Abstract
Urinary matrix metalloproteinase-7 (uMMP-7) levels consistently reflect the activity of intrarenal Wnt/β-catenin, which is activated in AKI models. To test the hypothesis that uMMP-7 is a predictor for severe AKI in patients after cardiac surgery, we performed a prospective, multicenter, two-stage cohort study in 721 patients undergoing cardiac surgery. In stage 1, we enrolled 323 children from three academic medical centers. In stage 2, we enrolled 398 adults at six centers. We analyzed levels of uMMP-7 and other injury biomarkers during the perioperative period. Severe AKI was defined as Kidney Disease Improving Global Outcomes stage 2 or 3. uMMP-7 level peaked within 6 hours after surgery in patients who subsequently developed severe AKI. After multivariate adjustment, the highest quintile of postoperative uMMP-7 level, compared with the lowest quintile, associated with 17-fold (in adults) and 36-fold (in children) higher odds of severe AKI. Elevated uMMP-7 level associated with increased risk of composite events (severe AKI, acute dialysis, and in-hospital death) and longer stay in the intensive care unit and hospital. For predicting severe AKI, uMMP-7 had an area under the receiver operating characteristic curve of 0.81 (in children) and 0.76 (in adults), outperforming urinary IL-18, angiotensinogen, neutrophil gelatinase-associated lipocalin, albumin-to-creatinine ratio, and tissue inhibitor of metalloproteinase-2·IGF-binding protein-7 and the clinical model. uMMP-7 significantly improved risk reclassification over the clinical model alone, as measured by net reclassification improvement and integrated discrimination improvement. In conclusion, uMMP-7 is a promising predictor for severe AKI and poor in-hospital outcomes in patients after cardiac surgery.
Keywords: Matrix Metalloproteinase-7, Acute Kidney Injury, Biomarker, Cardiac Surgery
Approximately 2 million cardiac surgeries are conducted each year around the world. AKI is a frequent complication of cardiac surgery.1 In those patients with severe AKI who double their serum creatinine or need acute dialysis, there is a two- to five-fold higher risk of death.2,3 In addition to increased short-term mortality, there is growing evidence that patients who survive an episode of AKI will have an increased risk of developing progressive CKD or even ESRD.4–6 Evidence is also mounting that the severity and frequency of AKI are major risk factors for subsequent development of CKD,7,8 suggesting that severe AKI may closely correlate with poor long-term outcome as well. The failure of previous clinical trials has, in part, been attributed to delays in diagnosis of AKI on the basis of rise in serum creatinine,9 which occurs several days after the initial injury.10,11 In this context, early detection of patients at high risk of severe AKI, before a detectable rise in serum creatinine, would afford clinicians a critical time window to halt or reverse the ongoing renal injury.
The matrix metalloproteinases (MMPs) are a family of zinc-containing enzymes with proteolytic activity against a wide range of extracellular proteins.12 MMP-7 is one of the smallest secreted MMPs, predominantly localized in renal tubular epithelium, and can be easily excreted into urine. Little or no MMP-7 expression is detected in the normal kidney. However, its expression is markedly induced in human and animal models of CKD, which is primary controlled transcriptionally by β-catenin, the principal downstream mediator of canonical Wnt signaling.13–15 We have previously shown that urinary matrix metalloproteinase-7 (uMMP-7) levels faithfully reflect renal Wnt/β-catenin activity,16 and the signal pathway is activated in AKI induced by ischemia-reperfusion injury or renal toxicity.16,17 We therefore hypothesized that uMMP-7 level might be used as a noninvasive biomarker for early prediction of AKI after cardiac surgery.
To test and validate the hypothesis, we conducted a prospective, multicenter, two-stage cohort study in 721 patients undergoing cardiac surgery to evaluate the performance of postoperative measures of uMMP-7 compared with five other reported biomarkers for predicting severe AKI in patients after cardiac surgery. In the first stage, 323 children receiving cardiac surgery were recruited from three academic medical centers. In the second stage, 398 adults were enrolled at six centers.
Results
Cohort Description
There were 721 patients receiving cardiac surgery included in this study. In stage 1, 323 children aged between 1 month and 18 years were enrolled from three academic medical centers, and in stage 2, 398 adults were included from six centers (Supplemental Figure 1). All surgeries were elective and used cardiopulmonary bypass (CPB).
One hundred and twenty six children (39.0%) and 164 adults (41.2%) developed AKI according to Kidney Disease Improving Global Outcomes (KDIGO) criteria26. Among these patients, 53 (16.4%) children and 50 (12.6%) adults developed severe AKI, defined as doubling of serum creatinine or a need for dialysis (consistent with KDIGO stage 2 or 3). Severe AKI occurred at a median of 24 (interquartile range [IQR], 24–48) hours after surgery in both children and adults. Children who developed severe AKI were younger, had longer CPB times, and higher surgery complexity scores (Table 1). Adult patients with severe AKI were older, more likely to have a lower preoperative eGFR, a history of CKD or hypertension, and longer CPB times (Table 2). The pediatric cohort had no preexisting comobilities, such as diabetes and hypertension, and less preoperative medications compared with the adult cohort (Tables 1 and 2).
Table 1.
Description of pediatric cohort after cardiac surgery
| Characteristics | All (n=323) | No AKI (n=197) | Mild AKIa (n=73) | Severe AKIa (n=53) | P Valueb |
|---|---|---|---|---|---|
| Age, mo | 21.8±31.5 | 23.5±33.0 | 22.9±31.5 | 14.6±24.6 | 0.01 |
| 1 mo to 2 yr, n (%) | 230 (71.2) | 134 (68.0) | 51 (69.9) | 45 (84.9) | 0.03 |
| 2–18 yr, n (%) | 93 (28.8) | 63 (32.0) | 22 (30.1) | 8 (15.1) | 0.06 |
| Men, n (%) | 201 (62.2) | 123 (62.4) | 47 (64.4) | 31 (58.5) | 0.72 |
| Height, cm | 77.3±23.8 | 78.1±23.4 | 78.0±25.7 | 68.1±20.4 | 0.004 |
| Weight, kg | 9.8±7.2 | 9.9±7.1 | 9.7±6.7 | 8.3±7.7 | 0.09 |
| Preoperative renal function | |||||
| eGFR, ml/min per 1.73 m2c | 101.6±35.0 | 92.0±28.9 | 112.5±32.0 | 122.5±45.5 | <0.001 |
| Serum creatinine, μmol/L | 29.3±9.8 | 32.4±9.7 | 26.0±7.1 | 22.2±8.5 | <0.001 |
| UACR, mg/g creatinine | 25 (14–66) | 24(12–65) | 27 (16–75) | 28 (20–84) | 0.16 |
| Preoperative medications, n (%) | |||||
| RAS inhibitors | 45 (13.9) | 30 (15.2) | 7 (9.6) | 8 (15.0) | 0.68 |
| Diuretics | 94 (29.1) | 58 (29.4) | 15 (20.5) | 21 (39.6) | 0.42 |
| Aspirin | 6 (1.9) | 1 (0.5) | 2 (2.7) | 3 (5.7) | 0.01 |
| β-Blockers | 5 (1.5) | 2 (1) | 1 (1.4) | 2 (3.8) | 0.19 |
| Operative characteristics | |||||
| CPB time, min | 95.1±45.6 | 89.4±23.5 | 102.3±47.6 | 106.7±54.0 | 0.02 |
| Crossclamp time, min | 53.7±33.1 | 51.9±31.1 | 55.7±34.3 | 57.8±38.4 | 0.44 |
| RACHS-1 scored | 2.2±0.6 | 2.2±0.5 | 2.2±0.6 | 2.4±0.6 | 0.003 |
| RACHS-1 score ≥3, n (%) | 81 (25.1) | 38 (19.3) | 19 (26.0) | 24 (45.3) | <0.001 |
| In-hospital outcomes | |||||
| Death, n (%) | 9 (2.8) | 2 (1.0) | 1 (1.4) | 6 (11.3) | <0.001 |
| Dialysis, n (%) | 4 (1.2) | 0 (0) | 0 (0) | 4 (7.5) | <0.001 |
| PICU stay, d | 2 (1–7) | 1 (1–5) | 2 (1–5) | 3 (1–10) | <0.001 |
| Hospital stay, d | 17 (12–25) | 15 (11–22) | 17 (12–23) | 23 (15–34) | <0.001 |
Continuous variables are expressed as mean±SD or median (25th percentile–75th percentile). Categorical variables were expressed as n (%). RAS, renin-angiotensin system; RACHS-1, risk adjustment for congenital heart surgery 1; PICU, pediatric intensive care unit.
Mild AKI was defined as an increase in serum creatinine level to ≥0.3 mg/dl within 48 hours or ≥50% in 7 days. Severe AKI was defined as an increase in serum creatinine level to ≥2.0 times baseline or acute dialysis.
P for global comparisons among groups by Kruskal–Wallis and chi-squared tests for continuous and categorical variables, respectively.
eGFR was calculated using the updated equation from Schwartz et al.30
The RACHS-1 consensus-based score system categorizes the complexity of surgery.
Table 2.
Description of adult cohort after cardiac surgery
| Characteristics | All (n=398) | No AKI (n=234) | Mild AKIa (n=114) | Severe AKIa (n=50) | P Valueb |
|---|---|---|---|---|---|
| Age, yr | 46.2±15.1 | 41.7±15.6 | 53.0±10.9 | 51.8±13.6 | <0.01 |
| Men, n (%) | 170 (42.7) | 89 (38.0) | 54 (47.3) | 27 (54.0) | 0.02 |
| Preexisting clinical conditions, n (%) | |||||
| Diabetes | 20 (5.0) | 8 (3.4) | 9 (7.9) | 3 (6.0) | 0.16 |
| Hypertension | 69 (17.3) | 22 (9.4) | 30 (26.3) | 17 (34.0) | <0.001 |
| CKD | 33 (8.3) | 10 (4.3) | 9 (7.9) | 14 (28.0) | <0.001 |
| Congestive heart failure | 203 (51.0) | 123 (52.6) | 57 (50.0) | 23 (46.0) | 0.38 |
| Preoperative characteristics | |||||
| eGFR, ml/min per 1.73 m2c | 93.5±23.7 | 99.7±24.0 | 85.5±17.8 | 83.5±25.1 | <0.001 |
| UACR, mg/g creatinine | 26 (14–68) | 26 (14–18) | 34 (18–67) | 51 (26–150) | 0.01 |
| Serum creatinine, μmol/L | 76.6±24.2 | 72.2±19.3 | 80.6±23.9 | 88.0±37.2 | <0.001 |
| Serum albumin, g/L | 38.6±5.3 | 39.3±5.0 | 37.9±5.2 | 36.9±6.0 | 0.004 |
| Preoperative medication n (%) | |||||
| RAS inhibitors | 120 (30.2) | 69 (29.5) | 35 (30.7) | 16 (32.0) | 0.70 |
| Diuretics | 255 (64.1) | 151 (64.5) | 69 (60.5) | 35 (70.0) | 0.80 |
| Aspirin | 238 (59.8) | 137 (58.5) | 70 (61.4) | 31 (62.0) | 0.56 |
| β-Blockers | 161 (40.5) | 91 (38.9) | 48 (42.1) | 22 (44.0) | 0.64 |
| Operative variables | |||||
| CABG alone, n (%) | 7 (1.7) | 4 (1.7) | 1 (0.9) | 2 (4.0) | 0.50 |
| Valve alone, n (%) | 297 (74.6) | 181 (77.3) | 80 (70.2) | 36 (72.0) | 0.05 |
| CABG and valve surgery, n (%) | 21 (5.3) | 13 (5.6) | 4 (3.5) | 4 (8.0) | 0.89 |
| CPB time, min | 118.9±59.1 | 107.4±56.8 | 129.3±53.6 | 149.0±67.1 | <0.001 |
| Crossclamp time, min | 81.7±45.2 | 77.5±47.5 | 86.7±39.3 | 90.3±44.1 | 0.08 |
| Postoperative outcomes | |||||
| Death, n (%) | 8 (2.0) | 3 (1.3) | 1 (0.9) | 4 (8.0) | 0.02 |
| Dialysis, n (%) | 5 (1.3) | 0 (0) | 0 (0) | 5 (10.0) | <0.001 |
| ICU stay, d | 4 (2–14) | 3(1–14) | 3 (2–14) | 9 (3–16) | <0.01 |
| Hospital stay, d | 20 (16–28) | 18 (15–23) | 23 (18–32) | 27 (20–42) | <0.001 |
Continuous variables were expressed as mean±SD or median (25th percentile–75th percentile). Categorical variables were expressed as n (%). RAS, renin-angiotensin system; CABG, coronary artery bypass grafting.
Mild AKI was defined as an increase in serum creatinine level to ≥0.3 mg/dl within 48 hours or ≥50% in 7 days. Severe AKI was defined as an increase in serum creatinine level to ≥2.0 times baseline or acute dialysis.
P for global comparisons among groups by Kruskal–Wallis and chi-squared tests for continuous and categorical variables, respectively.
eGFR was determined by the Chronic Kidney Disease Epidemiology Collaboration Equation (2009).31
uMMP-7 as a Predictor for Development of Severe AKI after Cardiac Surgery
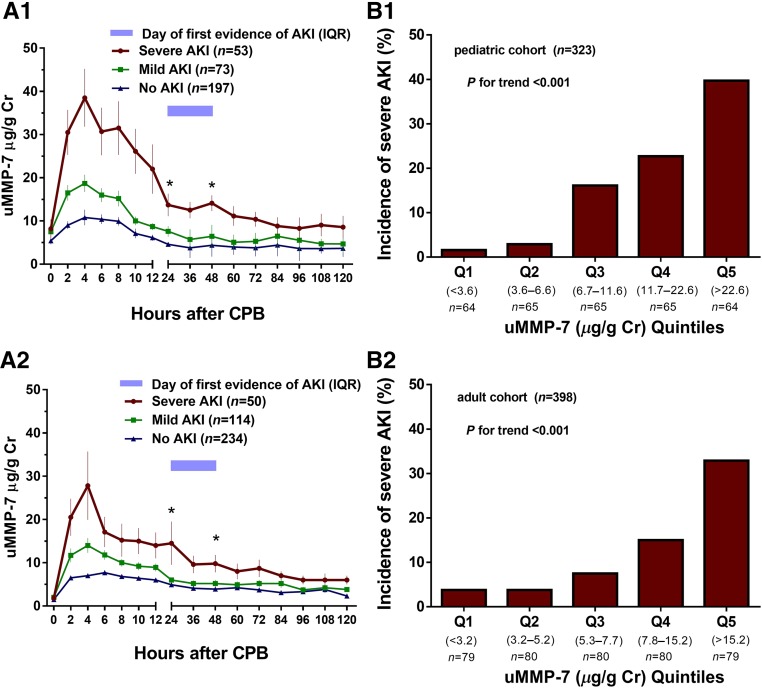
uMMP-7 levels were measured every 2 hours for the first 12 hours after CPB and then every 12 hours for 5 days. Figure 1A displayed serial measurements of uMMP-7 over the first 5 days after cardiac surgery. Preoperative uMMP-7 levels were not significantly different between patients who did and did not develop AKI in both children (Figure 1A1) and adults (Figure 1A2). However, the average levels of uMMP-7 within the first 6 hours were significantly higher in patients who subsequently developed severe AKI compared with those who developed mild AKI or had no AKI. The uMMP-7 level pattern in those with severe AKI was characterized by a peak within 6 hours after surgery and remained significantly elevated until 48 hours in both cohorts, whereas the peak in serum creatinine rise occurred after 24 hours of surgery. uMMP-7 increased in all individuals after cardiac surgery, but the magnitude of these changes over time (within the first 48 hours) differed significantly across groups (interaction P<0.01 from repeated-measures ANOVA in both children and adults).
Figure 1.
uMMP-7 level peaked within 6 hours after surgery (A) and quintiles of uMMP-7 levels within the first 6 hours had a graded relationship with the incidence of severe AKI (B). (A) uMMP-7 levels in patients with no AKI, mild AKI, and severe AKI in children (A1) and adults (A2). Data are shown as mean±SEM. *ANOVA P<0.01. (B) Quintiles of uMMP-7 levels within the first 6 hours after surgery are associated with incident severe AKI in children (B1) and adults (B2). There is no difference in the preoperative uMMP-7 levels in the three groups (P=0.15 in children and P=0.10 in adults) but a significant difference in changes over time (interaction P<0.01 from repeated-measures ANOVA), with increases in the uMMP-7 levels correlating with severity of AKI (P<0.001) in both children (A1) and adults (A2). Cr, creatinine; Q, quintile.
In the adult cohort with AKI, elevation in uMMP-7 level was noted in both subgroups with and without preexisting CKD, but the level was significantly higher in those with prior CKD compared with those without (Supplemental Figure 2). The best cutoff value of uMMP-7 for predicting severe AKI was significantly higher in patients with prior CKD (16.9 μg/g creatinine) compared with those with preserved eGFR before surgery (7.6 μg/g creatinine) (Supplemental Tables 1 and 2). Interestingly, patients who had higher uMMP-7 levels within the first 6 hours post-CPB tended to have unrecovered renal dysfunction at discharge (Supplemental Table 3).
Unlike uMMP-7, plasma MMP-7 levels did not change significantly in patients undergoing cardiac surgery with or without AKI. There was no significant difference in plasma MMP-7 levels between children and adult (Supplemental Figure 3).
uMMP-7 levels were associated with incident severe AKI in both pediatric and adult cohorts (Figure 1B). After adjustment for center and for clinical variables (including age, sex, preoperative confounders, preoperative renal function, preoperative urinary albumin-to-creatinine ratio (UACR), CPB time, and surgery complexity scores), higher quintiles of the postoperative uMMP-7 levels within the first 6 hours were independently associated with increasing risk of severe AKI in both children and adults. The highest quintile of mean uMMP-7 within the first 6 hours was associated with increased odds for severe AKI by 36-fold in children and 17-fold in adults compared with the lowest quintile (Table 3). Elevation of uMMP-7 level also predicted development of AKI (KDIGO stage 1–3) in both children and adults (Supplemental Table 1).
Table 3.
Multivariate logistic regression analyses of uMMP-7 for predicting severe AKI and outcomes after cardiac surgerya
| Primary Outcome: Severe AKI | Secondary Outcomes | |||||
|---|---|---|---|---|---|---|
| uMMP-7 (µg/g creatinine, n) | AKI, % | Unadjusted OR (95% CI) | Adjusted ORb (95% CI) | Composite Events, % | Length of Stay in ICU, d, Median (IQR) | Length of Stay in Hospital, d, Median (IQR) |
| Pediatric cohort (n=323) | ||||||
| Q1 (<3.6, 64) | 1.6 | 1.0 (Referent) | 1.0 (Referent) | 1.6 | 1 (1–2) | 12 (9–19) |
| Q2 (3.6–6.6, 65) | 2.9 | 1.9 (0.2 to 21.6) | 1.8 (0.2 to 21.4) | 5.9 | 2 (1–7) | 14 (11–22) |
| Q3 (6.7–11.6, 65) | 16.1 | 12.1 (1.5 to 97.7) | 6.7 (0.8 to 58.4) | 16.1 | 2 (1–5) | 18 (12–23) |
| Q4 (11.7–22.6, 65) | 22.7 | 18.5 (2.4 to 145.0) | 13.7 (1.6 to 119.3) | 22.7 | 2 (1–5) | 19 (12–25) |
| Q5 (>22.6, 64) | 39.7 | 41.4 (5.4 to 318.4) | 36.2 (4.0 to 325.6) | 41.3 | 4 (2–12) | 20 (15–26) |
| P for trend | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Adult cohort (n=398) | ||||||
| Q1 (<3.2, 79) | 3.8 | 1.0 (Referent) | 1.0 (Referent) | 3.8 | 3 (1–13) | 18 (15–25) |
| Q2 (3.2–5.2, 80) | 3.8 | 1.0 (0.2 to 5.0) | 1.2 (0.2 to 7.1) | 3.8 | 4 (1–15) | 18 (15–21) |
| Q3 (5.3–7.7, 80) | 7.5 | 2.1 (0.5 to 8.5) | 3.0 (0.6 to 15.2) | 7.5 | 3 (1–15) | 20 (16–25) |
| Q4 (7.8–15.2, 80) | 15.0 | 4.5 (1.2 to 16.5) | 6.0 (1.3 to 28.2) | 18.8 | 3 (1–12) | 23 (17–30) |
| Q5 (>15.2, 79) | 32.9 | 12.4 (3.6 to 43.2) | 17.0 (3.5 to 81.8) | 34.2 | 5 (3–15) | 27 (20–35) |
| P for trend | <0.001 | <0.001 | <0.001 | <0.001 | ||
Q, quintile.
uMMP-7 expressed as the mean levels within the first 6 hours after CPB.
In children, adjusted for age, preoperative eGFR, preoperative UACR, risk adjustment for congenital heart surgery 1 score ≥3, CPB time >120 minutes, and sites18; in adults, adjusted for age, sex, diabetes, hypertension, preoperative eGFR, preoperative UACR, preoperative serum albumin, CPB time >120 minutes, and sites.19
When the mean of the uMMP-7 level within the first 6 hours post-CPB was analyzed as a continuous variable, higher uMMP-7 level was also associated with the development of severe AKI (children: adjusted odds ratio [OR] per SD, 5.4; 95% confidence interval [95% CI], 3.1 to 9.3; P<0.001, adults: adjusted OR per SD, 3.1; 95% CI, 2.0 to 4.8; P<0.001) in a multivariable model.
When duration of AKI was evaluated as an outcome, higher uMMP-7 level was also associated with persistent AKI (AKI persisted ≥3 days) (children: adjusted OR per SD, 3.9; 95% CI, 2.0 to 7.7; P<0.001, adults: adjusted OR per SD, 3.7; 95% CI, 2.3 to 5.7; P<0.001).
uMMP-7 as a Predictor for Outcomes after Cardiac Surgery
Of 323 children in the stage 1 study, four (1.2%) received acute dialysis and nine (2.8%) died during hospitalization. In the stage 2 study, five (1.3%) adults received dialysis and eight (2.0%) adults died before discharge. The composite outcomes (severe AKI, acute dialysis, or in-hospital death) were observed in 56 (17.3%) patients in the pediatric cohort and 54 (13.6%) patients in adult cohort. The median lengths of intensive care unit (ICU) stay were 2 days (IQR, 1–7) in children and 4 days (IQR, 2–14) in adults. Median lengths of hospital stay were 17 days (IQR, 12–25) in children and 20 days (IQR, 16–28) in adults. After multivariable adjustment, higher uMMP-7 levels soon after surgery were significantly associated with increased risk of composite events and a longer stay in the ICU and in hospital in both children and adults (adjusted P for trend <0.001 in children and <0.001 in adults; Table 3).
Comparing the Performance of uMMP-7 with Other Biomarkers
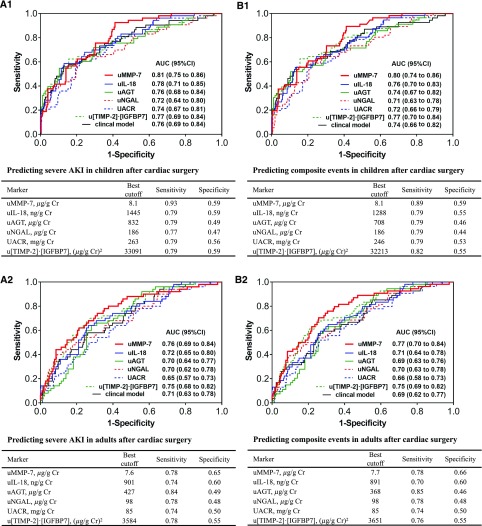
For predicting severe AKI, the area under the receiver-operating characteristic curve (AUC) of uMMP-7 within 6 hours postsurgery were 0.81 in children and 0.76 in adults. As shown in Figure 2A, the AUC of uMMP-7 for predicting severe AKI was the greatest among the tested biomarkers, such as urinary IL-18 (uIL-18), urinary angiotensinogen (uAGT), urinary neutrophil gelatinase-associated lipocalin (uNGAL), urinary tissue inhibitor of metalloproteinase-2·IGF-binding protein-7 (u[TIMP-2]·[IGFBP7]), UACR, and the clinical risk factor model. Moreover, uMMP-7 outperformed these tested biomarkers in predicting composite events in children and adults with AUCs of 0.80 and 0.77, respectively (Figure 2B). For predicting KDIGO stage 1–3 AKI, the AUCs of uMMP-7 were 0.75 in children and 0.72 in adults, which also outperformed the other tested biomarkers (Supplemental Figure 4). We also assessed the best time point at 2, 4, and 6 hours for each biomarker and compared them. We found that the AUC of uMMP-7 remained the greatest compared with the other tested biomarkers (Supplemental Figure 5).
Figure 2.
Performance of uMMP-7 and other biomarkers for predicting severe AKI and composite events. (A) The AUCs of biomarkers (mean level within the first 6 hours after surgery) and the clinical model for predicting severe AKI in children (A1) and adults (A2). (B) The AUCs of biomarkers (mean level within the first 6 hours after surgery) and the clinical model for predicting composite events in children (B1) and adults (B2). In children, the clinical model is comprised of age, risk adjustment for congenital heart surgery 1 score, preoperative eGFR, and CPB time; in adults, the clinical model is comprised of age, sex, diabetes, hypertension, preoperative eGFR, preoperative serum albumin, and CPB time. For predicting severe AKI, the AUC of uMMP-7 was the greatest among tested biomarkers and significantly greater than UACR (P=0.01 in children; P=0.02 in adults) and uNGAL (P=0.03 in children). However, the difference in the AUCs between uMMP-7 and other urinary biomarkers did not reach statistical significance (P>0.05). Cr, creatinine.
We further evaluated the performance of a combination of urinary biomarkers within the first 6 hours after surgery. The combination of uMMP-7 with uIL-18 yielded the largest AUC for predicting severe AKI (0.83 in children and 0.79 in adults) and composite events (0.82 in children and 0.79 in adults). When these two biomarkers were added to the clinical risk model, the performance for predicting severe AKI (AUC of 0.91 in children and 0.85 in adults) and composite events (AUC of 0.88 in children and 0.84 in adults) were further improved (Table 4).
Table 4.
Performance of combination of urinary biomarkers and clinical model for predicting severe AKI and composite events after cardiac surgerya
| AUC (95% CI) | |||
|---|---|---|---|
| Biomarkers | Biomarkers | Biomarkers and Clinical Modelb | P Valuec |
| Predicting severe AKI | |||
| Children (n=323) | |||
| uMMP-7+uIL-18 | 0.83 (0.78 to 0.89) | 0.91 (0.87 to 0.94) | <0.001 |
| uMMP-7+uAGT | 0.82 (0.76 to 0.88) | 0.90 (0.85 to 0.94) | <0.001 |
| Adults (n=398) | |||
| uMMP-7+uIL-18 | 0.79 (0.72 to 0.85) | 0.85 (0.80 to 0.91) | <0.01 |
| uMMP-7+uAGT | 0.79 (0.72 to 0.85) | 0.84 (0.78 to 0.90) | 0.01 |
| Predicting composite events | |||
| Children (n=323) | |||
| uMMP-7+uIL-18 | 0.82 (0.77 to 0.88) | 0.88 (0.83 to 0.93) | <0.001 |
| uMMP-7+uAGT | 0.81 (0.75 to 0.87) | 0.87 (0.82 to 0.93) | <0.001 |
| Adults (n=398) | |||
| uMMP-7+uIL-18 | 0.79 (0.72 to 0.85) | 0.84 (0.78 to 0.89) | 0.004 |
| uMMP-7+uAGT | 0.79 (0.73 to 0.85) | 0.84 (0.78 to 0.89) | <0.01 |
Severe AKI was defined as an increase in serum creatinine level to ≥2.0 times baseline or acute dialysis.
Biomarkers were expressed as the mean levels within the first 6 hours after CPB.
In children, clinical model is comprised of age, preoperative eGFR, risk adjustment for congenital heart surgery 1 score ≥3, and CPB time >120 minutes18; in adults, clinical model is comprised of age, sex, diabetes, hypertension, preoperative eGFR, preoperative serum albumin, and CPB time >120 minutes.19
Biomarkers and clinical model versus clinical model only.
Effect of uMMP-7 on Risk Reclassification of Severe AKI and Composite Events
Addition of uMMP-7, uIL-18, uAGT, uNGAL, UACR, and u[TIMP-2]·[IGFBP7] to the clinical risk model significantly improved risk reclassification over the clinical model alone, with uMMP-7 displaying the largest category-free net reclassification improvement (NRI) and integrated discrimination improvement (IDI) for both severe AKI and composite events in children and adults (Tables 5 and 6).
Table 5.
Risk reclassification of severe AKI and composite events in children after cardiac surgerya
| Outcomes | Category-Free NRI (95% CI) | P Value | Category-Free NRI (95% CI) | IDI (95% CI) | P Value | |||
|---|---|---|---|---|---|---|---|---|
| With Event | P Value | Without Event | P Value | |||||
| Severe AKI | ||||||||
| Clinical risk factorsb | Referent | Referent | ||||||
| Clinical risk factors plus uMMP-7 | 0.98 (0.70 to 1.26) | <0.001 | 0.51 (0.27 to 0.75) | <0.001 | 0.47 (0.36 to 0.57) | <0.001 | 0.17 (0.11 to 0.23) | <0.001 |
| Clinical risk factors plus uIL-18 | 0.81 (0.53 to 1.09) | <0.001 | 0.36 (0.10 to 0.62) | <0.01 | 0.45 (0.34 to 0.56) | <0.001 | 0.15 (0.09 to 0.21) | <0.001 |
| Clinical risk factors plus uAGT | 0.70 (0.42 to 0.98) | <0.001 | 0.40 (0.14 to 0.65) | 0.003 | 0.30 (0.18 to 0.41) | <0.001 | 0.12 (0.08 to 0.20) | <0.001 |
| Clinical risk factors plus uNGAL | 0.60 (0.32 to 0.88) | <0.001 | 0.25 (−0.02 to 0.52) | 0.07 | 0.35 (0.24 to 0.46) | <0.001 | 0.07 (0.03 to 0.11) | 0.001 |
| Clinical risk factors plus UACR | 0.66 (0.38 to 0.94) | <0.001 | 0.36 (0.10 to 0.62) | <0.01 | 0.30 (0.18 to 0.41) | <0.001 | 0.11 (0.05 to 0.17) | <0.001 |
| Clinical risk factors plus u[TIMP-2]·[IGFBP7] | 0.81 (0.53 to 1.09) | <0.001 | 0.47 (0.23 to 0.72) | <0.001 | 0.34 (0.23 to 0.46) | <0.001 | 0.13 (0.09 to 0.20) | <0.001 |
| Composite events | ||||||||
| Clinical risk factorsb | Referent | Referent | ||||||
| Clinical risk factors plus uMMP-7 | 0.88 (0.60 to 1.16) | <0.001 | 0.46 (0.23 to 0.70) | <0.001 | 0.42 (0.31 to 0.53) | <0.001 | 0.17 (0.11 to 0.23) | <0.001 |
| Clinical risk factors plus uIL-18 | 0.75 (0.47 to 1.03) | <0.001 | 0.32 (0.07 to 0.58) | 0.02 | 0.43 (0.32 to 0.54) | <0.001 | 0.15 (0.09 to 0.21) | <0.001 |
| Clinical risk factors plus uAGT | 0.63 (0.35 to 0.91) | <0.001 | 0.36 (0.07 to 0.58) | 0.02 | 0.27 (0.15 to 0.39) | <0.001 | 0.11 (0.05 to 0.17) | <0.001 |
| Clinical risk factors plus uNGAL | 0.50 (0.22 to 0.78) | <0.001 | 0.18 (−0.09 to 0.44) | 0.18 | 0.32 (0.20 to 0.43) | <0.001 | 0.06 (0.02 to 0.10) | 0.001 |
| Clinical risk factors plus UACR | 0.61 (0.33 to 0.89) | <0.001 | 0.29 (0.03 to 0.54) | 0.03 | 0.32 (0.20 to 0.43) | <0.001 | 0.10 (0.06 to 0.14) | <0.001 |
| Clinical risk factors plus u[TIMP-2]·[IGFBP7] | 0.81 (0.53 to 1.09) | <0.001 | 0.46 (0.23 to 0.70) | <0.001 | 0.35 (0.24 to 0.47) | <0.001 | 0.12 (0.08 to 0.20) | <0.001 |
Severe AKI was defined as an increase in serum creatinine level to ≥2.0 times baseline or acute dialysis. The composite events were defined as development of severe AKI or acute dialysis or in-hospital death.
The clinical risk factors are comprised of age, risk adjustment for congenital heart surgery 1 score, preoperative eGFR, and CPB time.18
Table 6.
Risk reclassification of severe AKI and composite events in adults after cardiac surgerya
| Outcomes | Category-Free NRI (95% CI) | P Value | Category-Free NRI (95% CI) | IDI (95% CI) | P Value | |||
|---|---|---|---|---|---|---|---|---|
| With Event | P Value | Without Event | P Value | |||||
| Severe AKI | ||||||||
| Clinical risk factorsb | Referent | Referent | ||||||
| Clinical risk factors plus uMMP-7 | 0.65 (0.37 to 0.93) | <0.001 | 0.35 (0.07 to 0.61) | <0.01 | 0.30 (0.20 to 0.40) | <0.001 | 0.09 (0.05 to 0.13) | <0.001 |
| Clinical risk factors plus uIL-18 | 0.62 (0.34 to 0.90) | <0.001 | 0.31 (0.05 to 0.59) | 0.02 | 0.31 (0.21 to 0.41) | <0.001 | 0.07 (0.04 to 0.09) | <0.001 |
| Clinical risk factors plus uAGT | 0.53 (0.25 to 0.81) | 0.001 | 0.40 (0.14 to 0.66) | 0.004 | 0.17 (0.07 to 0.28) | 0.001 | 0.04 (0.02 to 0.06) | 0.02 |
| Clinical risk factors plus uNGAL | 0.41 (0.12 to 0.70) | 0.003 | 0.12 (−0.17 to 0.41) | 0.40 | 0.29 (0.19 to 0.38) | <0.001 | 0.04 (0.02 to 0.06) | 0.03 |
| Clinical risk factors plus UACR | 0.43 (0.14 to 0.72) | 0.003 | 0.24 (−0.04 to 0.52) | 0.09 | 0.19 (0.09 to 0.29) | <0.001 | 0.03 (0.01 to 0.05) | 0.04 |
| Clinical risk factors plus u[TIMP-2]·[IGFBP7] | 0.63 (0.35 to 0.91) | <0.001 | 0.30 (0.04 to 0.58) | 0.02 | 0.33 (0.22 to 0.43) | <0.001 | 0.07 (0.04 to 0.09) | <0.001 |
| Composite events | ||||||||
| Clinical risk factorsb | Referent | Referent | ||||||
| Clinical risk factors plus uMMP-7 | 0.67 (0.39 to 0.95) | <0.001 | 0.37 (0.11 to 0.63) | <0.01 | 0.30 (0.20 to 0.40) | <0.001 | 0.09 (0.05 to 0.13) | <0.001 |
| Clinical risk factors plus uIL-18 | 0.55 (0.27 to 0.83) | <0.001 | 0.26 (−0.01 to 0.53) | 0.05 | 0.29 (0.19 to 0.39) | <0.001 | 0.06 (0.03 to 0.07) | 0.001 |
| Clinical risk factors plus uAGT | 0.52 (0.24 to 0.80) | 0.001 | 0.37 (0.11 to 0.63) | <0.01 | 0.15 (0.05 to 0.26) | <0.01 | 0.04 (0.02 to 0.06) | 0.02 |
| Clinical risk factors plus uNGAL | 0.45 (0.17 to 0.73) | 0.003 | 0.15 (−0.12 to 0.42) | 0.28 | 0.30 (0.21 to 0.40) | <0.001 | 0.04 (0.02 to 0.06) | 0.02 |
| Clinical risk factors plus UACR | 0.42 (0.14 to 0.70) | <0.001 | 0.22 (−0.05 to 0.49) | 0.10 | 0.20 (0.09 to 0.30) | <0.001 | 0.03 (0.01 to 0.05) | 0.04 |
| Clinical risk factors plus u[TIMP-2]·[IGFBP7] | 0.58 (0.31 to 0.86) | <0.001 | 0.23 (−0.04 to 0.50) | 0.10 | 0.35 (0.25 to 0.44) | <0.001 | 0.07 (0.04 to 0.09) | <0.001 |
Severe AKI was defined as an increase in serum creatinine level to ≥2.0 times baseline or acute dialysis. The composite events were defined as development of severe AKI or acute dialysis or in-hospital death.
The clinical risk factors are comprised of age, sex, diabetes, hypertension, preoperative eGFR, preoperative serum albumin, and CPB time.19
Discussion
In this prospective, multicenter study of pediatric and adult cohorts receiving cardiac surgery, we demonstrate for the first time that uMMP-7, measured within the first 6 hours after surgery, is a powerful predictor for severe AKI and in-hospital adverse outcomes in patients undergoing cardiac surgery. A postoperative level of uMMP-7>22.6 µg/g creatinine in children denoted >36-fold risk of severe AKI compared with those in the lowest quintile, whereas a postoperative level of uMMP-7>15.2 µg/g creatinine in adults denoted a 17-fold risk of severe AKI. Higher uMMP-7 levels shortly after surgery were associated with increased risk of poor clinical outcomes, including increased composite events (severe AKI, acute dialysis, and in-hospital death) and longer stay in the ICU and in hospital. The AUC of uMMP-7 for predicting severe AKI and composite events was greatest when compared with previously reported biomarkers, such as uAGT, uIL-18, uNGAL, UACR, u[TIMP-2]·[IGFBP7], and the clinical model. uMMP-7 significantly improved risk reclassification, as measured by NRI and IDI, for both severe AKI and composite events. These data suggest that immediate postoperative measurement of uMMP-7 could predict severe AKI and provide important prognostic information.
For the past decade, there have been a host of studies surrounding renal injury biomarkers of AKI because they may facilitate patient care and the development of therapies. IL-18 and NGAL are the most frequently studied, promising AKI predictors to date. Consistent with previous reports,18,19 the performance of uIL-18 for predicting severe AKI in adults and children in this study had an AUC of 0.72 and 0.78, respectively. uMMP-7 improved the AUC to 0.76 (in adults) and 0.81 (in children). uNGAL in this study population performed only modestly (AUC of 0.70 in adults and 0.72 in children). uAGT has been recognized as a strong predictor for AKI in the setting of acute decompensated heart failure.20 Elevated uAGT levels are associated with the risk of AKI progression in patients with cardiac surgery21 and acute heart failure.22 The ability of uAGT for predicting severe AKI was good in this study, but its AUC was not as great as uMMP-7. The predictive performance of uMMP-7 was better than UACR, a marker that is associated with risk of postoperative AKI.23 Interestingly, the AUC of uMMP-7 was also greater than u[TIMP-2]·[IGFBP7], a cell-cycle biomarker approved by the US Food and Drug Administration (FDA),24 although the difference in AUCs between these two biomarkers did not reach statistical significance.
Although uMMP-7 level is reported to be correlated with proteinuria in patients with CKD,25 elevation of uMMP-7 is an independent predictor of severe postoperative AKI even after adjusting for preoperative UACR and eGFR. The risk reclassification, as measured by category-free NRI and IDI, was significantly improved through addition of uMMP-7 to the clinical model. Furthermore, our results confirmed that the addition of uMMP-7 to uIL-18 or uAGT significantly improved their predictive performance as biomarkers. Addition of these combined biomarkers to the clinical model further increased the accuracy of predicting severe AKI and composite events, as demonstrated by the AUC results. These data support the concept that a single biomarker is not sufficient for the evaluation of severe AKI. A multimarker approach is therefore more likely to be of greater use.
We simultaneously measured both urine and plasma MMP-7 in this study. Unlike uMMP-7, plasma MMP-7 did not significantly change in patients receiving cardiac surgery with or without AKI. uMMP-7 is a marker reflecting the activity of intrarenal Wnt/β-catenin and dependably mirrors its expression in renal parenchyma, particularly in the tubular epithelium.13 In a murine model of severe ischemia-reperfusion injury, tubular MMP-7 expression is dramatically induced,17 which is primarily regulated by Wnt/β-catenin, a signaling pathway that is activated in AKI as a defensive response of the kidney.16,17 Consistent with the experimental data, uMMP-7 level was markedly increased in patients after cardiac surgery who subsequently developed severe AKI. Although the potential role of increased renal MMP-7 in human AKI remains to be investigated, early measurement of uMMP-7 after cardiac surgery to identify patients at high risk of severe AKI may offer clinicians a critical time window to halt or reverse ongoing kidney injury.
Our study has several strengths. First, we tested the performance of uMMP-7 in both adult and pediatric cohorts as biomarker performance may differ among these groups because of differences in preexisting CKD and underlying comorbidities. Moreover, the study participants were enrolled from six centers, representing patients in various clinical settings. Second, the samples were prospectively collected under standardized conditions in consecutive patients undergoing cardiac surgery across multiple centers. We tested levels of biomarkers from 2 hours after surgery for five consecutive days, and these measurements were performed in a central laboratory to ensure high quality. Third, we compared the performance of uMMP-7 with five most frequently studied biomarkers and the clinical model, and demonstrated that uMMP-7 outperformed uIL-18, uAGT, uNGAL, UACR, u[TIMP-2]·[IGFBP7], and the clinical model. Using a two-stage design, we show consistent results in both cohorts.
The study also has limitations. First, we were not able to use urine output for AKI diagnosis because an indwelling urine catheter was not present in most of the patients. Second, uMMP-7 but not blood MMP-7 was predictive of severe AKI. Although urinary markers have several advantages, including noninvasive sample collection and few interfering proteins, some disadvantages also exist, such as difficulty in collecting samples from patients with severe oliguria and potential changes in urinary biomarker levels induced by fluid status and use of diuretics. Finally, our study population was Chinese; future studies should consider whether these results are the same in other races.
In summary, uMMP-7 is a novel promising predictor for development of severe AKI and poor in-hospital outcomes in patients after cardiac surgery. If further confirmed, early measurement of uMMP-7 level would help clinicians to identify those at high risk of severe postoperative AKI and help them to plan and initiate the appropriate management strategies for patients undergoing cardiac surgery.
Concise Methods
Patients
This is a prospective, two-stage, multicenter cohort study approved by the Institutional Review Board of the National Clinical Research Center for Kidney Disease. The study participants provided written informed consent. The stage 1 study (child cohort) was conducted in three academic medical centers across two cities (Guangzhou and Guiyang) from September of 2013 to September of 2014. Sample collection for the stage 2 study (adult cohort) was performed in six centers across five cities (Guangzhou, Guiyang, Dalian, Zhanjiang, and Chengdu) from February of 2014 to December of 2015. The patients in both study stages were enrolled according to the same inclusion and exclusion criteria, except for age.
Eligible participants were patients receiving elective cardiac surgery aged 1 month to 18 years old (in stage 1) or 19–80 years old (in stage 2). Exclusion criteria included exposure to nephrotoxin (i.e., contrast media, aminoglycoside antibiotics, vancomycin, and nonsteroidal anti-inflammatory drugs except aspirin) within 4 weeks before surgery, preexisting advanced CKD (chronic dialysis, renal transplantation, or preoperative eGFR <30 ml/min per 1.73 m2), and urinary tract infection or obstruction.
Procedures
We collected spot urine and blood samples before operation and at frequent intervals for 5 days after surgery. Urine samples were collected every 2 hours for the first 12 hours after CPB, and then every 12 hours for the first 5 days after surgery. Blood samples were obtained at 2 and 12 hours after surgery, and then every 24 hours for 5 days after surgery. When the CPB time exceeded 2 hours, the first postoperative urine sample was obtained at the end of CPB, and this sample was regarded as the 2-hour sample. The urine samples were centrifuged at 3000 g for 10 minutes and the supernatants were stored at −80°C. Serum creatinine was measured to determined changes in renal function.
Laboratory Measurements
Urine and blood samples collected from the participating hospitals were shipped by commercial cold chain transportation. All of the biomarkers were measured in a central laboratory using a standard protocol, and all of the samples were labeled using study identification numbers without personal identifiers or clinical conditions.
Urinary and plasma MMP-7 levels were measured by ELISA Kit (DMP700; R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. uAGT, uNGAL, and uIL-18 levels were quantified using the ELISA kits (AGT: Immuno-Biologic Laboratories Co., Ltd., Fujioka, Japan; NGAL: Bioporto; IL-18: Ray Biotech Inc.) according to the manufacturer’s instructions. u[TIMP-2]·[IGFBP7] levels were tested by ELISA kit (DTM200 and DY1334–05; R&D Systems). The NEPHROCHECK for TIMP-2 and IGFBP7 measuring approved by the US FDA was not available in China. All biomarkers were measured in triplicate. Investigator-calculated intra- and interassay variability ranged between 2%–6% and 5%–9% on the basis of blinded replicate samples from study patients. Urinary albumin was measured using an automatic analyzer (BNPro Spec; Siemens). Urinary and serum creatinine was measured using an automatic biochemical analyzer (AU480; Olympus, Tokyo, Japan). All of the urinary biomarkers were normalized for urinary creatinine and expressed as micrograms per gram of creatinine or nanograms per gram of creatinine.
Outcome Definitions
The primary outcome was the development of severe AKI, defined as the peak level of serum creatinine after surgery being double that of the preoperative level, or as receiving acute dialysis (consistent with KDIGO stage 2 or 3 AKI). Mild AKI was defined as an increase in serum creatinine by 26.5 μmol/L (0.3 mg/dl) within 48 hours or a 50% increase in serum creatinine from the preoperative level within 7 days (KDIGO criteria).26 We did not use urine output criteria (<0.5 ml/kg per hour for 6 hours) for AKI diagnosis because an indwelling urinary catheter was not present in the majority of participants.
The secondary outcome was the development of composite events, including severe AKI, acute dialysis, and in-hospital death. We also used length of stay in the ICU and in hospital as the in-hospital outcomes.
Statistical Analyses
SPSS 17.0 software was used for all analyses. To compare continuous variables, we used the ANOVA test or the Kruskal–Wallis test. To compare categorical variables, we used the chi-squared test. For an overall comparison of change over time between groups, a repeated-measures ANOVA was performed, with particular focus on the interaction between group and time.
We categorized the uMMP-7 quintile essentially as a continuous variable and then performed logistic regression on created variables. We determined the adjusted ORs of severe AKI with multiple logistic regression analysis with random intercepts for each center. The selection of covariates for adjusted models was on the basis of known risk factors that predict severe AKI in children after cardiac surgery, including age, preoperative eGFR, preoperative UACR, risk adjustment for congenital heart surgery 1 score ≥3, and CPB time >120 minutes. In adults undergoing cardiac surgery, selected risk factors for severe AKI included age, sex, diabetes, hypertension, preoperative eGFR, preoperative UACR, preoperative serum albumin, and CPB time >120 minutes. In these analyses, the mean of the uMMP-7 level within the first 6 hours post-CPB was modeled both as a categorical variable (categorized into quintiles) and a continuous variable (log-transformed).
To compare the performance of uMMP-7 and other biomarkers at different cutoff value, an AUC was generated.27 To evaluate the utility of the biomarkers on risk reclassification, we determined the category-free NRI and IDI, as previously described.28,29
Disclosures
None.
Supplementary Material
Acknowledgments
This study was supported by the National Key Technology Support Program of China (grants 2013BAI09B06 and 2015BAI12B07 to F.F.H.), the State Key Program of National Natural Science Foundation of China (grant 81430016 to F.F.H.), the Foundation for Innovation Research Groups of the National Natural Science Foundation of China (grant 81521003 to Y.L.), the Major International (Regional) Joint Research Project of the National Natural Science Foundation of China (grant 81620108003 to F.F.H.), the National Natural Science Foundation of China (grants 81670636 to X.Y. and 81671963 to C.C.), the Science and Information Technology of Guangzhou Key Project (grant 201508020260 to J.N.), and the Major Scientific and Technological Planning Project of Guangzhou (grant 201504010027 to F.F.H.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017020142/-/DCSupplemental.
References
- 1.Rosner MH, Okusa MD: Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 1: 19–32, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J: Independent association between acute renal failure and mortality following cardiac surgery. Am J Med 104: 343–348, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Zanardo G, Michielon P, Paccagnella A, Rosi P, Caló M, Salandin V, Da Ros A, Michieletto F, Simini G: Acute renal failure in the patient undergoing cardiac operation. Prevalence, mortality rate, and main risk factors. J Thorac Cardiovasc Surg 107: 1489–1495, 1994 [PubMed] [Google Scholar]
- 4.Belayev LY, Palevsky PM: The link between acute kidney injury and chronic kidney disease. Curr Opin Nephrol Hypertens 23: 149–154, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leung KC, Tonelli M, James MT: Chronic kidney disease following acute kidney injury-risk and outcomes. Nat Rev Nephrol 9: 77–85, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Bonventre JV, Basile D, Liu KD, McKay D, Molitoris BA, Nath KA, Nickolas TL, Okusa MD, Palevsky PM, Schnellmann R, Rys-Sikora K, Kimmel PL, Star RA; Kidney Research National Dialogue (KRND) : AKI: A path forward. Clin J Am Soc Nephrol 8: 1606–1608, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coca SG, Singanamala S, Parikh CR: Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int 81: 442–448, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE: The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int 79: 1361–1369, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park M, Coca SG, Nigwekar SU, Garg AX, Garwood S, Parikh CR: Prevention and treatment of acute kidney injury in patients undergoing cardiac surgery: A systematic review. Am J Nephrol 31: 408–418, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P: Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365: 1231–1238, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Wagener G, Jan M, Kim M, Mori K, Barasch JM, Sladen RN, Lee HT: Association between increases in urinary neutrophil gelatinase-associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology 105: 485–491, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Brinckerhoff CE, Matrisian LM: Matrix metalloproteinases: A tail of a frog that became a prince. Nat Rev Mol Cell Biol 3: 207–214, 2002 [DOI] [PubMed] [Google Scholar]
- 13.He W, Tan RJ, Li Y, Wang D, Nie J, Hou FF, Liu Y: Matrix metalloproteinase-7 as a surrogate marker predicts renal Wnt/β-catenin activity in CKD. J Am Soc Nephrol 23: 294–304, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Surendran K, Simon TC, Liapis H, McGuire JK: Matrilysin (MMP-7) expression in renal tubular damage: Association with Wnt4. Kidney Int 65: 2212–2222, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Zhou D, Tan RJ, Fu H, Liu Y: Wnt/β-catenin signaling in kidney injury and repair: A double-edged sword. Lab Invest 96: 156–167, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou D, Li Y, Lin L, Zhou L, Igarashi P, Liu Y: Tubule-specific ablation of endogenous β-catenin aggravates acute kidney injury in mice. Kidney Int 82: 537–547, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao L, Zhou D, Tan RJ, Fu H, Zhou L, Hou FF, Liu Y: Sustained activation of Wnt/β-catenin signaling drives AKI to CKD progression. J Am Soc Nephrol 27: 1727–1740, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parikh CR, Devarajan P, Zappitelli M, Sint K, Thiessen-Philbrook H, Li S, Kim RW, Koyner JL, Coca SG, Edelstein CL, Shlipak MG, Garg AX, Krawczeski CD; TRIBE-AKI Consortium : Postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric cardiac surgery. J Am Soc Nephrol 22: 1737–1747, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parikh CR, Coca SG, Thiessen-Philbrook H, Shlipak MG, Koyner JL, Wang Z, Edelstein CL, Devarajan P, Patel UD, Zappitelli M, Krawczeski CD, Passik CS, Swaminathan M, Garg AX; TRIBE-AKI Consortium : Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol 22: 1748–1757, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang X, Chen C, Tian J, Zha Y, Xiong Y, Sun Z, Chen P, Li J, Yang T, Ma C, Liu H, Wang X, Hou FF: Urinary angiotensinogen level predicts AKI in acute decompensated heart failure: A prospective, two-stage study. J Am Soc Nephrol 26: 2032–2041, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alge JL, Karakala N, Neely BA, Janech MG, Tumlin JA, Chawla LS, Shaw AD, Arthur JM; SAKInet Investigators : Urinary angiotensinogen and risk of severe AKI. Clin J Am Soc Nephrol 8: 184–193, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen C, Yang X, Lei Y, Zha Y, Liu H, Ma C, Tian J, Chen P, Yang T, Hou FF: Urinary biomarkers at the time of AKI diagnosis as predictors of progression of AKI among patients with acute cardiorenal syndrome. Clin J Am Soc Nephrol 11: 1536–1544, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molnar AO, Parikh CR, Sint K, Coca SG, Koyner J, Patel UD, Butrymowicz I, Shlipak M, Garg AX: Association of postoperative proteinuria with AKI after cardiac surgery among patients at high risk. Clin J Am Soc Nephrol 7: 1749–1760, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vijayan A, Faubel S, Askenazi DJ, Cerda J, Fissell WH, Heung M, Humphreys BD, Koyner JL, Liu KD, Mour G, Nolin TD, Bihorac A; American Society of Nephrology Acute Kidney Injury Advisory Group : Clinical use of the urine biomarker [TIMP-2] x [IGFBP7] for acute kidney injury risk assessment. Am J Kidney Dis 68: 19–28, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou D, Tian Y, Sun L, Zhou L, Xiao L, Tan RJ, Tian J, Fu H, Hou FF, Liu Y: Matrix metalloproteinase-7 is a urinary biomarker and pathogenic mediator of kidney fibrosis. J Am Soc Nephrol 28: 598–611, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kidney Disease Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group : KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 1–138, 2012 [Google Scholar]
- 27.DeLong ER, DeLong DM, Clarke-Pearson DL: Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 44: 837–845, 1988 [PubMed] [Google Scholar]
- 28.Cook NR: Statistical evaluation of prognostic versus diagnostic models: Beyond the ROC curve. Clin Chem 54: 17–23, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Pencina MJ, D’Agostino Sr. RB, D’Agostino Jr. RB, Vasan RS: Evaluating the added predictive ability of a newmarker: From area under the ROC curve to reclassification and beyond. Stat Med 27: 157–172; discussion 207–112, 2008 [DOI] [PubMed]
- 30.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.