., Dickson D., Matsuoka Y., Ahlijanian M., Lau L.F., Duff K. Cdk5 is a key factor in tau aggregation and tangle formation in vivo. Neuron 2003; 38(4): 555–65. [DOI] [PubMed] [Google Scholar]
- 15.Baumann K., Mandelkow E.M., Biernat J., Piwnica-Worms H., Mandelkow E. Abnormal Alzheimer-like phosphorylation of tau-protein by cyclin-dependent kinases cdk2 and cdk5. FEBS Lett. 1993; 336(3): 417–24. [DOI] [PubMed] [Google Scholar]
- 16.Bennecib M., Gong C.X., Grundke-Iqbal I., Iqbal K. Role of protein phosphatase-2A and -1 in the regulation of GSK-3, cdk5 and cdc2 and the phosphorylation of tau in rat fore-brain. FEBS Lett. 2000; 485(1): 87–93. [DOI] [PubMed] [Google Scholar]
- 17.Paudel H.K. Phosphorylation by neuronal cdc2-like protein kinase promotes dimerization of Tau protein in vitro. J Biol Chem. 1997; 272(45): 28328–34. [DOI] [PubMed] [Google Scholar]
- 18.Arendt T., Bruckner M.K., Mosch B., Losche A. Selective cell death of hyperploid neurons in Alzheimer's disease. Am J Pathol. 2010; 177(1): 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silva A.R., Santos A.C., Farfel J.M., Grinberg L.T., Ferretti R.E., Campos A.H., Cunha I.W., Begnami M.D., Rocha R.M., Carraro D.M., de Braganca Pereira C.A., Jacob-Filho W., Brentani H. Repair of oxidative DNA damage, cell-cycle regulation and neuronal death may influence the clinical manifestation of Alzheimer's disease. PLoS One 2014; 9(6): e99897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swerdlow R.H., Burns J.M., Khan S.M. The Alzheimer's disease mitochondrial cascade hypothesis. J Alzheimers Dis. 2010; 20(suppl 2): S265–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Webber K.M., Raina A.K., Marlatt M.W., Zhu X., Prat M.I., Morelli L., Casadesus G., Perry G., Smith M.A. The cell cycle in Alzheimer disease: A unique target for neuropharmacology. Mech Ageing Dev. 2005; 126(10): 1019–25. [DOI] [PubMed] [Google Scholar]
- 22.Zivkovic L., Spremo-Potparevic B., Siedlak S.L., Perry G., Plecas-Solarovic B., Milicevic Z., Bajic V.P. DNA damage in Alzheimer disease lymphocytes and its relation to premature centromere division. Neurodegener Dis. 2013; 12(3): 156–63. [DOI] [PubMed] [Google Scholar]
- 23.Behrens M.I., Lendon C., Roe C.M. A common biological mechanism in cancer and Alzheimer's disease? Curr Alzheimer Res. 2009; 6(3): 196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakanishi A., Minami A., Kitagishi Y., Ogura Y., Matsuda S. BRCA1 and p53 tumor suppressor molecules in Alzheimer 's disease. Int J Mol Sci. 2015; 16(2): 2879–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaghefi H., Neet K.E. Deacetylation of p53 after nerve growth factor treatment in PC12 cells as a post-translational modification mechanism of neurotrophin-induced tumor suppressor activation. Oncogene 2004; 23(49): 8078–87. [DOI] [PubMed] [Google Scholar]
- 26.Badea T.C., Niculescu F.I., Soane L., Shin M.L., Rus H. Molecular cloning and characterization of RGC-32, a novel gene induced by complement activation in oligodendrocytes. J Biol Chem. 1998; 273(41): 26977–81. [DOI] [PubMed] [Google Scholar]
- 27.Saigusa K., Imoto I., Tanikawa C., Aoyagi M., Ohno K., Nakamura Y., Inazawa J. RGC32, a novel p53-inducible gene, is located on centrosomes during mitosis and results in G2/M arrest. Oncogene 2007; 26(8): 1110–21. [DOI] [PubMed] [Google Scholar]
- 28.Vlaicu S.I., Cudrici C., Ito T., Fosbrink M., Tegla C.A., Rus V., Mircea P.A., Rus H. Role of response gene to complement 32 in diseases. Arch Immunol Ther Exp. (Warsz) 2008; 56(2): 115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Badea T., Niculescu F., Soane L., Fosbrink M., Sorana H., Rus V., Shin M.L., Rus H. RGC-32 increases p34CDC2 kinase activity and entry of aortic smooth muscle cells into S-phase. J Biol Chem. 2002; 277(1): 502–8. [DOI] [PubMed] [Google Scholar]
- 30.Fosbrink M., Cudrici C., Tegla C.A., Soloviova K., Ito T., Vlaicu S., Rus V., Niculescu F., Rus H. Response gene to complement 32 is required for C5b-9 induced cell cycle activation in endothelial cells. Exp Mol Pathol. 2009; 86(2): 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oram S.W., Liu X.X., Lee T.L., Chan W.Y., Lau Y.F. TSPY potentiates cell proliferation and tumorigenesis by promoting cell cycle progression in HeLa and NIH3T3 cells. BMC Cancer 2006; 6: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tegla C.A., Cudrici C.D., Nguyen V., Danoff J., Kruszewski A.M., Boodhoo D., Mekala A.P., Vlaicu S.I., Chen C., Rus V., Badea T.C., Rus H. RGC-32 is a novel regulator of the T-lymphocyte cell cycle. Exp Mol Pathol. 2015; 98(3): 328–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bennett D.A., Wilson R.S., Schneider J.A., Evans D.A., Beckett L.A., Aggarwal N.T., Barnes L.L., Fox J.H., Bach J. Natural history of mild cognitive impairment in older persons. Neurology 2002; 59(2): 198–205. [DOI] [PubMed] [Google Scholar]
- 34.Counts S.E., Nadeem M., Lad S.P., Wuu J., Mufson E.J. Differential expression of synaptic proteins in the frontal and temporal cortex of elderly subjects with mild cognitive impairment. J Neuropathol Exp Neurol. 2006; 65(6): 592–601. [DOI] [PubMed] [Google Scholar]
- 35.Mufson E.J., Chen E.Y., Cochran E.J., Beckett L.A., Bennett D.A., Kordower J.H. Entorhinal cortex beta-amyloid load in individuals with mild cognitive impairment. Exp Neurol. 1999; 158(2): 469–90. [DOI] [PubMed] [Google Scholar]
- 36.Perez S.E., He B., Nadeem M., Wuu J., Scheff S.W., Abrahamson E.E., Ikonomovic M.D., Mufson E.J. Resilience of precuneus neurotrophic signaling pathways despite amyloid pathology in prodromal Alzheimer's disease. Biol Psychiatry 2015; 77(8): 693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984; 34(7): 939–44. [DOI] [PubMed] [Google Scholar]
- 38.Petersen R.C., Doody R., Kurz A., Mohs R.C., Morris J.C., Rabins P.V., Ritchie K., Rossor M., Thal L., Winblad B. Current concepts in mild cognitive impairment. Arch Neurol. 2001; 58(12): 1985–92. [DOI] [PubMed] [Google Scholar]
- 39.Ginsberg S.D., Alldred M.J., Counts S.E., Cataldo A.M., Neve R.L., Jiang Y., Wuu J., Chao M.V., Mufson E.J., Nixon R.A., Che S. Microarray analysis of hippocampal CA1 neurons implicates early endosomal dysfunction during Alzheimer's disease progression. Biol Psychiatry 2010; 68(10): 885–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mufson E.J., Chen E.Y., Cochran E.J., Beckett L.A., Bennett D.A., Kordower J.H. Entorhinal cortex beta-amyloid load in individuals with mild cognitive impairment. Exp Neurol. 1999; 158(2): 469–90. [DOI] [PubMed] [Google Scholar]
- 41.Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991; 82(4): 239–59. [DOI] [PubMed] [Google Scholar]
- 42.Hyman B.T., Phelps C.H., Beach T.G., Bigio E.H., Cairns N.J., Carrillo M.C., Dickson D.W., Duyckaerts C., Frosch M.P., Masliah E., Mirra S.S., Nelson P.T., Schneider J.A., Thal D.R., Thies B., Trojanowski J.Q., Vinters H.V., Montine T.J. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement. 2012; 8(1): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mirra S.S., Heyman A., McKeel D., Sumi S.M., Crain B.J., Brownlee L.M., Vogel F.S., Hughes J.P., van Belle G., Berg L. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 1991; 41(4): 479–86. [DOI] [PubMed] [Google Scholar]
- 44.Alldred M.J., Che S., Ginsberg S.D. Terminal continuation (TC) RNA amplification without second strand synthesis. J Neurosci Methods 2009; 177(2): 381–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Counts S.E., He B., Che S., Ikonomovic M.D., DeKosky S.T., Ginsberg S.D., Mufson E.J. Alpha7 nicotinic receptor up-regulation in cholinergic basal forebrain neurons in Alzheimer disease. Arch Neurol. 2007; 64(12): 1771–6. [DOI] [PubMed] [Google Scholar]
- 46.Ginsberg S.D. Transcriptional profiling of small samples in the central nervous system. Methods Mol Biol. 2008; 439: 147–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beck J.S., Mufson E.J., Counts S.E. Evidence for mitochondrial UPR gene activation in familial and sporadic Alzheimer's disease. Curr Alzheimer Res. 2016; 13(6): 610–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weinberg R.B., Mufson E.J., Counts S.E. Evidence for a neuroprotective microRNA pathway in amnestic mild cognitive impairment. Front Neurosci. 2015; 9: 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Counts S.E., Nadeem M., Wuu J., Ginsberg S.D., Saragovi H.U., Mufson E.J. Reduction of cortical TrkA but not p75(NTR) protein in early-stage Alzheimer's disease. Ann Neurol. 2004; 56(4): 520–31. [DOI] [PubMed] [Google Scholar]
- 50.Counts S.E., Lah J.J., Levey A.I. The regulation of presenilin-1 by nerve growth factor. J Neurochem. 2001; 76(3): 679–89. [DOI] [PubMed] [Google Scholar]
- 51.Greene L.A. Nerve growth factor prevents the death and stimulates the neuronal differentiation of clonal PC12 pheochromocytoma cells in serum-free medium. J Cell Biol. 1978; 78(3): 747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mesner P.W., Epting C.L., Hegarty J.L., Green S.H. A timetable of events during programmed cell death induced by trophic factor withdrawal from neuronal PC12 cells. J Neurosci. 1995; 15(11): 7357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bianco M.R., Berbenni M., Amara F., Viggiani S., Fragni M., Galimberti V., Colombo D., Cirillo G., Papa M., Alberghina L., Colangelo A.M. Cross-talk between cell cycle induction and mitochondrial dysfunction during oxidative stress and nerve growth factor withdrawal in differentiated PC12 cells. J Neurosci Res. 2011; 89(8): 1302–15. [DOI] [PubMed] [Google Scholar]
- 54.Gao C.Y., Zelenka P.S. Induction of cyclin B and H1 kinase activity in apoptotic PC12 cells. Exp Cell Res. 1995; 219(2): 612–8. [DOI] [PubMed] [Google Scholar]
- 55.Yan G.Z., Ziff E.B. NGF regulates the PC12 cell cycle machinery through specific inhibition of the Cdk kinases and induction of cyclin D1. J Neurosci. 1995; 15(9): 6200–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee S., Christakos S., Small M.B. Apoptosis and signal transduction: Clues to a molecular mechanism. Curr Opin Cell Biol. 1993; 5(2): 286–91. [DOI] [PubMed] [Google Scholar]
- 57.Ucker D.S. Death by suicide: One way to go in mammalian cellular development? New Biol. 1991; 3(2): 103–9. [PubMed] [Google Scholar]
- 58.Smith M.A., Nunomura A., Zhu X., Takeda A., Perry G. Metabolic, metallic, and mitotic sources of oxidative stress in Alzheimer disease. Antioxid Redox Signal. 2000; 2(3): 413–20. [DOI] [PubMed] [Google Scholar]
- 59.Katsel P., Tan W., Fam P., Purohit D.P., Haroutunian V. Cell cycle checkpoint abnormalities during dementia: A plausible association with the loss of protection against oxidative stress in Alzheimer's disease [corrected]. PLoS One 2013; 8(7): e68361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Audas T.E., Audas D.E., Jacob M.D., Ho J.J., Khacho M., Wang M., Perera J.K., Gardiner C., Bennett C.A., Head T., Kryvenko O.N., Jorda M., Daunert S., Malhorta A., Trinkle-Mulcahy L., Gonzalgo M.L., Lee S. Adaptation to stressors by systemic protein amyloidogenesis. Dev Cell 2016; 39(2): 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bennett D.A., Leurgans S. Is there a link between cancer and Alzheimer disease? Neurology 2010; 74(2): 100–1. [DOI] [PubMed] [Google Scholar]
- 62.Driver J.A. Inverse association between cancer and neurodegenerative disease: Review of the epidemiologic and biological evidence. Biogerontology 2014; 15(6): 547–57. [DOI] [PubMed] [Google Scholar]
- 63.Harris R.A., Tindale L., Cumming R.C. Age-dependent metabolic dysregulation in cancer and Alzheimer's disease. Biogerontology 2014; 15(6): 559–77. [DOI] [PubMed] [Google Scholar]
- 64.Vilchez D., Saez I., Dillin A. The role of protein clearance mechanisms in organismal ageing and age-related diseases. Nat Commun. 2014; 5: 5659. [DOI] [PubMed] [Google Scholar]
- 65.Heintz N. Cell death and the cell cycle: A relationship between transformation and neurodegeneration? Trends Biochem Sci. 1993; 18(5): 157–9. [DOI] [PubMed] [Google Scholar]
- 66.An X., Jin Y., Guo H., Foo S.Y., Cully B.L., Wu J., Zeng H., Rosenzweig A., Li J. Response gene to complement 32, a novel hypoxia-regulated angiogenic inhibitor. Circulation 2009; 120(7): 617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cui X.B., Luan J.N., Ye J., Chen S.Y. RGC32 deficiency protects against high-fat diet-induced obesity and insulin resistance in mice. J Endocrinol. 2015; 224(2): 127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tang R., Zhang G., Chen S.Y. Response gene to complement 32 protein promotes macrophage phagocytosis via activation of protein kinase C pathway. J Biol Chem. 2014; 289(33): 22715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu R., Shang C., Zhao J., Han Y., Liu J., Chen K., Shi W. Knockdown of response gene to complement 32 (RGC32) induces apoptosis and inhibits cell growth, migration, and invasion in human lung cancer cells. Mol Cell Biochem. 2014; 394(1–2): 109–18. [DOI] [PubMed] [Google Scholar]
- 70.Zhao P., Gao D., Wang Q., Song B., Shao Q., Sun J., Ji C., Li X., Li P., Qu X. Response gene to complement 32 (RGC-32) expression on M2-polarized and tumor-associated macrophages is M-CSF-dependent and enhanced by tumor-derived IL-4. Cell Mol Immunol. 2015; 12(6): 692–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Culmsee C., Mattson M.P. p53 in neuronal apoptosis. Biochem Biophys Res Commun. 2005; 331(3): 761–77. [DOI] [PubMed] [Google Scholar]
- 72.Lanni C., Racchi M., Memo M., Govoni S., Uberti D. p53 at the crossroads between cancer and neurodegeneration. Free Radic Biol Med. 2012; 52(9): 1727–33. [DOI] [PubMed] [Google Scholar]
- 73.Buizza L., Cenini G., Lanni C., Ferrari-Toninelli G., Prandelli C., Govoni S., Buoso E., Racchi M., Barcikowska M., Styczynska M., Szybinska A., Butterfield D.A., Memo M., Uberti D. Conformational altered p53 as an early marker of oxidative stress in Alzheimer's disease. PLoS One 2012; 7(1): e29789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cenini G., Sultana R., Memo M., Butterfield D.A. Elevated levels of pro-apoptotic p53 and its oxidative modification by the lipid peroxidation product, HNE, in brain from subjects with amnestic mild cognitive impairment and Alzheimer's disease. J Cell Mol Med. 2008; 12(3): 987–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Perluigi M., Barone E., Di Domenico F., Butterfield D.A. Aberrant protein phosphorylation in Alzheimer disease brain disturbs pro-survival and cell death pathways. Biochim Biophys Acta 2016; 1862(10): 1871–82. [DOI] [PubMed] [Google Scholar]
- 76.Altar C.A., Cai N., Bliven T., Juhasz M., Conner J.M., Acheson A.L., Lindsay R.M., Wiegand S.J. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature 1997; 389(6653): 856–60. [DOI] [PubMed] [Google Scholar]
- 77.Counts S.E., Mufson E.J. The role of nerve growth factor receptors in cholinergic basal forebrain degeneration in prodromal Alzheimer disease. J Neuropathol Exp Neurol. 2005; 64(4): 263–72. [DOI] [PubMed] [Google Scholar]
- 78.Sofroniew M.V., Howe C.L., Mobley W.C. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 2001; 24: 1217–81. [DOI] [PubMed] [Google Scholar]
- 79.Mufson E.J., Counts S.E., Perez S.E., Ginsberg S.D. Cholinergic system during the progression of Alzheimer's disease: Therapeutic implications. Expert Rev Neurother. 2008; 8(11): 1703–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barbacid M. Structural and functional properties of the TRK family of neurotrophin receptors. Ann NY Acad Sci. 1995; 766: 442–58. [DOI] [PubMed] [Google Scholar]
- 81.Lee R., Kermani P., Teng K.K., Hempstead B.L. Regulation of cell survival by secreted proneurotrophins. Science 2001; 294(5548): 1945–8. [DOI] [PubMed] [Google Scholar]
- 82.Peng S., Wuu J., Mufson E.J., Fahnestock M. Increased proNGF levels in subjects with mild cognitive impairment and mild Alzheimer's disease. J Neuropathol Exp Neurol. 2004; 63(6): 641–9. [DOI] [PubMed] [Google Scholar]
- 83.Peng S., Wuu J., Mufson E.J., Fahnestock M. Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer's disease. J Neurochem. 2005; 93(6): 1412–21. [DOI] [PubMed] [Google Scholar]
- 84.Bloom G.S. Amyloid-beta and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014; 71(4): 505–8. [DOI] [PubMed] [Google Scholar]
- 85.Seward M.E., Swanson E., Norambuena A., Reimann A., Cochran J.N., Li R., Roberson E.D., Bloom G.S. Amyloid-beta signals through tau to drive ectopic neuronal cell cycle reentry in Alzheimer's disease. J Cell Sci. 2013; 126(Pt 5): 1278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Varvel N.H., Bhaskar K., Patil A.R., Pimplikar S.W., Herrup K., Lamb B.T. Abeta oligomers induce neuronal cell cycle events in Alzheimer's disease. J Neurosci. 2008; 28(43): 10786–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aloyz R.S., Bamji S.X., Pozniak C.D., Toma J.G., Atwal J., Kaplan D.R., Miller F.D. p53 is essential for developmental neuron death as regulated by the TrkA and p75 neurotrophin receptors. J Cell Biol. 1998; 143(6): 1691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]