Abstract
Whether dietary nitrate supplementation improves exercise performance or not is still controversial. While redistribution of sufficient oxygen from inactive to active muscles is essential for optimal exercise performance, no study investigated the effects of nitrate supplementation on muscle oxygenation profiles between active and inactive muscles. Nine healthy males performed 25 min of submaximal (heart rate ~140 bpm; EXsub) and incremental cycling (EXmax) until exhaustion under three conditions: (A) normoxia without drink; (B) hypoxia (FiO2 = 13.95%) with placebo (PL); and (c) hypoxia with beetroot juice (BR). PL and BR were provided for 4 days. Oxygenated and deoxygenated hemoglobin (HbO2 and HHb) were measured in vastus lateralis (active) and biceps brachii (inactive) muscles, and the oxygen saturation of skeletal muscle (StO2; HbO2/total Hb) were calculated. During EXsub, BR suppressed the HHb increases in active muscles during the last 5 min of exercise. During EXmax, time to exhaustion with BR (513 ± 24 sec) was significantly longer than with PL (490 ± 39 sec, P < 0.05). In active muscles, BR suppressed the HHb increases at moderate work rates during EXmax compared to PL (P < 0.05). In addition, BR supplementation was associated with greater reductions in HbO2 and StO2 at higher work rates in inactive muscles during EXmax. Collectively, these findings indicate that short‐term dietary nitrate supplementation improved hypoxic exercise tolerance, perhaps, due to suppressed increases in HHb in active muscles at moderate work rates. Moreover, nitrate supplementation caused greater reductions in oxygenation in inactive muscle at higher work rates during hypoxic exercise.
Keywords: Blood flow, muscle O2 extraction, sympathetic vasoconstriction, tissue oxygenation
Introduction
It has been suggested that nitric oxide (NO) is a major factor in hypoxic‐induced vasodilation (Casey et al. 2010). Accumulating evidences revealed that dietary inorganic nitrate (NO3 −) has been linked to many physiological benefits, including improvement of oxygen supply to peripheral tissue (Hord et al. 2009). It is also widely accepted that NO3 − is reduced to nitrite (NO2 −) and further to NO, generally known as NO3 −‐NO2 −‐NO pathway (Lundberg and Weitzberg 2010). Given these biological effects, recent studies have investigated whether dietary nitrate (NO3 −) supplementation (e.g., beetroot juice rich with NO3 −), which is a potent vasodilator, improves exercise performance or has other ergogenic effects under hypoxia. Some studies showed that that dietary NO3 − supplementation reduced oxygen cost and/or improved exercise tolerance under hypoxia (Vanhatalo et al. 2011, 2014; Masschelein et al. 2012; Kelly et al. 2014; Muggeridge et al. 2014; Shannon et al. 2016, 2017); others, however, had contradictory findings (Arnold et al. 2015; Bourdillon et al. 2015; MacLeod et al. 2015; Carriker et al. 2016; Nyback et al. 2017). One possible explanation to account for these discrepancies may relate to differential study settings. For example, previous studies (e.g., Kelly et al. 2014) used various range of oxygen levels, fraction of inspiratory oxygen (FiO2) = 0.11–0.16, and it should be noted that subjects in most of previous studies were exposed to hypoxic condition for a relative short duration, ~15 min before the main exercise test except one study (Masschelein et al. 2012).
At high‐altitude, initial compensatory physiological responses is an increase in pulmonary ventilation to deliver sufficient oxygen into peripheral tissues (Dempsey and Foster 1982) and this immediate increases in ventilation showed a stable about after 1 h hypoxic exposure (Easton et al. 1986). These may indicate that a short duration of resting baseline in most of previous studies (e.g., Kelly et al. 2014; ~10 min hypoxic exposure) may cause different resting condition between subjects, resulted in controversial findings. In this regard, Masschelein et al. (2012) took 1 h resting hypoxic exposure before exercise, and found that dietary nitrate supplementation improves muscle oxygenation status, but not cerebral oxygenation status during hypoxic exercise. As it is well known that blood flow redistribution from inactive to active muscles is essential to optimize exercise performance (Rowell 1993), it may be required to investigate interaction in oxygenation status between these muscles for further understanding to elucidate potential mechanisms of nitrate effects on exercise performance in hypoxia.
In resting muscles during exercise, sympathetic vasoconstriction is known to be well preserved under either normoxia or hypoxia (Remensnyder et al. 1962; Hansen et al. 2000), a phenomenon for which Remensnyder et al. (1962) coined the term functional sympatholysis. Exercise training‐induced improvement of functional sympatholysis increases the maximal oxygen uptake of animal models (Mizuno et al. 2014). These results suggest that the balance between metabolic vasodilation in active muscles and sympathetic vasoconstriction in inactive muscles may play an important role in optimizing exercise performance. Thus, enhanced sympathetic vasoconstriction in inactive muscles may support oxygen supply via vasodilation in active muscles, and thus improve performance in hypoxia. Conversely, if nitrate supplementation may cause a vasodilation in inactive muscles as well as in active muscles, this may counteract improvement of exercise performance in hypoxia.
The aim of this study was to examine the potential impact of dietary nitrate supplementation on hypoxic exercise performance, as well as the interaction between metabolic vasodilation in active and sympathetic vasoconstriction in inactive muscles during submaximal and maximal incremental exercise in healthy young humans. We used near‐infrared spectroscopy (NIRS) to evaluate tissue oxygenation with high temporal resolution, since changes in tissue oxygenation have been shown to be a reliable measure of sympathetic vasoconstriction in resting and exercising skeletal muscles (Hansen et al. 1996; Fadel et al. 2004; Ogata et al. 2007, 2008; Horiuchi et al. 2014, 2015). In addition, muscle deoxygenation has been suggested to be an indicator of muscle O2 extraction (a‐v O2 difference) (DeLorey et al. 2003; Grassi et al. 2003). We evaluated muscle O2 extraction at active muscles and sympathetic vasoconstriction at inactive muscles using NIRS. We hypothesized that dietary NO3 − supplementation would improve exercise performance under hypoxia via lower exercise‐induced increases in muscle deoxygenation in active muscles (Masschelein et al. 2012) despite higher muscle oxygenation levels in inactive muscles.
Methods and Material
Subjects
Nine healthy male subjects with a mean age of 21 ± 3 years, height of 176 ± 5 cm, and body mass of 73 ± 9 kg (mean ± SD) participated in this study. Subjects engaged in regular physical activity (1–2 h per day, 3–5 days per week). None of the subjects had been exposed to an altitude higher than 1500 m within 6 months prior to the study. After receiving a detailed description of all study procedures and the possible risks and benefits of participation, each subject signed an informed consent form. All procedures were approved by the ethical committee of Mt. Fuji Research Institute in Japan and were performed in accordance with the guidelines of the Declaration of Helsinki (ECMFRI‐01‐2014).
Experimental procedures
Subjects were requested to abstain from caffeinated beverages for 12 h, and from strenuous exercise and alcohol for a minimum of 24 h before each session. All studies were performed at a temperature of 24 ± 1°C, and external stimuli were minimized. All subjects performed three trials: (1) normobaric normoxic exercise without any drink (Norm); (2) normobaric hypoxic exercise (FiO2 = 0.1395) with a placebo drink (PL), and (3) normobaric hypoxic exercise (FiO2 = 0.1395) with beetroot juice (BR). Each subject performed the Norm trial first. The two hypoxic trials were performed afterward in a random order. All three trials were performed with at least a 2‐week washout period. For 3 days prior to the BR and PL trials, subjects consumed 140 mL/day of NO3 −‐rich BR or 140 mL/day of NO3 −‐depleted BR concentrate as a placebo drink (Beet It; James White Drinks, Ltd., Ipswich, UK) (Kelly et al. 2014). Both subjects and researchers were blinded to the drink contents until the completion of the study. Subjects were also provided with a list of foods rich in NO3 − and instructed to avoid the consumption of these foods and to maintain their normal dietary intake for the duration of the study. In addition, they were asked to abstain from the use of antibacterial mouthwash, which eliminates the oral bacteria that reduce NO3 − to NO2 − for the duration of the study (Govoni et al. 2008).
On each study day (PL or BR trails), subjects consumed their final dose of BR or PL upon arrival at the laboratory, 2.5 h prior to the start of their submaximal exercise. Venous blood was sampled 30 min after the final dose (see “Blood samples and analysis” section below). This ingestion time was previously shown to achieve a peak increase in plasma nitrates or nitrite concentration that improved exercise tolerance (Wylie et al. 2013).
In all three trials (Norm, PL, and BR), 10 min normoxic resting condition was set at first while breathing room air. During the PL and BR trials, after 10 min normoxic exposure hypoxic gas was supplied via a commercial tent (about 4000 L) in tandem with a hypoxic gas generator system (YHS‐B05S: YKS, Nara, Japan; and Hypoxico Everest Summit II: Will Co., Ltd., Tokyo, Japan). Hypoxic baseline values were measured over the last 10 min of a 75‐min resting hypoxic exposure. Inspired oxygen concentration was verified before and after each experiment (AE‐300; Minato Medical Science, Osaka, Japan). After baseline measurements were acquired, subjects performed submaximal exercise on a cycle ergometer (Ergomedic 828 E; Monark, Stockholm, Sweden) in a semi‐recumbent position for 25 min at a target heart rate (HR) of 140 bpm. The pedaling rate was set as 60 rpm. Briefly, the starting work rate was 30 W, and it was increased by 30 W every minute, up to 5 min, until the subject's HR reached the target of 140 bpm. After reaching this HR, the subjects continued to cycle at a constant pedaling rate of 60 rpm for 25 min and exercise intensity was manually adjusted to maintain the target HR while the 25‐min cycling (Ogoh et al. 2014; Komiyama et al. 2015). Subjects then rested for 25 min before performing a maximal incremental exercise test (the starting work rate was 30 W, and increased by 30 W/min) until exhaustion. Subjects were asked to maintain a pedal frequency of 60 rpm throughout the exercise. The criteria for exhaustion was were as follows: (1) No increase in VO2 despite a further increase in work rate; (2) HR at 90% of the age‐predicted maximal value (220‐participant's age); (3) A rating of 19 on the Borg's scale of perceived exertion; or (4) Failure to maintain pedaling frequency of 60 rpm despite strong verbal encouragement. When participants met at least two of the above criteria, the test was terminated (Dobashi et al. 2016). Study procedures are shown in Figure 1.
Figure 1.
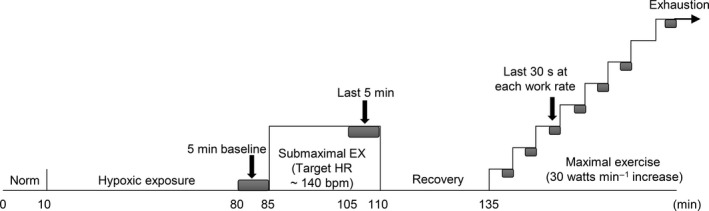
Experimental protocol of hypoxic trails (placebo and beetroot) in this study. Norm, normoxia; EX, exercise; HR, heart rate; bpm, beats per minute. Gray squares indicate the values at baseline, the last 5 min during submaximal exercise, and the last 30 sec at each work rate during maximal exercise. Data for the last 5 min and last 30 sec were used to calculate relative changes from the baseline values in near infrared spectroscopy signals (see Data analysis in the text).
Measurements
Pulmonary ventilation (V E) and gas exchange variables were measured with a breath‐by‐breath metabolic measurement system (AE‐310S; Minato Medical Science, Osaka, Japan), which assessed inspired and expired gas volumes via hot‐wire respirometry. Flow signals were electrically integrated for the duration of each breath, and summed to calculate minute ventilation. The expired fractions of O2 and CO2 were analyzed using a zirconium solid electrolyte oxygen analyzer and an infrared carbon dioxide analyzer, respectively. The standard gases (O2 15.23%, CO2 4.999%, and N2 balance) and room air were used to calibrate the gas analyzer.
Throughout the study, participants' HR were recorded with a wireless HR monitor (Polar RC800X; Polar Electro Japan, Tokyo, Japan). Arterial O2 saturation (SpO2) was monitored by a pulse oximeter on the right middle finger every 1 min throughout the study (Pulsox‐3; Minolta, Tokyo, Japan). Local tissue oxygenation profiles of the vastus lateralis (active) and biceps brachii (inactive) muscles were measured using NIRS (BOM‐L1TRW; Omegawave, Tokyo, Japan), as previously described (Horiuchi et al. 2014, 2015). In this study, each subject performed cycling in a semi‐recumbent position, and their arms were placed on side tables rather than on the handlebars. In addition, their arms were completely fixed by a vacuum pack to prevent arm movement (Vacuform, Muranaka Medical Instruments Co., Ltd., Osaka, Japan), and thus their biceps brachii muscles could be classified as inactive.
The NIRS instrument used three laser diodes (780, 810, and 830 nm) and calculated relative tissue levels of oxygenated and deoxygenated hemoglobin (HbO2 and HHb) according to the Modified‐Beer‐Lambert law. Total Hb was calculated as the sum of HbO2 and HHb, and the oxygen saturation of skeletal muscle (StO2) was expressed as (HbO2/total Hb) × 100 (i.e., as a percentage). NIRS optodes were placed over the lower third of the vastus lateralis muscle (10–12 cm above the knee joint) (Koga et al. 2007), and the belly of the biceps brachii muscle on the upper left arm of each subject (Ogata et al. 2007). The probe holder contained one light source probe, and two detectors were placed 2 cm (detector 1) and 4 cm (detector 2) away from the source. Hb concentrations received by detector 1 were subtracted from those received by detector 2. This procedure minimized the influence of skin blood flow (SkBF) (Ando et al. 2010). In this device, path wave length was set as 4.0; however, as modified beer lambert method cannot assess actual path wave length (Van der Zee et al. 1992), meaning that provisional absolute values, for example, μmol/L, are estimated values, we showed the NIRS values as arbitrary unit in representative recordings (see in Fig. 3), and relative changes from baseline values in mean values (see in data analysis and Figs. 4, 5).
Figure 3.
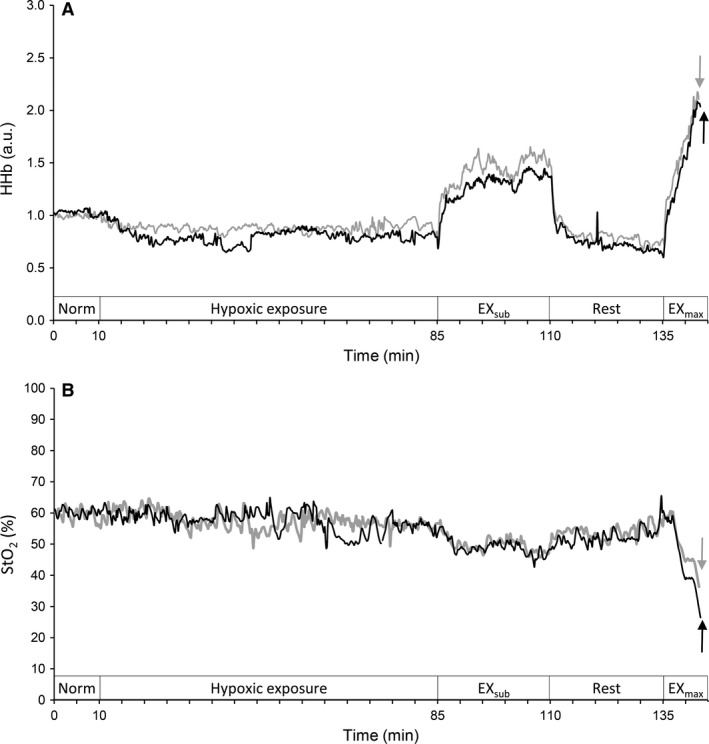
Time course changes in muscle deoxygenation (HHb) status in active muscles (vastus lateralis) and saturation of skeletal muscle (StO2) in inactive muscles (biceps brachii) for a typical single subject. Gray lines indicate PL and black lines indicate BR trials, respectively. a.u., arbitrary unit; EXsub, submaximal leg cycling exercise; EXmax, incremental leg cycling exercise. Arrows indicate at the point of exhaustion.
Figure 4.
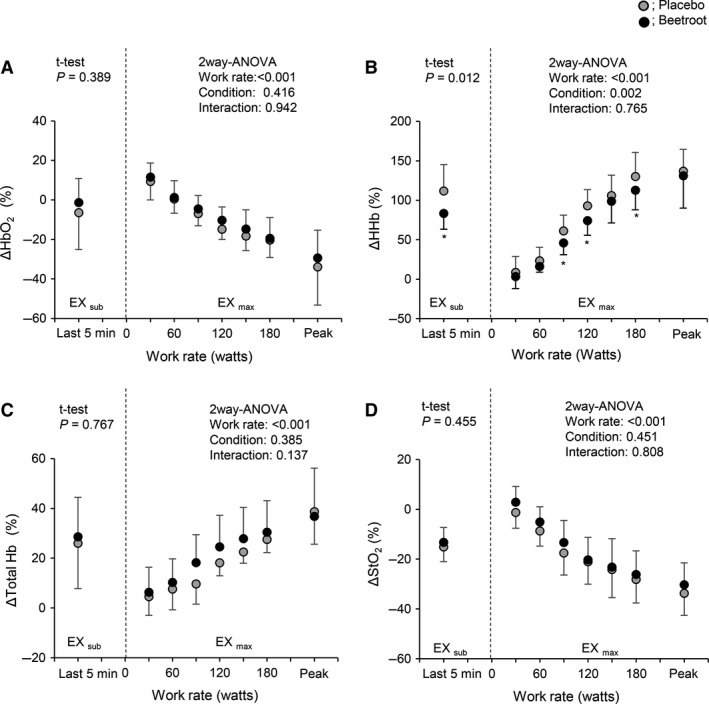
Relative changes from baseline values in each near infrared spectroscopy signal in active muscle during EXsub and EXmax between PL and BR trials. HbO2, muscle oxygenation; Total Hb, total hemoglobin. Peak indicates the individual peak work rate. Values are the means ± SD. *P < 0.05 between PL and BR within the same work rates.
Figure 5.
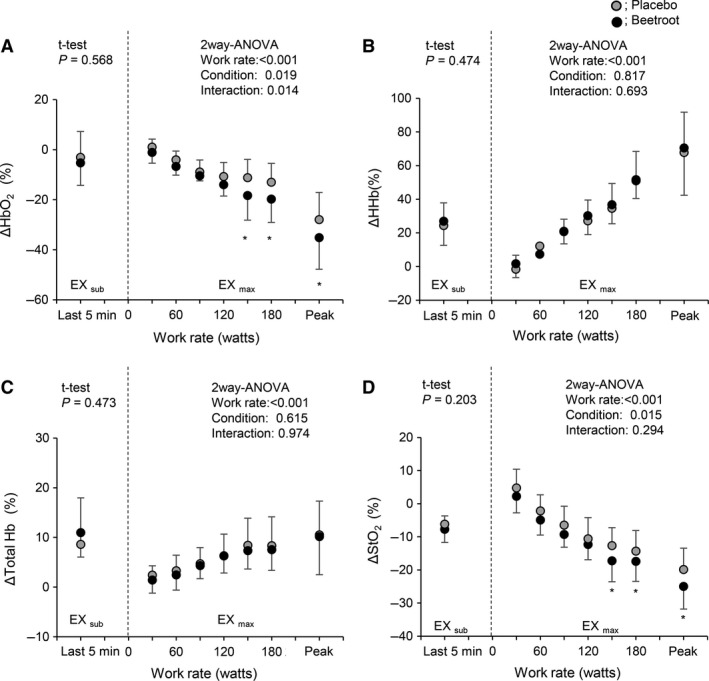
Relative changes from baseline values in each near infrared spectroscopy signal in inactive muscle during EXsub and EXmax between PL and BR trials. Values are the means ± SD. * P < 0.05 between PL and BR within the same work rates.
It has been reported that NIRS signals can reach half the depth of the distance between the probe and detector (Patterson et al. 1989). With this in mind, we used a distance of 4 cm between probes, which provided an NIRS signal traversing approximately 20 mm. This allowed the appropriate depth for sample muscles, since the sum of the skinfold plus the muscle thickness in the biceps brachii and vastus lateralis muscle was over 20 mm. Indeed, the measured skinfold thicknesses of the biceps brachii and vastus lateralis muscles were 3.8 ± 1.2 mm and 4.1 ± 1.0 mm, respectively (P = 0.073), whereas the muscle thickness was 28.4 ± 3.8 mm for the biceps brachii and 30.7 ± 5.3 mm (mean ± SD) for the vastus lateralis (P = 0.084), as assessed by B‐mode ultrasound (logic‐e; GE Healthcare, Tokyo, Japan). These numbers indicate that when NIRS probes were placed over the skin of each muscle, the NIR light was indeed transmitted to the desired muscle bed. Both muscles with attached optodes and covering were wrapped with an elastic bandage to minimize optode movement, while permitting mobility for cycling. SkBF at the vastus lateralis muscle was recorded using the laser Doppler method (ATBF‐LC1; Unique Medical Co., Ltd., Tokyo, Japan). The electrodes were briefly placed over the skin, about 2‐cm away from the NIRS probe. Pen marks were made on the skin to indicate the margins of the probe holder and electrodes. All subjects were asked to mark themselves again between trials before washing so that the optodes could be positioned at exactly the same place for each test.
Blood pressure (BP) was measured using two different methods. At rest, BP was measured with the oscillometric method using a digital BP monitor (HEM‐907; Omron, Tokyo, Japan) on the upper portion of each patient's right arm. BP was measured at least three times with a 1‐min interval between measurements. If the difference between the measurements of either systolic or diastolic BP was >5 mmHg, the measurements were repeated. The average BP values of measurement pairs were taken as the BP values, excluding those that were >5 mmHg. Under the PL and BR conditions, BP was measured under both normoxia and hypoxia during the resting periods. During exercise, beat‐by‐beat BP was also measured using finger photoplethysmography at the middle or index finger of the left hand (MUB‐101; Medisens Inc., Tokyo, Japan) throughout the study.
Data on the beat‐by‐beat BP, NIRS signals, and SkBF were stored with a sampling frequency of 200 Hz by a field data recorder (es8; TEAC, Tokyo, Japan), and transferred to a laptop computer for further analysis.
Blood samples and analysis
Resting venous blood samples (10 mL) were taken from the antecubital vein and immediately centrifuged at 1000 g for 15 min at 4°C (MX‐300; Tomy Seiko Co., Ltd., Tokyo, Japan) to separate serum and plasma. The serum samples were frozen at −80°C for further analysis of NO3 − by SRL Co., Ltd. (Tokyo, Japan). Capillary blood samples (0.3 μL) were taken from the finger for blood lactate concentration (LA) measures. Using an automated lactate analyzer, LA was analyzed immediately at rest under normoxia (Norm) and hypoxia (PL and BR), after 5 min of submaximal exercise, and after 5 min of maximal leg cycling (Lactate Pro 2LT‐1730; Arkray, Tokyo, Japan).
Data analysis
At rest, all physiological values (i.e., mean values for gas exchange variables, HR, SpO2, and the NIRS signals) were calculated over the last 5 min before the submaximal exercise in each trial (resting baseline values). Data were averaged over the last 5 min of submaximal exercise as the work rate was held to be constant during the last 5 min. During the maximal exercise, data were averaged over the last 30 sec at each work rate up to 180 W, and the last 30 sec just prior to exhaustion. To compare NIRS signals between subjects, the changes in HbO2, HHb, and total Hb were quantified as percentages from the resting baseline values. Because our NIRS device can represent each NIRS signal as an arbitrary unit, resting baseline values were defined as 100% and differences were shown as relative changes (Horiuchi et al. 2016) (Fig. 1). Using the oscillometric method, mean arterial pressure (MAP) was calculated as ([2 × diastolic pressure] + systolic pressure)/3, while beat‐by‐beat MAP was measured as the time averaged from the beat‐by‐beat pressure wave.
Statistics
Data were presented as the means ± SD. Based on the study design, separate analyses using two‐tailed paired t‐tests were performed to compare the effects of dietary NO3 − supplementation (BR vs. PL) and the general effects of hypoxia (Norm vs. PL) for comparison of cardiorespiratory variables and NIRS signals during submaximal exercise (Masschelein et al. 2012). A two‐way repeated measures ANOVA was also used to compare exercise‐induced relative changes in NIRS signals during maximal exercise. A P < 0.05 was considered statistically significant.
Results
General effects of hypoxia (Norm vs. PL)
At rest, 75 min of hypoxia reduced resting SpO2 and increased MAP (P < 0.05), while there were no differences in other variables (Table 1). During EXsub, the values of gas exchange variables and SpO2 were lower under the PL than the Norm condition (P < 0.05). Similar results were also observed during EXmax (P < 0.05, Table 1). In addition, hypoxic conditions significantly impaired exercise tolerance, time‐to‐exhaustion (574 ± 47 in Norm vs. 490 ± 39 sec in PL, P < 0.001), and maximal work rate (287 ± 34 in Norm vs. 233 ± 25 W in PL, P = 0.002) (Fig. 2).
Table 1.
Cardiorespiratory and hemodynamic variables under normoxia, placebo and beetroot trials at rest and during submaximal and maximal exercise
| Normoxia | Placebo | Beetroot | P value | ||
|---|---|---|---|---|---|
| Norm vs. PL | PL vs. BR | ||||
| Rest | |||||
| VO2, mL/min | 297 ± 33 | 305 ± 47 | 296 ± 40 | 0.622 | 0.431 |
| VCO2, mL/min | 257 ± 30 | 277 ± 42 | 273 ± 38 | 0.257 | 0.788 |
| RER | 0.87 ± 0.07 | 0.92 ± 0.04 | 0.92 ± 0.06 | 0.207 | 0.807 |
| V E, L/min | 10.0 ± 1.4 | 10.8 ± 1.4 | 10.9 ± 1.7 | 0.183 | 0.919 |
| HR, bpm | 60 ± 7 | 64 ± 7 | 65 ± 5 | 0.058 | 0.757 |
| MAP, mmHg | 85 ± 5 | 88 ± 6b | 86 ± 7 | <0.001 | 0.085 |
| LA, mmol/L | 1.4 ± 0.3 | 1.6 ± 0.2 | 1.7 ± 0.2 | 0.206 | 0.122 |
| SpO2, % | 98.3 ± 0.8 | 88.1 ± 2.0b | 87.6 ± 1.5 | <0.001 | 0.590 |
| Submaximal exercise | |||||
| VO2, mL/min | 1908 ± 305 | 1442 ± 183b | 1382 ± 225 | <0.001 | 0.209 |
| VCO2 mL/min | 1744 ± 286 | 1384 ± 161b | 1277 ± 207a | <0.001 | 0.012 |
| RER | 0.91 ± 0.02 | 0.97 ± 0.07 | 0.93 ± 0.07 | 0.063 | 0.069 |
| VE, L/min | 48.5 ± 8.9 | 46.3 ± 5.7 | 44.4 ± 10.0 | 0.371 | 0.344 |
| HR, bpm | 140 ± 2 | 140 ± 4 | 141 ± 4 | 0.715 | 0.560 |
| MAP, mmHg | 100 ± 10 | 93 ± 5 | 92 ± 8 | 0.080 | 0.843 |
| SkBF, % | 544 ± 223 | 538 ± 222 | 560 ± 321 | 0.953 | 0.744 |
| LA, mmol/L | 2.8 ± 1.1 | 4.0 ± 2.1b | 3.8 ± 1.6 | 0.040 | 0.759 |
| SpO2, % | 96.6 ± 1.3 | 74.6 ± 5.2b | 73.8 ± 5.1 | < 0.001 | 0.592 |
| Maximal exercise | |||||
| VO2 peak, mL/min | 3127 ± 361 | 2450 ± 277b | 2612 ± 337 | <0.001 | 0.066 |
| VCO2 peak, mL/min | 3688 ± 457 | 3152 ± 416b | 3222 ± 301 | 0.003 | 0.516 |
| RER peak | 1.18 ± 0.06 | 1.29 ± 0.17 | 1.23 ± 0.09 | 0.105 | 0.254 |
| V E peak, L/min | 111.3 ± 20.1 | 117.5 ± 17.0 | 118.0 ± 16.6 | 0.210 | 0.914 |
| HR peak, bpm | 180 ± 9 | 178 ± 6 | 182 ± 5a | 0.352 | 0.031 |
| MAP, mmHg | 149 ± 14 | 133 ± 20 | 130 ± 16 | 0.071 | 0.441 |
| SkBF, % | 529 ± 287 | 535 ± 287 | 568 ± 222 | 0.950 | 0.832 |
| LA peak, mmol/L | 12.1 ± 1.7 | 10.3 ± 2.6 | 12.3 ± 2.8 | 0.064 | 0.078 |
| SpO2 nadir,% | 95.3 ± 1.5 | 69.4 ± 5.9b | 68.9 ± 6.1 | <0.001 | 0.683 |
Values are the means ± standard deviation (SD).
Norm, normoxia; PL, placebo; BR, beetroot; VO2, oxygen uptake; VCO2, carbon dioxide output; RER, respiratory gas exchange ratio; V E, ventilation; HR, heart rate; MAP, mean arterial pressure; LA, blood lactate concentrations; SpO2, arterial O2 saturation SkBF, skin blood flow in active muscle. Note that MAP was measured by the oscillometric method at rest.
P < 0.05 between placebo and beetroot.
P < 0.05 between normoxia and placebo.
Figure 2.
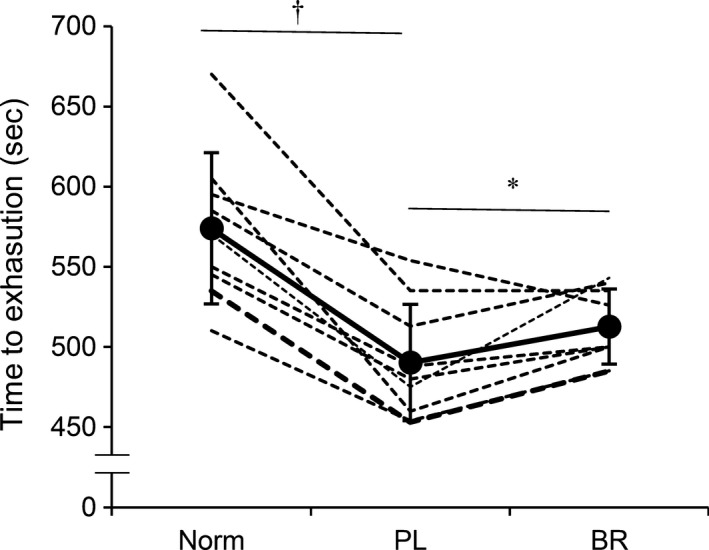
Time‐to‐exhaustion during maximal incremental exercise under normoxia (Norm), after placebo (PL) and beetroot (BR) supplementation. Dotted lines indicate an individual data, and the solid line indicates averaged values. Values are the means ± standard deviation (SD). *P < 0.05 between PL and BR, †P < 0.05 between Norm and PL.
Nitrate concentration and resting cardiorespiratory variables (PL vs. BR)
Nitrate supplementation (BR) significantly increased NO3 − concentration compared to PL (NO3 −37.2 ± 23.9 with PL vs. 220.0 ± 79.5 mmol/L with BR, P < 0.001), while BR had no observable effect on cardiorespiratory variables (all P > 0.05, Table 1).
Effects of nitrate supplementation during EXsub (PL vs. BR)
During EXsub, the work rate in the last 5 min was similar between PL and BR (105 ± 13 under PL vs. 105 ± 17 W under BR, P = 0.924). VCO2 with BR was significantly lower during EXsub compared to PL (P = 0.016, Table 1), while the other cardiorespiratory variables were unchanged during EXsub between BR and PL (all P > 0.05). Time course changes in HHb in active muscles (Fig. 3A) and StO2 in inactive muscles (Fig. 3B) for a typical single subject are shown. During EXsub, HHb in active muscles with BR appeared to be lower, while, StO2 in inactive muscles showed similar values. Figure 4 shows mean values of relative changes in each NIRS signal in active muscle during the last 5 min of EXsub. BR supplementation suppressed delta increases in HHb from the baseline during the last 5 min of EXsub (111.7 ± 33.7% with PL vs. 83.3 ± 19.9% with BR, P = 0.012). In contrast, there were no significant differences in the changes of HbO2, total Hb, and StO2 in active muscle between PL and BR (all P > 0.05). Mean values of the relative changes in each NIRS signal in inactive muscle during the last 5 min of EXsub are shown in Figure 5. There were no differences in all NIRS metrics between PL and BR during EXsub (all P > 0.05).
Effects of nitrate supplementation during EXmax
Nitrate supplementation (BR) significantly improved the time‐to‐exhaustion compared to PL (513 ± 24 in BR vs. 490 ± 39 sec in PL, P = 0.036, Fig. 2); no differences were observed in maximal work rate between BR (257 ± 22 W) and PL (233 ± 25 W, P = 0.133).
HRpeak was significantly higher (P = 0.031) and VO2peak was slightly but not significantly higher (P = 0.066) under the BR versus the PL condition during EXmax (Table 1). There were no statistically significant differences in other variables between PL and BR during EXmax (all P > 0.05, Table 1).
HHb in active muscles during EXmax seems to be slightly lower with BR (Fig. 3A) and StO2 in inactive muscles decreased greater with BR compared to PL (Fig. 3B). Mean values of the relative changes in each NIRS signal in active (Fig. 4) and inactive (Fig. 5) muscle during the last 30 sec at each work rate of EXmax are shown. In active muscle, HHb showed significantly lower values at the work rates of 90, 120 and 180 W with BR supplementation (~ 18% lower with BR, P < 0.05). In contrast, there were no significant differences in the changes of HbO2, total Hb, and StO2 in active muscle between PL and BR during EXmax (all P > 0.05). In inactive muscles, relative decreases in HbO2 and StO2 were significantly greater by ~7% in HbO2, and ~5% in StO2 with BR supplementation compared to PL at higher work rates (>150 W) with statistical differences between conditions (P < 0.05). No differences were observed in HHb and total Hb between PL and BR (all P > 0.05).
Discussion
This study is the first to investigate the effects of dietary nitrate supplementation on muscle oxygenation profiles between active and inactive muscles during hypoxic exercise. Our results indicate that, compared to PL, BR supplementation improved exercise tolerance, and reduced HHb in active muscles at moderate work rates. Moreover, reductions in tissue oxygenation in inactive muscles were greater during EXmax with BR supplementation than with PL.
Effects of nitrate supplementation during EXsub (PL vs. BR)
According to the Fick equation, oxygen uptake can be determined as a function of O2 delivery and O2 extraction, and HHb has been suggested to be an indicator of muscle O2 extraction (a‐v O2 difference) (DeLorey et al. 2003; Grassi et al. 2003). In this study, because HR was set as constant value of around 140 bpm during exercise, lower values in HHb in active muscles and/or lower stroke volume with BR may have produced lower VO2. However, a recent study demonstrated that BR supplementation did not change stroke volume during exercise (Hirai et al. 2017) even though different study settings between their study and our study, that is, heart failure patients versus healthy active subjects, and normoxia versus hypoxia. Thus, it may be still possible that lower VO2 values ~5% with BR compared to PL may relate to lower HHb. Indeed, similar low VO2 values of ~5%, were also observed in previous studies during hypoxic exercise (Masschelein et al. 2012; Kelly et al. 2014; Muggeridge et al. 2014) and lower HHb was also observed (Masschelein et al. 2012). These results may indicate that sufficient O2 delivery with nitrate supplementation, resulted in a lower rate of muscle O2 extraction. We also found lower VCO2 with BR supplementation. As it has been reported that increases in VCO2 accounted for the anaerobically‐generated excess‐CO2 (Kisaka et al. 2015), lower VCO2 might indicate no increases in anaerobic glycolysis to energy turnover with BR supplementation.
Effects of nitrate supplementation during EXmax (PL vs. BR)
In agreement with previous studies, we found that dietary nitrate supplementation significantly improved hypoxic exercise tolerance (Masschelein et al. 2012; Kelly et al. 2014). Other previous studies also demonstrated that nitrate supplementation significantly improved exercise tolerance under normoxia (Bailey et al. 2009, 2010; Lansley et al. 2011; Breese et al. 2013). In this study, BR supplementation suppressed the increases in HHb at moderate work rates compared to PL. Although we can only speculate, these results also suggested that muscle O2 extraction was restrained likewise during EXsub, perhaps, due to enhanced blood flow to active muscles with nitrate supplementation. In partly support this, total Hb was slightly higher with BR compared to PL though no statistical differences were observed. Because total Hb which was calculated by the sum of HbO2 and HHb, that is, blood volume, was correlated with changes in tissue blood flow (Van Beekvelt et al. 2001). While some authors have reported that a rightward shift (Bohr effect) in the oxyhemoglobin dissociation curve accelerates the rate of deoxygenation at or near lactate or ventilatory thresholds (Belardinelli et al. 1995; Grassi et al. 1999). The results of lower HHb at moderate work rate during EXmax appeared to have more muscle O2 extraction reserve, which resulted in the extension of time‐to‐exhaustion.
Contrary to our hypothesis, BR supplementation caused greater reductions in StO2 (P = 0.001) and HbO2 (P = 0.040) at exhaustion in inactive muscles compared to PL. Tissue oxygenation reflects the local balance between O2 supply and O2 consumption, and thus the decreased HbO2 and StO2 in inactive muscles may reflect a reduction in O2 supply to the inactive muscles under the assumption that O2 consumption would be constant in resting muscles (Ogata et al. 2002). Moreover, our results may indicate that sympathetic vasoconstriction was augmented by BR supplementation, as reductions in tissue oxygenation have been shown to be an indicator of sympathetic vasoconstriction with acute sympathetic stimulation during small muscle exercise (Hansen et al. 1996; Fadel et al. 2004; Horiuchi et al. 2014, 2015). With regard to whole body exercise, one also found that tissue oxygenation decreased at higher intensity exercise, for example, above anaerobic threshold, during incremental leg cycling (Ogata et al. 2007). Although direct and enough evidence may be required if reductions in tissue oxygenation in inactive muscles during whole body exercise is related to sympathetic vasoconstriction, Sheel et al. (2002) reported that inactive leg blood flow at rest was reduced when the fatiguing inspiratory muscle work was loaded. They suggested that the sympathetic vasoconstriction can be accounted for the decrease in the inactive leg blood flow during the inspiratory work because St Croix et al. (2000) found that the sympathetic nerve activity (SNA) was increased by the same inspiratory muscle work. It has also been reported that fatigue of the diaphragm occurs during whole body endurance exercise in excess of 85% of maximal oxygen uptake (Johnson et al. 1993). Together, the results in this study which showed greater reductions in HbO2 and StO2 in inactive muscles at higher exercise intensity with BR may relate to enhanced SNA‐induced vasoconstriction. Although we have no direct evidence to explain this, our recent study showed that ischemic preconditioning (IPC) caused a greater reduction in muscle oxygenation in resting muscles with acute sympathetic stimulation (cold pressor test). Moreover, the subjects with greater reduction in oxygenation in resting muscles showed greater increases in oxygenation in exercising muscles, suggesting that sympathetic vasoconstriction in resting muscles may have an important role to optimize exercise performance (Horiuchi et al. 2015). In addition, this IPC protocol could prevent exercise‐induced decrease in endothelial function, probably, due to enhanced nitric oxide bioavailability (Bailey et al. 2012). When relating such findings to our observations, benefits of experimental manipulation for increased NO bioavailability, that is, nitrate supplementation, might improve exercise performance via greater reductions in oxygenation in resting muscles. Indeed, greater reductions in oxygenation in even small inactive muscles (i.e., arm muscles) may cause higher peak VO2 during leg cycling (Yano et al. 2005), and a given increased in sympathetic nerve activity in inactive muscles may be required for optimal blood flow redistribution to active muscles for maintenance during knee extension exercise (Keller et al. 2003, 2004).
In addition, if increases in HR broadly reflect leg blood flow during cycling, as has been suggested for a knee‐extension exercise (MacPhee et al. 2005), higher HR with BR in this study may relate to effective blood redistributions to active muscles, perhaps, due to greater reductions in inactive muscle oxygenation, resulted in higher trend of VO2peak (Yano et al. 2005). However, we must acknowledge that we can only speculate this hypothesis. Future studies should be examined to further examine potential underlying mechanisms that could explain the impact of BR supplementation on hypoxic exercise performance.
Methodological considerations
Several limitations should be considered in the interpretation of our results. First, we must acknowledge the relatively small sample size. Based on our power analyses, we estimated that 9 subjects were necessary to achieve the appropriate statistical power for NIRS comparisons (HHb in active muscle during EXsub, StO2 in inactive muscle during EXmax), while 10–13 would have been required for time‐to‐exhaustion and cardiorespiratory variables (VCO2 during EXsub and HRpeak during EXmax) (G*Power 3.1). However, 8 of the 9 subjects showed an improvement in exercise tolerance (Fig. 2), and the statistical power was high enough for NIRS signals. Although future studies are needed, it is unlikely that such additional data would strongly affect our conclusion. Second, we cannot completely rule out the contamination of the NIRS signal by skin perfusion (Davis et al. 2006). To correct for this, we measured SkBF in the active muscles during exercise. As a result, no differences were observed in SkBF between PL and BR conditions. Moreover, there were no differences in MAP during exercise between the conditions. These results indicate that the effects of cutaneous circulation on NIRS signals were similar between the conditions. Third, we did not perform ischemia calibration to normalize the NIRS signals to evaluate maximal physiological changes; however, we found no differences in arbitrary unit values at baseline between PL and BR, moreover, our analysis has been conducted in our recent study (Horiuchi et al. 2016). In our preliminary test, arterial occlusion above 200 mmHg for 10–15 min caused strenuous pain and nausea for subset of subjects, possibly, due to after exhaustion under hypoxic exercise. Thus, we were not able to perform arterial occlusion method for ethical problems. In addition, a previous study demonstrated that muscle oxygenation and deoxygenation status at vastus lateralis during leg cycling showed similar values in comparison between modified beer lambert and time resolved spectroscopy method that can allow to assess absolute values (Saitoh et al. 2010).
Similarly, NIRS can represent only the balance between O2 delivery and utilization, not blood flow, and therefore it could not explain the actual blood flow redistribution between active and inactive skeletal muscles. While this may be one of the limitations to interpreting our results, NIRS also has several important advantages. For example, this technique provides continuous measurement of oxygen availability at the level of microcirculation, the part of the vascular tree most accessible to metabolic products of contraction (Mancini et al. 1994). In addition, optodes placement is sufficiently stable to acquire measurements even during high intensity dynamic exercise with large muscle groups. Finally, as we did not assess the effect of nitrate supplementation on normoxia trial, it remains unclear how nitrate supplementation affect muscle oxygenation status under normoixa. However, the main aim of this study is to investigate effects of dietary nitrate supplementation on exercise performance and muscle oxygenation status between active and inactive muscles under hypoxic exercise, and our results clearly demonstrated significant differences in these outcomes. Nonetheless, we must acknowledge that our results is limited under hypoxic exercise, and that future studies are required.
In conclusion, our results demonstrated that 4 days of dietary nitrate supplementation improved exercise tolerance during hypoxic exercise. Additionally, nitrate supplementation suppressed the increases of HHb in active muscles at moderate work rate. Meanwhile, accentuated reductions in HbO2 and StO2 were observed in inactive muscles at higher work rates during EXmax. Collectively, these findings indicate that short‐term dietary nitrate supplementation improved hypoxic exercise tolerance, perhaps, due to suppressed increases in HHb in active muscles at moderate work rates. Moreover, nitrate supplementation caused greater reductions in oxygenation in inactive muscle at higher work rates during hypoxic exercise.
Conflict of Interest
None declared.
Data Accessibility
Acknowledgments
We thank all the participants for their time and effort.
Horiuchi M., Endo J., Dobashi S., Handa Y., Kiuchi M., Koyama K.. Muscle oxygenation profiles between active and inactive muscles with nitrate supplementation under hypoxic exercise. Physiol Rep, 5 (20), 2017, e13475, https://doi.org/10.14814/phy2.13475
Funding Information
This study was supported in part by grant Japan Societry for the Promotion of Science JP. (No. 26440268 to M.H.).
References
- Ando, S. , Yamada Y., and Kokubu M.. 2010. Reaction time to peripheral visual stimuli during exercise under hypoxia. J. Appl. Physiol. 108:1210–1216. [DOI] [PubMed] [Google Scholar]
- Arnold, J. T. , Oliver S. J., Lewis‐Jones T. M., Wylie L. J., and Macdonald J. H.. 2015. Beetroot juice does not enhance altitude running performance in well‐trained athletes. Appl. Physiol. Nutr. Metab. 40:590–595. [DOI] [PubMed] [Google Scholar]
- Bailey, S. J. , Winyard P., Vanhatalo A., Blackwell J. R., Dimenna F. J., Wilkerson D. P., et al. 2009. Dietary nitrate supplementation reduces the O2 cost of low‐intensity exercise and enhances tolerance to high‐intensity exercise in humans. J. Appl. Physiol. 107:1144–1155. [DOI] [PubMed] [Google Scholar]
- Bailey, S. J. , Fulford J., Vanhatalo A., Winyard P. G., Blackwell J. R., DiMenna F. J., et al. 2010. Dietary nitrate supplementation enhances muscle contractile efficiency during knee‐extensor exercise in humans. J. Appl. Physiol. 109:135–148. [DOI] [PubMed] [Google Scholar]
- Bailey, T. G. , Birk G. K., Cable N. T., Atkinson G., Green D. J., Jones H., et al. 2012. Remote ischemic preconditioning prevents reduction in brachial artery flow‐mediated dilation after strenuous exercise. Am. J. Physiol. Heart Circ. Physiol. 303:H533–H538. [DOI] [PubMed] [Google Scholar]
- Belardinelli, R. , Barstow T. J., Porszasz J., and Wasserman K.. 1995. Changes in skeletal muscle oxygenation during incremental exercise measured with near infrared spectroscopy. Eur. J. Appl. Physiol. Occup. Physiol. 70:487–492. [DOI] [PubMed] [Google Scholar]
- Bourdillon, N. , Fan J. L., Uva B., Muller H., Meyer P., and Kayser B.. 2015. Effect of oral nitrate supplementation on pulmonary hemodynamics during exercise and time trial performance in normoxia and hypoxia: a randomized controlled trial. Front. Physiol. 6:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese, B. C. , McNarry M. A., Marwood S., Blackwell J. R., Bailey S. J., and Jones A. M.. 2013. Beetroot juice supplementation speeds O2 uptake kinetics and improves exercise tolerance during severe‐intensity exercise initiated from an elevated metabolic rate. Am. J. Physiol. Regul. Integr. Comp. Physiol. 305:R1441–R1450. [DOI] [PubMed] [Google Scholar]
- Carriker, C. R. , Mermier C. M., McLain T. A., Johnson K. E., Beltz N. M., Vaughan R. A., et al. 2016. Effect of acute dietary nitrate consumption on oxygen consumption during submaximal exercise in hypobaric hypoxia. Int J Sport Nutr Exerc. Metab. 26:315–322. [DOI] [PubMed] [Google Scholar]
- Casey, D. P. , Madery B. D., Curry T. B., Eisenach J. H., Wilkins B. W., and Joyner M. J.. 2010. Nitric oxide contributes to the augmented vasodilatation during hypoxic exercise. J. Physiol. 588:373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, S. L. , Fadel P. J., Cui J., Thomas G. D., and Crandall C. G.. 2006. Skin blood flow influences near‐infrared spectroscopy‐derived measurements of tissue oxygenation during heat stress. J. Appl. Physiol. 100:221–224. [DOI] [PubMed] [Google Scholar]
- DeLorey, D. S. , Kowalchuk J. M., and Paterson D. H.. 2003. Relationship between pulmonary O2 uptake kinetics and muscle deoxygenation during moderate‐intensity exercise. J. Appl. Physiol. 95:113–120. [DOI] [PubMed] [Google Scholar]
- Dempsey, J. A. , and Foster H. V.. 1982. Mediation of ventilatory adaptations. Physiol. Rev. 62:262–346. [DOI] [PubMed] [Google Scholar]
- Dobashi, S. , Horiuchi M., Endo J., Kiuchi M., and Koyama K.. 2016. Cognitive function and cerebral oxygenation during prolonged exercise under hypoxia in healthy young males. High Alt. Med. Biol. 17:214–221. [DOI] [PubMed] [Google Scholar]
- Easton, P. A. , Slykerman L. J., and Anthonisen N. R.. 1986. Ventilatory response to sustained hypoxia in normal adults. J. Appl. Physiol. 61:906–911. [DOI] [PubMed] [Google Scholar]
- Fadel, P. J. , Keller D. M., Watanabe H., Raven P. B., and Thomas G. D.. 2004. Noninvasive assessment of sympathetic vasoconstriction in human and rodent skeletal muscle using near‐infrared spectroscopy and Doppler ultrasound. J. Appl. Physiol. 96:1323–1330. [DOI] [PubMed] [Google Scholar]
- Govoni, M. , Jansson E. A., Weitzberg E., and Lundberg J. O.. 2008. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide 19:333–337. [DOI] [PubMed] [Google Scholar]
- Grassi, B. , Quaresima V., Marconi C., Ferrari M., and P., Cerretelli . 1999. Blood lactate accumulation and muscle deoxygenation during incremental exercise. J. Appl. Physiol. 87:348–355. [DOI] [PubMed] [Google Scholar]
- Grassi, B. , Pogliaghi S., Rampichini S., Quaresima V., Ferrari M., Marconi C., et al. 2003. Muscle oxygenation and pulmonary gas exchange kinetics during cycling exercise on‐transitions in humans. J. Appl. Physiol. 95:149–158. [DOI] [PubMed] [Google Scholar]
- Hansen, J. , Thomas G. D., Harris S. A., Parsons W. J., and Victor R. G.. 1996. Differential sympathetic neural control of oxygenation in resting and exercising human skeletal muscle. J. Clin. Invest. 98:584–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, J. , Sander M., Hald C. F., Victor R. G., and Thomas G. D.. 2000. Metabolic modulation of sympathetic vasoconstriction in human skeletal muscle: role of tissue hypoxia. J. Physiol. 527:387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai, D. M. , Zelt J. T., Jones J. H., Castanhas L. G., Bentley R. F., Earle W., et al. 2017. Dietary nitrate supplementation and exercise tolerance in patients with heart failure with reduced ejection fraction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 312:R13–R22. [DOI] [PubMed] [Google Scholar]
- Hord, N. G. , Tang Y., and Bryan N. S.. 2009. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am. J. Clin. Nutr. 90:1–10. [DOI] [PubMed] [Google Scholar]
- Horiuchi, M. , Fadel P. J., and Ogoh S.. 2014. Differential effect of sympathetic activation on tissue oxygenation in gastrocnemius and soleus muscles during exercise in humans. Exp. Physiol. 99:348–358. [DOI] [PubMed] [Google Scholar]
- Horiuchi, M. , Endo J., and Thijssen D. H.. 2015. Impact of ischemic preconditioning on functional sympatholysis during handgrip exercise in humans. Physiol. Rep. 3:e12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi, M. , Handa Y., Abe D., and Fukuoka Y.. 2016. Walking economy at simulated high altitude in human healthy young male lowlanders. Biol. Open 5:1408–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, B. D. , Babcock M. A., O. E., Suman , and J. A., Dempsey . 1993. Exercise‐induced diaphragmatic fatigue in healthy humans. J. Physiol. 460:385–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, D. M. , Wasmund W. L., Wray D. W., Ogoh S., Fadel P. J., Smith M. L., et al. 2003. Carotid baroreflex control of leg vascular conductance at rest and during exercise. J. Appl. Physiol. 94:542–548. [DOI] [PubMed] [Google Scholar]
- Keller, D. M. , Fadel P. J., Ogoh S., Brothers R. M., Hawkins M., Olivencia‐Yurvati A., et al. 2004. Carotid baroreflex control of leg vasculature in exercising and non‐exercising skeletal muscle in humans. J. Physiol. 561:283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, J. , Vanhatalo A., Bailey S. J., Wylie L. J., Tucker C., List S., et al. 2014. Dietary nitrate supplementation: effects on plasma nitrite and pulmonary O2 uptake dynamics during exercise in hypoxia and normoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 307:R920–R930. [DOI] [PubMed] [Google Scholar]
- Kisaka, T. , Cox T. A., Dumitrescu D., and Wasserman K.. 2015. CO2 pulse and acid‐base status during increasing work rate exercise in health and disease. Respir. Physiol. Neurobiol. 218:46–56. [DOI] [PubMed] [Google Scholar]
- Koga, S. , Poole D. C., Ferreira L. F., Whipp B. J., Kondo N., Saitoh T., et al. 2007. Spatial heterogeneity of quadriceps muscle deoxygenation kinetics during cycle exercise. J. Appl. Physiol. 103:2049–2056. [DOI] [PubMed] [Google Scholar]
- Komiyama, T. , Sudo M., Higaki Y., Kiyonaga A., Tanaka H., and Ando S.. 2015. Does moderate hypoxia alter working memory and executive function during prolonged exercise? Physiol. Behav. 139:290–296. [DOI] [PubMed] [Google Scholar]
- Lansley, K. E. , Winyard P. G., Bailey S. J., Vanhatalo A., Wilkerson D. P., Blackwell J. R., et al. 2011. Acute dietary nitrate supplementation improves cycling time trial performance. Med. Sci. Sports Exerc. 43:1125–1131. [DOI] [PubMed] [Google Scholar]
- Lundberg, J. O. , and Weitzberg E.. 2010. NO‐synthase independent NO generation in mammals. Biochem. Biophys. Res. Commun. 396:39–45. [DOI] [PubMed] [Google Scholar]
- MacLeod, K. E. , Nugent S. F., Barr S. I., Koehle M. S., Sporer B. C., and MacInnis M. J.. 2015. Acute beetroot juice supplementation does not improve cycling performance in normoxia or moderate hypoxia. Int J Sport Nutr Exerc. Metab. 25:359–366. [DOI] [PubMed] [Google Scholar]
- MacPhee, S. L. , Shoemaker J. K., Paterson D. H., and Kowalchuk J. M.. 2005. Kinetics of O2 uptake, leg blood flow, and muscle deoxygenation are slowed in the upper compared with lower region of the moderate‐intensity exercise domain. J. Appl. Physiol. 99:1822–1834. [DOI] [PubMed] [Google Scholar]
- Mancini, D. M. , Bolinger L., Li H., Kendrick K., Chance B., and Wilson J. R.. 1994. Validation of near‐infrared spectroscopy in humans. J. Appl. Physiol. 77:2740–2747. [DOI] [PubMed] [Google Scholar]
- Masschelein, E. , Van Thienen R., Wang X., Van Schepdael A., Thomis M., and Hespel P.. 2012. Dietary nitrate improves muscle but not cerebral oxygenation status during exercise in hypoxia. J. Appl. Physiol. 113:736–745. [DOI] [PubMed] [Google Scholar]
- Mizuno, M. , Iwamoto G. A., Vongpatanasin W., Mitchell J. H., and Smith S. A.. 2014. Exercise training improves functional sympatholysis in spontaneously hypertensive rats through a nitric oxide‐dependent mechanism. Am. J. Physiol. Heart Circ. Physiol. 307:H242–H251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muggeridge, D. J. , Howe C. C., Spendiff O., Pedlar C., James P. E., and Easton C.. 2014. A single dose of beetroot juice enhances cycling performance in simulated altitude. Med. Sci. Sports Exerc. 46:143–150. [DOI] [PubMed] [Google Scholar]
- Nyback, L. , Glannerud C., Larsson G., Weitzberg E., Shannon O. M., and McGawley K.. 2017. Physiological and performance effects of nitrate supplementation during roller‐sking in normoxia and normobaric hypoxia. Nitric Oxide 70:1–8. [DOI] [PubMed] [Google Scholar]
- Ogata, H. , Yunoki T., and Yano T.. 2002. Effect of arm cranking on the NIRS‐determined blood volume and oxygenation of human inactive and exercising vastus lateralis muscle. Eur. J. Appl. Physiol. 86:191–195. [DOI] [PubMed] [Google Scholar]
- Ogata, H. , Arimitsu T., Matsuura R., Yunoki T., Horiuchi M., and Yano T.. 2007. Relationship between oxygenation in inactive biceps brachii muscle and hyperventilation during leg cycling. Physiol. Res. 56:57–65. [DOI] [PubMed] [Google Scholar]
- Ogata, H. , Akai M., and Nakazawa K.. 2008. Metaboreceptor‐mediated muscle oxygen saturation during recovery following isometric handgrip exercise. J. Physiol. Anthropol. 27:83–91. [DOI] [PubMed] [Google Scholar]
- Ogoh, S. , Tsukamoto H., Hirasawa A., Hasegawa H., Hirose N., and Hashimoto T.. 2014. The effect of changes in cerebral blood flow on cognitive function during exercise. Physiol. Rep. 2:e12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson, M. S. , Chance B., and Wilson B. C.. 1989. Time resolved reflectance and transmittance for the non‐invasive measurement of tissue optical properties. Appl. Opt. 28:2331–2336. [DOI] [PubMed] [Google Scholar]
- Remensnyder, J. P. , Mitchell J. H., and Sarnoff S. J.. 1962. Functional sympatholysis during muscular activity. Observations on influence of carotid sinus on oxygen uptake. Circ. Res. 11:370–380. [DOI] [PubMed] [Google Scholar]
- Rowell, L. B. 1993. Human Cardiovascular Control. Oxford University Press, NY. [Google Scholar]
- Saitoh, T. , Ooue A., Kondo N., Niizeki K., and Koga S.. 2010. Active muscle oxygenation dynamics measured during high‐intensity exercise by using two near‐infrared spectroscopy methods. Adv. Exp. Med. Biol. 662:225–230. [DOI] [PubMed] [Google Scholar]
- Shannon, O. M. , Duckworth L., Barlow M., Woods D., Lara J., Siervo M., et al. 2016. Dietary nitrate supplementation enhances high‐intensity running performance in moderate normobaric hypoxia, independedn of arobic fitness. Nitric Oxide 59:63–70. [DOI] [PubMed] [Google Scholar]
- Shannon, O. M. , Duckworth L., Barlow M. J., Deighton K., Matu J., Williams E. L., et al. 2017. Effects of dietary nitrate supplementation on physiological responses, cognitive function, and exercise performance at moderate and very‐high simulated alttiude. Front. Physiol. 8:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheel, A. W. , Derchak P. A., Pegelow D. F., and Dempsey J. A.. 2002. Threshold effects of respiratory muscle work on limb vascular resistance. Am. J. Physiol. Heart Circ. Physiol. 282:H1732–H1738. [DOI] [PubMed] [Google Scholar]
- St Croix, C. M. , Morgan B. J., Wetter T. J., and Dempsey J. A.. 2000. Fatiguing inspiratory muscle work causes reflex sympathetic activation in humans. J. Physiol. 529:493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beekvelt, M. C. , Colier W. N., Wevers R. A., and Van Engelen B. G.. 2001. Performance of near‐infrared spectroscopy in measuring local O2 consumption and blood flow in skeletal muscle. J. Appl. Physiol. 90:511–519. [DOI] [PubMed] [Google Scholar]
- Van der Zee, P. , Cope M., Arridge S. R., Essenpreis M., Potter L. A., Edwards A. D., et al. 1992. Experimentally measured optical pathlengths for the adult head, calf and forearm and the head of the newborn infant as a function of inter optode spacing. Adv. Exp. Med. Biol. 316:143–153. [DOI] [PubMed] [Google Scholar]
- Vanhatalo, A. , Fulford J., Bailey S. J., Blackwell J. R., Winyard P. G., and Jones A. M.. 2011. Dietary nitrate reduces muscle metabolic perturbation and improves exercise tolerance in hypoxia. J. Physiol. 589:5517–5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhatalo, A. , Jones A. M., Blackwell J. R., Winyard P. G., and Fulford J.. 2014. Dietary nitrate accelerates postexercise muscle metabolic recovery and O2 delivery in hypoxia. J. Appl. Physiol. 117:1460–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie, L. J. , Kelly J., Bailey S. J., Blackwell J. R., Skiba P. F., Winyard P. G., et al. 2013. Beetroot juice and exercise: pharmacodynamic and dose‐response relationships. J. Appl. Physiol. 115:325–336. [DOI] [PubMed] [Google Scholar]
- Yano, T. , Horiuchi M., Yunoki T., Matsuura R., and Ogata H.. 2005. Relationship between maximal oxygen uptake and oxygenation level in inactive muscle at exhaustion in incremental exercise in humans. Physiol. Res. 54:679–685. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


