Abstract
Thrombospondin type 1 domain-containing 7A (THSD7A) is a target for autoimmunity in patients with membranous nephropathy (MN). Circulating autoantibodies from patients with THSD7A-associated MN have been demonstrated to cause MN in mice. However, THSD7A-associated MN is a rare disease, preventing the use of patient antibodies for larger experimental procedures. Therefore, we generated antibodies against the human and mouse orthologs of THSD7A in rabbits by coimmunization with the respective cDNAs. Injection of these anti-THSD7A antibodies into mice induced a severe nephrotic syndrome with proteinuria, weight gain, and hyperlipidemia. Immunofluorescence analyses revealed granular antigen-antibody complexes in a subepithelial location along the glomerular filtration barrier 14 days after antibody injection, and immunohistochemistry for rabbit IgG and THSD7A as well as ultrastructural analyses showed the typical characteristics of human MN. Mice injected with purified IgG from rabbit serum that was taken before immunization failed to develop any of these changes. Notably, MN developed in the absence of detectable complement activation, and disease was strain dependent. In vitro, anti-THSD7A antibodies caused cytoskeletal rearrangement and activation of focal adhesion signaling. Knockdown of the THSD7A ortholog, thsd7aa, in zebrafish larvae resulted in altered podocyte differentiation and impaired glomerular filtration barrier function, with development of pericardial edema, suggesting an important role of THSD7A in glomerular filtration barrier integrity. In summary, our study introduces a heterologous mouse model that allows further investigation of the molecular events that underlie MN.
Keywords: membranous nephropathy, podocyte, glomerular disease
Membranous nephropathy (MN) is a major cause of nephrotic syndrome in adults and affects both native and transplanted kidneys. About one third of patients experience spontaneous remission, whereas another 20%–30% develop ESRD within 10 years.1 The clinical characteristic is a moderate to heavy proteinuria, often with nephrotic syndrome. Histology shows granular IgG depositions along the capillary wall of the glomerulus in immunostaining and subepithelial electron-dense deposits in electron microscopy. Three podocyte-expressed antigens have been identified in patients with MN so far: neutral endopeptidase in neonates, the phospholipase A2 receptor 1, and thrombospondin type 1 domain-containing 7A (THSD7A) in adults.2–4 The measurement of anti-phospholipase A2 receptor 1 antibody titers is already part of clinical practice, both in the diagnosis of MN as well as the stratification and immunologic monitoring of affected patients.5–8 On the other hand, the role of anti-THSD7A antibody measurement for diagnosis and treatment of MN is currently being established and validated.9 Importantly, patients with THSD7A-associated MN have concurrent malignant tumors in about 20% of cases, suggesting a possible etiologic link between malignancy and the development of MN.10 The pathogenesis of MN has been extensively studied in a rat model of Heymann nephritis.11–13 In this model, the transfer of sheep antibodies against the podocyte membrane protein megalin results in subepithelial immune complex formation, activation of the complement system, and development of proteinuria.14–16 However, megalin is not expressed on human podocytes and therefore does not serve as a disease mediator in human MN. In a recent study, we demonstrated that anti-THSD7A antibodies from patients with MN can bind to mouse THSD7A (mTHSD7A) in vitro and in vivo.17 After passive transfer of the antibodies, mice developed proteinuria and the morphologic hallmarks of MN. This was the first description of an animal model involving a podocyte antigen that is relevant in human MN. However, patients with THSD7A-associated MN represent only 2%–5% of total MN cases, which hampers the use of human anti-THSD7A antibodies for more systematic investigations.9 For this reason, we have generated anti-THSD7A antibodies in rabbits and consequently evaluated these antibodies for their potential to induce MN in mice.
Results
Generation of Rabbit Anti-THSD7A Antibodies
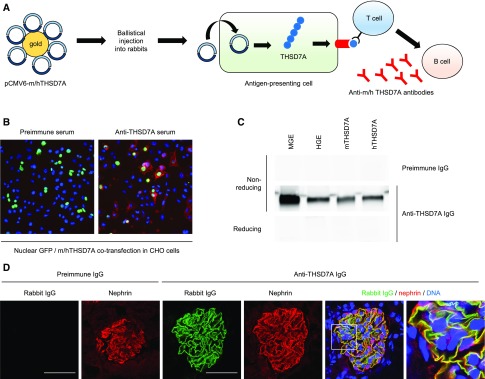
Two expression constructs encoding full-length mTHSD7A and human THSD7A (hTHSD7A) were conjugated to 1-μm gold particles and ballistically injected into three rabbits. It is known that after this type of immunization, antigen-presenting cells of the skin take up the constructs, produce the encoded proteins in their native conformation, and present peptides of the antigen on MHC molecules to the adaptive immune system.18 Upon stimulation of antigen-presenting cells, T cells activate B cells to produce antibodies against the presented antigen (scheme Figure 1A). Four months after the first injection, animals were euthanized and serum was collected. Rabbits did not develop renal failure or signs of nephrotic syndrome, such as hypoalbuminemia and hyperlipidemia (Supplemental Figure 1). Chinese hamster ovary (CHO) cells were cotransfected with nuclear GFP and mTHSD7A or hTHSD7A, whereby nuclear GFP served as a transfection control. The serum collected before immunization (preimmune serum) did not recognize any protein expressed on the transfected CHO cells in immunofluorescence analysis, whereas IgG present in the serum after immunization was bound at the cell membrane of cells that simultaneously expressed nuclear GFP, suggesting the presence of THSD7A-specific antibodies in the immunized rabbits (Figure 1B). We next purified the IgG fraction from the rabbit serum before and after immunization with THSD7A (referred to as preimmune IgG and anti-THSD7A IgG, respectively, see Supplemental Figure 2) and evaluated the antigen-binding characteristics of the purified IgG with mouse glomerular extracts, human glomerular extracts, and recombinant mTHSD7A and hTHSD7A in Western blot analysis. We found that preimmune IgG did not bind to any protein present in the tested material under nonreducing or reducing conditions (Figure 1C and data not shown). In contrast, IgG from rabbits after immunization recognized a protein of 250 kDa, present in both mouse glomerular extracts and human glomerular extracts, as well as recombinant mTHSD7A and hTHSD7A exclusively under nonreducing conditions (Figure 1C). Purified rabbit IgG did not self-react with isolated rabbit glomeruli by Western blot or immunofluorescence analysis, indicating that rabbit antibodies target epitopes present in mTHSD7A and hTHSD7A but not in the rabbit ortholog of the protein (Supplemental Figure 3).
Figure 1.
Generation of anti-THSD7A antibodies in rabbits. (A) Scheme of rabbit immunization with two constructs encoding mTHSD7A and hTHSD7A. (B) Immunofluorescence analysis of reactivity of rabbit sera before and after immunization with membrane-bound mTHSD7A expressed in CHO cells. Bound rabbit IgG was visualized with an AF568 anti-rabbit IgG (red); nuclear GFP (green) was used to indicate transfected cells. (C) Reactivity of purified preimmune IgG and anti-THSD7A IgG with mouse glomerular extracts (MGE), human glomerular extracts (HGE), mTHSD7A, and hTHSD7A in Western blot analysis under nonreducing and reducing conditions. (D) Immunofluorescence staining of rabbit IgG and nephrin 2 hours after intravenous injection of preimmune IgG or anti-THSD7A IgG into male BALB/c mice (Rabbit [AF488], Nephrin [CY3], DNA [Draq5]). Scale bars indicate 50 µm.
In order to test the in vivo binding capacity of the generated antibodies, we injected 500 µg of preimmune IgG and anti-THSD7A IgG into male BALB/c mice. Two hours after injection, rabbit IgG was bound along the GFB in colocalization with nephrin in the mouse that received anti-THSD7A IgG, whereas no binding was seen in the mouse that received preimmune IgG (Figure 1D). Taken together, these results suggest the presence of specific antibodies in the rabbit serum that bind conformation-dependent epitopes of THSD7A, similar to patient anti-THSD7A antibodies.
Disease Development in Mice Receiving Anti-THSD7A Antibodies
As three different rabbits were immunized to generate anti-THSD7A antibodies, we initially tested the effect of the different rabbit IgGs on 15-week-old male BALB/c mice. Two mice were injected with 1.4 mg total rabbit IgG purified from serum of the three immunized rabbits as well as from one serum that was taken before immunization. Mice that were injected with anti-THSD7A IgG from the three immunized rabbits developed a similar degree of proteinuria and showed binding of rabbit IgG along the GFB (Supplemental Figure 4, A and B). Therefore, the anti-THSD7A antibody–containing rabbit sera were pooled for further experiments in order to be able to investigate a larger number of animals.
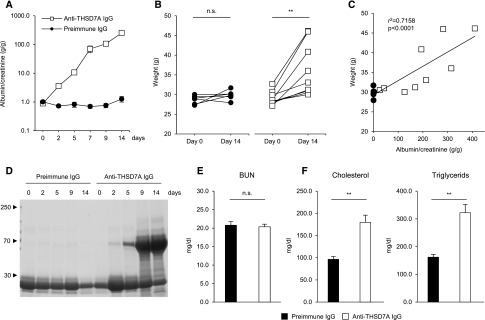
Two days after intravenous injection of anti-THSD7A IgG, mice tripled urinary albumin excretion compared with their baseline values that were measured before antibody treatment (Figure 2A). Proteinuria increased gradually over the observation period of 14 days, reaching a mean albuminuria of 217 g/g, with some mice reaching top values of around 400 g/g albumin-to-creatinine ratio. Mice that received preimmune IgG did not increase urinary albumin excretion (Figure 2A). Moreover, some of the mice that were injected with anti-THSD7A IgG developed severe ascites and a significant weight gain of 24% over all animals (Figure 2B). Body weight significantly correlated with degree of proteinuria after 14 days (Figure 2C). Proteinuria was nonselective, as seen in a Coomassie blue–stained gel after electrophoretic separation of the proteins present in the urine (Figure 2D). All mice had levels of BUN that were within the normal range after 14 days, suggesting that no acute kidney failure had developed despite the gross proteinuria (Figure 2E). On the other hand, serum levels of both cholesterol and triglycerides increased in mice that were injected with anti-THSD7A IgG compared with the control group (Figure 2F). Because of the severe ascites in some of the mice, we tested whether disease could also be initiated with injection of lower amounts of antibody. We found that the injection of 1, 0.7, and 0.35 mg of rabbit IgG still resulted in an early onset of proteinuria, but the increase in proteinuria and the final levels of proteinuria were dose-dependent (Supplemental Figure 4C). In summary, these results describe the development of a severe nephrotic syndrome after exposure to anti-THSD7A antibodies.
Figure 2.
Mice develop a nephrotic syndrome after exposure to anti-THSD7A antibodies. Proteinuria as measured by albumin-to-creatinine ratio (A) and weight change (B) in mice after injection of preimmune or anti-THSD7A IgG. (C) Correlation of mouse body weight with proteinuria 14 days after exposure to preimmune IgG or anti-THSD7A antibodies. (D) Coomassie blue staining of urine containing 0.5 µg of creatinine from one mouse that was exposed to preimmune IgG and one mouse that was exposed to anti-THSD7A IgG. Serum values of BUN (E) as well as cholesterol and triglycerides (F) 2 weeks after injection of preimmune or anti-THSD7A IgG. Data depict mean±SEM. **P<0.01, two-tailed nonparametric Mann–Whitney U test.
Histologic and Ultrastructural Changes in Mice Receiving Anti-THSD7A Antibodies
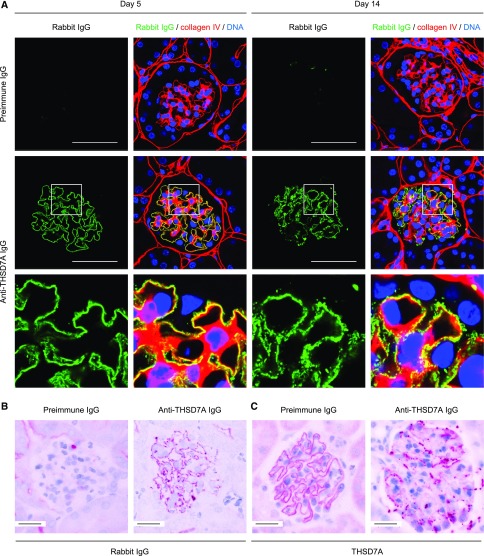
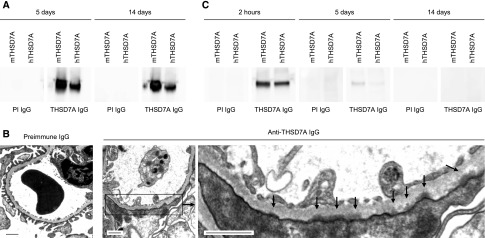
We next investigated the histologic changes in mice after antibody injection. Light microscopic evaluation of the glomerular architecture using PAS staining showed only mild alterations. Podocytes were swollen and exhibited a vacuolated cytoplasm and the glomerular basement membrane (GBM) was slightly thickened in comparison to preimmune IgG–injected mice. No alterations of the mesangium were noted in anti-THSD7A IgG–injected mice (Supplemental Figure 5). Although no rabbit IgG was bound along the GFB 5 days after injection of preimmune IgG, mice that were injected with anti-THSD7A IgG had a pseudolinear binding pattern of rabbit IgG along the GBM in immunofluorescence analysis (Figure 3A). Thereby, the rabbit IgG was located on the outer (subepithelial) aspect of the GBM, suggesting binding to a podocyte antigen. However, after 14 days, the staining for rabbit IgG was granular and in a strictly subepithelial location (Figure 3A). This granular deposition of rabbit IgG along the GFB was also seen in immunohistochemical analyses after 14 days in the mice that were injected with anti-THSD7A IgG (Figure 3B). Moreover, the rabbit IgG colocalized with THSD7A in granular immune complexes after 14 days (Supplemental Figure 6). Interestingly, the staining for the antigen changed toward a fine granular pattern with enhanced intensity in the mice that received anti-THSD7A IgG in immunohistochemical analyses after 14 days (Figure 3C), resembling the situation seen in patients with THSD7A-associated MN.4,9 We next eluted IgG from frozen kidney sections of injected animals and tested for IgG reactivity with recombinant mTHSD7A and hTHSD7A by Western blot analysis. IgG eluted from mice that received anti-THSD7A IgG, but not from mice that received preimmune IgG, recognized recombinant mTHSD7A and hTHSD7A, suggesting antigen-specificity of the bound antibodies (Figure 4A). Furthermore, in electron microscopic analyses after 14 days, anti-THSD7A IgG–injected mice showed electron-dense deposits in a strictly subepithelial location and effacement of podocyte foot processes (Figure 4B). Circulating anti-THSD7A IgG was still detected in the serum of these mice after 5 days, but not after 14 days, suggesting that all circulating THSD7A-specific antibodies were either bound to their target antigen or lost because of the massive proteinuria (Figure 4C). Exposure of mice to foreign IgG is usually followed by the generation of antibodies against this foreign IgG. We did not find any deposition of mouse IgG in our mice after 5 days, but sparse mouse IgG was bound along the GFB after 14 days in the mice that were injected with anti-THSD7A IgG (Supplemental Figure 7). Taken together, these results demonstrate that mice develop the typical histomorphologic signs of human MN after exposure to rabbit anti-THSD7A antibodies.
Figure 3.
Mice develop the histologic signs of MN after exposure to anti-THSD7A antibodies. (A) Immunofluorescence staining for rabbit IgG and collagen type 4 at 5 days and 2 weeks after injection of preimmune or anti-THSD7A IgG (Rabbit IgG [AF488], collagen type IV [CY3], DNA [Draq5]). Scale bars represent 50 µm. Lower panels represent 4× enlargements of the boxed areas above. (B and C) Immunohistochemical staining for rabbit IgG (B) and THSD7A (Rabbit IgG [AF488]) (C) 2 weeks after injection of preimmune or anti-THSD7A IgG (THSD7A [Neufuchsin]).
Figure 4.
Antibody elution and ultrastructural changes after exposure to anti-THSD7A IgG. (A) Western blot analysis of reactivity of IgG eluted from frozen kidney sections 5 and 14 days after injection of preimmune or anti-THSD7A IgG (PI IgG and THSD7A IgG, respectively) with recombinant mTHSD7A and hTHSD7A. (B) Electron microscopic studies of mice 2 weeks after injection of preimmune or anti-THSD7A IgG. Arrows in the lower left panel indicate subepithelial electron-dense deposits. Scale bars indicate 1 µm. (C) Western blot analysis of reactivity of mouse sera with mTHSD7A and hTHSD7A 2 hours, 5 days, and 14 days after exposure to PI IgG and THSD7A IgG. An HRP-conjugated anti-rabbit IgG antibody was used as the secondary antibody to detect circulating rabbit anti-THSD7A IgG.
Rabbit Anti-THSD7A Antibodies and Complement Activation
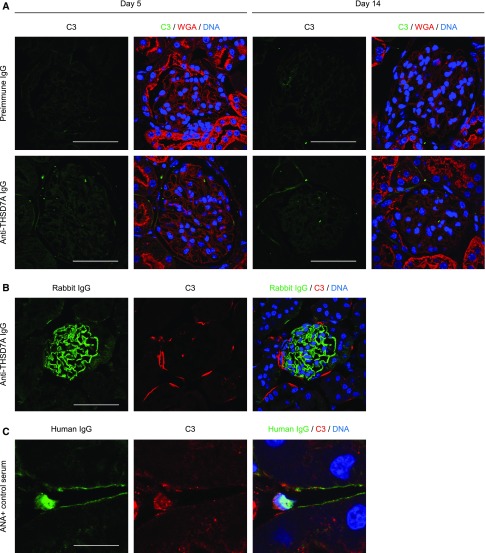
In order to evaluate a potential activation of the complement system, which is considered to be crucial in the pathogenesis of MN, we stained for complement component 3 (C3) in our mice 5 and 14 days after induction of anti-THSD7A MN. Surprisingly, no C3 deposition was seen in the preimmune IgG– or anti-THSD7A–injected mice (Figure 5A). So as not to miss a possible activation of the complement system at a very early time point, we injected another three mice each with preimmune IgG and anti-THSD7A IgG, and mice were euthanized after 3 days, i.e., shortly after the development of proteinuria. In line with the above results and our previous observations,17 we could not detect any significant deposition of C3 at this early time point (data not shown).
Figure 5.
Anti-THSD7A antibodies and complement activation. (A) Immunofluorescence staining for C3, wheat germ agglutinin (WGA), and DNA in mice 5 days and 14 days after injection of preimmune IgG or anti-THSD7A IgG (C3 [FITC], WGA [Rhodamine], DNA [Draq5]). Scale bars indicate 50 µm. (B) Immunofluorescence staining for rabbit IgG, C3, and DNA after incubation of cryosections from naïve murine kidneys with anti-THSD7A IgG and complement-containing full human serum (Rabbit IgG [AF488], C3 [CY3], DNA [Draq5]). Scale bars indicate 50 µm. (C) Immunofluorescence staining for human IgG, C3, and DNA after incubation of cryosections from naïve murine kidneys with ANA-containing human serum and complement-containing full human serum (Human IgG [CY2], C3 [CY3], DNA [Draq5]). Scale bars indicate 12.5 µm.
We next investigated whether IgG purified from our rabbits before or after immunization was capable of activating the complement cascade in vitro.19 Paraffin sections from naïve murine kidneys were incubated with either preimmune or anti-THSD7A IgG diluted in complement activation buffer supplemented with complement-containing or heat-inactivated (complement-depleted) full human or mouse serum. Linear binding of rabbit IgG to the GFB was observed after incubation with anti-THSD7A IgG only (Figure 5B and data not shown). However, staining for complement did not exhibit complement activation secondary to anti-THSD7A binding in the presence of complement-containing human serum (Figure 5B). Consequently, no complement activation was seen when complement-depleted human serum was used (Supplemental Figure 8A). As an assay positive control, we incubated naïve mouse kidney paraffin sections with serum from patients with high anti-nuclear antibody (ANA) titers diluted in complement activation buffer that was supplemented with complement-containing or complement-depleted full human or mouse serum. Exposure to ANA-containing serum resulted in a positive nuclear binding of human IgG in tubular, endothelial, and glomerular cells. Furthermore, nuclear complement deposition was detected in the presence of complement-containing human serum (Figure 5C). In contrast, nuclear complement deposition was absent in ANA-containing serum diluted in heat-inactivated human serum (Supplemental Figure 8A). Identical results were obtained when experiments were performed with complement-containing or complement-depleted mouse sera (Supplemental Figure 8B). Together, these data demonstrate that, in contrast to human ANAs, purified rabbit anti-THSD7A IgG binds to THSD7A and induces clinical and morphologic MN, but lacks the capability of activating complement in mice.
Podocyte Alterations after Exposure to Anti-THSD7A Antibodies
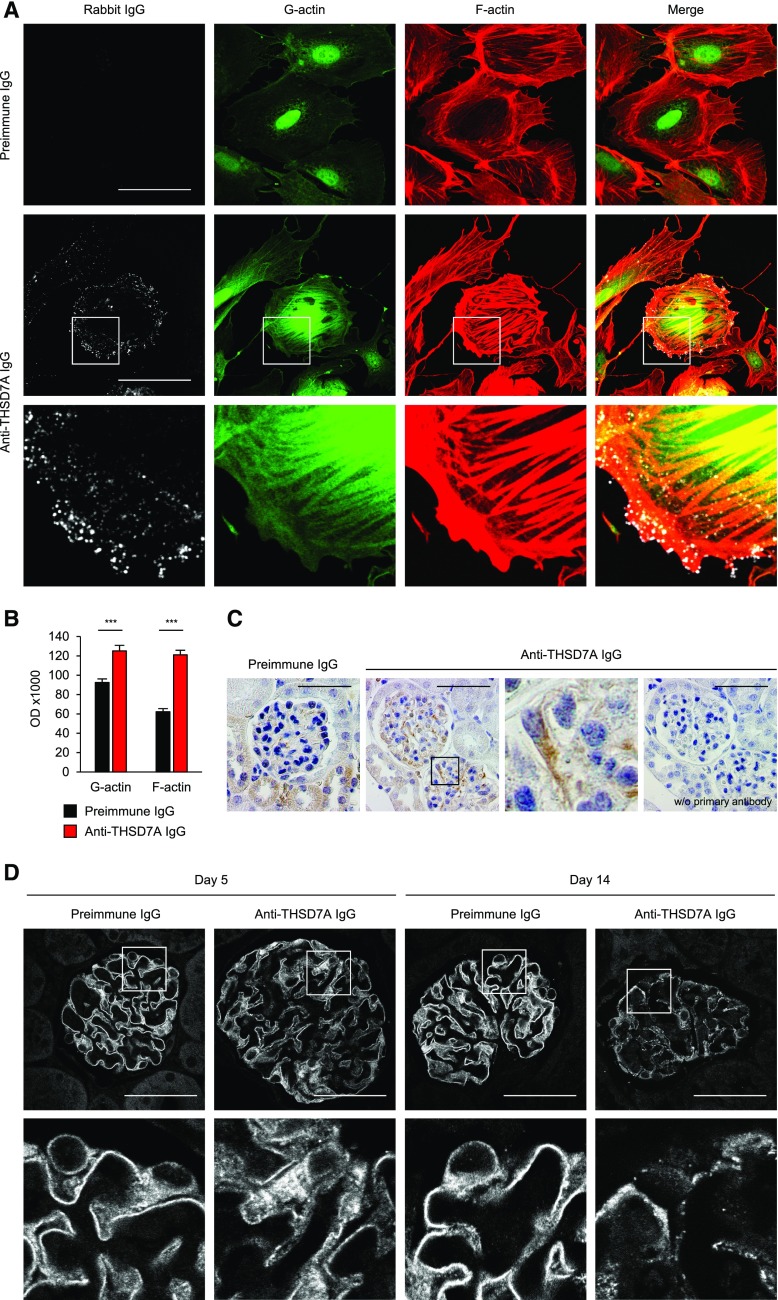
We next examined whether rabbit anti-THSD7A antibodies could directly affect the cellular architecture of THSD7A-expressing cells. We generated primary glomerular epithelial cells (GECs) by culturing isolated murine glomeruli on collagen 1–coated plates. These cells are known to express THSD7A for at least 5 days.17 A 1-hour treatment of GECs with rabbit anti-THSD7A IgG resulted in binding of rabbit IgG to the cell membrane, a contraction of cells, and marked cytoskeletal rearrangement with formation of stress fibers and enhanced G-and F-actin staining (Figure 6A). Quantification of G- and F-actin optical density in cells treated with anti-THSD7A IgG compared with cells treated with preimmune IgG exhibited a 1.4- and a two-fold increase, respectively (Figure 6B). No rabbit IgG binding or cytoskeletal rearrangement was observed in response to incubation with preimmune IgG.
Figure 6.
Anti-THSD7A antibodies and podocyte damage. (A) Immunofluorescence staining for rabbit IgG, G-actin, and F-actin (phalloidin) in primary cultured GECs after a 1-hour incubation with 1:100 preimmune or anti-THSD7A IgG (Rabbit IgG [AF488], G-actin [FITC], F-actin [AF568]). Lower panels represent 4× enlargements of the boxed areas in the middle panels. Scale bars indicate 50 µm. (B) Quantification of stress fiber formation after treatment of GECs with preimmune or anti-THSD7A IgG. One hundred images from three independent experiments were analyzed. Data indicate mean±SEM of actin optical density of individually circled cells. ***P<0.001, two-tailed nonparametric Mann–Whitney U test. (C) Immunohistochemical staining for SOD2 in mice 14 days after injection of preimmune or anti-THSD7A IgG (SOD2 [AF488]). The third panel represents a 4× enlargement of the boxed area in the second panel. As the antibody against SOD2 is derived from rabbits, we performed an additional control using the secondary anti-rabbit antibody only on a consecutive section of the diseased mouse (right panel). Scale bars indicate 50 µm. (D) Immunofluorescence staining for nephrin in mice 5 days and 14 days after injection of preimmune IgG or anti-THSD7A IgG. Scale bars indicate 50 µm (Nephrin [C2]). Lower panels represent 4× enlargements of the boxed areas above.
In order to investigate whether the changes in cytoskeletal organization were accompanied by altered signaling between the extracellular matrix and the actin cytoskeleton, we performed staining for phosphorylated paxillin.20,21 We found an increase in phosphorylated paxillin–positive focal adhesions in GECs exposed to anti-THSD7A IgG only (Supplemental Figure 9).
Oxidative stress and an upregulation of superoxide dismutase 2 (SOD2) are features of podocyte injury in MN.22 Accordingly, we found SOD2 expression to be increased in mice that were exposed to anti-THSD7A IgG compared with preimmune IgG (Figure 6C). Additionally, podocyte injury was indicated by a slight disruption of nephrin localization after 5 days and a severe disintegration after 14 days in the mice that received anti-THSD7A IgG but not controls (Figure 6D). In order to examine whether the high-level proteinuria and disruption of podocyte architecture was accompanied by a decline in actual podocyte number, we stained for the podocyte-specific protein WT1 and counted positive cells per glomerular area (Supplemental Figure 10A). We did not find a significant difference in WT1-positive podocytes between anti-THSD7A IgG–injected and control mice after 5 or 14 days (Supplemental Figure 10B). Moreover, as cellular infiltration is not a classic feature of MN, we investigated whether inflammatory cells infiltrated the kidneys after disease induction. Although there were no differences in the number of Ly6G-positive granulocytes between the two groups after 5 or 14 days in either the tubular or glomerular region (Supplemental Figure 11), there were a higher number of CD3-positive T cells after 14 days in both the tubular and glomerular compartment in mice that received anti-THSD7A IgG compared with mice that received preimmune IgG (Supplemental Figure 12). However, proteinuria and MN morphology developed before the immigration of T cells, strongly suggesting that the development of MN in these mice is independent of T cells and that the cellular infiltrate is a consequence of the tubulointerstitial damage, secondary to the severe damage at the GFB induced by anti-THSD7A antibodies.
Anti-THSD7A IgG Injection into Other Rodents
In order to evaluate whether our model also works in other rodents, we injected 1.4 mg preimmune or anti-THSD7A IgG into male C57BL/6 and DBA/J1 mice, as well as 10 mg preimmune or anti-THSD7A IgG into male Sprague–Dawley rats. After intravenous injection of preimmune or anti-THSD7A IgG, C57BL/6 mice developed a very low degree of proteinuria after 3 days. This proteinuria was not statistically significant and only transient. After 9 days, mice had a urinary albumin excretion that was comparable to preimmune IgG–injected control mice (Supplemental Figure 13A). In immunofluorescence analyses, C57BL/6 mice exposed to anti-THSD7A IgG had granular staining for rabbit IgG after 30 days, which was absent in control mice (Supplemental Figure 13B). Similarly, DBA/J1 mice injected with anti-THSD7A IgG also did not develop significant proteinuria, but still had rabbit IgG bound along the GFB (Supplemental Figure 13, C and D).
A total of eight 6-month-old male Sprague–Dawley rats that weighed around 500 g were injected with 10 mg preimmune or anti-THSD7A IgG. None of the rats demonstrated an increased albumin excretion (data not shown). However, rats that received anti-THSD7A IgG showed linear staining for rabbit IgG after 30 days (Supplemental Figure 13E). Taken together, these data show that anti-THSD7A IgG binds to the GFB in different rodent species. However, the genetic background seems crucial for the induction of proteinuria in our model of THSD7A-associated MN.
Knockdown of thsd7aa in Zebrafish Larvae Leads to an Impairment of the Glomerular Filtration Barrier
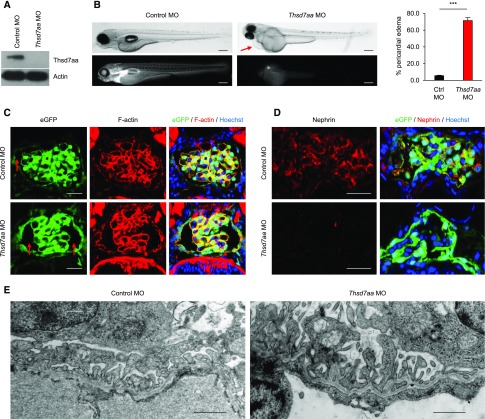
The zebrafish ortholog of THSD7A, thsd7aa, shares 69% and 68% of homology with hTHSD7A and mTHSD7A, respectively, and has been suggested to promote angiogenesis in zebrafish.23 In order to investigate whether thsd7aa itself has a function in maintenance of GFB integrity, we aimed to elucidate the role of thsd7aa for GFB development and function in zebrafish larvae. By injection of morpholinos against thsd7aa (thsd7aa MO) into fertilized eggs, we performed a targeted gene knockdown in two different zebrafish strains. First, we used transgenic CADE zebrafish larvae, which express the GFP-labeled, group-specific component (GC-GFP) in the blood. The molecular mass of GC-GFP is 78 kDa, and thus it cannot pass an intact GFB.24 A decrease in circulating GC-GFP is indicative of a compromised GFB in zebrafish.25 Second, we used ET zebrafish larvae, which express eGFP under the specific control of the wt1a promotor in podocytes and parietal epithelial cells of the pronephros.26 We confirmed the knockdown of thsd7aa in zebrafish larvae by Western blot (Figure 7A). Compared with control MO-treated CADE larvae, the majority of thsd7aa MO-treated larvae developed marked pericardial edema (Figure 7B), a hallmark of a compromised GFB in zebrafish. Moreover, the thsd7aa MO-treated larvae had a marked decrease in vascular fluorescence, suggesting loss of GC-GFP into the tank water (Figure 7B, lower panels). Histologic examination of the glomeruli of ET zebrafish larvae revealed morphologic changes associated with a dilated Bowman’s space and malformation of the glomerular tuft consisting of a decreased capillarization, and a reduction of podocytes per glomerular tuft in thsd7aa MO-treated animals but not controls (Figure 7C). Additionally, we found a downregulation of the expression of the podocyte-specific protein nephrin in podocytes of thsd7aa MO-treated larvae (Figure 7D). Electron microscopy revealed focal areas of effaced podocyte foot processes in thsd7aa MO-treated animals but not controls (Figure 7E). Interestingly, podocytes consistently developed filopodia-like projections into the urinary space at their apical membrane (Figure 7E). Together, these results suggest an important role of THSD7A for the integrity and function of the podocyte and of the GFB in zebrafish.
Figure 7.
Knockdown of thsd7aa in zebrafish disturbs glomerular filtration barrier integrity. (A) Western blot analysis for thsd7aa after injection of a control morpholino (control MO) or a morpholino against thsd7aa (thsd7aa MO) into zebrafish larvae. (B) Phase contrast microscopic images (upper panels) and fluorescence microscopic images (lower panels) of CADE zebrafish larvae expressing the GC-GFP in the bloodstream after injection of either control MO or thsd7aa MO. Arrow indicates the pericardial area. Scale bars indicate 1 mm. ***P<0.001, two-tailed nonparametric Mann–Whitney U Test. (C and D) Immunofluorescence staining for eGFP and F-actin (eGFP, F-actin [AF568]) (C) or nephrin and eGFP (Nephrin [CY3]) (D) in zebrafish injected with control MO or thsd7aa MO. Arrows in (C) indicate Bowman’s space. Scale bars indicate 20 µm. (E) Electron microscopic studies in zebrafish larvae after injection of control MO or thsd7aa MO. Scale bars indicate 1 µm.
Discussion
Membranous nephropathy is the most common cause of nephrotic syndrome in white adults. In 3%–5% of all cases, MN is associated with autoantibodies to THSD7A, a podocyte-specific, high molecular weight membrane protein. Here, we introduce a reproducible murine model of THSD7A-associated MN that does not rely on the availability of human serum containing anti-THSD7A antibodies.
In a recent study,17 we demonstrated that hTHSD7A autoantibodies are sufficient to induce MN in wild-type BALB/c mice, which constitutively express THSD7A on podocytes.27 Injection of human anti-THSD7A autoantibodies into mice resulted in MN with granular subepithelial IgG deposition, subepithelial immune deposits, broadening of podocyte foot processes, and development of proteinuria. This proteinuria, however, was subnephrotic.17 In accordance with these previous findings, the injection of rabbit anti-THSD7A antibodies in this study also resulted in MN, yet with the development of more pronounced granular subepithelial IgG deposits, subepithelial electron-dense immune deposits, and extensive foot process effacement in BALB/c mice. Additionally, mice developed a full-blown nephrotic syndrome with proteinuria, hyperlipidemia, weight gain, and ascites. The onset of proteinuria after the injection of rabbit anti-THSD7A antibodies was comparable to the time point after the injection of human anti-THSD7A autoantibodies.17 In both models, BALB/c mice developed early proteinuria after 2–3 days, supposedly a direct effect of the heterologous rabbit or human antibodies on the antigen. This proteinuria was then sustained, likely because of the autologous reaction with generation of mouse anti-rabbit IgG antibodies, which occurs about 1 week after exposure to foreign IgG.19 Interestingly, proteinuria was more prominent in mice after injection of rabbit anti-THSD7A antibodies than after injection of affinity-purified human anti-THSD7A autoantibodies or anti-THSD7A antibody-positive serum from patients with THSD7A-associated MN in the same strain.17 This could be the result of a higher anti-THSD7A antibody dose achieved with the injection of purified rabbit anti-THSD7A antibodies compared with the human anti-THSD7A autoantibodies. However, another possible explanation could be that the polyclonal rabbit and human anti-THSD7A antibodies bound to multiple and likely different epitopes present in THSD7A. This could result in an enhanced deposition of rabbit IgG in the subepithelial space and/or in a more pronounced alteration of the hitherto unknown function of THSD7A in podocytes. Furthermore, the autologous reaction could differ in mice injected with rabbit IgG in comparison with human IgG because of stronger antigenicity of rabbit IgG in mice. Of note, the induction of MN in mice with rabbit anti-THSD7A antibodies was strain-dependent. C57BL/6 mice and DBA/J1 mice exhibited only a weak heterologous phase after the injection of rabbit anti-THSD7A antibodies, which we cannot sufficiently explain at this time. However, some issues may be relevant. Considering a direct effect of the antibody on the biologic function of the antigen as a potential mechanism of proteinuria, differences in GFB architecture, such as GBM composition28 or Fc receptor expression on podocytes,29 could represent one explanation for the differential reaction to anti-THSD7A antibodies across murine strains. Moreover, BALB/c mice are known to exert a predominant Th2 response, whereas C57BL/6 mice promote a Th1 response to Igs,30–33 which could influence the extent of the autologous phase. Additionally, the quantity of injected rabbit anti-THSD7A antibodies modulated the severity of proteinuria in BALB/c mice. Consequently, the failure of rats to develop proteinuria despite bound rabbit anti-THSD7A antibodies could be (besides species-dependent differences in THSD7A protein sequence and GFB composition) secondary to a quantitative problem.
Histologically, deposition of complement is a hallmark of human MN and is also present in THSD7A-associated MN.4 Remarkably, the human IgG isotype deposited in glomeruli of patients with primary MN, including THSD7A-associated MN, belongs to the IgG4 subclass and is considered to be noncomplement-fixing.34 There is considerable experimental evidence from studies in passive Heymann nephritis, an established rat model of experimental MN,11 that proteinuria in this model depends on the activation of the complement system and that the complement membrane attack complex C5b-9 is, in part, responsible for podocyte injury and the development of proteinuria in the heterologous and autologous phase.16,35 Other studies using genetically modified rodents suggest that proteinuria can develop in the absence of certain complement factors in the passive Heymann nephritis model36,37 and in the murine antipodocyte nephritis model.38 We therefore analyzed mouse kidneys from our experiments for the deposition of complement components in the subepithelial space. We could not detect C3 in mice that received rabbit anti-THSD7A antibodies in both the heterologous and the autologous phase of the disease. These findings were in accordance with the absence of complement deposition after the injection of purified human anti-THSD7A autoantibodies.17 There may be several reasons for this. First, rabbit anti-THSD7A antibodies did not activate the complement system in an in vitro complement activation assay that was established by Salant and Cybulsky in 1988,19 suggesting that these antibodies may be noncomplement-fixing. This could possibly be related to the use of acid elution during the antibody purification procedure. Also, the rabbit anti-THSD7A antibodies may be, similar to human IgG4 or murine IgG1,39,40 of a noncomplement-activating subclass. Second, complement activation in the autologous phase of the disease may rely on the presence of large amounts of murine IgG, which was deposited only barely in the subepithelial space of the GFB. However, in light of our results, it seems possible that complement activation is not vital in the initiation of podocyte injury and proteinuria in this THSD7A-dependent mouse model of MN. As complement increases immune complex solubility and elimination, it is also interesting to think of the activation of the complement system in MN as an attempt of the immune system to limit glomerular damage.41
The function of THSD7A in podocytes remains unknown. Soluble THSD7A has been shown to promote endothelial cell migration during angiogenesis through activation of focal adhesion kinase signaling in human umbilical vein endothelial cell sprouts, and through subintestinal vessel angiogenic assays with downstream phosphorylation and activation of ERK1/2 in zebrafish.23 On the other hand, overexpression of a carboxy-terminal fragment of THSD7A in human umbilical vein endothelial cells was found to inhibit endothelial cell migration and suppression of THSD7A expression to promote endothelial cell migration.42 In accordance with these findings in endothelial cells, we could demonstrate that human17 and rabbit anti-THSD7A antibodies were directly pathogenic through alterations in podocyte architecture with increased stress fiber formation and activation of signaling at focal adhesions.
Loss of THSD7A expression in zebrafish by morpholino knockdown resulted in a strong podocyte phenotype with GFB leakiness and consecutive protein loss. These results strengthen the idea that THSD7A is important for normal podocyte function and that perturbation of THSD7A function through antibody binding constitutes a potential mechanism of proteinuria.
In conclusion, this study presents a novel mouse model of THSD7A-dependent MN that strongly resembles human MN regarding proteinuria and histopathologic changes. The use of this mouse model will possibly help to better understand the pathophysiology of MN and may be applied in the search for new therapeutic strategies.
Concise Methods
Generation of Antibodies against Native mTHSD7A and hTHSD7A
Three rabbit antisera (rabbit 1, rabbit 2, and rabbit 3) recognizing native mTHSD7A and hTHSD7A were produced by cDNA immunization essentially as described previously.43,44 Briefly, two expression constructs encoding full-length mTHSD7A and hTHSD7A were conjugated to 1-µm gold particles (Bio-Rad Laboratories, München, Germany). These were ballistically injected into three rabbits at the antibody core unit of the University Medical Center Hamburg-Eppendorf. The rabbits received four immunizations in 3–6 week intervals, each with 12 shots of plasmid-conjugated gold particles (1 µg DNA/mg gold per shot). Hence, rabbits were immunized with a combination of mTHSD7A and hTHSD7A cDNA. Serum was obtained 3 weeks after the last DNA immunization. The specificity of the antiserum for native mTHSD7A and hTHSD7A was verified using immunofluorescence staining of transfected CHO cells with serial dilutions of the antiserum 24 hours after transient cotransfection of cells with expression constructs for nuclear GFP and full-length THSD7A, and by Western blotting with native THSD7A from glomerular extracts and recombinant THSD7A. Bound antibodies were detected with phycoerythrin-conjugated anti-rabbit IgG (Dianova, Hamburg, Germany).
Purification of Rabbit IgG before and after Immunization
Rabbit serum was decomplemented by heating for 30 minutes at 56°C in a water bath. Debris was removed by centrifugation at 14,000 rpm for 15 minutes. For the purification of preimmune and anti-THSD7A IgG from rabbit sera, we used Protein G columns (Thermo Fisher Scientific, Waltham, MA). Columns were washed with 25 ml PBS and rabbit serum that was taken before or after immunization was added to the column. After complete drainage, columns were washed with 20 ml PBS and then IgG was eluted in three steps, each time using 4.5 ml 100 mM glycine (pH 3). The eluate was captured in Tris (pH 9) to neutralize. The buffer was exchanged for PBS by size exclusion chromatography on PD-10 columns (GE Healthcare, Chalfont St. Giles, UK). Purification of IgG was verified using SDS-PAGE and Coomassie blue staining. As around 95% of IgG was in the first two eluates, eluates were pooled and concentrated using Amicon Ultra-15 centrifugal filters (Millipore, Billerica, MA). The final concentration of purified rabbit IgG was determined with a Bradford assay.
Western Blot Analysis
Protein samples were prepared for Western blot analysis by addition of 5× Laemmli buffer (1.5 M Tris-HCl [pH 6.8], 50% glycerol, 10% SDS, 1% bromophenol blue) and subsequent heating to 95°C for 10 minutes. If reducing conditions were desired, 20% β-mercaptoethanol was added to the 5× loading buffer. Proteins were separated by electrophoresis in precast gels (Bio-Rad, Hercules, CA) containing SDS in an electrophoresis chamber (Bio-Rad) in the presence of a migration buffer (25 mM Tris, 192 mM glycine, 0.1% SDS; Amresco, Solon, OH). Proteins were then transferred to methanol-soaked PVDF membranes (Millipore) under semidry conditions in the presence of 25 mM Tris (pH 8.5), 192 mM glycine, and ethanol 20% using Transblot Turbo (Bio-Rad) at 25 V constant for 35 minutes. Membranes were blocked overnight at 4°C in 5% dry milk with PBS, and then incubated with primary and secondary antibodies for 2 hours at room temperature. Purified rabbit IgG and whole mouse serum were used as primary antibodies and were diluted at 1:1000 and 1:100, respectively, with 0.5% dry milk in PBS and horseradish peroxidase (HRP)-conjugated secondary antibodies in PBS alone. Membranes were washed three times for 5 minutes in PBS-Tween 0.05% after incubation with primary and secondary antibodies. Secondary antibodies were HRP-conjugated goat anti-mouse IgG and goat anti-rabbit IgG (all 1:20,000; SouthernBiotech, Birmingham, AL).
Animal Care
Wild-type male BALB/c mice (10–14 weeks old) were bred in the animal facility of the University Medical Center Hamburg-Eppendorf. C57BL/6, DBA/J1, and Sprague–Dawley rats were purchased from Charles Rivers. Animals had free access to water and standard animal chow.
Animal Experiments
Fifteen-week-old male BALB/c mice were injected intravenously with 1.4 mg of either preimmune or anti-THSD7A IgG. For the initial experiments and analyses, two mice each were injected with IgG purified from serum from rabbit 1, 2, and 3, as well as from serum that was taken from rabbit 1 before immunization. IgG from the three rabbits was then pooled for further experiments and 17 mice were injected with anti-THSD7A IgG and nine mice were injected with preimmune IgG. Development of proteinuria was monitored using metabolic cages. Three mice from the preimmune and seven mice from the anti-THSD7A IgG group were euthanized after 5 days. Because of the severe nephrotic syndrome with weight gain due to ascites in most animals, with some animals developing signs of respiratory distress, the remaining animals had to be euthanized after 14 days. Histologic analyses were performed in all animals. For experiments with C57BL/6 and DBA/J1 mice, five (C57BL/6) and three (DBA/J1) male wild-type animals of 14 weeks of age were injected with 1.4 mg of either preimmune or anti-THSD7A IgG. As mice did not develop severe proteinuria, animals were observed for 30 days and then euthanized and analyzed. For rat experiments, four male Sprague–Dawley wild-type rats were used per group and injected with 10 mg preimmune or anti-THSD7A IgG. Animals were euthanized and analyzed after 30 days.
All animal experiments were performed according to national and institutional animal care and ethical guidelines and were approved by the veterinarian agency of Hamburg and the local animal care committee.
Albumin ELISA
Urine albumin content was quantified using a commercially available ELISA system (Bethyl, Montgomery, AL) according to the manufacturer’s instructions. Briefly, 96-well plates were coated at 1:100 with goat anti-mouse albumin in binding buffer (0.05 M carbonate-bicarbonate, pH 9.6) for 60 minutes at room temperature. After washes in 50 mM Tris, 0.14 M NaCl, and 0.05% Tween-20 (pH 8.0), the plates were blocked for 30 minutes at room temperature with 50 mM Tris, 0.14 M NaCl, and 1% BSA (pH 8.0) and rewashed. Diluted urine was incubated for 60 minutes at room temperature. After washes, the secondary antibody (1:40,000; HRP goat anti-mouse albumin) was applied for 60 minutes at room temperature. After washes, enzyme substrate supplied by the kit was added and the color development was stopped after 5 minutes with 2 M sulfuric acid. Extinction was measured at 450 nm in an ELISA plate reader (EL 808; BioTek). The urinary albumin concentration was calculated according to the formula absorption = (A−D)/1+(x/C)B+D, where A and D are values from the standard curve. Regression values for the standard curve were calculated to assess the accuracy of the measured values. Standard curves with r values >0.99 were used. The urinary albumin values were standardized against urinary creatinine values of the same sample.
Immunofluorescence Analyses
For immunolocalization of nephrin (guinea pig pAB, 1:100; Acris Antibodies), laminin (rabbit pAB, 1:1000; Sigma-Aldrich, St. Louis, MO), THSD7A (goat pAB, 1:200; Santa Cruz Biotechnology), WT-1 (rabbit pAB, 1:600; Santa Cruz Biotechnology), rabbit IgG (F488-rbIgG H+L, 1:200; Jackson ImmunoResearch Laboratories), murine IgG (Cy2-mouse IgG H+L, 1:400; Jackson ImmunoResearch Laboratories), C3 (FITC-goat pAB, 1:100; Cappel), or SOD2 (rabbit pAB, 1:100; Acris Antibodies), 2 µM paraffin sections of experimental mouse kidneys were deparaffinized and rehydrated to water. Antigen retrieval was obtained by boiling in citrate buffer at pH 6.1 (60 minutes at constant 98°C), or by digestion with protease XXIV (5 µg/ml; Sigma-Aldrich) for 15 minutes at 37°C. Unspecific binding was blocked with 5% horse serum (Vector Laboratories, Burlingame, CA) with 0.05% Triton X-100 (Sigma-Aldrich) in PBS for 30 minutes at room temperature before incubation at 4°C overnight with primary antibodies in blocking buffer. Staining was visualized with fluorochrome-conjugated secondary antibodies (all affinity purified from Jackson ImmunoResearch Laboratories; 1:400) for 30 minutes at room temperature in 5% horse serum with 0.05% Triton X-100. To control for crossreaction of the anti-rabbit secondary antibodies with the injected and bound rabbit anti-THSD7A IgG, intrinsic IgG was destroyed by excessive boiling. Furthermore, TrueBlot HRP-coupled anti-rabbit antibodies (1:200; eBioscience) were used, which only bind to native rabbit IgG. Additionally, every individual mouse was controlled by omitting the primary antibody. Nuclei were counterstained with DRAQ5 (1:1000; Molecular Probes).
Stainings were evaluated with a confocal LSM 510 meta microscope using the LSM software (all Carl Zeiss GmbH, Jena, Germany), or by an Axioskop using the Axiovision software for light microscopy (all Carl Zeiss GmbH).
Immunohistochemical Analyses
Paraffin sections (1-µM thick) of mouse kidneys were deparaffinized and rehydrated. Antigen retrieval was obtained for rabbit IgG by digestion with protease XXIV (5 µg/ml; Sigma-Aldrich) for 15 minutes at 37°C, for THSD7A by boiling in DAKO antigen retrieval buffer at pH 9 (60 minutes at 98°C), and subsequent cooling. Nonspecific binding was blocked with 5% horse serum (Vector Laboratories) with 0.05% Triton X-100 (Sigma-Aldrich) in PBS for 30 minutes at room temperature before incubation at 4°C overnight with rabbit anti-THSD7A (1:400; Atlas) or with biotinylated anti-rabbit IgG (1:400) in blocking buffer. Staining was visualized with the ZytochemPlus AP Polymer kit (Zytomed Systems, Berlin, Germany) or with the ABC kit (Vector Laboratories) according to the manufacturer’s instruction, with new fuchsin (Merck) as a color substrate. Nuclei were counterstained with hemalum (Merck) and sections were mounted with gum Arabic (Sigma-Aldrich). Negative controls were performed by omitting primary antibodies. Stainings were evaluated with an Axioskop using the Axiovision software (all Carl Zeiss GmbH).
Electron Microscopy
Electron microscopic analyses were performed on kidneys that were fixed in 4% buffered paraformaldehyde. Tissue was postfixed with 1% osmium in 0.1 M sodium-cacodylate buffer, stained with 1% uranyl acetate and embedded in epoxy-resin (Serva, Heidelberg, Germany). Ultrathin sections were cut (Ultramicrotome; Reichert-Jung, Nussloch, Germany) and contrasted with uranyl acetate in methanol followed by lead citrate. Micrographs were generated with a transmission electron microscope (JEM 1010, JEOL).
Antibody Elution
A total of 200–220 sections (10-µm thick) from frozen renal tissue were resuspended in 1 ml sterile PBS and centrifuged at low speed for 1 minute. The pellet was then washed three times in 1 ml PBS. After the last wash, the pellet was resuspended in 150 µl 25 mM citrate (pH 3.2) and left on ice for 20 minutes with intermediate shaking. Subsequently, the sample was centrifuged again and the supernatant was added to 150 µl 1 M Tris (pH 8). The pellet was resuspended in 150 µl 25 mM citrate (pH 2.5) and left on ice for 20 minutes with intermediate shaking. The sample was then centrifuged for 5 minutes at 14,000 rpm and 4°C. Next, the supernatant was added to the first elution and diluted to 2 ml using 0.05% dry milk in PBS-T. The resulting sample was used as the primary antibody in immunoblot analyses, as described above.
GEC Culture
For the isolation of glomeruli, mice were perfused with Dynabeads (Dynal, Oslo, Norway) as described previously.45,46 Decapsulated glomeruli were plated on collagen 1–coated 10-cm culture plates (Biocoat) in RPMI 1640 supplemented with 10% FCS, 10 mmol/L HEPES, 1 mmol/L sodium pyruvate, 100 U/ml penicillin, 100 mg/ml streptomycin at 38°C, and 5% CO2. Four days after initial glomerular isolation, primary culture GECs were challenged with 1:100 rabbit preimmune or anti-THSD7A IgG. Immunofluorescence analyses were performed 60 minutes after serum challenge. For immunofluorescence analyses, GECs were fixed with 4% paraformaldehyde for 8 minutes at room temperature. Staining was performed as described for frozen tissue sections. Primary antibodies used were rabbit anti-phosphorylated (Y118) paxillin (Life Technologies, coupled with AF568-labeling kit from Life Technologies, according to the manufacturer’s instructions), AF568- or AF488-Phalloidin (both 1:400; Molecular Probes), AF488-Deoxyribonuclease 1 (1:400; Molecular Probes), and Cy5 anti-rabbit (1:200; Jackson ImmunoResearch Laboratories).
G- and F-actin quantification was performed using Fiji software. For analysis, at least 15 greyscale images from each of the experiments were used at a 1000-fold magnification. Individual cell areas were circled and optical density was analyzed.
In Vitro Fixation of Complement Assay
Cryosections (5-µm thick) of naïve murine kidneys were fixed for 10 minutes at −20°C in 10% acetone and air-dried. The procedure was carried out as described previously.19 Briefly, sections were incubated for 30 minutes at room temperature with preimmune IgG, anti-THSD7A IgG, or patient serum with high ANA titers (all 1:100) in fresh complement activation buffer (145 mM NaCl, 5 mM KCl, 0.5 mM MgSO4, 1 mM Na2HPO4, 5 mM glucose, 20 mM HEPES, 0.5 mM CaCl2, pH 7.4) containing fresh or heat-inactivated human or mouse serum (1:20). Serum was heat-inactivated by boiling for 30 minutes at 57°C in a water bath. After washing with PBS (three times for 5 minutes), sections were stained for 30 minutes at room temperature with goat anti–C3-FITC (1:8; Cappel) for samples incubated with mouse complement or with goat anti-human C3 (1:100; Thermo Fisher Scientific) followed by Cy3–anti-goat IgG (1:400) for samples incubated with human complement or with donkey anti–rabbit-AF488 (for detection of the rabbit IgG) or with donkey anti-human IgG-Cy2 (for detection of the human ANA antibodies). Sections incubated with preimmune rabbit IgG and stained exactly as above served as negative controls.
Zebrafish Experiments
Zebrafish were grown, mated, and maintained at 28.5°C, according to standard protocols as previously described.24,26 Zebrafish husbandry and experiments on larvae were performed in accordance with German animal protection law overseen by the agencies of the Federal State of Mecklenburg-Pomerania. Embryos were kept and handled in E3 solution.
The following zebrafish strains were used: CADE [Tg(−3.5 fabp10a:gc-eGFP) mitfaw2/w2; roya9/a9], the larvae express a 78-kDa GC-eGFP fusion protein in the blood47; ET [Tg(mitfaw2/w2;roya9/a9);Tg(−35.1wt1a:eGFP)], the transparent larvae express eGFP in the cytosol of podocytes and parietal epithelial cells under control of the wt1a promotor.26
The following morpholinos were used: thsd7aa MO: 5′-GTAAAGCAGAAGAAG CTGACCTG AT-3′; Crtl MO: 5′-CCTCTTACCTCAGTTACAATTTATA-3′. MOs were diluted to a concentration of 0.3 mM. A volume of approximately 3 nl per zebrafish egg was injected into the yolk at the one- to four-cell stage using a microinjector (Transjector 5246; Eppendorf). For histologic and immunofluorescence staining we followed our previously published protocols.47 An anti-nephrin antibody (zebrafish; Innovagen) was used. For transmission electron microscopy, we used our standard protocol.47
For in vivo imaging of the glomerular morphology, zebrafish larvae at 5 days post fertilization were imaged by two-photon microscopy with an excitation wavelength of 860 nm, as described before.26 Z-stacks, three-dimensional reconstruction, and exportation were done using a Zeiss LSM710MP system equipped with the ZEN 2010 Software (Carl Zeiss GmbH).
Statistical Analyses
Data are expressed as mean±SEM. For statistical analyses for comparison of mouse treatment groups, we performed the nonparametric Mann–Whitney U test to enable robust conclusions on effect significance in case of departures from normality associated with small sample sizes. For comparison of more than two conditions, nonparametric one-way ANOVA was performed with Dunn multiple comparison post-test. Replicates used were biologic replicates, which were measured using different samples derived from distinct mice. All animals were wild-type mice and were blindly assigned to the experimental groups. No inclusion or exclusion criteria were defined for animals and no animals were excluded from the analysis.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Silke Dehde and Marlies Sachs for excellent technical assistance. Furthermore, we thank Fabienne Seyfried, Gudrun Dubberke, and Sarah Hewald for technical assistance with cDNA immunization and transfection of CHO cells, Ursula Kneißler for technical assistance with electron microscopic studies in mice, and Anja Blumenthal for performing electron microscopic studies in zebrafish.
This study was supported by grants from the Deutsche Forschungsgemeinschaft as part of the Sonderforschungsbereich 1192 (project B1 to R.A.K.S. and E.H., project B2 to N.M.T. and G.Z., project B3 to C.M.-S., and project B5 to F.K.-N.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017010030/-/DCSupplemental.
References
- 1.Cattran D: Management of membranous nephropathy: When and what for treatment. J Am Soc Nephrol 16: 1188–1194, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Debiec H, Guigonis V, Mougenot B, Decobert F, Haymann JP, Bensman A, Deschênes G, Ronco PM: Antenatal membranous glomerulonephritis due to anti-neutral endopeptidase antibodies. N Engl J Med 346: 2053–2060, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Beck LH Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ: M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomas NM, Beck LH Jr, Meyer-Schwesinger C, Seitz-Polski B, Ma H, Zahner G, Dolla G, Hoxha E, Helmchen U, Dabert-Gay AS, Debayle D, Merchant M, Klein J, Salant DJ, Stahl RA, Lambeau G: Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 371: 2277–2287, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoxha E, Harendza S, Zahner G, Panzer U, Steinmetz O, Fechner K, Helmchen U, Stahl RA: An immunofluorescence test for phospholipase-A2-receptor antibodies and its clinical usefulness in patients with membranous glomerulonephritis. Nephrol Dial Transplant 26: 2526–2532, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Hoxha E, Harendza S, Pinnschmidt H, Panzer U, Stahl RA: M-type phospholipase A2 receptor autoantibodies and renal function in patients with primary membranous nephropathy. Clin J Am Soc Nephrol 9: 1883–1890, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hofstra JM, Beck LH Jr, Beck DM, Wetzels JF, Salant DJ: Anti-phospholipase A2 receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin J Am Soc Nephrol 6: 1286–1291, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoxha E, Harendza S, Pinnschmidt HO, Tomas NM, Helmchen U, Panzer U, Stahl RA: Spontaneous remission of proteinuria is a frequent event in phospholipase A2 receptor antibody-negative patients with membranous nephropathy. Nephrol Dial Transplant 30: 1862–1869, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Hoxha E, Beck LH Jr, Wiech T, Tomas NM, Probst C, Mindorf S, Meyer-Schwesinger C, Zahner G, Stahl PR, Schopper R, Panzer U, Harendza S, Helmchen U, Salant DJ, Stahl RA: An indirect immunofluorescence method facilitates detection of thrombospondin type 1 domain-containing 7A-specific antibodies in membranous nephropathy. J Am Soc Nephrol 28: 520–531, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoxha E, Wiech T, Stahl PR, Zahner G, Tomas NM, Meyer-Schwesinger C, Wenzel U, Janneck M, Steinmetz OM, Panzer U, Harendza S, Stahl RA: A mechanism for cancer-associated membranous nephropathy. N Engl J Med 374: 1995–1996, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Heymann W: III. Nephrotic syndrome induced by injection of anti-kidney serum. Methods Med Res 5: 264–267, 1952 [PubMed] [Google Scholar]
- 12.Heymann W, Hackel DB, Harwood S, Wilson SG, Hunter JL: Production of nephrotic syndrome in rats by Freund’s adjuvants and rat kidney suspensions. Proc Soc Exp Biol Med 100: 660–664, 1959 [DOI] [PubMed] [Google Scholar]
- 13.Kerjaschki D, Neale TJ: Molecular mechanisms of glomerular injury in rat experimental membranous nephropathy (Heymann nephritis). J Am Soc Nephrol 7: 2518–2526, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Kerjaschki D, Farquhar MG: The pathogenic antigen of Heymann nephritis is a membrane glycoprotein of the renal proximal tubule brush border. Proc Natl Acad Sci USA 79: 5557–5561, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farquhar MG, Saito A, Kerjaschki D, Orlando RA: The Heymann nephritis antigenic complex: Megalin (gp330) and RAP. J Am Soc Nephrol 6: 35–47, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Salant DJ, Belok S, Madaio MP, Couser WG: A new role for complement in experimental membranous nephropathy in rats. J Clin Invest 66: 1339–1350, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomas NM, Hoxha E, Reinicke AT, Fester L, Helmchen U, Gerth J, Bachmann F, Budde K, Koch-Nolte F, Zahner G, Rune G, Lambeau G, Meyer-Schwesinger C, Stahl RA: Autoantibodies against thrombospondin type 1 domain-containing 7A induce membranous nephropathy. J Clin Invest 126: 2519–2532, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koch-Nolte F, Glowacki G, Bannas P, Braasch F, Dubberke G, Ortolan E, Funaro A, Malavasi F, Haag F: Use of genetic immunization to raise antibodies recognizing toxin-related cell surface ADP-ribosyltransferases in native conformation. Cell Immunol 236: 66–71, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Salant DJ, Cybulsky AV: Experimental glomerulonephritis. Methods Enzymol 162: 421–461, 1988 [DOI] [PubMed] [Google Scholar]
- 20.Turner CE, Glenney JR Jr, Burridge K: Paxillin: A new vinculin-binding protein present in focal adhesions. J Cell Biol 111: 1059–1068, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burridge K, Turner CE, Romer LH: Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: A role in cytoskeletal assembly. J Cell Biol 119: 893–903, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prunotto M, Carnevali ML, Candiano G, Murtas C, Bruschi M, Corradini E, Trivelli A, Magnasco A, Petretto A, Santucci L, Mattei S, Gatti R, Scolari F, Kador P, Allegri L, Ghiggeri GM: Autoimmunity in membranous nephropathy targets aldose reductase and SOD2. J Am Soc Nephrol 21: 507–519, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuo MW, Wang CH, Wu HC, Chang SJ, Chuang YJ: Soluble THSD7A is an N-glycoprotein that promotes endothelial cell migration and tube formation in angiogenesis. PLoS One 6: e29000, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotb AM, Müller T, Xie J, Anand-Apte B, Endlich K, Endlich N: Simultaneous assessment of glomerular filtration and barrier function in live zebrafish. Am J Physiol Renal Physiol 307: F1427–F1434, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hentschel DM, Mengel M, Boehme L, Liebsch F, Albertin C, Bonventre JV, Haller H, Schiffer M: Rapid screening of glomerular slit diaphragm integrity in larval zebrafish. Am J Physiol Renal Physiol 293: F1746–F1750, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Endlich N, Simon O, Göpferich A, Wegner H, Moeller MJ, Rumpel E, Kotb AM, Endlich K: Two-photon microscopy reveals stationary podocytes in living zebrafish larvae. J Am Soc Nephrol 25: 681–686, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer-Schwesinger C, Lambeau G, Stahl RA: Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 372: 1074–1075, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Randles MJ, Woolf AS, Huang JL, Byron A, Humphries JD, Price KL, Kolatsi-Joannou M, Collinson S, Denny T, Knight D, Mironov A, Starborg T, Korstanje R, Humphries MJ, Long DA, Lennon R: Genetic background is a key determinant of glomerular extracellular matrix composition and organization. J Am Soc Nephrol 26: 3021–3034, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akilesh S, Huber TB, Wu H, Wang G, Hartleben B, Kopp JB, Miner JH, Roopenian DC, Unanue ER, Shaw AS: Podocytes use FcRn to clear IgG from the glomerular basement membrane. Proc Natl Acad Sci USA 105: 967–972, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsieh CS, Macatonia SE, O’Garra A, Murphy KM: T cell genetic background determines default T helper phenotype development in vitro. J Exp Med 181: 713–721, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosmann TR, Coffman RL: TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 7: 145–173, 1989 [DOI] [PubMed] [Google Scholar]
- 32.Reiner SL, Locksley RM: The regulation of immunity to Leishmania major. Annu Rev Immunol 13: 151–177, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Güler ML, Gorham JD, Hsieh CS, Mackey AJ, Steen RG, Dietrich WF, Murphy KM: Genetic susceptibility to Leishmania: IL-12 responsiveness in TH1 cell development. Science 271: 984–987, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Hofstra JM, Debiec H, Short CD, Pellé T, Kleta R, Mathieson PW, Ronco P, Brenchley PE, Wetzels JF: Antiphospholipase A2 receptor antibody titer and subclass in idiopathic membranous nephropathy. J Am Soc Nephrol 23: 1735–1743, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baker PJ, Ochi RF, Schulze M, Johnson RJ, Campbell C, Couser WG: Depletion of C6 prevents development of proteinuria in experimental membranous nephropathy in rats. Am J Pathol 135: 185–194, 1989 [PMC free article] [PubMed] [Google Scholar]
- 36.Spicer ST, Tran GT, Killingsworth MC, Carter N, Power DA, Paizis K, Boyd R, Hodgkinson SJ, Hall BM: Induction of passive Heymann nephritis in complement component 6-deficient PVG rats. J Immunol 179: 172–178, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Leenaerts PL, Hall BM, Van Damme BJ, Daha MR, Vanrenterghem YF: Active Heymann nephritis in complement component C6 deficient rats. Kidney Int 47: 1604–1614, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Meyer-Schwesinger C, Dehde S, Klug P, Becker JU, Mathey S, Arefi K, Balabanov S, Venz S, Endlich KH, Pekna M, Gessner JE, Thaiss F, Meyer TN: Nephrotic syndrome and subepithelial deposits in a mouse model of immune-mediated anti-podocyte glomerulonephritis. J Immunol 187: 3218–3229, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Davies AM, Sutton BJ: Human IgG4: A structural perspective. Immunol Rev 268: 139–159, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barrington R, Zhang M, Fischer M, Carroll MC: The role of complement in inflammation and adaptive immunity. Immunol Rev 180: 5–15, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Sheerin NS, Springall T, Abe K, Sacks SH: Protection and injury: The differing roles of complement in the development of glomerular injury. Eur J Immunol 31: 1255–1260, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Wang CH, Chen IH, Kuo MW, Su PT, Lai ZY, Wang CH, Huang WC, Hoffman J, Kuo CJ, You MS, Chuang YJ: Zebrafish Thsd7a is a neural protein required for angiogenic patterning during development. Dev Dyn 240: 1412–1421, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Adriouch S, Dubberke G, Diessenbacher P, Rassendren F, Seman M, Haag F, Koch-Nolte F: Probing the expression and function of the P2X7 purinoceptor with antibodies raised by genetic immunization. Cell Immunol 236: 72–77, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Möller S, Jung C, Adriouch S, Dubberke G, Seyfried F, Seman M, Haag F, Koch-Nolte F: Monitoring the expression of purinoceptors and nucleotide-metabolizing ecto-enzymes with antibodies directed against proteins in native conformation. Purinergic Signal 3: 359–366, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu X, Fan Q, Yang G, Liu N, Chen D, Jiang Y, Wang L: Isolating glomeruli from mice: A practical approach for beginners. Exp Ther Med 5: 1322–1326, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C: A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kotb AM, Simon O, Blumenthal A, Vogelgesang S, Dombrowski F, Amann K, Zimmermann U, Endlich K, Endlich N: Knockdown of apoL1 in zebrafish larvae affects the glomerular filtration barrier and the expression of Nephrin. PLoS One 11: e0153768, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.