Abstract
Endothelin-1, a marker of endothelial dysfunction, is a potent vasoconstrictor released by endothelial cells and an important regulator of renal physiology. It is not known whether elevated serum levels of endothelin-1 indicate future risk of kidney disease in the general population. In participants in the Jackson Heart Study, a community-based observational study of cardiovascular risk in black adults, we measured serum endothelin-1 level at baseline (2000–2004; n=3538). We defined incident CKD as eGFR<60 ml/min per 1.73 m2 and ≥30% eGFR decline at the third visit (2009–2013) relative to baseline among those participants with baseline eGFR ≥60 ml/min per 1.73 m2. At baseline, mean age was 55 years old, 37% of participants were men, and mean eGFR was 94 ml/min per 1.73 m2. Over a median follow-up of 8 years, 228 (6.4%) cases of incident CKD occurred in participants. Participants with baseline endothelin-1 levels in higher quartiles had a greater incidence of CKD in the fully adjusted model (odds ratio for fourth versus first quartile, 1.81; 95% confidence interval, 1.11 to 2.96; Ptrend=0.04). Endothelin-1 positively associated with all-cause mortality (hazard ratio for fourth versus first quartile, 1.64; 95% confidence interval, 1.24 to 2.16; Ptrend<0.001). In conclusion, higher baseline serum endothelin-1 levels associated with incident CKD and all-cause mortality during follow-up in this general population sample of blacks.
Keywords: Chronic kidney disease, endothelin-1, Epidemiology and outcomes
Endothelin-1 is an important regulator of volume homeostasis in normal physiologic conditions and, at pathologic levels, a potent vasoconstrictor.1,2 This 21-amino acid peptide released by endothelial cells exerts its biologic effects by binding to endothelin receptor A or endothelin receptor B.3 Endothelin-1 as well as the endothelin receptors are expressed in various cell types in the kidney.4 Basic science and animal research suggest that endothelin-1 exerts a variety of biologic effects in the kidney, such as constricting renal vasculature, inhibiting sodium and water reabsorption, and leading to glomerular and tubular damage.5,6 However, there is limited knowledge about the association between endothelin-1 and kidney function in humans.
In the United States, blacks experience a disproportionate burden of kidney disease and a greater risk of progression to ESRD compared with their white counterparts.7–10 Thus, blacks constitute an important segment of the United States population in whom to study kidney disease.
The objective of this study was to characterize the cross-sectional association between serum levels of endothelin-1 and kidney function assessed using eGFR among participants in the Jackson Heart Study. In addition, we sought to characterize the prospective association between baseline serum levels of endothelin-1 and kidney function decline. These findings could allow for the detection of early, subclinical disease on the basis of elevated serum levels of endothelin-1 and provide a better understanding of kidney disease pathogenesis.
Results
In the overall study population at baseline, mean age was 55 years old, 37% were men, and mean eGFR was 94 ml/min per 1.73 m2. Participants with higher quartiles of serum endothelin-1 levels were older and more likely to be men; be smokers; have hypertension, diabetes, and a history of cardiovascular disease; have higher body mass index (BMI), systolic BP, total cholesterol, C-reactive protein, and hematocrit; and have moderately or severely increased albuminuria (Table 1).
Table 1.
Baseline characteristics of the Jackson Heart Study participants by quartile of endothelin-1 (n=5200)
| Characteristic | Quartile of Endothelin-1 (Range) | P Value for Trend | |||
|---|---|---|---|---|---|
| Quartile 1: 0.1–1.0 pg/ml, n=1680 | Quartile 2: 1.1–1.3 pg/ml, n=1305 | Quartile 3: 1.4–1.6 pg/ml, n=979 | Quartile 4: 1.7–9.6 pg/ml, n=1236 | ||
| Age, yr | 52.7 (12.5) | 55.0 (12.9) | 56.4 (12.9) | 58.6 (12.4) | <0.001 |
| Men, % | 32.6 (547) | 38.1 (497) | 38.9 (381) | 39.2 (485) | <0.001 |
| BMI, kg/m2 | 31.2 (6.5) | 31.5 (7.1) | 32.2 (7.7) | 32.4 (7.8) | <0.001 |
| Smoking, % | 8.2 (137) | 11.0 (143) | 15.8 (153) | 20.4 (249) | <0.001 |
| Systolic BP, mmHg | 123.5 (16.6) | 127.1 (18.7) | 128.5 (18.7) | 130.5 (19.5) | <0.001 |
| Hypertension, % | 53.5 (899) | 59.3 (773) | 64.8 (634) | 71.9 (886) | <0.001 |
| Antihypertensive medication use, % | 53.8 (723) | 59.4 (625) | 64.1 (513) | 71.9 (746) | <0.001 |
| Hemoglobin A1c, % | 6.0 (1.4) | 5.9 (1.2) | 6.0 (1.2) | 6.1 (1.4) | 0.01 |
| Diabetes, % | 20.6 (346) | 19.8 (258) | 23.3 (228) | 24.2 (299) | 0.006 |
| Total cholesterol, mg/dl | 197.3 (38.9) | 200.6 (39.8) | 199.1 (39.4) | 200.9 (42.6) | 0.04 |
| HDL cholesterol, mg/dl | 52.5 (14.3) | 51.1 (14.3) | 51.7 (14.9) | 51.6 (15.2) | 0.17 |
| History of CVD, % | 7.7 (129) | 10.0 (131) | 11.0 (108) | 15.3 (189) | <0.001 |
| C-reactive protein, mg/dl | 0.46 (0.73) | 0.48 (0.72) | 0.50 (0.66) | 0.64 (1.32) | <0.001 |
| Hematocrit, % | 39.0 (4.2) | 39.2 (4.2) | 39.6 (4.2) | 39.7 (4.5) | <0.001 |
| eGFR, ml/min per 1.73 m2 | 97.3 (20.3) | 94.4 (21.1) | 93.2 (20.6) | 90.1 (25.2) | <0.001 |
| Urine albumin-to-creatinine ratio,a mg/g, % | |||||
| <30 | 91.6 (691) | 87.9 (574) | 87.0 (428) | 79.2 (471) | |
| 30–300 | 6.9 (52) | 9.5 (62) | 10.4 (51) | 15.1 (90) | |
| >300 | 1.5 (11) | 2.6 (17) | 2.6 (13) | 5.7 (34) | <0.001 |
| Dietary protein intake, g/d | 82.0 (37.9) | 80.0 (36.8) | 80.2 (37.6) | 80.1 (37.1) | 0.21 |
| Dietary sodium intake, mg/d | 3471 (1509) | 3437 (1527) | 3397 (1521) | 3461 (1549) | 0.69 |
| Dietary acid load, mEq/d | 11.8 (21.0) | 11.2 (20.8) | 11.1 (22.8) | 9.5 (21.9) | 0.007 |
Mean (SD) for continuous variables and percentage (n) for categorical variables. CVD, cardiovascular disease.
Measurements for urine albumin-to-creatinine ratio were only available for 48% of the study population (2494 of 5200).
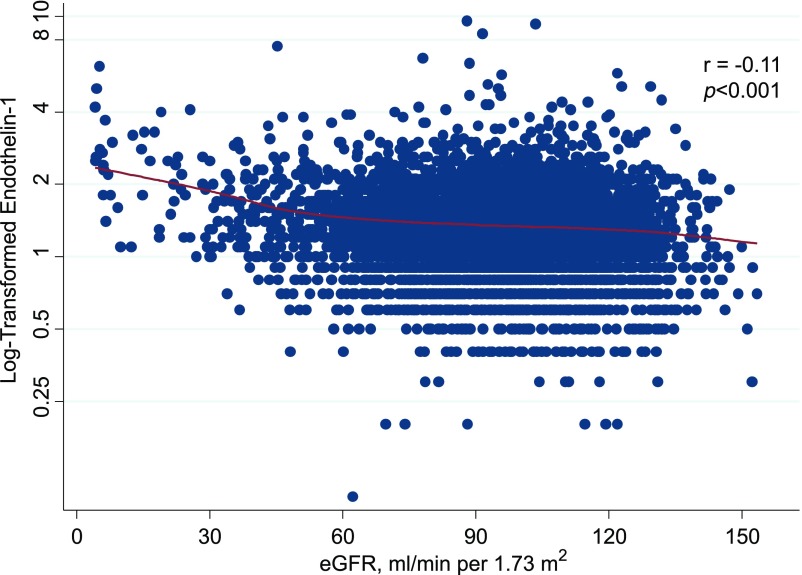
In cross-sectional analyses, baseline levels of endothelin-1 were inversely correlated with eGFR (r=−0.11; P<0.001) (Figure 1). This correlation was moderate among those with reduced baseline eGFR defined as eGFR<60 ml/min per 1.73 m2 (r=−0.26; P<0.001), and there was a poor correlation among those with normal eGFR at baseline, defined as eGFR≥60 ml/min per 1.73 m2, although it remained statistically significant (r=−0.07; P<0.001). For those participants with the lowest kidney function at baseline (eGFR<30 ml/min per 1.73 m2), there was a stronger correlation between eGFR and endothelin-1 (r=−0.45; P<0.001). The age- and sex-adjusted prevalence estimates of reduced eGFR at baseline were higher among participants with higher quartiles of endothelin-1 (8.8% in quartile 4 versus 4.9% in quartile 1; P value for trend across quartiles =0.003).
Figure 1.
Log-transformed baseline serum endothelin-1 is inversely associated with eGFR. The solid red line represents the locally weighted scatterplot smoothing curve.
A total of 228 participants with incident CKD were observed (6.4%) over a median follow-up of 8 years. Incidence of CKD was greater among those with higher quartiles of baseline endothelin-1 (8.7% in quartile 4 versus 4.1% in quartile 1; P for trend <0.001) (Table 2). In the unadjusted analysis, the odds of developing CKD were over twofold greater for those in the highest versus lowest endothelin-1 quartile, and there was a statistically significant trend across quartiles (odds ratio [OR] for quartile 2 versus 1, 1.72; 95% confidence interval [95% CI], 1.17 to 2.52; OR for quartile 3 versus 1, 1.93; 95% CI, 1.29 to 2.88; OR for quartile 4 versus 1, 2.23; 95% CI, 1.53 to 3.27; P for trend <0.001). The association between endothelin-1 and incident CKD remained after accounting for age, sex, BMI, smoking, hypertension, antihypertensive medication use, hemoglobin A1c, diabetes, total cholesterol, HDL cholesterol, history of cardiovascular disease, C-reactive protein, hematocrit, and baseline eGFR in the fully adjusted model, although the fully adjusted results were no longer monotonic (OR for quartile 2 versus 1, 1.75; 95% CI, 1.08 to 2.85; OR for quartile 3 versus 1, 1.63; 95% CI, 0.98 to 2.73; OR for quartile 4 versus 1, 1.81; 95% CI, 1.11 to 2.96; P for trend =0.04). For the continuous analysis, one SD higher baseline endothelin-1 was associated with 22% greater odds of incident CKD in the fully adjusted model (OR, 1.22; 95% CI, 1.06 to 1.40; P=0.004). Further adjustment for systolic BP in a sensitivity analysis yielded similar results (OR per one SD higher endothelin, 1.20; 95% CI, 1.05 to 1.38; P=0.008; hazard ratio [HR] for quartile 2 versus 1, 1.66; 95% CI, 1.02 to 2.71; HR for quartile 3 versus 1, 1.57; 95% CI, 0.94 to 2.63; HR for quartile 4 versus 1, 1.72; 95% CI, 1.05 to 2.83; P for trend =0.05).
Table 2.
Frequency and OR (95% CI) for incident CKD by quartile and per SD higher serum endothelin-1 level
| Variable/Model | Quartile of Endothelin-1 (Range) | Continuous Endothelin-1 | |||||
|---|---|---|---|---|---|---|---|
| Quartile 1: 0.1–1.0 pg/ml | Quartile 2: 1.1–1.3 pg/ml | Quartile 3: 1.4–1.6 pg/ml | Quartile 4: 1.7–9.6 pg/ml | P Value for Trend | Frequency or OR (95% CI)a | P Value | |
| Incident CKD, % (n/N)b | 4.1 (50/1219) | 6.9 (62/905) | 7.6 (51/669) | 8.7 (65/745) | <0.001 | 6.4 (228/3538) | — |
| Unadjusted | 1 (Reference) | 1.72 (1.17 to 2.52) | 1.93 (1.29 to 2.88) | 2.23 (1.53 to 3.27) | <0.001 | 1.28 (1.14 to 1.44) | <0.001 |
| Adjusted for age and sex | 1 (Reference) | 1.49 (1.01 to 2.20) | 1.50 (0.99 to 2.27) | 1.64 (1.11 to 2.43) | 0.02 | 1.18 (1.05 to 1.33) | 0.007 |
| Adjusted for age, sex, and risk factorsc | 1 (Reference) | 1.70 (1.05 to 2.75) | 1.63 (0.98 to 2.71) | 1.79 (1.10 to 2.91) | 0.04 | 1.21 (1.06 to 1.38) | 0.005 |
| Adjusted for age, sex, risk factors,c and eGFR | 1 (Reference) | 1.75 (1.08 to 2.85) | 1.63 (0.98 to 2.73) | 1.81 (1.11 to 2.96) | 0.04 | 1.22 (1.06 to 1.40) | 0.004 |
—, not applicable.
OR (95% CI) for incident CKD per one SD higher endothelin-1 level (0.62 pg/ml).
Incident CKD = visit 3 eGFR <60 ml/min per 1.73 m2 and ≥30% eGFR decline from visit 1 to visit 3; N = all participants without CKD at baseline with nonmissing endothelin-1 values within each endothelin-1 quartile.
Risk factors: BMI, smoking, hypertension, antihypertensive medication use, hemoglobin A1c, diabetes, total and HDL cholesterol, history of cardiovascular disease, C-reactive protein, and hematocrit.
For the secondary outcome of all-cause mortality, a total of 604 (11.6%) deaths were observed over a median follow-up of 10 years. Higher levels of endothelin-1 were significantly and independently associated with a higher risk of all-cause mortality (Table 3).
Table 3.
Frequency and HR (95% CI) for all-cause mortality by quartile and per SD higher serum endothelin-1 level
| Variable/Model | Quartile of Endothelin-1 (Range) | Continuous Endothelin-1 | |||||
|---|---|---|---|---|---|---|---|
| Quartile 1: 0.1–1.0 pg/ml | Quartile 2: 1.1–1.3 pg/ml | Quartile 3: 1.4–1.6 pg/ml | Quartile 4: 1.7–9.6 pg/ml | P Value for Trend | Frequency or HR (95% CI)a | P Value | |
| All-cause mortality, % (n/N)b | 7.2 (121/1680) | 11.3 (147/1305) | 10.8 (106/979) | 18.6 (230/1236) | <0.001 | 11.6 (604/5200) | — |
| Unadjusted | 1 (Reference) | 1.62 (1.27 to 2.06) | 1.55 (1.20 to 2.02) | 2.83 (2.27 to 3.52) | <0.001 | 1.29 (1.24 to 1.35) | <0.001 |
| Adjusted for age and sex | 1 (Reference) | 1.32 (1.04 to 1.68) | 1.13 (0.87 to 1.47) | 1.90 (1.52 to 2.37) | <0.001 | 1.22 (1.16 to 1.28) | <0.001 |
| Adjusted for age, sex, and risk factorsc | 1 (Reference) | 1.21 (0.90 to 1.63) | 1.18 (0.86 to 1.61) | 1.68 (1.27 to 2.21) | <0.001 | 1.17 (1.10 to 1.25) | <0.001 |
| Adjusted for age, sex, risk factors,c and eGFR | 1 (Reference) | 1.20 (0.89 to 1.62) | 1.18 (0.86 to 1.61) | 1.64 (1.24 to 2.16) | <0.001 | 1.17 (1.10 to 1.25) | <0.001 |
—, not applicable.
HR (95% CI) for all-cause mortality per one SD higher endothelin-1 level (0.62 pg/ml).
N = all participants with nonmissing endothelin-1 values within each endothelin-1 quartile.
Risk factors: BMI, smoking, hypertension, antihypertensive medication use, hemoglobin A1c, diabetes, total and HDL cholesterol, history of cardiovascular disease, C-reactive protein, and hematocrit.
The distribution of serum endothelin-1 levels was slightly higher among men (median: 1.3 pg/dl; 25th percentile: 1.0 pg/dl; 75th percentile: 1.7 pg/dl) than women (median: 1.2 pg/dl; 25th percentile: 0.9 pg/dl; 75th percentile: 1.6 pg/dl) as shown by a right shift in the kernel density plot (P<0.001) (Supplemental Figure 1). The association between endothelin-1 and incident CKD was stronger among men but not significant in fully adjusted models for either men or women (Supplemental Table 1). Tests of interaction of the association between endothelin-1 and CKD by sex were not statistically significant (P=0.52).
After stratifying by diabetes status, there was a stronger association between endothelin-1 and CKD among those without diabetes at baseline, and there was no significant association for those with diabetes at baseline (Supplemental Table 2). The test for interaction of the association between endothelin-1 and CKD by diabetes was not significant (P=0.32). There was also no statistical evidence of interaction by hypertension status (P=0.74). The association between endothelin-1 and CKD was stronger, but estimates were less precise and not statistically significant for those without hypertension compared with those with hypertension at baseline (Supplemental Table 3).
Discussion
In this general population sample of black men and women living in the area of Jackson, Mississippi, higher serum concentrations of endothelin-1 were cross-sectionally associated with poorer kidney function. Furthermore, elevated levels of endothelin-1 were independently associated with greater odds of incident CKD over a median follow-up of 8 years after accounting for demographics, kidney disease risk factors (BMI, smoking, hypertension, antihypertensive medication use, hemoglobin A1c, diabetes, total cholesterol, HDL cholesterol, history of cardiovascular disease, C-reactive protein, and hematocrit), and baseline kidney function. Greater odds for eGFR decline and mortality were apparent at endothelin-1 levels >1.0 pg/ml and most pronounced in the highest endothelin-1 quartile (≥1.7 pg/ml).
There are at least two lines of evidence from basic science research that suggest the role of endothelin-1 on kidney disease pathogenesis. In particular, endothelin-1 can increase the permeability of the glomerular filtration barrier by disrupting actin cytoskeleton in podocytes.11–13 Furthermore, endothelin-1 induces endoplasmic reticulum stress and apoptosis in renal tubular cells, resulting in kidney injury.6,14–17 Expression of endothelin-1 and its receptors is ubiquitous in all kidney cell types.18 The actions of endothelin-1 in the kidney are variable depending on the site of autocrine or paracrine signaling. It is possible that alterations in serum concentrations of endothelin-1 might signify extant kidney disease due to upregulation of endothelin and endothelin receptor production in the setting of ongoing kidney damage. Another likely mechanism through which endothelin-1 contributes to kidney disease development is related to the main role of endothelin-1 as a vasoconstrictor, thereby resulting in arterial stiffness and hypertension.19
Clinical trials have evaluated the potential utility of targeting the endothelin pathway for the treatment of kidney disease and other conditions. Antagonists that selectively block endothelin receptor A or endothelin receptor B and nonselective endothelin receptor antagonists have been tested in clinical trials for a wide array of cardiovascular diseases and are used to treat pulmonary arterial hypertension.20 In A Randomised, Double Blind, Placebo Controlled, Parallel Group Study to Assess the Effect of the Endothelin Receptor Antagonist Avosentan on Time to Doubling of Serum Creatinine, End Stage Renal Disease or Death in Patients with Type 2 Diabetes Mellitus and Diabetic Nephropathy (ASCEND) Trial, avosentan, a predominant endothelin receptor A antagonist, was shown to reduce proteinuria (a secondary outcome) in participants with diabetic nephropathy (CKD stages 3 and 4), but there was no difference in the primary composite outcome of doubling of serum creatinine, ESRD, or death by treatment group.21 The trial was terminated prematurely as a result of an increased risk of congestive heart failure due to fluid retention. Avosentan can increase sodium reabsorption and body weight, which may be due to it being only weakly selective for endothelin receptor A.22–24 In the Reducing Residual Albuminuria in Subjects with Diabetes and Nephropathy with Atrasentan (RADAR) Trial of atrasentan, a highly selective endothelin receptor A blocker, proteinuria was reduced in participants with diabetes and eGFR of 30–75 ml/min per 1.73 m2 (CKD stages 2 and 3), and there was no significant difference in heart failure between treatment groups.25 Atrasentan is currently being investigated in the Study of Diabetic Nephropathy with Atrasentan (SONAR) (phase 3) Trial. There is ongoing research attempting to improve the risk-benefit ratio for therapeutic agents targeting the endothelin pathway to delay kidney disease progression.26 Our study findings add to the substantial mechanistic evidence from basic science research and clinical trials on the endothelin pathway and kidney disease.
There are several strengths and limitations of our study to be considered when interpreting our study findings. A major strength of the Jackson Heart Study is the representation of blacks in the southern region of the United States, a segment of our population that experiences a disproportionate burden of kidney disease. The length of the follow-up period (median of 8 years) provided enough time for the enumeration of a sufficient number of participants with incident CKD (n=228). The prospective study design allowed for us to establish temporality between baseline serum endothelin-1 and future onset of kidney disease in a study population with preserved kidney function initially. Furthermore, detailed information was ascertained about the study participants as a result of the extensive examination at the baseline study visit, and we were, therefore, able to adjust for multiple important confounding factors in multivariable regression models, including demographic characteristics, established risk factors for kidney disease (BMI, smoking, hypertension, antihypertensive medication use, hemoglobin A1c, diabetes, total cholesterol, HDL cholesterol, history of cardiovascular disease, C-reactive protein, and hematocrit), and baseline kidney function. Nonetheless, given the observational nature of the study, residual confounding due to unmeasured or imprecisely measured factors could in part explain the observed association. This study included measurements of endothelin-1 in serum specimens, and elevated serum levels of endothelin-1 may reflect decreased renal clearance, which is supported by our finding of a stronger inverse correlation between endothelin-1 and eGFR among those with reduced kidney function (eGFR<60 ml/min per 1.73 m2). It is worth noting that our study was conducted in a population-based study of participants with preserved eGFR at baseline (excluding those with baseline eGFR <60 ml/min per 1.73 m2), and our findings for the prospective association between endothelin-1 and CKD persisted, even after adjusting for baseline eGFR. Urine measurements of endothelin-1 were not available, which are thought to represent kidney injury. Further research is warranted to examine urine endothelin-1 levels in association with kidney disease risk. Study participants were enrolled from three different counties from a single metropolitan area and may not be representative of all black men and women in the United States. It is warranted to replicate these findings in other study populations, including other race/ethnic groups and other geographic regions. Kidney function was assessed infrequently in the Jackson Heart Study at planned study visits. Selection bias due to loss to follow-up and death may be present, but the response rate (79.5% after accounting for 617 deaths) was acceptable for a cohort study with a median follow-up of 8 years.27 It is possible that some individuals had transient kidney function decline, which subsequently resolved. However, we defined CKD not only by falling below the threshold of 60 ml/min per 1.73 m2 but also as having substantial decline in kidney function relative to baseline (≥30%), which reduces the likelihood of outcome misclassification.
In conclusion, serum endothelin-1 was inversely associated with eGFR and positively associated with incident CKD and all-cause mortality in this cohort of blacks.
Concise Methods
Study Design
The Jackson Heart Study is a community-based observational study of 5301 black men and women 21–94 years of age from three counties in the Jackson, Mississippi metropolitan area (Hinds, Madison, and Rankin).28–31 The overall objective of the Jackson Heart Study was to investigate risk factors for cardiovascular disease development and progression in blacks. Study participants were recruited, were enrolled into the study, and completed the baseline examination in 2000–2004. There have been two subsequent follow-up examinations in 2005–2008 (study visit 2) and 2009–2013 (study visit 3). The study protocol was approved by the University of Mississippi Medical Center Institutional Review Board, and study participants provided written informed consent. Study procedures were followed in accordance with the Declaration of Helsinki.
Study Population
Study participants without measurements of serum endothelin-1 levels at baseline were excluded from this study (n=101). In prospective analyses of incident CKD, additional exclusions were: those without measurements of serum creatinine at baseline (n=20), those with reduced eGFR at baseline (eGFR<60 ml/min per 1.73 m2; n=328), those who died before visit 3 (n=401), those who were alive but did not attend visit 3 (n=868), and those with missing measurements of serum creatinine at visit 3 (n=45). After these exclusions, the analytic sample size for prospective analyses was 3538. Overall, the study participants who were excluded from the prospective analysis were older; were more likely to be smokers; were more likely to have hypertension, diabetes, and a history of cardiovascular disease; were more likely to have moderately or severely increased albuminuria; and had a lower baseline eGFR compared with those who were included in the prospective analysis (Supplemental Table 4).
Measurement of Endothelin-1
Fasting blood samples were collected at all study visits and stored at −80°C.32 Levels of endothelin-1 were measured in serum specimens collected at baseline (2000–2004) by ELISA (QuantiGlo Human ET-1 Immunoassay; R&D Systems, Minneapolis, MN). The coefficient of variation for this assay was 8.9%. Endothelin-1 levels were analyzed continuously as well as according to quartiles of the distribution.
Outcome Ascertainment
Serum creatinine levels were measured at baseline (2000–2004) and visit 3 (2009–2013) using a multipoint enzymatic spectrophotometric assay with the Vitros Ortho-Clinical Diagnostics Analyzer (Raritan, NJ). In 2006, as part of a calibration study, measurements of serum creatinine were repeated for a random sample of 206 study participants using the enzymatic method on the Roche Modular P Chemistry Analyzer (Roche Diagnostics Corporation, Indianapolis, IN).33 Baseline (visit 1) and visit 3 measurements of serum creatinine were harmonized using the isotope dilution mass spectrometry–traceable method. eGFR was calculated using the 2009 Chronic Kidney Disease Epidemiology Collaboration equation on the basis of serum creatinine.34 For cross-sectional analyses, reduced eGFR was defined as baseline eGFR <60 ml/min per 1.73 m2. For prospective analyses, incident CKD was defined conservatively as the development of eGFR<60 ml/min per 1.73 m2 accompanied by ≥30% eGFR decline at study visit 3 relative to baseline among study participants with baseline eGFR ≥60 ml/min per 1.73 m2.35 For the secondary outcome of all-cause mortality, vital status was ascertained by annual follow-up telephone calls with next of kin, medical record abstraction, and review of death certificates from the Mississippi State Department of Health.36
Assessment of Other Covariates
A structured questionnaire was administered at the baseline study visit to obtain information on demographics, lifestyle factors, health history, medication use, and other health parameters. Certified technicians and nurses measured anthropometrics and vital signs. BMI was determined as weight in kilograms divided by height in meters squared. BP was measured twice at rest while seated with a standardized random zero sphygmomanometer, and the average of the two measurements was used. Hypertension was defined as BP≥140/90 mmHg or use of antihypertensive medication. Smoking status was determined on the basis of self-reported current cigarette smoking. Diabetes was defined as physician diagnosis, use of diabetes medication, fasting glucose ≥126 mg/dl, or hemoglobin A1c ≥6.5%.
A 158-item food frequency questionnaire, adapted from the Lower Mississippi Delta Nutrition Intervention Research Initiative food frequency questionnaire, was administered to participants at baseline.37–39 Dietary intake of macronutrients and micronutrients was estimated by multiplying the frequency of consumption of each food item on the food frequency questionnaire by its nutritional content. Potential renal acid load, a measure of dietary acid load, was calculated using dietary intake of protein, phosphorus, potassium, magnesium, and calcium.40
Blood samples were collected according to standard procedures and analyzed at the University of Minnesota Central Laboratory. Fasting glucose was measured on a Vitros 950 Ortho-Clinical Diagnostics Analyzer (Raritan, NJ). Glycosylated hemoglobin A1c concentrations were measured using an HPLC system (Tosoh Corporation, Tokyo, Japan). C-reactive protein was measured by the immunoturbidimetric, high-sensitivity C-reactive protein Latex assay (Kamiya Biomedical Company, Seattle, WA) using a Hitachi 911 analyzer (Roche Diagnostics, Indianapolis, IN). Total cholesterol was assayed by the cholesterol oxidase method with assays from Boehringer Mannheim Diagnostics on the Roche COBAS Analyzer (Indianapolis, IN). HDL cholesterol was measured after precipitation of non-HDL cholesterol with magnesium-dextran. Random spot urine specimens were collected from study participants at baseline. Urine excretion of albumin was measured using the nephelometric method (Dade Behring BN 11 Nephelometer, Newark, DE), and urine excretion of creatinine was measured using the enzymatic method (Vitros 950 Ortho-Clinical Diagnostics Analyzer).32
Statistical Analyses
Descriptive statistics (mean, SD, and proportion) were used to characterize the study population according to endothelin-1 quartiles with respect to demographics, health behaviors, disease status, health history, and other clinical characteristics. Spearman rank correlation coefficients were calculated to quantify the association between baseline endothelin-1 and baseline eGFR in the overall study population and according to eGFR level. Scatterplots and locally weighted scatterplot smoothing (lowess) were used to visualize the cross-sectional association between endothelin-1 and eGFR.41 Marginal standardization was used to calculate age- and sex-adjusted prevalence estimates of reduced eGFR at baseline according to quartiles of endothelin-1.42
Logistic regression was used to estimate the association between endothelin-1 levels at baseline and incident CKD at study visit 3 (2009–2013). Cox proportional hazards regression was used to estimate the association between baseline endothelin-1 levels and all-cause mortality, incorporating time until death. Models were progressively adjusted for age, sex, BMI, smoking, hypertension, antihypertensive medication use, hemoglobin A1c, diabetes, HDL cholesterol, total cholesterol, history of cardiovascular disease, hematocrit, C-reactive protein, and baseline eGFR. In a sensitivity analysis, we additionally adjusted for systolic BP. Endothelin-1 levels were modeled as quartiles and continuously. ORs and HRs were expressed for the higher quartiles (2–4) relative to quartile 1 as well as per one-SD increase in endothelin-1 (0.62 pg/ml).
Prospective analyses were performed after stratifying the study population by sex and conducting the analyses separately for men and women. Tests of interaction by sex were proposed on the basis of a priori knowledge of sex as an important biologic variable in general and for endothelin-1 specifically. The tests of interaction were conducted by assessing the significance of an interaction term in the fully adjusted multivariable regression models for sex and endothelin-1 modeled as a continuous variable. Similarly, we stratified by diabetes status and hypertension status and tested for interaction in the fully adjusted multivariate model with endothelin-1 modeled as a continuous variable. A kernel density plot was created to visualize the distribution of log-transformed endothelin-1 levels by sex, and the Wilcoxon rank sum test was used to evaluate differences in the distribution of endothelin-1 levels by sex. Tests of linear trend across quartiles of endothelin-1 levels were conducted by assessing the statistical significance of endothelin-1 quartile as an ordinal variable in regression models. Stata statistical software version 14.1 was used (StataCorp LP, College Station, TX).
Disclosures
None.
Supplementary Material
Acknowledgments
We thank all of participants and data collection staff of the Jackson Heart Study for their time, effort, and dedication toward this study.
C.M.R. is supported by National Institute of Diabetes and Digestive and Kidney Diseases grant K01 DK107782. B.A.Y. is supported by National Institute of Diabetes and Digestive and Kidney Diseases grant R01 DK102134 and the Veterans Affairs Puget Sound Health Care System. The Jackson Heart Study is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, and HHSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities, with additional support from the National Institute on Biomedical Imaging and Bioengineering.
Parts of this study were presented in abstract form at the American Society of Nephrology Kidney Week 2014, which was held in Philadelphia, Pennsylvania on November 11–16, 2014.
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the US Department of Health and Human Services. The Veterans Affairs does not endorse any of the statements or opinions advocated by this manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016111236/-/DCSupplemental.
References
- 1.De Miguel C, Speed JS, Kasztan M, Gohar EY, Pollock DM: Endothelin-1 and the kidney: New perspectives and recent findings. Curr Opin Nephrol Hypertens 25: 35–41, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rabelink TJ, Kaasjager KA, Boer P, Stroes EG, Braam B, Koomans HA: Effects of endothelin-1 on renal function in humans: Implications for physiology and pathophysiology. Kidney Int 46: 376–381, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Yanagisawa M, Inoue A, Ishikawa T, Kasuya Y, Kimura S, Kumagaye S, Nakajima K, Watanabe TX, Sakakibara S, Goto K, Masaki T: Primary structure, synthesis, and biological activity of rat endothelin, an endothelium-derived vasoconstrictor peptide. Proc Natl Acad Sci U S A 85: 6964–6967, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vignon-Zellweger N, Heiden S, Miyauchi T, Emoto N: Endothelin and endothelin receptors in the renal and cardiovascular systems. Life Sci 91: 490–500, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Kohan DE: The renal medullary endothelin system in control of sodium and water excretion and systemic blood pressure. Curr Opin Nephrol Hypertens 15: 34–40, 2006 [DOI] [PubMed] [Google Scholar]
- 6.De Miguel C, Pollock DM, Pollock JS: Endothelium-derived ET-1 and the development of renal injury. Am J Physiol Regul Integr Comp Physiol 309: R1071–R1073, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flessner MF, Wyatt SB, Akylbekova EL, Coady S, Fulop T, Lee F, Taylor HA, Crook E: Prevalence and awareness of CKD among African Americans: The Jackson Heart Study. Am J Kidney Dis 53: 238–247, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) : Prevalence of chronic kidney disease and associated risk factors--United States, 1999-2004. MMWR Morb Mortal Wkly Rep 56: 161–165, 2007 [PubMed] [Google Scholar]
- 9.Muntner P, Newsome B, Kramer H, Peralta CA, Kim Y, Jacobs DR Jr, Kiefe CI, Lewis CE: Racial differences in the incidence of chronic kidney disease. Clin J Am Soc Nephrol 7: 101–107, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarver-Carr ME, Powe NR, Eberhardt MS, LaVeist TA, Kington RS, Coresh J, Brancati FL: Excess risk of chronic kidney disease among African-American versus white subjects in the United States: A population-based study of potential explanatory factors. J Am Soc Nephrol 13: 2363–2370, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Saleh MA, Boesen EI, Pollock JS, Savin VJ, Pollock DM: Endothelin-1 increases glomerular permeability and inflammation independent of blood pressure in the rat. Hypertension 56: 942–949, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saleh MA, Sandoval RM, Rhodes GJ, Campos-Bilderback SB, Molitoris BA, Pollock DM: Chronic endothelin-1 infusion elevates glomerular sieving coefficient and proximal tubular albumin reuptake in the rat. Life Sci 91: 634–637, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin J, Sison K, Li C, Tian R, Wnuk M, Sung HK, Jeansson M, Zhang C, Tucholska M, Jones N, Kerjaschki D, Shibuya M, Fantus IG, Nagy A, Gerber HP, Ferrara N, Pawson T, Quaggin SE: Soluble FLT1 binds lipid microdomains in podocytes to control cell morphology and glomerular barrier function. Cell 151: 384–399, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Taniguchi M, Yoshida H: Endoplasmic reticulum stress in kidney function and disease. Curr Opin Nephrol Hypertens 24: 345–350, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Yang D, Yang D, Jia R, Ding G: Role of reactive oxygen species-mediated endoplasmic reticulum stress in contrast-induced renal tubular cell apoptosis. Nephron Exp Nephrol 128: 30–36, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Jesmin S, Shimojo N, Yamaguchi N, Mowa CN, Oki M, Zaedi S, Sultana SN, Rahman A, Islam M, Sawamura A, Gando S, Kawano S, Miyauchi T, Mizutani T: Effects of protease activated receptor (PAR)2 blocking peptide on endothelin-1 levels in kidney tissues in endotoxemic rat mode. Life Sci 102: 127–133, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Sagar SK, Zhang C, Guo Q, Yi R, Lin-Tang: Role of expression of endothelin-1 and angiotensin-II and hypoxia-inducible factor-1α in the kidney tissues of patients with diabetic nephropathy. Saudi J Kidney Dis Transpl 24: 959–964, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Kohan DE: Role of collecting duct endothelin in control of renal function and blood pressure. Am J Physiol Regul Integr Comp Physiol 305: R659–R668, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Böhm F, Pernow J: The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc Res 76: 8–18, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Rich S, McLaughlin VV: Endothelin receptor blockers in cardiovascular disease. Circulation 108: 2184–2190, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Mann JF, Green D, Jamerson K, Ruilope LM, Kuranoff SJ, Littke T, Viberti G; ASCEND Study Group : Avosentan for overt diabetic nephropathy. J Am Soc Nephrol 21: 527–535, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Jose PA, Zeng C: Regulation of sodium transport in the proximal tubule by endothelin. Contrib Nephrol 172: 63–75, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Battistini B, Berthiaume N, Kelland NF, Webb DJ, Kohan DE: Profile of past and current clinical trials involving endothelin receptor antagonists: The novel "-sentan" class of drug. Exp Biol Med (Maywood) 231: 653–695, 2006 [PubMed] [Google Scholar]
- 24.Smolander J, Vogt B, Maillard M, Zweiacker C, Littke T, Hengelage T, Burnier M: Dose-dependent acute and sustained renal effects of the endothelin receptor antagonist avosentan in healthy subjects. Clin Pharmacol Ther 85: 628–634, 2009 [DOI] [PubMed] [Google Scholar]
- 25.de Zeeuw D, Coll B, Andress D, Brennan JJ, Tang H, Houser M, Correa-Rotter R, Kohan D, Lambers Heerspink HJ, Makino H, Perkovic V, Pritchett Y, Remuzzi G, Tobe SW, Toto R, Viberti G, Parving HH: The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J Am Soc Nephrol 25: 1083–1093, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandrashekar K, Juncos LA: Endothelin antagonists in diabetic nephropathy: Back to basics. J Am Soc Nephrol 25: 869–871, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kristman V, Manno M, Côté P: Loss to follow-up in cohort studies: How much is too much? Eur J Epidemiol 19: 751–760, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Taylor HA Jr, Wilson JG, Jones DW, Sarpong DF, Srinivasan A, Garrison RJ, Nelson C, Wyatt SB: Toward resolution of cardiovascular health disparities in African Americans: Design and methods of the Jackson Heart Study. Ethn Dis 15[4 Suppl 6]: S6-4–S6-17, 2005 [PubMed] [Google Scholar]
- 29.Taylor HA, Jr: The Jackson Heart Study: An overview. Ethn Dis 15[4 Suppl 6]: S6-1–S6-3, 2005 [PubMed] [Google Scholar]
- 30.Fuqua SR, Wyatt SB, Andrew ME, Sarpong DF, Henderson FR, Cunningham MF, Taylor HA Jr.: Recruiting African-American research participation in the Jackson Heart Study: Methods, response rates, and sample description. Ethn Dis 15[4 Suppl 6]: S6-18–S6-29, 2005 [PubMed] [Google Scholar]
- 31.Taylor HA, Jr: The Jackson Heart Study of the future. Ethn Dis 22[3 Suppl 1]: S1-49–S1-54, 2012 [PubMed] [Google Scholar]
- 32.Carpenter MA, Crow R, Steffes M, Rock W, Heilbraun J, Evans G, Skelton T, Jensen R, Sarpong D: Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci 328: 131–144, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Wang W, Young BA, Fülöp T, de Boer IH, Boulware LE, Katz R, Correa A, Griswold ME: Effects of serum creatinine calibration on estimated renal function in african americans: The Jackson heart study. Am J Med Sci 349: 379–384, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levey AS, Inker LA, Matsushita K, Greene T, Willis K, Lewis E, de Zeeuw D, Cheung AK, Coresh J: GFR decline as an end point for clinical trials in CKD: A scientific workshop sponsored by the national kidney foundation and the US food and drug administration. Am J Kidney Dis 64: 821–835, 2014 [DOI] [PubMed] [Google Scholar]
- 36.Keku E, Rosamond W, Taylor HA Jr, Garrison R, Wyatt SB, Richard M, Jenkins B, Reeves L, Sarpong D: Cardiovascular disease event classification in the Jackson Heart Study: Methods and procedures. Ethn Dis 15[4 Suppl 6]: S6-62–S6-70, 2005 [PubMed] [Google Scholar]
- 37.Carithers T, Dubbert PM, Crook E, Davy B, Wyatt SB, Bogle ML, Taylor HA Jr, Tucker KL: Dietary assessment in African Americans: Methods used in the Jackson Heart Study. Ethn Dis 15[4 Suppl 6]: S6-49–S6-55, 2005 [PubMed] [Google Scholar]
- 38.Carithers TC, Talegawkar SA, Rowser ML, Henry OR, Dubbert PM, Bogle ML, Taylor HA Jr, Tucker KL: Validity and calibration of food frequency questionnaires used with African-American adults in the Jackson Heart Study. J Am Diet Assoc 109: 1184–1193, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tucker KL, Maras J, Champagne C, Connell C, Goolsby S, Weber J, Zaghloul S, Carithers T, Bogle ML: A regional food-frequency questionnaire for the US Mississippi Delta. Public Health Nutr 8: 87–96, 2005 [PubMed] [Google Scholar]
- 40.Remer T, Manz F: Potential renal acid load of foods and its influence on urine pH. J Am Diet Assoc 95: 791–797, 1995 [DOI] [PubMed] [Google Scholar]
- 41.Cleveland WS: Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc 74: 829–836, 1979 [Google Scholar]
- 42.Muller CJ, MacLehose RF: Estimating predicted probabilities from logistic regression: Different methods correspond to different target populations. Int J Epidemiol 43: 962–970, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.