Abstract
Glutathione S-transferase mu 1 (GSTM1) encodes an enzyme that catalyzes the conjugation of electrophilic compounds with glutathione to facilitate their degradation or excretion. The loss of one or both copies of GSTM1 is common in many populations and has been associated with CKD progression. With the hypothesis that the loss of GSTM1 is also associated with incident kidney failure and heart failure, we estimated GSTM1 copy number using exome sequencing reads in the Atherosclerosis Risk in Communities (ARIC) Study, a community-based prospective cohort of white and black participants. Overall, 51.2% and 39.8% of white participants and 25.6% and 48.5% of black participants had zero or one copy of GSTM1, respectively. Over a median follow-up of 24.6 years, 256 kidney failure events occurred in 5715 participants without prevalent kidney failure, and 1028 heart failure events occurred in 5368 participants without prevalent heart failure. In analysis adjusted for demographics, diabetes, and hypertension, having zero or one copy of GSTM1 associated with higher risk of kidney failure and heart failure (adjusted hazard ratio [95% confidence interval] for zero or one versus two copies of GSTM1: kidney failure, 1.66 [1.27 to 2.17]; heart failure, 1.16 [1.04 to 1.29]). Risk did not differ significantly between participants with zero and one copy of GSTM1 (P>0.10). In summary, the loss of GSTM1 was significantly associated with incident kidney and heart failure, independent of traditional risk factors. These results suggest GSTM1 function is a potential treatment target for the prevention of kidney and heart failure.
Keywords: cardiovascular disease, chronic heart failure, chronic renal failure, Epidemiology and outcomes, human genetics
Oxidative stress has been proposed as a key driver in the pathogenesis of both kidney and heart disease.1,2 An important mechanism in the defense against oxidative stress is the conjugation of pro-oxidant electrophiles with glutathione to facilitate their degradation or excretion.3 Glutathione S-transferase mu 1 (GSTM1) encodes one of the cytoplasmic isoenzymes that facilitates this conjugation.4,5 The loss of GSTM1 is common in many populations. Among white Americans, approximately 50% have zero copies, and 40% have one copy. Among black Americans, approximately 27% have zero copies, and 50% have one copy.6 Having zero or one copy of GSTM1 has been associated with CKD progression in black Americans with CKD attributed to hypertension.7 Having zero copies of GSTM1 has also been associated with ESRD in two other nonwhite populations.8,9 The consequences of the loss of GSTM1 in white and black Americans in the general population have not yet been evaluated.
The antioxidant activity conferred by GSTM1 may also be critical for the maintenance of normal cardiac function. In a mouse model of renal vascular injury, Gstm1 knockout mice exhibited vascular smooth muscle cell hypertrophy and hyperplasia in the renal vasculature.4,10 Similar vascular phenotypes have often been observed in the myocardium of patients with heart failure.11 Kidney disease and heart failure often coexist and may share common pathways, including inflammation and oxidative stress.12,13 Higher levels of acrolein, a substrate of GSTM1 and a product of lipid peroxidation, have been linked to kidney and cardiac damage in animal models.14,15 Thus, we evaluated the association of the loss of GSTM1 with kidney failure and heart failure in the Atherosclerosis Risk in Communities (ARIC) Study, a community-based cohort including both white and black participants. Incident CKD was analyzed as a secondary outcome to determine whether the loss of GSTM1 might be associated with earlier stages of kidney function decline.
Results
The ARIC Study enrolled 15,792 participants at baseline. This study included 5715 participants (3461 white and 2254 black participants) with whole exome sequencing reads in the Cohorts for Heart & Aging Research in Genomic Epidemiology Sequencing (CHARGE-S) project, available from the database of Genotype and Phenotypes (dbGaP study accession: phs000668.v1.p1).16 There were no major differences between included study participants and those missing exome sequencing, with the exception that included participants had slightly lower prevalent diabetes (both white and black participants) and prevalent hypertension and slightly higher eGFR (black participants only) (Supplemental Table 1).
Among the 5715 participants with available data, 61% were white and 39% were black (Table 1). In white participants, the mean age was 55 years, the mean baseline eGFR was 99 ml/min per 1.73 m2, prevalent diabetes was 8.3%, and prevalent hypertension was 27.9%. In black participants, the mean age was 53, baseline eGFR was 112 ml/min per 1.73 m2, prevalent diabetes was 17.7%, and prevalent hypertension was 53.0%. In white participants, 51.2% had zero copies of GSTM1, and 39.8% had one copy. In black participants, 25.6% had zero copies of GSTM1 and 48.5% had one copy. The distributions of the GSTM1 copy number in both groups did not have significant deviation from Hardy–Weinberg equilibrium (whites: P=0.08, blacks: P=0.16).
Table 1.
Baseline characteristics of participants by GSTM1 copy number (n=5715)
| Characteristic | White | Black | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 Copy | 1 Copy | 2 Copies | P Value | 0 Copy | 1 Copy | 2 Copies | P Value | |
| N | 1772 | 1379 | 310 | 576 | 1093 | 585 | ||
| Age, yr, mean (SD) | 54.7 (5.7) | 54.4 (5.6) | 55.0 (5.6) | 0.19 | 54.0 (5.9) | 53.4 (5.8) | 53.8 (5.7) | 0.12 |
| Men, n (%) | 821 (46.3) | 639 (46.3) | 151 (48.7) | 0.73 | 211 (36.6) | 406 (37.1) | 207 (35.4) | 0.77 |
| BMI, mean (SD) | 27.1 (4.7) | 27.0 (4.8) | 27.0 (4.6) | 0.97 | 29.6 (5.8) | 29.6 (6.3) | 29.9 (6.6) | 0.57 |
| Smoking, n (%) | 0.92 | 0.36 | ||||||
| Current smoker | 445 (25.1) | 328 (23.8) | 73 (23.5) | 178 (30.9) | 321 (29.4) | 159 (27.2) | ||
| Former smoker | 652 (36.8) | 521 (37.8) | 116 (37.4) | 151 (26.2) | 258 (23.6) | 146 (25.0) | ||
| Never smoked | 675 (38.1) | 530 (38.4) | 121 (39) | 247 (42.9) | 514 (47) | 280 (47.9) | ||
| Diabetes, n (%) | 153 (8.6) | 106 (7.7) | 27 (8.7) | 0.60 | 107 (18.6) | 197 (18) | 98 (16.8) | 0.70 |
| Hypertension, n (%) | 511 (28.8) | 370 (26.8) | 92 (29.7) | 0.38 | 322 (55.9) | 563 (51.5) | 309 (52.8) | 0.23 |
| Coronary heart disease, n (%) | 70 (4.0) | 71 (5.1) | 18 (5.8) | 0.16 | 27 (4.7) | 44 (4) | 18 (3.1) | 0.36 |
| eGFR, ml/min per 1.73 m2, mean (SD) | 98.9 (13.0) | 99.5 (12.8) | 98.6 (13.9) | 0.41 | 111.0 (19.0) | 112.6 (18.9) | 111.5 (18.4) | 0.20 |
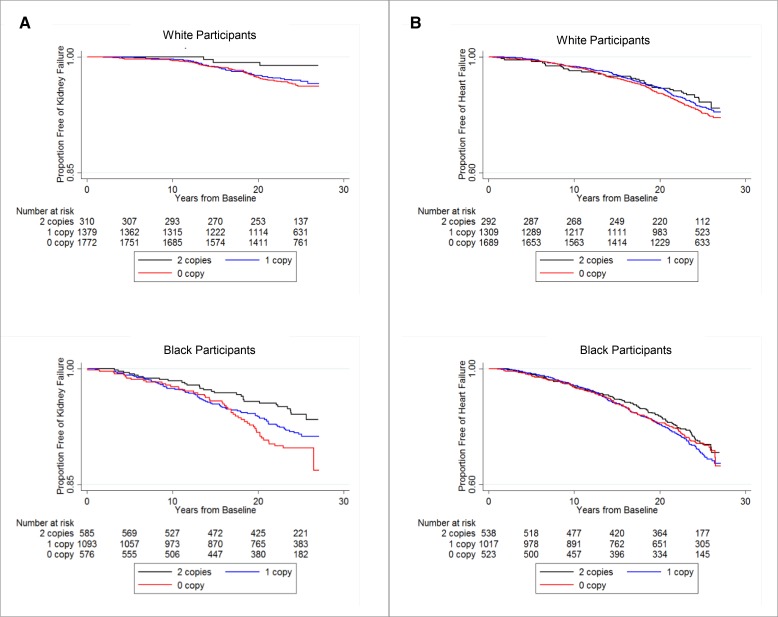
Over a median follow-up of 24.6 years, there were 256 incident kidney failure events (99 events in 3461 white participants and 157 events in 2254 black participants). In both white and black participants, those with two copies of GSTM1 had longer kidney failure–free survival than those with zero or one copy of GSTM1 (Figure 1A).
Figure 1.
In both white and black participants, those with two copies of GSTM1 had higher proportion of participants free of kidney failure than those with zero or one copy of GSTM1 during the follow-up period; a similar trend occurred for heart failure. Proportion free of kidney failure (A) and heart failure (B) in white and black participants by GSTM1 copy number.
In multivariable analysis including sampling weights to account for the case-cohort design of the CHARGE-S project, the increased risk for incident kidney failure in those with zero or one copy of GSTM1 persisted after adjusting for demographic factors and genetic principal components (model 1) as well as additional clinical risk factors, including age, sex, baseline eGFR, smoking status, body mass index (BMI), prevalent diabetes, hypertension, and coronary heart disease (model 2) (Table 2). Because the risk associations were similar between zero and one copy of GSTM1, we also included the dominant genetic model to compare those with zero or one copy of GSTM1 to those with two copies. Model fit was similar between the genotypic model (zero, one, and two GSTM1 copy numbers treated as separate categories) and the dominant genetic model (all models, likelihood ratio test P>0.1). Overall, with full adjustment including race, having zero or one copy of GSTM1 was associated with 66% higher risk for kidney failure (hazard ratio, 1.66; 95% confidence interval [95% CI], 1.27 to 2.17; P<0.001). There was no statistically significant difference by race in the association between GSTM1 copy number and incident kidney failure (all models, P for race interaction >0.10). There were 1647 incident CKD events (987 events in 3420 white participants and 660 events in 2214 black participants). No significant association between GSTM1 copy number and incident CKD was detected (Supplemental Table 2).
Table 2.
Risk of kidney failure associated with GSTM1 copy number (n=5715)
| Hazard Ratio (95% Confidence Interval) | |||||||
|---|---|---|---|---|---|---|---|
| Model | Genotypic Model (by GSTM1 Copy Number) | Dominant Genetic Model | |||||
| 0 Copy | 1 Copy | 2 Copies | P for Trend | 0 or 1 Copy versus 2 Copies | P Value | LRT P Value | |
| White cohort | |||||||
| Model 1 | 2.52 (1.32 to 4.82) | 2.54 (1.32 to 4.89) | Reference | 0.04 | 2.53 (1.34 to 4.79) | 0.004 | 0.96 |
| Model 2 | 2.45 (1.26 to 4.77) | 2.67 (1.36 to 5.23) | Reference | 0.12 | 2.54 (1.32 to 4.88) | <0.01 | 0.54 |
| Black cohort | |||||||
| Model 1 | 1.62 (1.18 to 2.22) | 1.38 (1.03 to 1.85) | Reference | 0.003 | 1.46 (1.11 to 1.92) | <0.01 | 0.23 |
| Model 2 | 1.55 (1.12 to 2.14) | 1.33 (0.99 to 1.78) | Reference | <0.01 | 1.40 (1.06 to 1.84) | 0.02 | 0.26 |
| Overall | |||||||
| Model 1 | 1.76 (1.35 to 2.31) | 1.58 (1.22 to 2.05) | Reference | 0.001 | 1.65 (1.29 to 2.12) | <0.001 | 0.13 |
| Model 2 | 1.76 (1.32 to 2.35) | 1.59 (1.20 to 2.11) | Reference | <0.001 | 1.66 (1.27 to 2.17) | <0.001 | 0.30 |
Model 1: age, sex, center, and ten genetic principal components. Model 2: model 1 plus prevalent diabetes, hypertension, coronary heart disease, smoking status, BMI, and eGFR. Race was included as a covariate in the combined analysis. Likelihood ratio test (LRT) was conducted to assess whether significant difference existed between the genotypic model and the dominant genetic model.
Over a median follow-up time of 24.4 years, there were 1028 incident heart failure events (532 events in 3290 white participants and 496 events in 2078 black participants). In both white and black participants, those with two copies of GSTM1 had a trend toward longer heart failure–free survival compared with those with zero or one copy of GSTM1 (Figure 1B). In multivariate analysis adjusting for demographic factors and genetic principal components (model 1) as well as additional clinical risk factors, including age, sex, baseline eGFR, smoking status, BMI, prevalent diabetes, hypertension, and coronary heart disease (model 2), there was a nonstatistically significant trend toward higher risk in those with zero or one copy of GSTM1 compared with participants with two copies in each race group (Table 3). When both race groups were combined, those with zero or one copy of GSTM1 had 16% higher risk for incident heart failure compared with those with two copies (hazard ratio, 1.16; 95% CI, 1.04 to 1.29; P<0.01; Table 3). The risk for heart failure in those with zero or one copy of GSTM1 was not significantly different between the two race groups (all P for race interaction >0.10), and all tests comparing the genotypic model versus the dominant genetic model were not significant, indicating similar model fit (all P>0.10).
Table 3.
Risk of heart failure associated with GSTM1 copy number (n=5368)
| Hazard Ratio (95% Confidence Interval) | |||||||
|---|---|---|---|---|---|---|---|
| Model | Genotypic Model (by GSTM1 Copy Number) | Dominant Genetic Model | |||||
| 0 Copy | 1 Copy | 2 Copies | P for Trend | 0 or 1 Copy versus 2 Copies | P Value | LRT P Value | |
| White cohort | |||||||
| Model 1 | 1.14 (0.96 to 1.35) | 1.17 (0.98 to 1.39) | Reference | 0.43 | 1.15 (0.97 to 1.36) | 0.11 | 0.62 |
| Model 2 | 1.16 (0.97 to 1.38) | 1.18 (0.99 to 1.42) | Reference | 0.31 | 1.17 (0.98 to 1.39) | 0.07 | 0.69 |
| Black cohort | |||||||
| Model 1 | 1.05 (0.88 to 1.25) | 1.15 (0.99 to 1.33) | Reference | 0.56 | 1.12 (0.97 to 1.28) | 0.12 | 0.22 |
| Model 2 | 1.04 (0.87 to 1.23) | 1.18 (1.02 to 1.36) | Reference | 0.63 | 1.13 (0.98 to 1.30) | 0.09 | 0.25 |
| Overall | |||||||
| Model 1 | 1.09 (0.97 to 1.23) | 1.15 (1.03 to 1.29) | Reference | 0.38 | 1.13 (1.01 to 1.25) | 0.03 | 0.24 |
| Model 2 | 1.12 (0.99 to 1.26) | 1.20 (1.07 to 1.34) | Reference | 0.32 | 1.16 (1.04 to 1.29) | <0.01 | 0.13 |
Model 1: age, sex, center, and ten genetic principal components. Model 2: model 1 plus prevalent diabetes, hypertension, coronary heart disease, smoking status, BMI, and eGFR. Race was included as a covariate in the combined analysis. Likelihood ratio test (LRT) was conducted to assess whether significant difference existed between the genotypic model and the dominant genetic model.
To ensure that the risk of kidney failure associated with having zero or one copy of GSTM1 was not driven by a higher risk of incident heart failure or vice versa, we repeated the analyses treating the alternative outcome as a time-varying covariate. Associations were not significantly changed. For example, participants with zero or one copy of GSTM1 had a 56% higher risk for kidney failure (95% CI, 1.21 to 2.01; P<0.001) after adjusting for prevalent and time-varying heart failure. In analysis adjusting for incident kidney failure, participants with zero or one copy of GSTM1 had a 16% higher risk of incident heart failure (95% CI, 1.03 to 1.28; P=0.01).
Discussion
In this large community-based cohort of middle-aged white and black American participants followed for 25 years, having zero or one copy of GSTM1 was associated with a 66% higher risk for kidney failure and 16% higher risk for heart failure independent of traditional risk factors. The association was robust to adjustment for the other outcome, suggesting that the risk of kidney failure is not mediated solely through the development of heart failure and vice versa. These results confirm findings of increased kidney risk associated with zero or one copy of GSTM1 in black Americans with CKD, and extend them to white and black Americans in the general population. Given the high population prevalence of zero or one copy of GSTM1 (>50%), a finding of increased and potentially modifiable risk could have substantial affect on public health.
GSTM1, located at 1p13.3, is a member of the cytosolic glutathione S-transferase gene family and is expressed in a wide range of tissues, including the kidney and the heart.17,18 These enzymes aid the detoxification function of glutathione by conjugating electrophiles to glutathione to facilitate their degradation or excretion. The substrates of GSTM1 include a wide range of aldehydes, hydrocarbons, and epoxides.5 For example, the enzyme encoded by GSTM1 participates in the elimination of acrolein and 4-hydroxynonenal, products of lipid peroxidation from both exogenous and endogenous sources.19,20 These compounds have been shown to cause cell damage in vitro20,21 and are linked to kidney and cardiac damage in animal models.14,15 Members of the glutathione transferase gene family are regulated by nuclear factor erythroid 2 like 2 (NFE2L2, also known as NRF2), an important transcription factor in the regulation of oxidative stress response.22
Previously, zero or one copy of GSTM1 compared with two copies was associated with CKD progression in the African American Study of Kidney Disease and Hypertension (AASK).7 Other previous studies of GSTM1 and kidney risk used the recessive genetic model, which compares zero copy of GSTM1 to one or two copies. For example, zero copies of GSTM1 was associated with ESRD in several case-control studies when compared with one and two copies combined.7–9,23 In addition, in patients on hemodialysis, zero copies was associated with higher levels of leukocyte 8-hydroxy-2′-deoxyguanosine (8-OHdG), a marker of oxidant-induced DNA damage, and with two-fold higher risk of all-cause mortality.24 This study adds to the existing literature by confirming the association between the loss of GSTM1 and kidney failure in a prospective community-based cohort of white and black Americans with largely normal kidney function at baseline, as well as establishing independent and parallel associations with incident heart failure.
In addition to confirming a link between GSTM1 and kidney failure, we identified a somewhat weaker association between the loss of GSTM1 and heart failure. Kidney dysfunction and heart failure often co-occur, and oxidative stress may be involved in the pathogenesis of both.13,25 Experimental evidence has supported the effect of oxidative stress on cardiac remodeling and hypertrophy.26 For example, Nfe2l2 knockout mice exhibited increased cardiac hypertrophy compared with wild-type mice after treatment with transverse aortic constriction.27 The transcription factor NFE2L2, which regulates GSTM1 expression, also defends against oxidative stress in the kidney.28,29 Our findings on the parallel association of the loss of GSTM1 with kidney and heart failure support the existence of common pathways.
The association between GSTM1 copy number and incident CKD was not significant in our study. The low sensitivity of the incident CKD outcome may have biased the results toward null. Another potential explanation is that the loss of GSTM1 may only be deleterious in the setting of high levels of uremic toxins, such as when GFR falls below certain levels. This is consistent with the observation that although most individuals (>70%) have zero or one copy of GSTM1, only a small proportion experienced the disease outcomes.
For kidney and heart failure in the ARIC Study, we found that the dominant model of GSTM1 deletion fit the data as well as the genotypic model. The dominant model was also reported as appropriate for CKD progression in the AASK cohort.30 This finding may suggest that two copies of GSTM1 is necessary to detoxify the substrates that cause kidney and heart damage. Alternatively, there may be complex interactions where the function of the GSTM1 enzyme depends not only on the number of copies but also on the overall dynamic of the metabolic system.31 For example, the dosage effect of GSTM1 copy number might be influenced by the levels of the substrates, intracellular glutathione levels, or the overlapping function of other members in glutathione S-transferase family.
The cytosolic glutathione S-transferase family has at least 17 members that are categorized into five classes. GSTM1 belongs to the mu class, which has five members located in close proximity at chromosome 1.18 These transferases share enzymatic function within and across families.5,19,32–34 For example, GSTP1 also metabolizes acrolein.35 In some individuals with zero copies of GSTM1, GSTM2 was found to be overexpressed leading to dosage compensation.36 As a result, similar total plasma glutathione transferase activities were observed in persons with and without GSTM1.36 Given that most individuals (>70%) in our study had zero or one copy of GSTM1 and only a small proportion experienced the disease outcomes, it is plausible that a second hit is required, such as increased toxic substrates or reduced dosage compensation from other transferases. Future studies on the dosage effect of GSTM1 copy number may benefit by measuring total glutathione transferase activity and considering dosage compensation from other genes in the glutathione transferase family. In addition, common toxic substrates of GSTM1 may be potential therapeutic targets for kidney and heart failure.
The strengths of this study include the use of a large community-based cohort including both white and black participants with a follow-up time of nearly 25 years. All three genotypes of GSTM1 were rigorously characterized. The definitions of kidney failure and heart failure had high sensitivity and specificity compared with physician chart review. However, some limitations warrant mentioning. First, our study population only included a subset of the ARIC Study participants with whole exome sequencing reads available from the CHARGE-S project. Those included appear to have a more favorable risk profile; for example, lower proportions of prevalent diabetes in both white and black participants. The significant association of GSTM1 in our analyzed sample was not likely due to the inclusion of high-risk individuals. In addition, we included sampling weights in our association analysis to account for the design of the CHARGE-S project (reported in the Concise Methods). Second, in white participants, there was a relatively low number with two copies of GSTM1. Future studies with a larger sample size may provide more precise estimates of the dosage effect for copy numbers. Third, the effect size of GSTM1 copy number on heart failure was small, which could preclude detection of dosage effects. Finally, the incident CKD outcome has low sensitivity, which may have biased the association between GSTM1 copy number and CKD toward null. In conclusion, the loss of GSTM1 (fewer than two copies) was associated with higher risk for kidney failure and heart failure in both white and black Americans, independent of traditional risk factors. Further studies on the GSTM1 detoxification pathways may uncover therapeutic targets for both kidney and heart failure.
Concise Methods
Study Population
The ARIC Study is a prospective study of 15,792 individuals, aged 45–65 years at visit 1 (1987–1989) from four communities in the United States.37 The National Heart, Lung, and Blood Institute CHARGE-S project includes 6491 ARIC Study participants (dbGaP study accession: phs000668.v1.p1).16 Of these participants, GSTM1 copy number was available for 6287 participants. Details on the quality control of exome sequencing reads are reported in the Supplemental Material. After further excluding those with eGFR<15 ml/min per 1.73 m2 or missing eGFR (n=89), and those having missing values in clinical covariates (n=159) or genetic principal components (n=324), the overall analyzed sample included 5715 participants for the study of incident kidney failure. For the analysis of incident CKD, we further excluded those with baseline eGFR <60 ml/min per 1.73 m2 or missing incident CKD status (n=81), resulting in a sample size of 5634. For the analysis of incident heart failure, from the overall analyzed sample of 5715, we excluded those with prevalent heart failure (n=150) or missing incident heart failure status (n=197), resulting in a sample size of 5368. Supplemental Table 1 reports a comparison of the key characteristics of participants who were included and excluded from the overall analyzed sample.
Estimation of GSTM1 Copy Number
Methods for whole exome sequencing are reported in the Supplemental Material. We estimated GSTM1 copy number by examining the distribution of the sum of the normalized coverage at GSTM1. First, after the quality control of the exome sequencing reads, we normalized the coverage at each exon of GSTM1 using the mean coverage of all captured regions at chromosome 1, which contains GSTM1. To combine all available information at GSTM1, we summed the normalized coverage of all eight exons of GSTM1. Because read length affects coverage distribution, sums of the normalized coverage were grouped by read length ranging from 50 to 101 bp. On the basis of the distribution of the sum of the normalized coverage, we determined empirical thresholds for determining GSTM1 copy number. Supplemental Table 3 reports the empirical thresholds for zero and one copy for each read length, and Supplemental Figure 1 presents the histogram of the sum of the normalized coverage for each read length. Previously, in the ARIC Study, a multiplex PCR assay was used to determine having zero copy versus one or two copies of GSTM1 in a subsample of 1211 participants.38,39 The number of participants with genotype from both PCR and exome sequencing reads was 853, and the agreement for zero copy of GSTM1 between the two methods was 99.3%.
Definitions of Outcomes
Kidney failure was defined as ESRD ascertained using linkage to the US Renal Data System (USRDS) or kidney failure on the basis of the International Classification of Diseases, Ninth or Tenth Revision, Clinical Modification (ICD-9-CM/ICD-10-CM) code for hospitalization or death.40 Compared with chart review by trained physicians, the sensitivity and specificity were 88% and 97% for kidney failure on the basis of ICD code, and 95% and 100% for ESRD.
Heart failure was defined as the first heart failure hospitalization or presence of heart failure code on death certificate (ICD-9 code 428×, ICD-10 code 150×). The percent agreement on heart failure event between adjudication by a standardized physician panel and ICD-9 code of 428× at any position was 73%.41
Incident CKD was defined as a composite outcome of (1) baseline eGFR ≥60 ml/min per 1.73 m2 at baseline with ≥25% decline to a level below 60 ml/min per 1.73 m2 at a follow-up visit, (2) CKD-related hospitalization identified by ICD-9-CM/ICD-10-CM codes, or (3) ESRD ascertained from the USRDS. Compared with chart review by trained physicians, the sensitivity and specificity of the hospital-based CKD events were 35.5% and 95.7%, respectively.42 All three outcomes were ascertained up to December 31, 2013.
Measures of Covariates
Race and smoking status were self-reported. Diabetes was defined as the use of diabetes medication, a fasting glucose level ≥126 mg/dl, or a random glucose ≥200 mg/dl, or self-reported physician diagnosis of diabetes. Hypertension was defined as a systolic BP ≥140 mm Hg, a diastolic BP ≥90 mm Hg, or the use of antihypertensive medications. Prevalent coronary heart disease was defined as a self-reported history of myocardial infarction or cardiac procedure. eGFR was calculated using the CKD Epidemiology Collaboration creatinine equation.43 Genetic principal components for controlling for population stratification were generated using genotypes from the Illuminia HumanExome BeadChip after quality control.44,45
Analyses
GSTM1 Genetic Models
We first analyzed the association between GSTM1 copy number and the outcomes using the genotypic model, i.e., using the three copy numbers as categorical variables. Having observed participants with zero or one copy of GSTM1 have similar and higher kidney failure risk than those with two copies, we further analyzed the association of GSTM1 copy number with kidney and heart failures using the dominant genetic model, i.e., combining zero and one copy as one group. We further performed likelihood ratio tests to compare the model fit of the genotypic model versus the dominant genetic model. In this paper, the loss of GSTM1 refers to those with zero or one copy of GSTM1.
Association Analysis
Baseline characteristics of participants were compared using t tests for nonskewed continuous variables, Wilcoxon tests for skewed continuous variable, and chi-squared tests for categorical variables. Kaplan–Meier estimates were plotted by GSTM1 copy number.
For the multivariate analysis of kidney failure, heart failure, and incident CKD, we used Cox regression including sampling weights on the basis of the CHARGE-S case-cohort design (details in Supplemental Material).16 In model 1, we adjusted for age, sex, study center, and the first ten genetic principal components. In model 2, we added other risk factors (prevalent diabetes, hypertension, coronary heart disease, smoking status, BMI, and eGFR at baseline). In the analysis combining both race groups, we added race as a covariate. For kidney and heart failure, we performed additional sensitivity analysis to investigate potential mediating effects of kidney failure for heart failure and vice versa. When incident kidney failure was the outcome, the sensitivity analysis included prevalent and incident heart failure as time-varying covariate. When incident heart failure was the outcome, the sensitivity analysis included kidney failure as time-varying covariate. Comparison of baseline characteristics was performed using R software version 3.2.5 and Cox regression were performed using SAS software version 9.4.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the Atherosclerosis Risk in Communities (ARIC) Study for their important contributions.
This work is supported by National Institute of Diabetes and Digestive and Kidney Diseases grant R21 DK112087. The ARIC Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
An abstract reporting the results of this study was presented at the American Society of Nephrology Kidney Week, in Chicago, IL, November 19, 2016.
Some of the data reported here have been supplied by the US Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US Government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017030228/-/DCSupplemental.
References
- 1.Roger VL: Epidemiology of heart failure. Circ Res 113: 646–659, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prakash S, O’Hare AM: Interaction of aging and chronic kidney disease. Semin Nephrol 29: 497–503, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dickinson DA, Moellering DR, Iles KE, Patel RP, Levonen AL, Wigley A, Darley-Usmar VM, Forman HJ: Cytoprotection against oxidative stress and the regulation of glutathione synthesis. Biol Chem 384: 527–537, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Yang Y, Parsons KK, Chi L, Malakauskas SM, Le TH: Glutathione S-transferase-micro1 regulates vascular smooth muscle cell proliferation, migration, and oxidative stress. Hypertension 54: 1360–1368, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayes JD, Flanagan JU, Jowsey IR: Glutathione transferases. Annu Rev Pharmacol Toxicol 45: 51–88, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Garte S, Gaspari L, Alexandrie AK, Ambrosone C, Autrup H, Autrup JL, Baranova H, Bathum L, Benhamou S, Boffetta P, Bouchardy C, Breskvar K, Brockmoller J, Cascorbi I, Clapper ML, Coutelle C, Daly A, Dell’Omo M, Dolzan V, Dresler CM, Fryer A, Haugen A, Hein DW, Hildesheim A, Hirvonen A, Hsieh LL, Ingelman-Sundberg M, Kalina I, Kang D, Kihara M, Kiyohara C, Kremers P, Lazarus P, Le Marchand L, Lechner MC, van Lieshout EM, London S, Manni JJ, Maugard CM, Morita S, Nazar-Stewart V, Noda K, Oda Y, Parl FF, Pastorelli R, Persson I, Peters WH, Rannug A, Rebbeck T, Risch A, Roelandt L, Romkes M, Ryberg D, Salagovic J, Schoket B, Seidegard J, Shields PG, Sim E, Sinnet D, Strange RC, Stücker I, Sugimura H, To-Figueras J, Vineis P, Yu MC, Taioli E: Metabolic gene polymorphism frequencies in control populations. Cancer Epidemiol Biomarkers Prev 10: 1239–1248, 2001 [PubMed] [Google Scholar]
- 7.Chang J, Ma JZ, Zeng Q, Cechova S, Gantz A, Nievergelt C, O’Connor D, Lipkowitz M, Le TH: Loss of GSTM1, a NRF2 target, is associated with accelerated progression of hypertensive kidney disease in the African American Study of Kidney Disease (AASK). Am J Physiol Renal Physiol 304: F348–F355, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutiérrez-Amavizca BE, Orozco-Castellanos R, Ortíz-Orozco R, Padilla-Gutiérrez J, Valle Y, Gutiérrez-Gutiérrez N, García-García G, Gallegos-Arreola M, Figuera LE: Contribution of GSTM1, GSTT1, and MTHFR polymorphisms to end-stage renal disease of unknown etiology in Mexicans. Indian J Nephrol 23: 438–443, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agrawal S, Tripathi G, Khan F, Sharma R, Baburaj VP: Relationship between GSTs gene polymorphism and susceptibility to end stage renal disease among North Indians. Ren Fail 29: 947–953, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Le TH, Fogo AB, Salzler HR, Vinogradova T, Oliverio MI, Marchuk DA, Coffman TM: Modifier locus on mouse chromosome 3 for renal vascular pathology in AT1A receptor-deficiency. Hypertension 43: 445–451, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Hasenfuss G, Just H: Myocardial phenotype changes in heart failure: Cellular and subcellular adaptations and their functional significance. Br Heart J 72[Suppl]: S10–S17, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shiba N, Shimokawa H: Chronic kidney disease and heart failure--Bidirectional close link and common therapeutic goal. J Cardiol 57: 8–17, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Schefold JC, Filippatos G, Hasenfuss G, Anker SD, von Haehling S: Heart failure and kidney dysfunction: Epidemiology, mechanisms and management. Nat Rev Nephrol 12: 610–623, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Fujii H, Nishijima F, Goto S, Sugano M, Yamato H, Kitazawa R, Kitazawa S, Fukagawa M: Oral charcoal adsorbent (AST-120) prevents progression of cardiac damage in chronic kidney disease through suppression of oxidative stress. Nephrol Dial Transplant 24: 2089–2095, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa N, Hasebe N, Sumitomo K, Fujino T, Fukuzawa J, Hirayama T, Kikuchi K: An oral adsorbent, AST-120, suppresses oxidative stress in uremic rats. Am J Nephrol 26: 455–461, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Building on GWAS for NHLBI-Diseases: The U.S. CHARGE Consortium (CHARGE-S): ARIC. Available at https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000668.v1.p1. Accessed: July 17, 2015
- 17.Rowe JD, Nieves E, Listowsky I: Subunit diversity and tissue distribution of human glutathione S-transferases: Interpretations based on electrospray ionization-MS and peptide sequence-specific antisera. Biochem J 325: 481–486, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins LG, Hayes JD: Mechanisms of induction of cytosolic and microsomal glutathione transferase (GST) genes by xenobiotics and pro-inflammatory agents. Drug Metab Rev 43: 92–137, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Singhal SS, Zimniak P, Awasthi S, Piper JT, He NG, Teng JI, Petersen DR, Awasthi YC: Several closely related glutathione S-transferase isozymes catalyzing conjugation of 4-hydroxynonenal are differentially expressed in human tissues. Arch Biochem Biophys 311: 242–250, 1994 [DOI] [PubMed] [Google Scholar]
- 20.Kehrer JP, Biswal SS: The molecular effects of acrolein. Toxicol Sci 57: 6–15, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Chapple SJ, Cheng X, Mann GE: Effects of 4-hydroxynonenal on vascular endothelial and smooth muscle cell redox signaling and function in health and disease. Redox Biol 1: 319–331, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chanas SA, Jiang Q, McMahon M, McWalter GK, McLellan LI, Elcombe CR, Henderson CJ, Wolf CR, Moffat GJ, Itoh K, Yamamoto M, Hayes JD: Loss of the Nrf2 transcription factor causes a marked reduction in constitutive and inducible expression of the glutathione S-transferase Gsta1, Gsta2, Gstm1, Gstm2, Gstm3 and Gstm4 genes in the livers of male and female mice. Biochem J 365: 405–416, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suvakov S, Damjanovic T, Stefanovic A, Pekmezovic T, Savic-Radojevic A, Pljesa-Ercegovac M, Matic M, Djukic T, Coric V, Jakovljevic J, Ivanisevic J, Pljesa S, Jelic-Ivanovic Z, Mimic-Oka J, Dimkovic N, Simic T: Glutathione S-transferase A1, M1, P1 and T1 null or low-activity genotypes are associated with enhanced oxidative damage among haemodialysis patients. Nephrol Dial Transplant 28: 202–212, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Lin YS, Hung SC, Wei YH, Tarng DC: GST M1 polymorphism associates with DNA oxidative damage and mortality among hemodialysis patients. J Am Soc Nephrol 20: 405–415, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hatamizadeh P, Fonarow GC, Budoff MJ, Darabian S, Kovesdy CP, Kalantar-Zadeh K: Cardiorenal syndrome: Pathophysiology and potential targets for clinical management. Nat Rev Nephrol 9: 99–111, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Takimoto E, Kass DA: Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension 49: 241–248, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Li J, Ichikawa T, Villacorta L, Janicki JS, Brower GL, Yamamoto M, Cui T: Nrf2 protects against maladaptive cardiac responses to hemodynamic stress. Arterioscler Thromb Vasc Biol 29: 1843–1850, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Shelton LM, Lister A, Walsh J, Jenkins RE, Wong MH, Rowe C, Ricci E, Ressel L, Fang Y, Demougin P, Vukojevic V, O’Neill PM, Goldring CE, Kitteringham NR, Park BK, Odermatt A, Copple IM: Integrated transcriptomic and proteomic analyses uncover regulatory roles of Nrf2 in the kidney. Kidney Int 88: 1261–1273, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruiz S, Pergola PE, Zager RA, Vaziri ND: Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int 83: 1029–1041, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bodonyi-Kovacs G, Ma JZ, Chang J, Lipkowitz MS, Kopp JB, Winkler CA, Le TH: Combined effects of GSTM1 null allele and APOL1 renal risk alleles in CKD progression in the African American Study of Kidney Disease and Hypertension Trial. J Am Soc Nephrol 27: 3140–3152, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kacser H, Burns JA: The molecular basis of dominance. Genetics 97: 639–666, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Comstock KE, Widersten M, Hao XY, Henner WD, Mannervik B: A comparison of the enzymatic and physicochemical properties of human glutathione transferase M4-4 and three other human Mu class enzymes. Arch Biochem Biophys 311: 487–495, 1994 [DOI] [PubMed] [Google Scholar]
- 33.Hansson LO, Mannervik B: Use of chimeras generated by DNA shuffling: Probing structure-function relationships among glutathione transferases. Methods Enzymol 328: 463–477, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Bogaards JJ, Venekamp JC, Salmon FG, van Bladeren PJ: Conjugation of isoprene monoepoxides with glutathione, catalyzed by alpha, mu, pi and theta-class glutathione S-transferases of rat and man. Chem Biol Interact 117: 1–14, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Berhane K, Widersten M, Engström A, Kozarich JW, Mannervik B: Detoxication of base propenals and other alpha, beta-unsaturated aldehyde products of radical reactions and lipid peroxidation by human glutathione transferases. Proc Natl Acad Sci U S A 91: 1480–1484, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhattacharjee P, Paul S, Banerjee M, Patra D, Banerjee P, Ghoshal N, Bandyopadhyay A, Giri AK: Functional compensation of glutathione S-transferase M1 (GSTM1) null by another GST superfamily member, GSTM2. Sci Rep 3: 2704, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.ARIC : The Atherosclerosis Risk in Communities (ARIC) study: Design and objectives. The ARIC investigators. Am J Epidemiol 129: 687–702, 1989 [PubMed] [Google Scholar]
- 38.Li R, Boerwinkle E, Olshan AF, Chambless LE, Pankow JS, Tyroler HA, Bray M, Pittman GS, Bell DA, Heiss G: Glutathione S-transferase genotype as a susceptibility factor in smoking-related coronary heart disease. Atherosclerosis 149: 451–462, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Olshan AF, Li R, Pankow JS, Bray M, Tyroler HA, Chambless LE, Boerwinkle E, Pittman GS, Bell DA: Risk of atherosclerosis: Interaction of smoking and glutathione S-transferase genes. Epidemiology 14: 321–327, 2003 [PubMed] [Google Scholar]
- 40.Rebholz CM, Coresh J, Ballew SH, McMahon B, Whelton SP, Selvin E, Grams ME: Kidney failure and ESRD in the Atherosclerosis Risk in Communities (ARIC) study: Comparing ascertainment of treated and untreated kidney failure in a cohort study. Am J Kidney Dis 66: 231–239, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agarwal SK, Chambless LE, Ballantyne CM, Astor B, Bertoni AG, Chang PP, Folsom AR, He M, Hoogeveen RC, Ni H, Quibrera PM, Rosamond WD, Russell SD, Shahar E, Heiss G: Prediction of incident heart failure in general practice: The Atherosclerosis Risk in Communities (ARIC) study. Circ Heart Fail 5: 422–429, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grams ME, Rebholz CM, McMahon B, Whelton S, Ballew SH, Selvin E, Wruck L, Coresh J: Identification of incident CKD stage 3 in research studies. Am J Kidney Dis 64: 214–221, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd , Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li M, Li Y, Weeks O, Mijatovic V, Teumer A, Huffman JE, Tromp G, Fuchsberger C, Gorski M, Lyytikainen LP, Nutile T, Sedaghat S, Sorice R, Tin A, Yang Q, Ahluwalia TS, Arking DE, Bihlmeyer NA, Boger CA, Carroll RJ, Chasman DI, Cornelis MC, Dehghan A, Faul JD, Feitosa MF, Gambaro G, Gasparini P, Giulianini F, Heid I, Huang J, Imboden M, Jackson AU, Jeff J, Jhun MA, Katz R, Kifley A, Kilpelainen TO, Kumar A, Laakso M, Li-Gao R, Lohman K, Lu Y, Magi R, Malerba G, Mihailov E, Mohlke KL, Mook-Kanamori DO, Robino A, Ruderfer D, Salvi E, Schick UM, Schulz CA, Smith AV, Smith JA, Traglia M, Yerges-Armstrong LM, Zhao W, Goodarzi MO, Kraja AT, Liu C, Wessel J, Boerwinkle E, Borecki IB, Bork-Jensen J, Bottinger EP, Braga D, Brandslund I, Brody JA, Campbell A, Carey DJ, Christensen C, Coresh J, Crook E, Curhan GC, Cusi D, de Boer IH, de Vries AP, Denny JC, Devuyst O, Dreisbach AW, Endlich K, Esko T, Franco OH, Fulop T, Gerhard GS, Glumer C, Gottesman O, Grarup N, Gudnason V, Harris TB, Hayward C, Hocking L, Hofman A, Hu FB, Husemoen LL, Jackson RD, Jorgensen T, Jorgensen ME, Kahonen M, Kardia SL, Konig W, Kooperberg C, Kriebel J, Launer LJ, Lauritzen T, Lehtimaki T, Levy D, Linksted P, Linneberg A, Liu Y, Loos RJ, Lupo A, Meisinger C, Melander O, Metspalu A, Mitchell P, Nauck M, Nurnberg P, Orho-Melander M, Parsa A, Pedersen O, Peters A, Peters U, Polasek O, Porteous D, Probst-Hensch NM, Psaty BM, Qi L, Raitakari OT, Reiner AP, Rettig R, Ridker PM, Rivadeneira F, Rossouw JE, Schmidt F, Siscovick D, Soranzo N, Strauch K, Toniolo D, Turner ST, Uitterlinden AG, Ulivi S, Velayutham D, Volker U, Volzke H, Waldenberger M, Wang JJ, Weir DR, Witte D, Kuivaniemi H, Fox CS, Franceschini N, Goessling W, Kottgen A, Chu AY: SOS2 and ACP1 loci identified through large-scale exome chip analysis regulate kidney development and function. J Am Soc Nephrol 28: 981–994, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grove ML, Yu B, Cochran BJ, Haritunians T, Bis JC, Taylor KD, Hansen M, Borecki IB, Cupples LA, Fornage M, Gudnason V, Harris TB, Kathiresan S, Kraaij R, Launer LJ, Levy D, Liu Y, Mosley T, Peloso GM, Psaty BM, Rich SS, Rivadeneira F, Siscovick DS, Smith AV, Uitterlinden A, van Duijn CM, Wilson JG, O’Donnell CJ, Rotter JI, Boerwinkle E: Best practices and joint calling of the HumanExome BeadChip: The CHARGE Consortium. PLoS One 8: e68095, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.