Abstract
High levels of bilirubin in infants can cause kernicterus, which includes basal ganglia damage and dystonia. Stem cell transplantation may be an effective treatment for this disease. In this study, we transplanted human neural progenitor cells differentiated toward propriospinal interneurons into the striatum of 20-day-old spontaneously jaundiced (jj) Gunn rats and nonjaundiced (Nj) littermates. Using immunohistochemical methods, we found that grafted cells survived and grew fibers in jj and Nj brains 3 weeks after transplantation. Grafted cells had a higher survival rate in jj than in Nj brains, suggesting that slightly elevated bilirubin may protect graft survival due to its antioxidative and immunosuppressive effects. Despite their survival, only a small portion of grafted neurons expressed GAD-6 or ChAT, which mark GABAergic and cholinergic neurons, respectively, and are the cells that we are attempting to replace in kernicterus. Thus, NPCs containing large populations of GABAergic and cholinergic neurons should be used for further study in this field.
Keywords: Bilirubin encephalopathy, Basal ganglia, Gunn rats, Xenotransplantation
Introduction
Bilirubin neurotoxicity, caused by excessive neonatal hyperbilirubinemia, targets specific brain regions and can lead to kernicterus1,2. The most vulnerable brain areas include basal ganglia [especially the globus pallidus (GP) and subthalamic nucleus], hippocampus, cerebellum, and inferior colliculus2. The symptoms of kernicterus, which include severe dystonia and athetosis, are permanent and extremely debilitating. Currently, there is no effective treatment for this disease.
Stem cell transplantation is a potential treatment for basal ganglia diseases such as Parkinson's disease (PD) and Huntington's disease (HD). Stem cells have been reported to improve motor function in animal models of PD3,4 and HD5,6, leading to clinical trials in PD (see Fu et al.7 for review). In the case of kernicterus, since unconjugated unbound bilirubin causes cell apoptosis and necrosis in the GP2,8, neural stem cell grafting is a promising therapeutic strategy for replacing these cells. For this purpose, neural progenitor cells (NPCs) arising from medial ganglionic eminence (MGE), as the majority of GP neurons do9, are a rational choice. However, the elevated bilirubin levels in the susceptible preadolescent GP could adversely affect NPCs having the same origin as the GP cells. An alternative approach is to use stem cells that arise from a region of the central nervous system (CNS) known to be more resistant to high levels of bilirubin in kernicterus, while having some potential to express neurotransmitters or other neurochemical characteristics of the GP neural population. NPCs derived from the WA09 human embryonic stem cell (hESC) line and differentiated with a novel protocol10 to resemble excitatory spinal cord interneurons and to express glutamate decarboxylase (GAD) and choline acetyltransferase (ChAT) in small proportions were chosen for this study. We transplanted these cells into the basal ganglia of jaundiced (jj) Gunn rats and their nonjaundiced (Nj) littermates. The main purpose of this experiment was to determine donor cell survival, fiber outgrowth, and neurochemical properties in the striatal region to assess the feasibility of stem cell transplantation as a therapeutic strategy for kernicterus. We demonstrated that grafted NPCs were able to survive and generate neurites in both jj and Nj brains.
Materials and Methods
Stem Cell Preparation and Differentiation
Undifferentiated WA09 hESCs obtained from WiCell (University of Wisconsin, Madison, WI, USA) were maintained on mTESR media (Stem Cell Technologies, Vancouver, Canada). For the differentiation to neural lineages, a novel protocol10 modified from Boulting et al.11 and Maury et al.12 was used. In brief, embryonic bodies (EBs) were aggregated and treated sequentially with small-molecule inhibitors. These EBs were fully dissociated with Accutase (Stem Cell Technologies), counted, and used in injection procedures. All embryonic stem cell procedures were approved by the Institutional Human Embryonic Stem Cell Committee and conform to National Institutes of Health (NIH) regulations.
Animals
All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals. The study was approved by the University of Kansas Medical Center's Institutional Animal Care and Use Committee (IACUC). Gunn rats used in this experiment were bred in our own colony by pairing homozygous jj males and heterozygous Nj females. Rats lived with their mothers before and after surgery and were weaned at 28 days of age. Animals were housed in a temperature- and humidity-controlled room maintained on 12/12-h light/dark cycles with food (Teklad laboratory animal diets 8604;Envigo, Madison, WI, USA) and water available ad libitum.
Surgery
At 20 days of age (P20), rats were anesthetized with isoflurane (3%-4% for induction and 2%-2.5% for maintenance with oxygen) and fixed in a stereotaxic instrument (Kopf, Tujunga, CA, USA). A small hole on the skull was drilled, a suspension of NPCs (10,000 in 2.5 μl) was injected into the striatum unilaterally with a 26-gauge, 10-μl Hamilton microsyringe at a rate of 0.5 μl/min; the needle was left in place for an additional 3 min to prevent cell efflux. Stereotaxic coordinates were anterior-posterior (AP), 6.5 mm; medial-lateral (ML), 2.8 mm; and dorsal-ventral (DV), 5.0 mm from the brain surface and using the interaural midpoint as the zero point13. After surgery, all rats received ketoprofen 5 mg/kg [subcutaneously (SC), 3 days]. Immunosuppressant was not used in this experiment.
Immunohistochemistry (IHC)
At 23 days after surgery (43 days of age), rats were deeply anesthetized with isoflurane and perfused with ice-cold phosphate-buffered saline (PBS) followed with 4% paraformaldehyde (PFA; Sigma-Aldrich, St. Louis, MO, USA). Brains were removed and processed for IHC analyses. Coronal frozen sections spanning the injection sites were cut at 30 μm. All sections were processed with standard floating section methods. Every 1:6 series of sections were stained with mouse monoclonal antibody specific for human cytoplasmic marker, STEM121 (1:500; Cellartis-Clontech, Mountain View, CA, USA), to identify grafted human cells and alternatively double stained with the following antibodies: rabbit anti-Ku80 (1:500; Abcam, Cambridge, MA, USA) for human cell nucleus, goat anti-ChAT (1:200; Chemicon, Temecula, CA, USA), or mouse immunoglobulin G (IgG)2A anti-glutamic acid decarboxylase (GAD-6; 1:100; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA, USA). A further set of 1:6 series sections was processed for double staining of mouse anti-STEM123 [1:300; specific for human glial fibrillary acidic protein (GFAP); Cellartis-Clontech] and Ku80. Immune reactions were visualized by corresponding secondary antibody labeling: goat anti-mouse IgG (H + L) Alexa Fluor 488 (1:2,000; Invitrogen, Waltham, MA, USA), goat anti-rabbit Alexa Fluor 555 (1:2,000; Invitrogen), cyanine 3 (Cy3) donkey anti-goat IgG (1:200; Jackson ImmunoResearch, West Grove, PA, USA), goat anti-mouse IgG2A Alexa Fluor 594 (1:1,000; Invitrogen), and goat anti-rabbit (1:2,000).
Quantification and Statistics
All sections were examined under a fluorescence microscope (Nikon Eclipse 80i; Nikon, Melville, NY, USA) and analyzed with CellSens Standard (Olympus, Center Valley, PA, USA) or NIS-Elements AR (Nikon) software. Images (4×, 10×, and 20×) of the grafted areas were used. Based on the STEM121 and Ku80 double labeling, the numbers of viable transplanted cells were manually counted with CellSens or NIH ImageJ (NIH, Bethesda, MD, USA) independently by two people. The mean was multiplied by 6 to estimate the total number of viable cells. The proportion of astrocytes to the total surviving cells was quantified by comparing the STEM123 and Ku80 double staining to the total Ku80 staining. The migration distances of grafted cells from the graft tract and the length of outgrowth axons were measured with CellSens Standard or NIH ImageJ based on the images of 10× magnification. In all cases, data were expressed as mean ± standard error of the mean (SEM), data were analyzed using Student's t-test with SYSTAT (Systat Software Inc., San Jose, CA, USA), and statistical significance was set at p < 0.05.
Results
Survivability of Transplanted Cells
Brains in which a survival graft was identified in the basal ganglia were processed for further IHC analyses. Cell survival rate was calculated according to the count of the number of Ku80-ir nuclei, which were surrounded by or apposite to STEM121-ir cells to total cells injected. Cell survival rate in jj rats was 21% (2,103 ± 9) and 12.4% in Nj rats (1,237 ± 167). This difference was statistically significant [t(4) = −3.395, p = 0.027]. See Figure 1 for representative images of graft survival in jj (A-C) and Nj (D-F) striatum.
Figure 1.
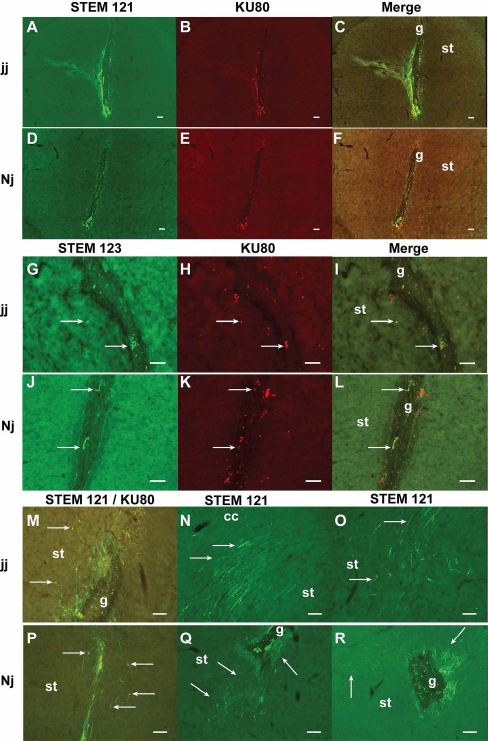
Grafted cell survival and fiber outgrowth in jaundiced (jj) and nonjaundiced (Nj) brain. (A-F) Overall graft survival, distribution, and the injection tract labeled with STEM121 and Ku80 separately and the merged image in a jj striatum (A-C) and an Nj striatum (D-F). Note the greater number of labeled cells in the jj brain. Grafts usually grow along the edge of the tract. Some necrotic tissues were seen in the dorsal part of the tract and inside the tract. (G-L) Distribution of grafted astrocytes identified with STEM123, total grafted cells identified with Ku80, and the merged image in jj (G-I) and Nj (J-L) brains. Note that the number of Ku80-labeled nuclei (H, K) was greater than STEM123 and Ku80 double-labeled cells (I, L). (M, P) Cell migration from the injection tract toward the host parenchyma and long fibers. (N, O, Q, R) Grafted neurons had abundant neurite outgrowth in jj and Nj brains. (N) Long fibers located in and ventral to the corpus callosum. (O, Q) Distinct fiber patterns were distributed in the midventral striatum, ventral to the graft site. (Q, R) Dense fibers located near the tract; the direction of axonal projection varied depending on the cell location. Note that few fibers grew into or traversed the tract. g, graft; arrow, cells, cell clusters, or fibers; st, striatum. Scale bars: 100 μm.
Astrocyte Population in Grafts
IHC for STEM123 (human GFAP) labeling revealed that a small portion of transplanted NPCs differentiated into astrocytes. When comparing the total number of Ku80-ir nuclei to STEM123 and Ku80 double-labeled cell number, the percentage of astrocytes was 10.7% in an Nj brain and 8.6% in a jj brain (Fig. 1G-I for jj and Fig. 1J-L for Nj).
Grafted Cell Distribution and Axonal Extension
The AP distribution of grafted cells was identified by cell staining for both STEM121 and Ku80. The range was 1,440 ± 540 μm in jj brains and 1,335 ± 282 μm in Nj brains. This difference was not statistically significant [t(4) = −0.196, p = 0.854]. The majority of grafted cells were located in the striatum. Robust STEM121 and Ku80 double-stained cells were usually seen near the injection tract, mostly within 50 μm. Cells that migrated between 100 and 200 μm away from the injection site were frequently observed. Cell clusters usually grew on the edge of the tract or inside the tract. However, some single cells were identified that migrated longer distances (up to 500 μm). In some cases, cells could be found in the cerebral cortex, in the corpus callosum (the dorsal part of the graft), and in the nucleus accumbens, GP, and claustrum (the ventral part of the graft). Cell distribution depended more on the site of injection than on jj versus Nj groups (Fig. 1M and P for cell migration in jj and Nj brains).
Grafted neurons had abundant neurite outgrowth in both jj and Nj brains 3 weeks after transplantation (Fig. 1M-R). The AP distances covered by the grafted fibers were 2,160 ± 720 μm in jj brains and 1,485 ± 306 μm in Nj brains. This difference did not reach statistical significance [t(4) = −1.060, p = 0.349]. Extended fibers distributed mostly in the cerebral cortex, striatum, and GP. The length of fibers varied widely; representative long fibers that could be clearly identified within a single plane (one brain section) were usually around 200 to 300 μm, with some as long as 400 μm. When axons of the graft tissue extended into the corpus callosum or the anterior commissure, they usually coursed in parallel with these projection fiber tracts for a very long distance (500-800 μm) (see Fig. 1N). The direction of axonal projection varied depending on the location of cells; usually, the closer to the injection tract, the denser the fibers were (Fig. 1M and P-R). Neurons with axons projecting across a very long distance were also frequently observed (Fig. 1R). Interestingly, most of the fibers grew toward the targets of the host brain from the graft sites, with only a minority of fibers growing into or traversing the tract (Fig. 1M, Q, and R). Some distance away from the graft site (usually ventral to the tract), grafted fibers forming distinct, brush-like patterns in the striatum were frequently identified in both jj and Nj brains (Fig. 1O and Q). These types of fibers were usually identified in the midventral region of the striatum, with some extending to the GP.
Neurochemical Characteristics of NPCs Grafted Into jj and Nj Rat Brains
IHC labeling for ChAT and STEM121 expression revealed very few ChAT-ir-transplanted neurons in either Nj or jj rat brains (Fig. 2A-C for jj and Fig. 2D-F for Nj). This result suggests that only a small population of progenitor cells differentiated into cholinergic neurons in vivo.
Figure 2.
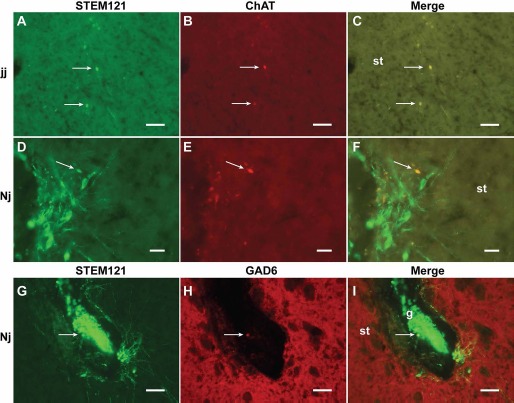
Choline acetyltransferase (ChAT) and anti-glutamic acid decarboxylase (GAD-6) expression in the grafted neurons. (A-F) Grafted cholinergic neurons identified with immunohistochemistry (IHC) labeling for STEM121 and ChAT expression in jaundice (jj; A-C) and nonjaundice (Nj; D-F) brains. Images show STEM121, Ku80, and merged. (G-I) GAD-6-labeled neuron within grafted tissue in an Nj brain. (G) STEM121; (H) GAD-6; (I) merge. There were limited numbers of cholinergic or GABAergic neurons presented in the graft. g, graft; arrow, cells; st, striatum. Scale bars: 50 μm (A-C, G-I), 20 μm (D-F).
Labeling for GAD-6 and STEM121 expression revealed that very few GAD-6+ grafted neurons were present in the Nj brain (Fig. 2G-I). There were no definitive GAD-6+ grafted neurons identified in the jj brain (data not shown).
Discussion
The principle finding in this study was that NPCs derived from a WA09 hESC line survived in both jj and Nj brains 3 weeks after transplantation. Although the transplantation surgery occurred at P20, after plasma bilirubin levels have peaked, yet still within the period of hyperbilirubinemia14,15, the risk of bilirubin toxicity was reduced. Interestingly, we found significantly greater cell survival in the jj than in the Nj brain. In addition to the fact that the cells were engineered to be different than GP cells, slightly elevated bilirubin levels may actually protect grafted cells and increase survival due to bilirubin's reported antioxidant properties16. Immunosuppression is often applied to the host in xenotransplantation studies to prevent rejection of grafted cells. Because of the brain's relatively immune-privileged status, the fact that the blood-brain barrier is still developing at the age of transplantation17, and to avoid side effects of the drug, immunosuppressant was not used in this experiment. However, the outcomes of graft survival (21% in jj and 12.4% in Nj, respectively) suggest some immune response. There was some necrotic tissue in all grafted jj and Nj brains, especially inside the tract and in areas adjacent to the dorsal part of the tracts (mainly in the cerebral cortex and corpus callosum). Nevertheless, this condition provided us an opportunity to observe the “clean” effects of cell transplantation in the jj brain. Bilirubin is a powerful immunomodulator, acting directly to induce immune tolerance and inhibit T-cell responses18. The greater survival of grafted cells suggests partial immunosuppression in jj rats.
Grafted cell migration was identified by STEM121 and Ku80 staining. The distance of migration was usually within 500 μm. A previous study reported the effects of grafting human neural stem cells derived from fetal cortex into the striatum in a rat HD model6. These authors reported wider and more even cell distribution throughout the striatum and migration of cells to GP, entopeduncular nucleus, and substantia nigra. Differences in transplanted cell types, number of cells injected (10,000 vs. 200,000), and notably, the posttransplant survival period (3 weeks vs. 8 weeks) may have played roles in these different results.
The IHC of STEM121 labeling demonstrated that grafted NPCs were able to grow numerous fibers that projected broadly. The graft-derived fiber distribution and patterns were similar to the description in another experiment in which human or mouse fetal NPCs were transplanted into the striatum of rats in an HD model19. The rich axonal outgrowth we observed suggests good integration of the graft into the host tissue. Whether these fibers formed synapses requires further investigation. We found that only a few fibers projected into the injection track or passed through the track to the other side of the brain. It is possible that necrotic tissue and microglia repelled fibers or prevented their growth into this area.
Immunocytochemistry (ICC) of neuronal developmental marker Tuj1 (for βIII-tubulin) and differentiated postmitotic neuronal marker neurofilament indicated that at least 75% of the cells used in this study differentiated into neurons10. Three weeks after grafting, the results of the IHC for STEM123 (staining human GFAP) suggest that a small percentage (about 10%) of transplanted cells were astrocytes. These complementary data match the ICC results and provide a more complete picture of the constitution of the grafted cells. Grafts containing a mixture of a high percentage of neurons and a low percentage of glial cells may better promote host-graft integration than grafts with pure neurons because they better resemble the cell environment of the host brain.
Based on the ICC of developmental markers, it has been demonstrated that the NPCs used in this study had small populations of cholinergic and GABAergic cells (about 5% and 2%, respectively)10. After 3 weeks of survival, only a small number of cholinergic neurons could be detected by ChAT labeling in the STEM121-ir grafted tissue in both jj and Nj brains. This finding was in agreement with the ICC result, suggesting cholinergic neurons were not common in NPCs differentiated using this protocol. A similar result was found in GABAergic neurons. The IHC for GAD-6 and STEM121 revealed only a few small STEM121-ir neurons were labeled by GAD-6 in the Nj brain, and none in the jj brain. Alternatively, the lack of GAD-6 expression in grafted cells in the jj brain may reflect greater susceptibility of GABAergic neurons to bilirubin toxicity, since most of the GP neurons and the cerebellar Purkinje cells are GABAergic. It could also be that the grafted neurons were not mature enough to express these specific neural markers, as in McBride et al.'s study6 reporting that none of their neuronal nuclei-positive (NeuN+) cells expressed GAD67, even after 8 weeks of transplantation.
In conclusion, this study demonstrates that transplanted human NPCs survived and formed neurites with extensive outgrowth within the basal ganglia of both jj and Nj rats. One limitation of this study was that we did not test the effects of these cells in a more severe kernicterus model15. Future studies should address this important issue. As a first step toward this goal, we wanted to determine NPC survival in the jaundiced brain. Importantly, cells grafted into the jj brain had greater survivability than those grafted into the Nj brain. These results suggest that cell type, graft site, and antioxidant and immunosuppressive effects of bilirubin all affected cell survival. Since very few neurons expressed GAD-6 or ChAT in the grafts, these NPCs may not be ideal for cell replacement in kernicterus. Future studies should also focus on using NPCs containing greater populations of GABAergic and cholinergic neurons for transplantation and optimizing the injection site to allow more neuronal growth and functional connections in the GP.
Acknowledgments
The authors acknowledge Sarah Tague, Ph.D., and Jing Huang, B.S., for their technical assistance and the Transgenic and Gene Targeting Institutional Facility at the University of Kansas Medical Center for the preparation of differentiated cells; this facility is supported by the NIH Center of Biomedical Research Excellence program project P20 GM103549. This work was supported by Children's Mercy Hospital, the Ronald D. Deffenbaugh Foundation, and the KIDDRC center grant HD 02528. The authors declare no conflicts of interest.
References
- 1.Hansen T.W. Bilirubin brain toxicity. J Perinatol. 2001; 21(suppl 1): S48–51; discussion S59–62. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro S.M. Definition of the clinical spectrum of kernicterus and bilirubin-induced neurologic dysfunction (BIND). J Perinatol. 2005; 25(1): 54–9. [DOI] [PubMed] [Google Scholar]
- 3.Bjorklund L.M., Sanchez-Pernaute R., Chung S., Andersson T., Chen I.Y., McNaught K.S., Brownell A.L., Jenkins B.G., Wahlestedt C., Kim K.S., Isacson O. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci USA 2002; 99(4): 2344–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindvall O. Treatment of Parkinson's disease using cell transplantation. Philos Trans R Soc Lond B Biol Sci. 2015; 370(1680): 20140370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maucksch C., Vazey E.M., Gordon R.J., Connor B. Stem cell-based therapy for Huntington's disease. J Cell Biochem. 2013; 114(4): 754–63. [DOI] [PubMed] [Google Scholar]
- 6.McBride J.L., Behrstock S.P., Chen E.Y., Jakel R.J., Siegel I., Svendsen C.N., Kordower J.H. Human neural stem cell transplants improve motor function in a rat model of Huntington's disease. J Comp Neurol. 2004; 475(2): 211–9. [DOI] [PubMed] [Google Scholar]
- 7.Fu M.H., Li C.L., Lin H.L., Chen P.C., Calkins M.J., Chang Y.F., Cheng P.H., Yang S.H. Stem cell transplantation therapy in Parkinson's disease. Springerplus 2015; 4: 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watchko J.F., Tiribelli C. Bilirubin-induced neurologic damage—Mechanisms and management approaches. N Engl J Med. 2013; 369(21): 2021–30. [DOI] [PubMed] [Google Scholar]
- 9.Nobrega-Pereira S., Gelman D., Bartolini G., Pla R., Pierani A., Marin O. Origin and molecular specification of globus pallidus neurons. J Neurosci. 2010; 30(8): 2824–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vivian J., Tague S., Agabas D., Draper J., Pace J., Paul S., Weiss M., Smith P. Differentiated human pluripotent stem cells for cell-based therapy of spinal cord injury. Proceedings of the 45th Annual Meeting of the Society for Neuroscience. 2015 Oct 17–21; Chicago, IL. [Abstract 380.24]
- 11.Boulting G.L., Kiskinis E., Croft G.F., Amoroso M.W., Oakley D.H., Wainger B.J., Williams D.J., Kahler D.J., Yamaki M., Davidow L., Rodolfa C.T., Dimos J.T., Mikkilineni S., MacDermott A.B., Woolf C.J., Henderson C.E., Wichterle H., Eggan K. A functionally characterized test set of human induced pluripotent stem cells. Nat Biotechnol. 2011; 29(3): 279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maury Y., Come J., Piskorowski R.A., Salah-Mohellibi N., Chevaleyre V., Peschanski M., Martinat C., Nedelec S. Combinatorial analysis of developmental cues efficiently converts human pluripotent stem cells into multiple neuronal subtypes. Nat Biotechnol. 2015; 33(1): 89–96. [DOI] [PubMed] [Google Scholar]
- 13.Sherwood N.M., Timiras P.S. A stereotaxic atlas of the developing rat brain. 1st ed. Berkeley (CA): University of California Press; 1970. p. 209. p. 14–203 illus. [Google Scholar]
- 14.Gazzin S., Zelenka J., Zdrahalova L., Konickova R., Zabetta C.C., Giraudi P.J., Berengeno A.L., Raseni A., Robert M.C., Vitek L., Tribelli C. Bilirubin accumulation and Cyp mRNA expression in selected brain regions of jaundiced Gunn rat pups. Pediatr Res. 2012; 71(6): 653–60. [DOI] [PubMed] [Google Scholar]
- 15.Schutta H.S., Johnson L. Clinical signs and morphologic abnormalities in Gunn rats treated with sulfadimethoxine. J Pediatr. 1969; 75(6): 1070–9. [DOI] [PubMed] [Google Scholar]
- 16.Sedlak T.W., Saleh M., Higginson D.S., Paul B.D., Juluri K.R., Snyder S.H. Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proc Natl Acad Sci USA 2009; 106(13): 5171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saunders N.R., Liddelow S.A., Dziegielewska K.M. Barrier mechanisms in the developing brain. Front Pharmacol. 2012; 3: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y., Li P., Lu J., Xiong W., Oger J., Tetzlaff W., Cynader M. Bilirubin possesses powerful immunomodulatory activity and suppresses experimental autoimmune encephalomyelitis. J Immunol. 2008; 181(3): 1887–97. [DOI] [PubMed] [Google Scholar]
- 19.Kelly C.M., Precious S.V., Penketh R., Amso N., Dunnett S.B., Rosser A.E. Striatal graft projections are influenced by donor cell type and not the immunogenic background. Brain 2007; 130(Pt 5): 1317–29. [DOI] [PubMed] [Google Scholar]


