Abstract
In damaged kidneys, increased extracellular matrix (ECM) and tissue stiffness stimulate kidney fibrosis through incompletely characterized molecular mechanisms. The transcriptional coactivators yes-associated protein (Yap) and transcriptional coactivator with PDZ-binding motif (Taz) function as mechanosensors in cancer cells and have been implicated in the regulation of myofibroblasts in the kidney. We hypothesized that the development of kidney fibrosis depends on Yap-induced activation and proliferation of kidney fibroblasts. In mice, Yap expression increased in renal fibroblasts after unilateral ureteral obstruction (UUO), in association with worsening of interstitial fibrosis. In cultured fibroblasts, inhibition of Yap/Taz signaling blocked TGF-β1–induced fibroblast-to-myofibroblast transformation and ECM production, whereas constitutive activation of Yap promoted fibroblast transformation and ECM production even in the absence of TGF-β1. Moreover, in the absence of TGF-β1, fibroblasts seeded on a stiffened ECM transformed into myofibroblasts in a process dependent on the activation of Yap. In mice with UUO, the Yap inhibitor verteporfin reduced interstitial fibrosis. Furthermore, Gli1+ cell-specific knockout of Yap/Taz in mice suppressed UUO-induced ECM deposition, myofibroblast accumulation, and interstitial fibrosis. In a UUO-release model, induction of Gli1+ cell-specific Yap/Taz knockout partially reversed the development of interstitial fibrosis. Thus, in the kidney, Yap is a tissue mechanosensor that can be activated by ECM and transforms fibroblasts into myofibroblasts; the interaction of Yap/Taz and ECM forms a feed-forward loop resulting in kidney fibrosis. Identifying mechanisms that interrupt this profibrotic cycle could lead to the development of anti-fibrosis therapy.
Keywords: fibrosis, obstructive nephropathy, stiffness, YAP
The development of interstitial fibrosis in the kidney is linked to progressive kidney damage and CKD.1,2 Hallmarks of interstitial fibrosis in the kidney include proliferation of myofibroblasts and excessive deposition of extracellular matrix (ECM).3 Although these changes have been considered to be a protective response to maintain tissue integrity, the presence of fibrosis markedly depresses the regenerative potential of the kidney and leads to a decline in kidney function.4 A key step in the development of tissue fibrosis is sustained activation of myofibroblasts, but the source of these cells is controversial: suggested origins include the bone marrow, activated interstitial fibroblasts, or fibroblasts that result from Epithelial-Mesenchymal Transition or from Endothelial-Mesenchymal Transition.5–8 The pathways activating myofibroblasts are also unsettled; multiple signaling pathways have been proposed to be activators of myofibroblasts. Proposed signaling processes include pathways arising from TGF-β1, Wnt, BMP, and/or Notch.9–11 Alternatively, myofibroblasts can be activated by mechanical forces as reportedly occurs in skin and mucosal wounds or after injury to heart, lung, liver, and kidney tissues.3 For example, it has been reported that stiffness of the ECM increases Yap expression, forming a feed-forward loop which leads to excessive deposition of ECM.12–14 Other investigators report that this loop is present in bleomycin-induced fibrosis in the lungs.14 It remains unclear how tissue stiffness in the damaged kidney activates myofibroblasts and the origin of a sensor that recognizes excess ECM to stimulate the feed-forward signaling pathway has not been identified.
The components of a Yap/Taz signaling pathway initiated by Hippo relay signals from events on the plasma membrane into the nucleus.15 The core of the Hippo signaling pathway includes the serine/threonine kinases MST1, MST2, LATS1 and LATS2, the scaffolding protein Salvador homolog 1 (SAV1), other scaffolding proteins, and the MOB kinase activator 1A (MOB1A).16 Targets of Hippo include Yap and Taz.17 These coactivators are uncovered in mammary epithelial cells and found to be unique mechanosensors when TEAD1 and TEAD4 bind to a specific domain in Yap and Taz. In cancer cells, the mechanosensor, Yap, reportedly functions as a fibroblast activator, and the investigators note that Yap mechanically promotes a feed-forward loop.13 In another study, persistent activation of Yap in cancer cells induces ECM production and facilitates transformation of myofibroblasts.18 The Hippo/SAV1 pathway regulates renal tubulointerstitial fibrosis through Taz-induced expression of TGF-β1 and TGF-β receptor II.19 Increased Yap expression stimulates myofibroblast accumulation after AKI20 and promotes myofibroblast formation during kidney development.21 A recent report showed that verteporfin,22 a small molecule that inhibits TEAD-Yap interactions and Yap signals, suppresses renal fibrosis.23
In this study, we explored whether Yap/Taz affect the development and progression of interstitial fibrosis in the mouse model of progressive tubulointerstitial fibrosis induced by unilateral ureteral obstruction (UUO). Our results show that Yap/Taz activation leads to ECM deposition which contributes to increased ECM stiffness. In a model of progressive kidney fibrosis, we provide evidence for a feed-forward loop consisting of Yap to ECM production and ECM to Yap activation.
Results
In Kidneys Damaged by UUO, Yap Is Expressed in Fibroblasts
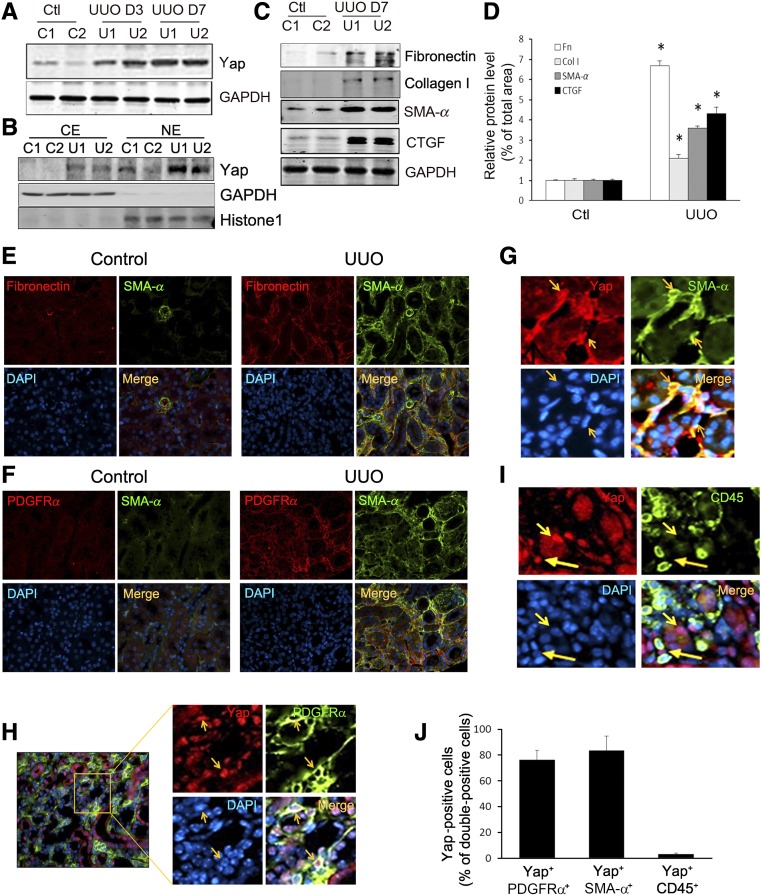
In mice with kidney damage induced by UUO, Yap expression increased significantly and was present as early as 3 days after UUO and even more pronounced at day 7 (Figure 1A). Yap upregulation was specific for the injured kidneys because there was only limited expression of Yap in contralateral kidneys (Figure 1A). Because activated Yap plays its protranscriptional function in the nucleus, we isolated cytoplasmic and nuclear proteins from kidneys and found that UUO increased Yap expression and most of the Yap was located in the nucleus (Figure 1B). Western blot analysis revealed increased accumulation of ECM, fibronectin, and collagen I; as well as expression of SMA-α and CTGF (targets of Yap) in UUO kidneys (Figure 1, C and D). Double immunostaining analysis indicated that UUO increased the accumulation of SMA-α–positive tubulointerstitial myofibroblasts and most of the SMA-α+ myofibroblasts costained with fibronectin and PDGFRα (Figure 1, E and F). The PDGFRα-expressing cells colocalized with another commonly used myofibroblast marker, PDGFRβ, positive cells in different areas (e.g., tubulointerstitial area in cortex and in medulla as well as in perivascular area) in obstructed kidneys (Supplemental Figure 1). We also examined which type of cells expressed nuclear Yap and found that nuclear Yap colocalized with cells that were positive for SMA-α (Figure 1G) and PDGFRα in the interstitium (Figure 1H). Yap is also located in the cytoplasm and nucleus of tubule cells (Figure 1, G and H). We did not find cells double-positive for CD45/Yap in UUO kidneys (Figure 1I). Notably, >80% of PDGFRα- or SMA-α–positive cells were Yap-positive but few CD45+ cells were found to express Yap (Figure 1J).
Figure 1.
Activated Yap colocalizes with interstitial cells in UUO kidneys. (A) UUO stimulates Yap expression. (B) UUO induces Yap nuclear translocation. After 7 days of UUO, the cytoplasmic and nuclear proteins were prepared and the levels of Yap in kidneys were detected by western blot analysis. GAPDH and histone 1 were used as loading controls for cytoplasm and nuclear, respectively. (C and D) UUO stimulates myofibroblast accumulation and ECM protein deposition. The myofibroblast marker (SMA-α) and ECM proteins were determined by western blot (C) with density analysis shown (D). (E and F) Double immunofluorescent staining of SMA-α with fibronectin (E) or PDGFRα (F) in control and obstructed kidneys after 7 days of UUO. (G and H) Double immunofluorescent staining of Yap in obstructed kidneys with myofibroblast marker SMA-α (G), PDGFRα (H), and inflammatory monocyte marker CD45 (I), respectively (arrowhead indicates Yap staining in interstitial cells and tubules). (J) The percentage of Yap-positive cells in the indicated double-positive cells was counted and calculated. *P<0.05 versus control; n=5. CE, cytoplasmic extract; NE, nuclear extract.
Yap Activation Induces Myofibroblast Differentiation and ECM Production
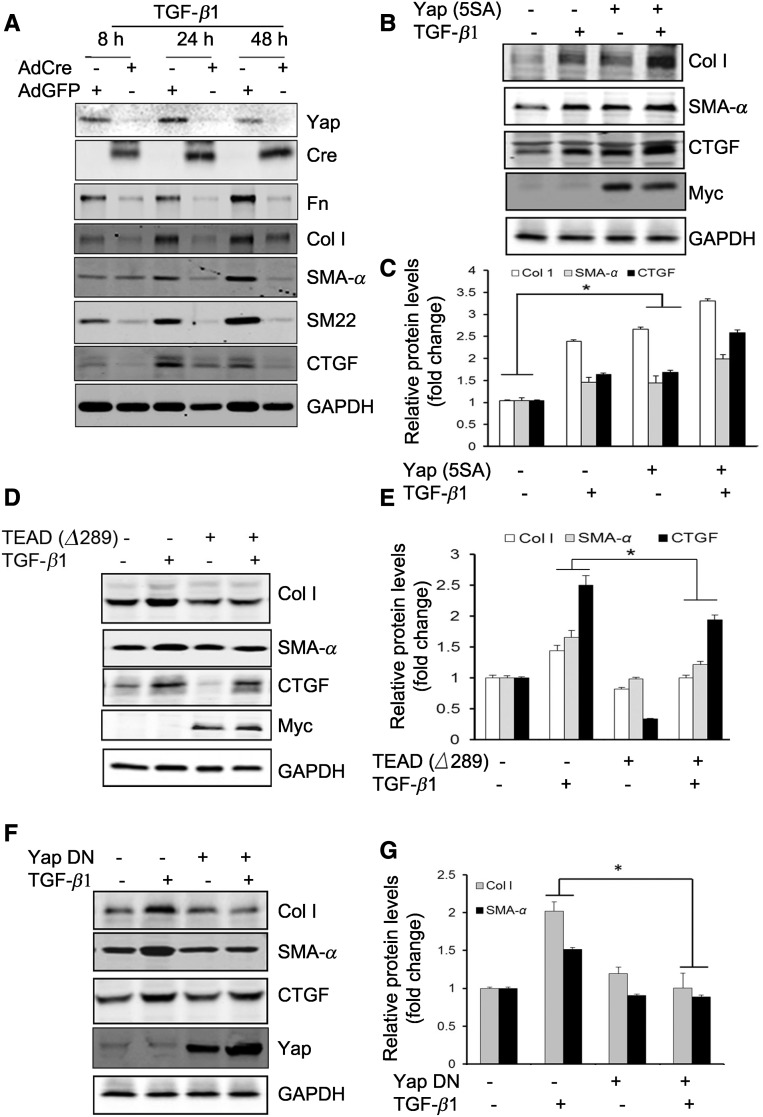
The differentiation of fibroblasts into myofibroblasts is intimately involved in the development of renal interstitial fibrosis.3 TGF-β1 is a potent stimulus of myofibroblast activation, and we found that the UUO stimulated TGF-β1 expression in the tubule cells (Supplemental Figure 2). Because convergence of Yap/TGF-β1 signaling has been proposed for the development of tissue fibrosis,14 we investigated the role of Yap in myofibroblast activation in the kidney. Firstly, we isolated mouse kidney fibroblasts from Yapf/f/Tazf/f transgenic mice. The isolated fibroblasts were characterized by different cell markers; these cells are positive for fibroblast markers (e.g., vimentin, SM-22, N-cadherin, and collagen I), but negative for markers for tubule cells (E-cadherin), endothelial cells (CD31), and vascular smooth muscle cells (SMMHC, smooth muscle myosin heavy chain) (Supplemental Figure 3). Yap and Taz in the isolated fibroblasts were knocked out by infection with Adenovirus-Cre (Figure 2A). TGF-β1 induced fibroblast activation and the expression of ECM proteins (fibronectin and collagen I) and myofibroblast markers (SMA-α and SM22), and these TGF-β1–induced responses were blocked in cells infected with Adenovirus-Cre (Figure 2A). These results indicate that KO of Yap/Taz dramatically inhibited TGF-β1–mediated profibrogenic responses. Secondly, we found that fibroblasts expressing constitutively active Yap (5SA) promoted fibroblast transformation, increased expression of ECM proteins, and SMA-α independent of TGF-β1, suggesting that Yap activation is sufficient to induce fibroblasts/myofibroblast transformation and activation even in the absence of TGF-β1 (Figure 2, B and C). We found that inhibition of Yap by dominant negative TEAD (TEAD ∆289) in fibroblasts blocked TGF-β1–induced expression of ECM proteins and SMA-α (Figure 2, D and E). As expected, the expression of cyclin D1 and production of CTGF induced by TGF-β1 were inhibited by TEAD (∆289) (Supplemental Figure 4, A and B). Overexpression of TEAD (∆289) inhibited Yap (5SA)-induced CTGF production, indicating that TEAD mediates Yap downstream signals (Supplemental Figure 4C). Thirdly, to further test the role of Yap/TEAD signaling in TGF-β1–stimulated fibrogenic responses, mouse fibroblasts were infected with lentivirus-Yap1 (∆60–89), which lacks TEAD binding domain.24 We found that overexpression of Yap1 (∆60–89) suppressed TGF-β1–induced expression of SMA-α and collagen I (Figure 2, F and G). Moreover, fibroblasts overexpressing Yap had increased expression of SMA-α filaments, whereas overexpression of TEAD (∆289) inhibited TGF-β1–induced SMA-α expression (Supplemental Figure 5). Thus, Yap is required for the activation of myofibroblasts.
Figure 2.
Yap mediates TGF-β1–induced fibroblast activation. (A) Mouse fibroblasts were isolated from Yapf/f/Tazf/f mice. The cells were infected with Adeno-Cre; the Adeno-GFP was used as control. After 48 hours, the cells were serum starved and treated with TGF-β1 (2 ng/ml), cell lysates were collected at the indicated time points, and the levels of indicated molecules were determined by western blots. (B and C) Mouse fibroblasts expressing constitutive active Yap (5SA) promoted fibroblast transformation into myofibroblasts. Density analysis of the western blots is shown (C). (D and E) Mouse fibroblasts overexpressing dominant negative TEAD (Δ289) suppressed the expression of collagen I, SMA-α, and Yap target CTGF (D). The corresponding density analyses of the western blots are shown (E). *P<0.05 versus TGF-β1–treated group; n=3 repeats. (F) Overexpression of dominant negative Yap (Δ60–89) suppressed TGF-β1–induced myofibroblast activation. The density analysis of the levels of collagen I and SMA-α are shown in (G). *P<0.05; n=3 repeats. AdCre, adenovirus overexpressing Cre; AdGFP, adenovirus overexpressing GFP; DN, dominant negative.
ECM Stiffness Activates Yap to Promote Fibroblast Activation
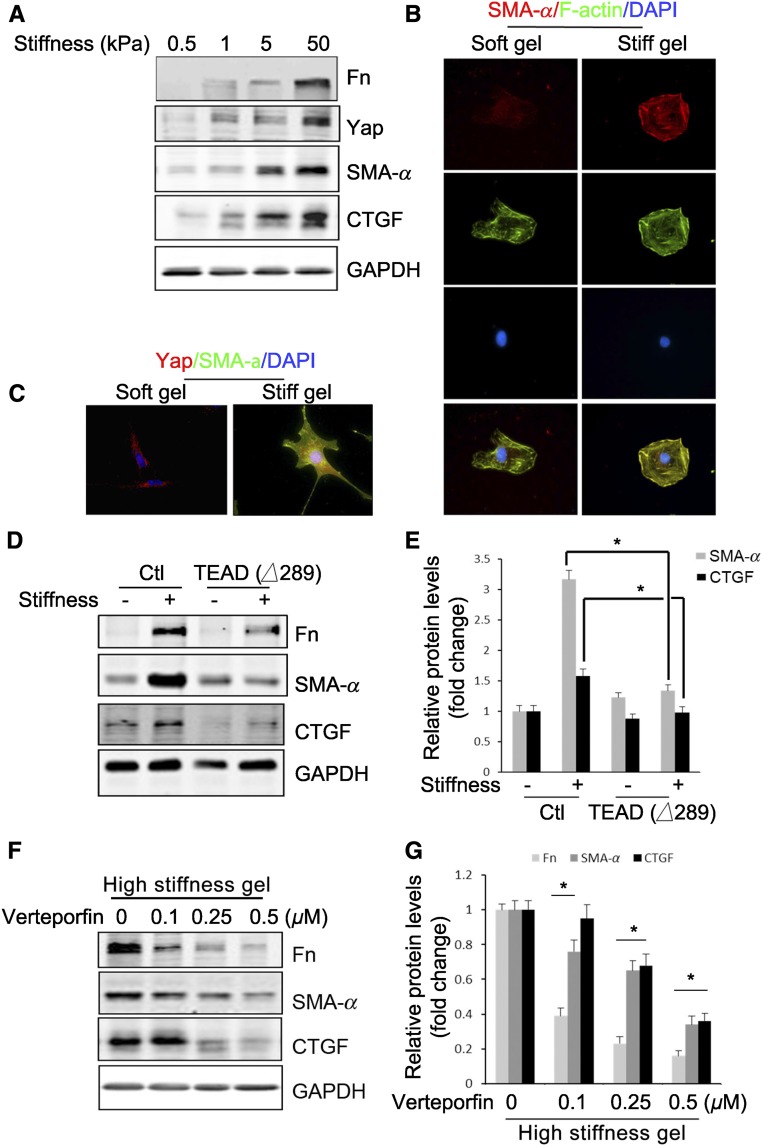
The stiffness of healthy lung or kidney tissue is in the approximately 5 kPa range.25,26 Fibroblasts cultured in hydrogels (approximately 20 kPa) are resistant to TGF-β1–induced fibroblast activation.27 We tested the stiffness on fibroblast activation.25,26 Fibroblasts were cultured on different matrices and we found that the expression of Yap and its downstream target CTGF were increased proportionately to the stiffness with a maximum effect at 50 kPa (Figure 3A). An increase in matrix stiffness induced expression of fibronectin and SMA-α (Figure 3A). Cultured fibroblasts also exhibited stiffness-dependent spreading, documented by stress fiber formation and flattening of cells that became positive for SMA-α, whereas cells on soft gel showed disorganized stress fiber formation and were negative for SMA-α (Figure 3B). Notably, cells cultured in gels with greater stiffness revealed localization of Yap to the nucleus in conjunction with the expression of myofibroblast marker (SMA-α) (Figure 3C). In contrast, fibroblasts expressing TEAD (Δ289) had reduced stiffness-induced expression of ECM protein, SMA-α, and CTGF (Figure 3, D and E). Similarly, pretreatment of cells with verteporfin, a Yap inhibitor, specifically inhibits Yap (5SA)-induced CTGF production (Supplemental Figure 6A). Cells cultured on a high stiffness gel exhibited activation of fibroblasts (expression of fibronectin, SMA-α, and CTGF), and these responses were inhibited by verteporfin (Figure 3, F and G). Verteporfin at doses of 0.1–0.5 µM, which inhibited Yap activation, did not cause cell apoptosis, and activated caspase 3 could only be detected at verteporfin doses >2 µM for 24 hours (Supplemental Figure 6B). Thus, matrix stiffness can activate fibroblasts in a Yap-dependent fashion.
Figure 3.
Matrix stiffness stimulates Yap-dependent fibroblast activation. (A–C) Stiff gel induces Yap expression and fibroblast transformation. Fibroblasts were incubated on polyacrylamide gels of varied stiffness but conjugated with the same collagen concentration on the top surface. This creates a microenvironment in which the mechanical properties are uncoupled from the biochemical properties. The expression of Yap and myofibroblast markers were detected by western blot (A), and the stress fiber formation (B) and Yap localization (C) and their costaining with SMA-α were determined by immunofluorescent staining (C). (D and E) Inhibition of Yap signaling suppresses stiffness-induced fibroblast activation. Control or fibroblasts expressing TEAD (Δ289) were seeded on soft or high-stiffness gel for 24 hours, the expression of myofibroblast markers were detected by Western blot (D), and the density was analyzed (E) (P<0.05, compared with stiffness-treated control group). (F and G) Fibroblasts on high-stiffness gel of 50 kPa were incubated with or without varied concentrations of verteporfin for 24 hours, the expression of myofibroblast markers was detected by western blot (F), and the density was analyzed (G). Data were presented from two repeated experiments. P<0.05, compared with control.
Yap Inhibition Suppresses UUO-Induced Fibrosis
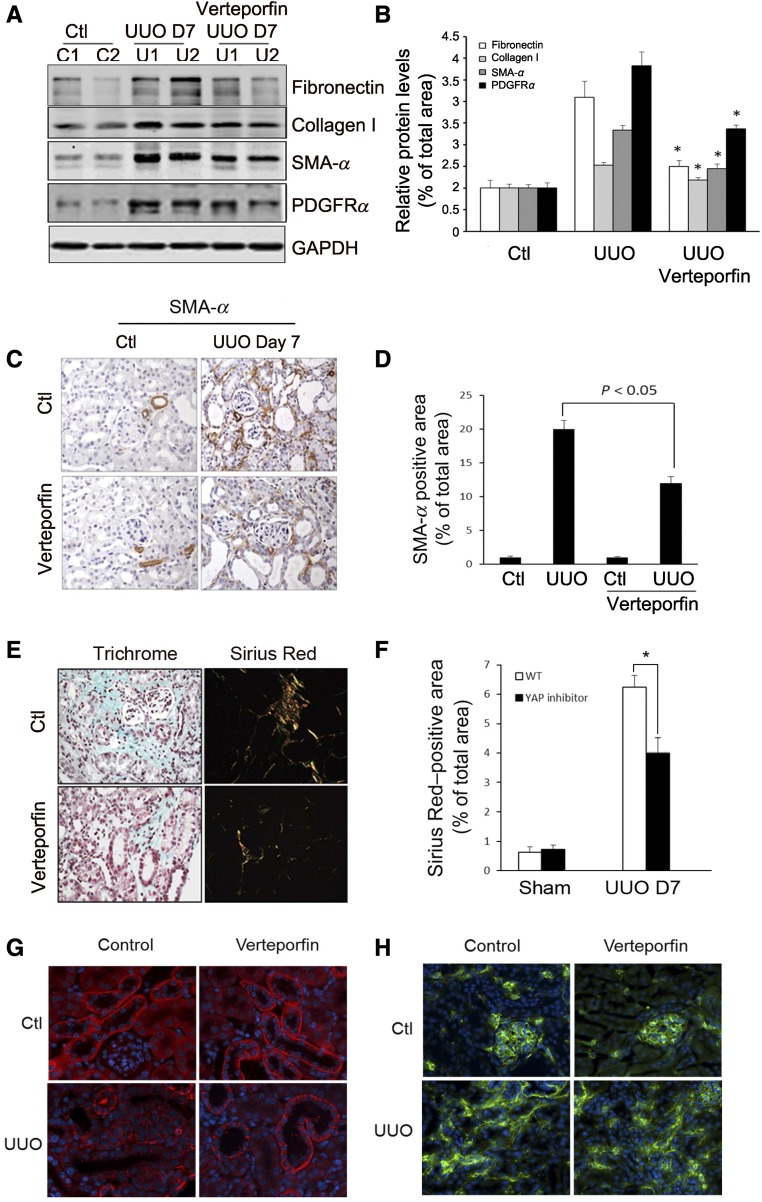
To determine whether Yap inhibition ameliorates fibroblast activation in vivo, we used Yap inhibitor verteporfin in a mouse UUO model. The expression of ECM proteins (fibronectin and collagen I) and fibroblast markers (SMA-α and PDGFRα) was partially attenuated by verteporfin treatment (Figure 4, A and B). Immunostaining revealed decreased numbers of SMA-α–positive cells in obstructed kidneys after treatment with verteporfin (Figure 4, C and D). Verteporfin also protected from UUO-induced ECM deposition and fibrosis (data for day 7 after UUO; Figure 4, E and F). There were no significant alterations in apoptotic cells (TUNEL assay) in obstructed kidneys from both control and verteporfin-treated mice (Supplemental Figure 7). To determine whether verteporfin treatment affected the vasculature and tubule cells, we tested for the expression of VE-cadherin (endothelial cell marker) and E-cadherin (tubule cell marker). UUO changed E-cadherin distribution from basal membrane to lateral membrane; verteporfin treatment attenuated E-cadherin translocation and was associated with a higher level of E-cadherin expression after UUO (Figure 4G). There were no significant changes in the levels of UUO-induced VE-cadherin between the two groups (Figure 4H).
Figure 4.
Yap inhibitor suppresses UUO-induced kidney fibrosis. (A and B) Yap inhibitor suppresses UUO-induced myofibroblast accumulation. Mice with UUO were treated with DMSO or verteporfin (100 mg/kg body wt, i.p. every other day for four times). Markers of myofibroblasts, SMA-α and PDGFRα, and ECM proteins, fibronectin, and collagen I, were determined by western blot (A), with density analysis of (A) shown in (B) (*P<0.05 versus UUO group). (C and D) Immunohistochemistry analysis of myofibroblast marker SMA-α (C) was performed and the positive-staining area (D) was measured. (E and F) Kidney fibrosis was determined by trichrome and Sirius Red staining; the area of positive staining, the green color of trichrome staining, and the red color in Sirius red staining were summarized (E). (G and H) The tubule cell marker, E-cadherin, (G) and the endothelial cell marker, VE-cadherin (H), were determined by immunofluorescent staining in control and verteporfin-treated groups. n=5; *P<0.05.
Yap/Taz Deletion in Gli-Derived Myofibroblasts Attenuates Fibrosis
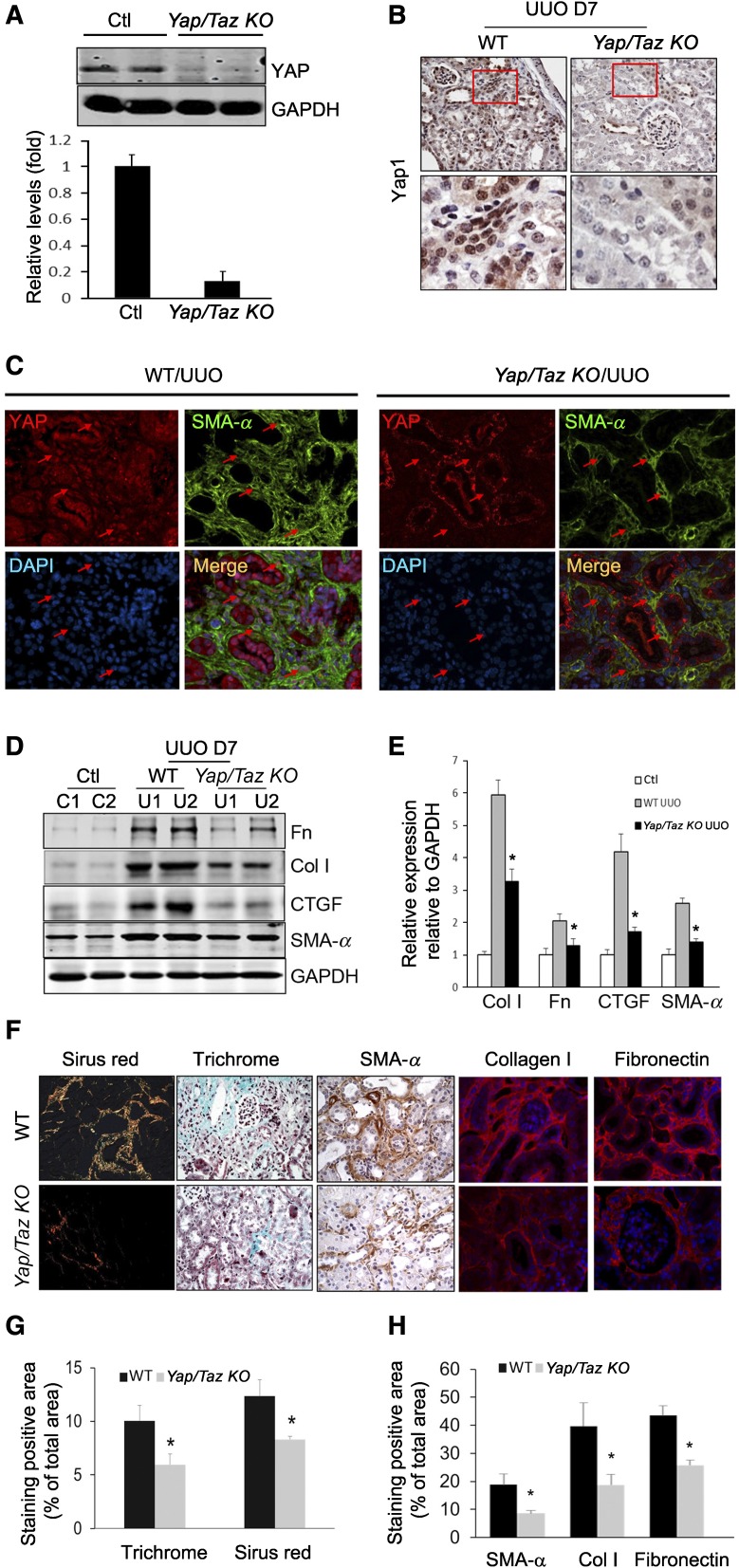
Gli2–positive fibroblasts in mediating tissue fibrosis were recently reported and both Gli1+ and Gli2+ cells can label kidney myofibroblasts.28–30 We created UUO in Gli1-LacZ reporter mice, and found that UUO stimulated accumulation of X-gal–positive cells in obstructed kidney, suggesting activation of Gli1 in myofibroblasts (Supplemental Figure 8). We next generated Gli1+ cell-specific Yap/Taz KO mice (Yapf/f/Tazf/f/Gli1-ERCre mice), where Yap and Taz were knocked out by tamoxifen treatment in Gli1+ cells and their lineages (Supplemental Figure 9). UUO was created in wild-type and Gli1 Yap/Taz KO mice after tamoxifen treatment, and kidney fibroblasts were isolated. Western blot and immunohistochemistry results showed that Yap expression level was significantly decreased in fibroblasts from Yap/Taz KO mice (Figure 5, A and B). Double immunostaining indicated that SMA-α was expressed in Yap-activated myofibroblasts in obstructed kidneys of wild-type mice; however, no Yap signals were detected in most SMA-α+ cells from Yap/Taz KO mice (Figure 5C). There was no significant difference in Yap expression level in kidney tubules of wild-type and Yap/Taz KO mice (Figure 5C). Notably, at day 7 after UUO, we found that specific deletion of Yap in mouse fibroblasts resulted in suppressed accumulation of SMA-α+ myofibroblasts and ECM proteins (fibronectin and collagen I) (Figure 5, F and G). ECM deposition and fibrosis (trichrome- and Sirius Red–positive signals) were reduced in the kidneys of Yap/Taz KO mice compared with wild-type mice (Figure 5, F and H).
Figure 5.
Yap/Taz KO in fibroblasts suppresses renal fibrogenesis. (A) Yap/Taz was knocked out in fibroblasts in Yapf/f/Tazf/f/Gli1-ERCre mice (Yap/Taz KO) after tamoxifen inoculation for 5 days (80 mg/kg body wt). Western blot analysis of Yap expression in fibroblasts from UUO kidneys of control and Yap/Taz KO mice. (B) Immunostaining showed that Yap was absent in interstitial cells of Yap/Taz KO mice. Lower panel represents magnified area of the red frame in the upper panel (arrowhead in panel indicates Yap staining in interstitium in WT). (C) Double immunostaining of Yap and SMA-α in obstructed kidneys of wild-type and Yap/Taz KO mice. Red arrows point to nuclei of interstitial fibroblasts. (D and E) Representative western blot and relative intensity analysis indicate levels of myofibroblast markers in UUO kidneys of WT and Yap/Taz KO mice. (F–H) Sections of obstructed kidneys of WT and Yap/Taz KO mice were immunostained with anti-fibronectin, collagen I, and SMA-α (F). Fibrosis was determined by trichrome and Sirius Red staining (F). The total positive areas were measured and statistically analyzed (G and H) (n=5; *P<0.05 versus WT UUO group).
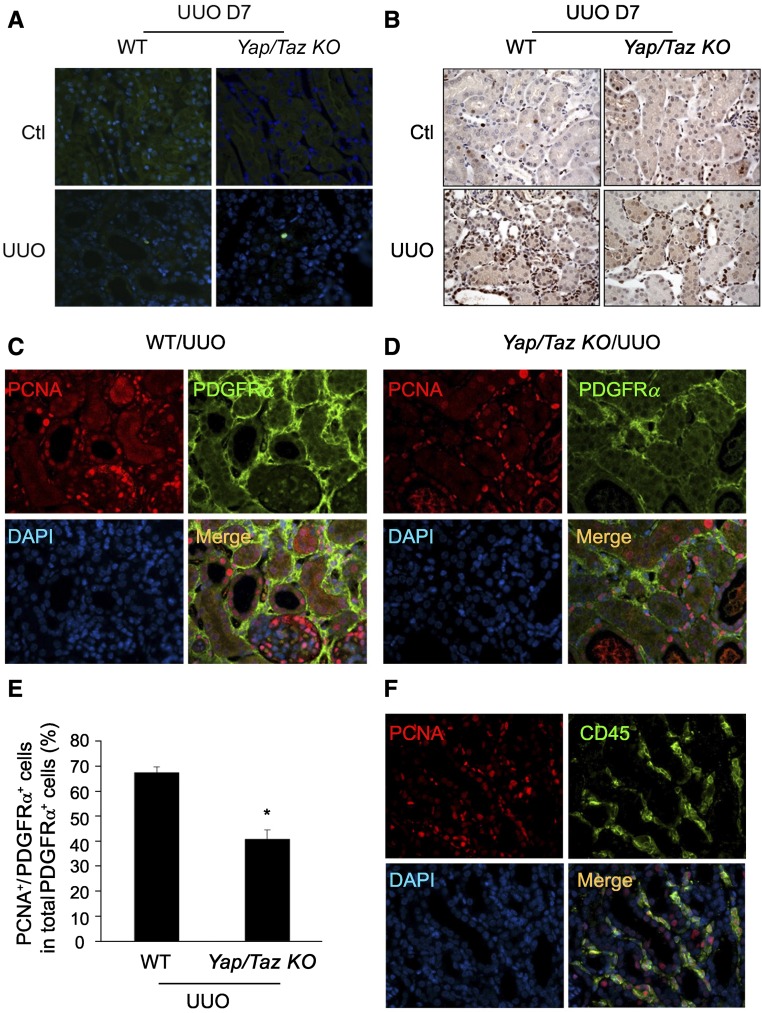
To determine whether Yap/Taz-mediated accumulation of myofibroblasts is associated with cell proliferation or apoptosis, TUNEL assay and the expression of proliferative markers were determined. We observed no significant difference in apoptotic cells between the two groups (Figure 6A). However, the amount of proliferating cell nuclear antigen (PCNA) was lower in obstructed kidneys of Yap/Taz KO compared with wild type (Figure 6B). We also found that the number of PCNA+/PDGFRα+ double-positive myofibroblasts was decreased in tubulointerstitium of obstructed kidneys in Yap/Taz KO mice versus that of WT mice (Figure 6, C–E). We also detected some PDGFRα−/PCNA+ cells which are CD45+ inflammatory cells (Figure 6F). Taken together, KO of Yap/Taz in Gli1+ cells suppresses UUO-induced fibrosis through inhibition of myofibroblast transformation and proliferation, with no significant effects in apoptosis of myofibroblasts.
Figure 6.
Yap/Taz KO inhibits the accumulation of proliferating cells in tubulointerstitium after UUO. (A) Yap/Taz KO had no effect on apoptotic cells in obstructed kidneys. Apoptosis was determined by TUNEL analysis; positive cells show green color in the nucleus. (B) Yap/Taz KO inhibits PCNA-positive cells in the tubulointerstitium of obstructed kidney. (C and D) Double immunostaining of PCNA and PDGFRα-positive cells in UUO kidney of WT and Yap/Taz KO mice. (E) The percentage of PCNA+/PDGFRα+ double-positive cells in total PDGFRα+ cells were summarized (n=5). (F) CD45 inflammatory cells are positively stained for PCNA in UUO kidney from YAP/Taz KO mice (n=5).
Yap/Taz Deletion in Gli-Derived Myofibroblasts Partially Reversed UUO-Induced Kidney Fibrosis
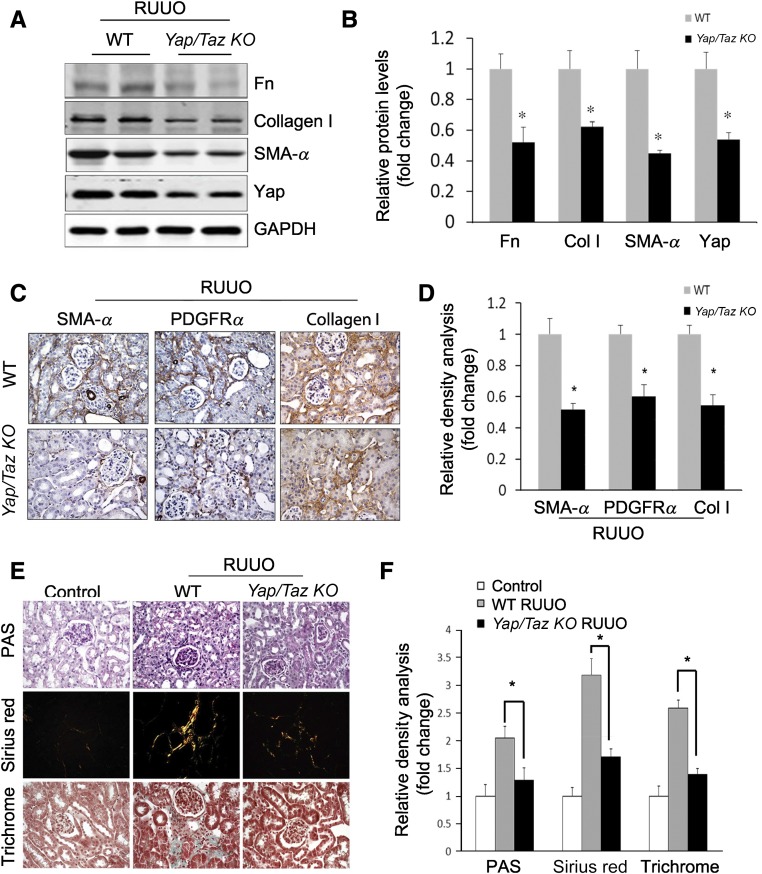
To test whether Yap is a therapeutic target, we studied Yapf/f/Tazf/f/Gli1-ERCre mice after creation of a release-UUO injury. This model can be used to determine if deletion of Yap can reverse UUO-induced kidney fibrosis. After releasing the obstruction (UUO for 3 days), wild-type and Yapf/f/Tazf/f/Gli1-ERCre mice were treated with tamoxifen for 5 days to delete Yap and Taz in fibroblasts in Yapf/f/Tazf/f/Gli1-ERCre mice. We found there was similar induction of SMA-α expression in UUO kidneys in both WT and Yapf/f/Tazf/f/Gli1-ERCre mice at day 3 UUO before tamoxifen treatment (Supplemental Figure 10). Elimination of Yap/Taz decreased the expression of myofibroblast markers (fibronectin, collagen, and SMA-α) (Figure 7, A and B) and their interstitial localization in obstructed kidneys 7 days after release of obstruction (Figure 7, C and D). The degree of UUO-induced interstitial fibrosis was markedly decreased by deletion of Yap/Taz (Figure 7E) and quantitative analysis showed an approximately 50% reduction in interstitial fibrosis in mice with fibroblast KO of Yap/Taz (Figure 7F).
Figure 7.
Inducible KO of Yap/Taz reverses UUO-induced kidney fibrosis. (A–D) Release-UUO model was used to determine whether Yap/Taz KO in fibroblasts ameliorates fibrosis. UUO was released after 3 days; Yap/Taz KO mice were induced by adding tamoxifen for 5 days. Wild-type mice that received tamoxifen treatment as Yap/Taz KO mice were taken as control group. After 7 days, kidneys were collected and the expression of myofibroblast markers was detected by western blot (A) and immunohistochemistry (C). The corresponding density analyses are shown (B and D), respectively. (n=5; *P<0.05 compared with WT control group). (E and F) Fibrosis was detected by PAS, Sirius Red, and trichrome staining (E) and the positive areas were statistically analyzed (F). (n=5; *P<0.05).
Discussion
Over the past two decades, there have been extensive efforts directed at understanding if the development of fibrosis is predictive of progression to renal insufficiency.31 In fibroblasts arising in response to UUO, we found that the activation and expression of Yap are induced in a matrix stiffness–dependent manner. We also found that Yap initiates transformation of fibroblasts into myofibroblasts and raises the stiffness of the ECM thereby maintaining Yap activation. These events form a feed-forward loop resulting in formation of scar tissue and fibrosis in the kidney. This forward cycle can be inhibited by knocking out Yap/Taz in fibroblasts. We and others23 demonstrated that a Yap inhibitor, verteporfin, suppresses the development of kidney fibrosis. Inducible knockout of Yap/Taz, specifically in fibroblasts, reversed the fibrogenesis that is induced by UUO.
Kidney myofibroblasts could be derived from a number of sources such as local renal fibroblasts, pericytes, epithelial-to-mesenchymal transition, endothelial-to-mesenchymal transition, and fibrocytes.5,8,32 Recently, Gli1-positive cells represent a subpopulation of interstitial myofibroblasts in kidney and other organs.29 Gli1-positive cells are present in the perivascular area in capillaries and arteries. They function as adventitial mesenchymal stem cell–like cells and are involved in calcification and neointima formation in arteries.33 In normal kidney, Gli1+ cells are quiescent and localized in the tubulointerstitial and perivascular areas; they function as pericytes to maintain endothelial barrier intact.29,34 In UUO, the perivascular Gli1+ cells are exposed to growth factors (e.g., TGF-β, PDGF-BB) which were derived from injured tubular cells or platelets, inducing ECM deposition.35,36 Once activated, the Gli1-positive cells express myofibroblast markers (SMA-α, PDGFRα/β, collagen I).34,37,38 We and others found that renal interstitial Gli1 is selectively induced in active fibroblasts in the Gli1-LacZ transgenic mice after UUO.28,39 Genetic depletion of Gli1-positive cells ameliorates fibrosis and rescues organ fibrosis.29 Gli1-ERCre mediated deletion of hedgehog signaling suppresses UUO-induced kidney fibrosis,28,30 and we selected Gli1-ERCre to delete Yap/Taz in kidney fibroblasts and found it blocks UUO-induced fibrosis.
Yap mediates TGF-β1 activation of fibroblasts.40 It is well known that TGF-β1/Smad signaling induces myofibroblast activation.41,42 Upon deletion of Yap in interstitial fibroblasts, we find an inhibition of TGF-β1/Smad signaling and myofibroblast activation. Yap is involved in regulating the TGF-β1 signaling pathway through two potential mechanisms: firstly, Yap can bind directly to Smad2/3 with an enhancement in the activation of fibroblasts.43 Secondly, activated Yap could potentially enhance TGF-β1/Smad2/3 signaling through the actions of CTGF. CTGF is one of the downstream targets of Yap-TEAD and has emerged as a prominent profibrotic growth factor that stimulates the growth of fibroblasts and ECM production.44 How could CTGF induce TGF-β1 signaling? Li et al. and Wahab et al. have shown that CTGF can suppress Smad7 (an inhibitor of TGF-β1 signaling).45,46 Thus, Yap/Taz can potentially enhance TGF-β1 signaling by interfering with Smad7 expression.
How is Yap/Taz activated in the UUO kidney? There is evidence that Yap expression is influenced by the Hippo pathway.47 For example, inhibition of Hippo/Lats1/2 or Mst1/2 in the Hippo pathway leads to nuclear translocation of Yap. However, Yap/Taz can also be activated via the Hippo-independent pathway.12,13,48 Matrix stiffness can induce Yap activation through a ROCK1-dependent cytoskeleton rearrangement; this leads to nuclear localization of Yap.16 Other procontractile stimuli, such as α-lysophosphatidic acid and TGF-β1, have been noted to promote nuclear accumulation of Yap.49 Increased Yap/Taz not only senses the ECM deposition/stiffness but also produces CTGF, leading to more ECM deposition. This forms a forward loop to induce transformation and proliferation of kidney fibroblasts. In addition, the ECM can induce Yap/Taz nuclear translocation through integrin-mediated outside-in signal and enhances cell proliferation.50 Thus, the results from others and our experiments indicate that Yap/Taz could be a mechanotransductor, transforming mechanical cues into biochemical signals and promoting Gli1+ cell survival and proliferation.
Kidney fibrosis is reversed when Yap expression is blocked. It has been thought that fibrosis is irreversible, but the renal fibrosis that is associated with chronic diabetes can be repaired by successful pancreas transplantation.51,52 In addition, cultured myofibroblasts can be redifferentiated into fibroblasts by treatment with FGF-2.53 Others have found that an inhibitor of ROCK1 (Y27632) reduced ECM remolding and blocked a feed-forward loop resulting in reversion of the behavior of cancer cells.12,18 Moreover, decreases in the modulus of the matrix stiffness from approximately 32 kPa to approximately 7 kPa led to myofibroblast deactivation and quiescence,54–56 indicating that matrix modulus alone can alter fibroblast fate. In the present experiments, we found that Yap activation in fibroblasts stimulated their proliferation and accelerated the process of kidney fibrosis. Inhibition of Yap/Taz signaling blocks a feedback loop leading to a fibrotic ECM that is both a cause and consequence of fibroblast activation. By deletion of Yap/Taz signaling, UUO-induced fibrosis could be at least partially reversed. These results demonstrate that blocking the function of Yap can suppress or reverse the process of fibrogenesis.
In summary, Yap can be activated by increasing the stiffness of the matrix to promote sustained activation of fibroblasts and their transformation into myofibroblasts. Accumulation of ECM promotes myofibroblast proliferation. These responses increase the synthesis of contractile proteins (SMA-α, vimentin, etc.), and in turn increase ECM stiffness and stimulate Yap activation. This feed-forward cycle leading to kidney fibrosis and elimination/suppression of Yap may provide a therapeutic target to inhibit fibrosis.
Concise Methods
Reagents
Penicillin, streptomycin, DMEM, and FBS were obtained from Invitrogen Life Technologies (Carlsbad, CA). Protein assay kit was purchased from Bio-Rad (Hercules, CA). NE-Per Nuclear and Cytoplasmic Extraction kit was purchased from ThermoFisher Scientific (Chicago, IL). DeadEnd Fluorometric TUNEL System was purchased from Promega (Madison, WI). The Nucleofector Kit for electroporation was from Lonza (Allendale, NJ); Monoclonal SMA-α-FITC, Cre antibodies, and verteporfin were from Sigma-Aldrich (St. Louis, MO); Yap (Goat), vimentin, PCNA, CTGF, and GAPDH antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA); Phalloidin-FITC and fluorescent secondary antibodies were from Invitrogen; antibodies for phosphorylated and total Yap (rabbit), caspase 3 and activated caspase 3, TEAD, and Histone 1 were from Cell Signaling Technology (Beverly, MA); antibodies against PDGFRα, PDGFRβ, TGF-β1, N-cadherin, E-cadherin, and Gli1 were from R&D (Minneapolis, MN); 4′,6-diamidino-2-phenylindole (DAPI) was from Southern Biotech (Birmingham, AL); CD31 and CD45 were from BD (Franklin Lakes, NJ); smooth muscle myosin heavy chain (SMMHC) was from DAKO (Glostrup, Denmark); antibodies for collagen I, fibronectin, and α-SMA were from Abcam (Cambridge, MA). X-gal staining kit was purchased from Genlantis (San Diego, CA), and performed as described.57
Animals
Wild-type (C57/B6), Gli1-CreER, and Gli1-LacZ transgenic mice were purchased from Jackson Laboratory (Bar Harbor, ME). Mice with Yapf/f/Tazf/f transgenic mice were generously provided by James Martin with permission from Eric Nelson.58 To generate mice with Yap/Taz knock out in the fibroblast, mice carrying a floxed Yap/Taz were bred with Gli1-CreER mice in which Cre is only expressed in interstitial fibroblasts.28,30 After backcrossing, Yapf/f/Tazf/f/Gli1-CreER+ mice were obtained, and Yap/Taz KO was achieved after tamoxifen treatment for 5 days with 80 mg/kg body wt (i.p.). Although whole-body KO of Yap is fatal and leads to embryonic death,59 we found that inducible KO of Yap in fibroblasts did not cause abnormality in gross appearance, fertility, or male/female ratio. Mice carrying this mutation were of normal size and weight and did not display any apparent renal pathology in our experiment period. Mice were housed in a conventional animal facility with a 12-hour light/dark cycle. Genotyping was performed using tail DNA. Yap/Taz KO and WT littermate control male mice aged 2–3 months were used in experiments. All animal experiments were conducted in accordance with accepted animal protocols approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine.
Cells, Plasmids, and Viruses
Primary kidney fibroblasts were isolated from wild-type kidney cortex as previously described.60 The method is on the basis of the outgrowth of cells from explanted renal cortex. The finely minced cortex was explanted into a cross-wise scratched, gelatin (2%)-coated petri dish. The tissue was then incubated in enriched medium (DMEM with 20% FCS and glutamine and penicillin/streptomycin) for 10–14 days until the monolayer had covered 75% of the dish. The cells were then lifted from the base of the petri dish by trypsinization, before being resuspended in enriched medium and transferred to culture flasks until confluent. After this stage, the isolated fibroblasts were kept in maintenance medium (DMEM with 10% FCS and glutamine and penicillin/streptomycin). Cells were characterized as fibroblasts on the basis of positive staining for SM22, vimentin, and collagen I in conjunction with an absence of CD31, SMMHC, and E-cadherin staining (Supplemental Figure 3). The cells were starved of growth factor supplement 24 hours before stimulation with reagents. All assays were performed between passages two to four. TGF-β1 (Sigma Aldrich, St. Louis, MO) was used at a dose of 2 ng/ml. To knockout Yap/Taz, mouse fibroblasts were isolated from Yapf/f/Tazf/f transgenic mice, and then infected with AdCre.61 The lentivirus vector pLX304-YAP1(S6A)24 (Yap constitutive active) and pLX304-Yap1-del 60–89,24 a Yap dominant negative vector with loss of TEAD binding domain, were purchased from Addgene. The lentivirus particles were packaged and used to infect mouse fibroblasts. Constitutively active Yap construct pQCXIH-Myc-Yap-5SA and TEAD DN construct TEAD1-del C were generously provided by Dr. Kunliang Guan (University of California, San Diego).62 Yap mutants (Yap5SA) have the LATS kinase–induced phosphorylation sites substituted to alanine, preventing their cytoplasmic sequestration and proteasomal degradation.44 Cell lysates were prepared 48–72 hours after lentivirus infection for western blot.
Renal Interstitial Fibrosis Model
UUO or sham surgery was performed on 2–3-month-old male mice as described previously.63,64 Male mice were anesthetized with an intraperitoneal injection of Rodent III Combo. Kidney tissue was harvested 3 or 7 days after UUO or sham surgery. Because previous studies showed no differences between these time points in sham-operated mice,64 a single time point (day 3 or 7) was used for sham controls in each experiment. Under anesthesia, the left ureter was isolated and ligated for 3 or 7 days. After 3 or 7 days, all animals were euthanized and the kidneys were analyzed. In the verteporfin-treated experiment, mice were treated with DMSO or verteporfin (100 mg/kg body wt, i.p. every other day for a total of four times).
Release of UUO
Yapf/f/Tazf/f/Gli1-CreER and WT male mice were used. Under anesthesia, the right ureter was isolated and clamped with a nontraumatic microvascular clip (5–15 g/mm2, 7 mm S&T Vascular Clamp; Fine Science Tools, Foster City, CA) for 3 days. UUO was released by removing the microvascular clamp. Success of the release of the obstruction was confirmed by observation of the renal pelvis. After release of the obstruction, KO of Yap/Taz was induced by exposure to tamoxifen for 5 days (80 mg/kg body wt, i.p.). Seven days later, animals were euthanized and the kidneys were analyzed. These time points were selected as we and others have found that the fibrogenic responses (increased expression of SMA-α and collagen I) persisted despite relief of obstruction.65,66 We tested whether KO of Yap/Taz can interrupt these processes and reverse renal fibrosis.
Histology and Immunohistochemistry
For histologic analysis, the kidneys were prepared by perfusion of the mice through the left ventricle and slides of the kidney were prepared as described.64 Histology scoring was performed by examining Masson trichrome-stained kidney sections of five mice in each group by an experienced pathologist on coded slides. Immunohistochemical staining was performed on kidney tissues using procedures established in our laboratory. For double immunofluorescence staining, after primary antibodies, fluorescent secondary antibodies were incubated. DAPI was used to stain the nuclear DNA. A Nikon Eclipse 80i fluorescence microscope (Melville, NY) was used to capture the images and the negative control was either an isotype-matched IgG or PBST. The areas of positive signal were measured using NIS-Elements BR 3.0 program. Images (×400) from each section were analyzed in a blinded manner and quantified using Image-Pro Plus software (Media Cybernetics, Silver Spring, MD). The results were expressed as the percentage of total tubulointerstitial area stained. Masson trichrome and Picro-Sirius Red staining were done as described.36
Preparation of Polyacrylamide Hydrogels
Hydrogels of low (0.5–5 kPa) and high (20–50 kPa) stiffness were used for the experiments. A detailed protocol for generating fibronectin-coated polyacrylamide hydrogels has been previously described.67,68 Briefly, cover slips were generated by incubation with NaOH followed by addition of 3-aminopropyltrimethosysilane (3-APTMS). Siliconized coverslips were dried by aspiration and placed reactive side up on strips of Parafilm. The coverslips were allowed to air dry completely. Six‐well culture dishes were filled with 2 ml of PBS to serve as receptacles for the polymerized gels. Glutaraldehyde was used to cross-link the 3-APTMS and the polyacrylamide gel. A polyacrylamide (40%), bis-acrylamide (2%), and ammonium persulfate were used for the polymerization of the hydrogel, as described.67 The acrylamide solution polymerizes very quickly; the droplets were placed on the coverslips immediately. As soon as the droplets were in place, a siliconized coverslip was carefully placed on top; the resulting capillary action resulted in a uniformly spread polyacrylamide gel. After 2–3 minutes, the gels were polymerized, the top (siliconized) coverslips were carefully removed, and the polymerized gels were placed in the prepared six‐well dishes containing PBS. N-hydroxysuccinimide was incorporated into the polyacrylamide solution to crosslink ECM protein to the hydrogel. After polymerization of the hydrogel, the gel surface was coated with collagen I (10 ng/ml). The calculation of the elastic moduli from concentration of polyacrylamide was on the basis of a previous publication.69
Western Blot Analysis
Cell extracts were prepared in RIPA buffer, protein concentration in the extracts was determined using Bradford protein assay kit (BioRad, Hercules, CA), and approximately 30 μg of protein was separated by SDS-PAGE. After transfer to nitrocellulose membranes, immunoblots were probed separately with various primary antibodies after blocking with 5% skimmed milk in TBS. Fluorescently labeled secondary antibodies were used for detection by the Odyssey Infrared Imaging System (LI-COR, Inc., Lincoln, Nebraska).
Statistical Analyses
All data are presented as mean±SEM. A total of five images were randomly collected from each sample. Comparison between groups was made using one-way ANOVA followed by pairwise comparisons with P value adjustment; P<0.05 was considered to be statistically significant.
Disclosure
None.
Supplementary Material
Acknowledgments
We acknowledge Dr. William E. Mitch and Dr. David Sheikh-Hamad for constructive suggestions.
This work was supported by grants from the American Heart Association (115GRNT25700209 and RO1 DK095867 [J.C.]), National Institutes of Health grants (R37DK37175 and T32-DK07656), National Natural Science Foundation of China (NSFC81570689 [M.L.]), Science & Technology Planning Project of Guangzhou (201707010290 [M.L.]) and a generous grant from Dr. and Mrs. Harold Selzman.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015121354/-/DCSupplemental.
References
- 1.Falke LL, Gholizadeh S, Goldschmeding R, Kok RJ, Nguyen TQ: Diverse origins of the myofibroblast—Implications for kidney fibrosis. Nat Rev Nephrol 11: 233–244, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Grande MT, López-Novoa JM: Fibroblast activation and myofibroblast generation in obstructive nephropathy. Nat Rev Nephrol 5: 319–328, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Rockey DC, Bell PD, Hill JA: Fibrosis--a common pathway to organ injury and failure. N Engl J Med 372: 1138–1149, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Liu Y: Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol 7: 684–696, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS: Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176: 85–97, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalluri R, Weinberg RA: The basics of epithelial-mesenchymal transition. J Clin Invest 119: 1420–1428, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R: Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol 19: 2282–2287, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LeBleu VS, Taduri G, O’Connell J, Teng Y, Cooke VG, Woda C, Sugimoto H, Kalluri R: Origin and function of myofibroblasts in kidney fibrosis. Nat Med 19: 1047–1053, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He W, Dai C, Li Y, Zeng G, Monga SP, and Liu Y. Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J AM Soc Nephrol 20: 765–776, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyle SC, Kim M, Valerius MT, McMahon AP, Kopan R: Notch pathway activation can replace the requirement for Wnt4 and Wnt9b in mesenchymal-to-epithelial transition of nephron stem cells. Development 138: 4245–4254, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, Kalluri R: BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med 9: 964–968, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI, Harrington K, Williamson P, Moeendarbary E, Charras G, Sahai E: Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol 15: 637–646, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S: Role of YAP/TAZ in mechanotransduction. Nature 474: 179–183, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Liu F, Lagares D, Choi KM, Stopfer L, Marinković A, Vrbanac V, Probst CK, Hiemer SE, Sisson TH, Horowitz JC, Rosas IO, Fredenburgh LE, Feghali-Bostwick C, Varelas X, Tager AM, Tschumperlin DJ: Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol Lung Cell Mol Physiol 308: L344–L357, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahoney JE, Mori M, Szymaniak AD, Varelas X, Cardoso WV: The hippo pathway effector Yap controls patterning and differentiation of airway epithelial progenitors. Dev Cell 30: 137–150, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, Fu XD, Mills GB, Guan KL: Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 150: 780–791, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yimlamai D, Christodoulou C, Galli GG, Yanger K, Pepe-Mooney B, Gurung B, Shrestha K, Cahan P, Stanger BZ, Camargo FD: Hippo pathway activity influences liver cell fate. Cell 157: 1324–1338, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson R, Halder G: The two faces of Hippo: Targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat Rev Drug Discov 13: 63–79, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seo E, Kim WY, Hur J, Kim H, Nam SA, Choi A, Kim YM, Park SH, Chung C, Kim J, Min S, Myung SJ, Lim DS, Kim YK: The Hippo-Salvador signaling pathway regulates renal tubulointerstitial fibrosis. Sci Rep 6: 31931, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu J, Li PX, Wu J, Gao YJ, Yin MX, Lin Y, Yang M, Chen DP, Sun HP, Liu ZB, Gu XC, Huang HL, Fu LL, Hu HM, He LL, Wu WQ, Fei ZL, Ji HB, Zhang L, Mei CL: Involvement of the Hippo pathway in regeneration and fibrogenesis after ischaemic acute kidney injury: YAP is the key effector. Clin Sci (Lond) 130: 349–363, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNeill H, Reginensi A: Lats1/2 regulate Yap/Taz to control nephron progenitor epithelialization and inhibit myofibroblast formation. J Am Soc Nephrol 28: 852–861, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee SJ, Anders RA, Liu JO, Pan D: Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev 26: 1300–1305, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szeto SG, Narimatsu M, Lu M, He X, Sidiqi AM, Tolosa MF, Chan L, De Freitas K, Bialik JF, Majumder S, Boo S, Hinz B, Dan Q, Advani A, John R, Wrana JL, Kapus A, Yuen DA: YAP/TAZ are mechanoregulators of TGF-β-Smad signaling and renal fibrogenesis. J Am Soc Nephrol 27: 3117–3128, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenbluh J, Nijhawan D, Cox AG, Li X, Neal JT, Schafer EJ, Zack TI, Wang X, Tsherniak A, Schinzel AC, Shao DD, Schumacher SE, Weir BA, Vazquez F, Cowley GS, Root DE, Mesirov JP, Beroukhim R, Kuo CJ, Goessling W, Hahn WC: β-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell 151: 1457–1473, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smithmyer ME, Sawicki LA, Kloxin AM: Hydrogel scaffolds as in vitro models to study fibroblast activation in wound healing and disease. Biomater Sci 2: 634–650, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gould ST, Anseth KS: Role of cell-matrix interactions on VIC phenotype and tissue deposition in 3D PEG hydrogels. J Tissue Eng Regen Med 10: E443–E453, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chia HN, Vigen M, Kasko AM: Effect of substrate stiffness on pulmonary fibroblast activation by TGF-β. Acta Biomater 8: 2602–2611, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Kramann R, Fleig SV, Schneider RK, Fabian SL, DiRocco DP, Maarouf O, Wongboonsin J, Ikeda Y, Heckl D, Chang SL, Rennke HG, Waikar SS, Humphreys BD: Pharmacological GLI2 inhibition prevents myofibroblast cell-cycle progression and reduces kidney fibrosis. J Clin Invest 125: 2935–2951, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, Henderson JM, Ebert BL, Humphreys BD: Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 16: 51–66, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou D, Li Y, Zhou L, Tan RJ, Xiao L, Liang M, Hou FF, Liu Y: Sonic hedgehog is a novel tubule-derived growth factor for interstitial fibroblasts after kidney injury. J Am Soc Nephrol 25: 2187–2200, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ko YA, Mohtat D, Suzuki M, Park AS, Izquierdo MC, Han SY, Kang HM, Si H, Hostetter T, Pullman JM, Fazzari M, Verma A, Zheng D, Greally JM, Susztak K: Cytosine methylation changes in enhancer regions of core pro-fibrotic genes characterize kidney fibrosis development. Genome Biol 14: R108, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duffield JS, Humphreys BD: Origin of new cells in the adult kidney: Results from genetic labeling techniques. Kidney Int 79: 494–501, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Kramann R, Goettsch C, Wongboonsin J, Iwata H, Schneider RK, Kuppe C, Kaesler N, Chang-Panesso M, Machado FG, Gratwohl S, Madhurima K, Hutcheson JD, Jain S, Aikawa E, Humphreys BD: Adventitial MSC-like cells are progenitors of vascular smooth muscle cells and drive vascular calcification in chronic kidney disease. Cell Stem Cell 19: 628–642, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kramann R, Wongboonsin J, Chang-Panesso M, Machado FG, Humphreys BD: Gli1+ pericyte loss induces capillary rarefaction and proximal tubular injury. J Am Soc Nephrol 28:776–784, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ho YY, Lagares D, Tager AM, Kapoor M: Fibrosis--a lethal component of systemic sclerosis. Nat Rev Rheumatol 10: 390–402, 2014 [DOI] [PubMed] [Google Scholar]
- 36.Liang M, Woodard LE, Liang A, Luo J, Wilson MH, Mitch WE, Cheng J: Protective role of insulin-like growth factor-1 receptor in endothelial cells against unilateral ureteral obstruction-induced renal fibrosis. Am J Pathol 185: 1234–1250, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y-T, Chang F-C, Wu C-F, Chou Y-H, Hsu H-L, Chiang W-C, Shen J, Chen Y-M, Wu K-D, Tsai T-J, Duffield JS, Lin S-L: Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int 80: 1170–1181, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Lin SL, Kisseleva T, Brenner DA, Duffield JS: Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol 173: 1617–1627, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ding H, Zhou D, Hao S, Zhou L, He W, Nie J, Hou FF, Liu Y: Sonic hedgehog signaling mediates epithelial-mesenchymal communication and promotes renal fibrosis. J Am Soc Nephrol 23: 801–813, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piersma B, de Rond S, Werker PM, Boo S, Hinz B, van Beuge MM, Bank RA: YAP1 is a driver of myofibroblast differentiation in normal and diseased fibroblasts. Am J Pathol 185: 3326–3337, 2015 [DOI] [PubMed] [Google Scholar]
- 41.Pohlers D, Brenmoehl J, Loffler I, Muller CK, Leipner C, Schultze-Mosgau S, Stallmach A, Kinne RW, and Wolf G. TGF-beta and fibrosis in different organs - molecular pathway imprints. Biochim Biophys Acta. 1792:746–756, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Wang S, Wilkes MC, Leof EB, Hirschberg R: Imatinib mesylate blocks a non-Smad TGF-beta pathway and reduces renal fibrogenesis in vivo. FASEB J 19: 1–11, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Varelas X, Sakuma R, Samavarchi-Tehrani P, Peerani R, Rao BM, Dembowy J, Yaffe MB, Zandstra PW, Wrana JL: TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat Cell Biol 10: 837–848, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, Zheng P, Ye K, Chinnaiyan A, Halder G, Lai ZC, Guan KL: Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 21: 2747–2761, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li JH, Zhu HJ, Huang XR, Lai KN, Johnson RJ, Lan HY: Smad7 inhibits fibrotic effect of TGF-Beta on renal tubular epithelial cells by blocking Smad2 activation. J Am Soc Nephrol 13: 1464–1472, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Wahab NA, Weston BS, Mason RM: Modulation of the TGFbeta/Smad signaling pathway in mesangial cells by CTGF/CCN2. Exp Cell Res 307: 305–314, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Wong J, Meliambro K, Ray J, and Campbell K.. Hippo signaling in the kidney: The good and the bad. Am J Physiol Renal Physiol 311: F241–F248, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Dupont S, Piccolo S: A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 154: 1047–1059, 2013 [DOI] [PubMed] [Google Scholar]
- 49.Pefani DE, Pankova D, Abraham AG, Grawenda AM, Vlahov N, Scrace S, O’ Neill E: TGF-β targets the hippo pathway scaffold RASSF1A to facilitate YAP/SMAD2 nuclear translocation. Mol Cell 63: 156–166, 2016 [DOI] [PubMed] [Google Scholar]
- 50.Wang L, Luo JY, Li B, Tian XY, Chen LJ, Huang Y, Liu J, Deng D, Lau CW, Wan S, Ai D, Mak KK, Tong KK, Kwan KM, Wang N, Chiu JJ, Zhu Y, Huang Y: Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow [published online ahead of print December 7, 2016]. Nature doi: 10.1038/nature20602 [DOI] [PubMed] [Google Scholar]
- 51.Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M: Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med 339: 69–75, 1998 [DOI] [PubMed] [Google Scholar]
- 52.Fioretto P, Sutherland DE, Najafian B, Mauer M: Remodeling of renal interstitial and tubular lesions in pancreas transplant recipients. Kidney Int 69: 907–912, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Desai VD, Hsia HC, Schwarzbauer JE: Reversible modulation of myofibroblast differentiation in adipose-derived mesenchymal stem cells. PLoS One 9: e86865, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kloxin AM, Benton JA, Anseth KS: In situ elasticity modulation with dynamic substrates to direct cell phenotype. Biomaterials 31: 1–8, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kloxin AM, Tibbitt MW, Anseth KS: Synthesis of photodegradable hydrogels as dynamically tunable cell culture platforms. Nat Protoc 5: 1867–1887, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang H, Haeger SM, Kloxin AM, Leinwand LA, Anseth KS: Redirecting valvular myofibroblasts into dormant fibroblasts through light-mediated reduction in substrate modulus. PLoS One 7: e39969, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liang M, Liang A, Wang Y, Jiang J, Cheng J: Smooth muscle cells from the anastomosed artery are the major precursors for neointima formation in both artery and vein grafts. Basic Res Cardiol 109: 431, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang J, Xiao Y, Hsu CW, Martinez-Traverso IM, Zhang M, Bai Y, Ishii M, Maxson RE, Olson EN, Dickinson ME, Wythe JD, Martin JF: Yap and Taz play a crucial role in neural crest-derived craniofacial development. Development 143: 504–515, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang H, von Gise A, Liu Q, Hu T, Tian X, He L, Pu W, Huang X, He L, Cai CL, Camargo FD, Pu WT, Zhou B: Yap1 is required for endothelial to mesenchymal transition of the atrioventricular cushion. J Biol Chem 289: 18681–18692, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grimwood L, Masterson R: Propagation and culture of renal fibroblasts. Methods Mol Biol 466: 25–37, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Wang Y, Liang A, Luo J, Liang M, Han G, Mitch WE, Cheng J: Blocking Notch in endothelial cells prevents arteriovenous fistula failure despite CKD. J Am Soc Nephrol 25: 773–783, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang CY, Chinnaiyan AM, Lai ZC, Guan KL: TEAD mediates YAP-dependent gene induction and growth control. Genes Dev 22: 1962–1971, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheng J, Truong LD, Wu X, Kuhl D, Lang F, Du J: Serum- and glucocorticoid-regulated kinase 1 is upregulated following unilateral ureteral obstruction causing epithelial-mesenchymal transition. Kidney Int 78: 668–678, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liang A, Wang Y, Han G, Truong L, Cheng J: Chronic kidney disease accelerates endothelial barrier dysfunction in a mouse model of an arteriovenous fistula. Am J Physiol Renal Physiol 304: F1413–F1420, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liang A, Wang Y, Woodard LE, Wilson MH, Sharma R, Awasthi YC, Du J, Mitch WE, Cheng J: Loss of glutathione S-transferase A4 accelerates obstruction-induced tubule damage and renal fibrosis. J Pathol 228: 448–458, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tapmeier TT, Brown KL, Tang Z, Sacks SH, Sheerin NS, Wong W: Reimplantation of the ureter after unilateral ureteral obstruction provides a model that allows functional evaluation. Kidney Int 73: 885–889, 2008 [DOI] [PubMed] [Google Scholar]
- 67.Cretu A, Castagnino P, Assoian R: Studying the effects of matrix stiffness on cellular function using acrylamide-based hydrogels. J Vis Exp 42: 2089, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klein EA, Yung Y, Castagnino P, Kothapalli D, Assoian RK: Cell adhesion, cellular tension, and cell cycle control. Methods Enzymol 426: 155–175, 2007 [DOI] [PubMed] [Google Scholar]
- 69.Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V, Janmey PA: Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton 60: 24–34, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.