Abstract
Despite more than two decades of use, the optimal maintenance dose of tacrolimus for kidney transplant recipients is unknown. We hypothesized that HLA class II de novo donor-specific antibody (dnDSA) development correlates with tacrolimus trough levels and the recipient’s individualized alloimmune risk determined by HLA-DR/DQ epitope mismatch. A cohort of 596 renal transplant recipients with 50,011 serial tacrolimus trough levels had HLA-DR/DQ eplet mismatch determined using HLAMatchmaker software. We analyzed the frequency of tacrolimus trough levels below a series of thresholds <6 ng/ml and the mean tacrolimus levels before dnDSA development in the context of HLA-DR/DQ eplet mismatch. HLA-DR/DQ eplet mismatch was a significant multivariate predictor of dnDSA development. Recipients treated with a cyclosporin regimen had a 2.7-fold higher incidence of dnDSA development than recipients on a tacrolimus regimen. Recipients treated with tacrolimus who developed HLA-DR/DQ dnDSA had a higher proportion of tacrolimus trough levels <5 ng/ml, which continued to be significant after adjustment for HLA-DR/DQ eplet mismatch. Mean tacrolimus trough levels in the 6 months before dnDSA development were significantly lower than the levels >6 months before dnDSA development in the same patients. Recipients with a high-risk HLA eplet mismatch score were less likely to tolerate low tacrolimus levels without developing dnDSA. We conclude that HLA-DR/DQ eplet mismatch and tacrolimus trough levels are independent predictors of dnDSA development. Recipients with high HLA alloimmune risk should not target tacrolimus levels <5 ng/ml unless essential, and monitoring for dnDSA may be advisable in this setting.
Keywords: kidney transplantation, acute allograft rejection, donor specific antibody, Human leukocyte antigen, tacrolimus, allograft survival
Allograft survival is limited by alloimmunity, recurrent disease, infection, medication toxicity, and death with a functioning graft.1,2 Because each of these is directly or indirectly influenced by immunosuppression, physicians prescribe the lowest effective dose to minimize serious adverse effects, while controlling the alloimmune response. Unfortunately, predictive biomarkers to facilitate personalized immunosuppression are lacking, resulting in most patients being treated with similar medication regimens.3 Specifically, 93% of United States transplant programs use tacrolimus/mycophenolate mofetil (MMF) maintenance immunosuppression on the basis of the ELITE-Symphony Study and target tacrolimus trough levels formally studied for only 1 year.4–6 Knowledge of effective tacrolimus trough levels long term is limited, and one cannot predict in whom immunosuppression can be safely minimized.3,4
Recent immunosuppression minimization trials attempted to use clinical/histologic stability to define low risk for enrolment.7–9 However, these trials resulted in increased rates of class II de novo donor-specific antibody (dnDSA) development, known to be a major cause of allograft loss. Given the role of class II dnDSA in mediating late graft loss, attention has focused on the targets of antibody allorecognition (i.e., HLA epitopes).10 The most frequently used computational method to evaluate HLA epitope mismatch, the HLA Matchmaker program, identifies small patches of surface-exposed amino acids named “eplets” on each HLA allele.10 Whereas traditional HLA mismatch is constrained by a limited range of possible values (zero, one, or two per locus) at the whole-antigen level, HLA eplet mismatch assessment enhances the precision by quantifying the degree of similarity between donor-recipient HLA at the molecular level. Over the last 4 years, class II HLA eplet mismatch determination has been shown to be a superior method of risk stratification compared with traditional HLA antigen mismatch for predicting class II dnDSA development, allograft rejection, transplant glomerulopathy, and allograft survival.11–15 Whether the degree of class II eplet mismatch can serve as a predictive biomarker to guide personalized immunosuppression has not been determined.
For the first time, this study sought to determine the optimal tacrolimus trough level required to limit class II dnDSA development and allograft loss in relation to an individual’s class II HLA-DR and -DQ eplet mismatch. Unique to this consecutive patient cohort is the strict exclusion of preexisting HLA DSA, the availability of serial sera to characterize the timing of dnDSA onset, >50,000 serial tacrolimus trough levels, histopathologic data obtained as part of surveillance or clinically indicated biopsies, and long-term graft outcomes. This unprecedented granularity identifies an individual’s risk of minimizing tacrolimus therapy below key thresholds in relation to their HLA class II eplet mismatch.
Results
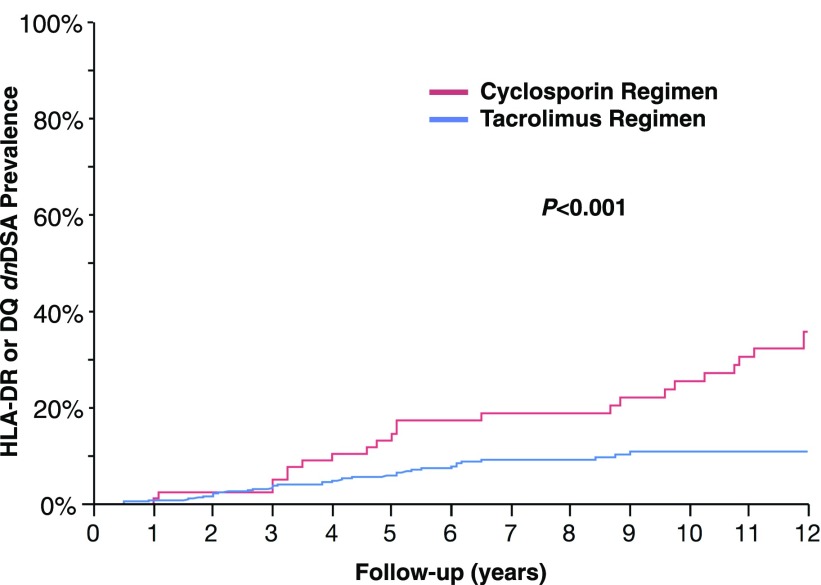
This consecutive cohort (n=596) represented a low-risk group (96% first transplant, <10% with cPRA>80%). Nevertheless, HLA-DR or -DQ dnDSA developed in 66 recipients (11%) at a median of 55 months (range of 6–170) post-transplant. At the time of dnDSA development, 15 of 66 (23%) had HLA-DR dnDSA alone, 37 of 66 (56%) had HLA-DQ dnDSA alone, and 14 of 66 (21%) had both HLA-DR and -DQ dnDSA. Significant correlates with class II dnDSA were younger recipient and donor ages, class II HLA-DR and -DQ eplet mismatch, longer cold ischemic time, calcineurin inhibitor (CNI) regimen (cyclosporin versus tacrolimus), nonadherence, CNI coefficient of variation (CV), and T cell–mediated rejection (TCMR) in the first year (Figure 1, Table 1). The incidence of class II dnDSA development was 2.7-fold higher in recipients treated with a cyclosporin regimen compared with a tacrolimus regimen (32 versus 12 per 1000 patients per year).
Figure 1.
Treatment with a tacrolimus regimen was associated with a lower prevalence of dnDSA development.
Table 1.
Recipient characteristics
| HLA-DR or -DQ dnDSA, n=66 | No HLA-DR or -DQ dnDSA, n=530 | P Value | |
|---|---|---|---|
| First transplant, % | 97 | 96 | 0.92 |
| Recipient age at transplant, yr | 33.6±17.6 | 44.6±15.6 | <0.001 |
| Donor age, yr | 36.6±14.9 | 40.7±14.7 | 0.04 |
| Living donor, % | 41 | 50 | 0.17 |
| Recipient ethnicity, white versus other, % | 76 | 65 | 0.07 |
| Cold ischemic time, h | 8.7±5.7 | 6.8±5.4 | 0.004 |
| Delayed graft function, % | 14 | 12 | 0.65 |
| Nonadherence, % | 41 | 11 | <0.001 |
| Cyclosporin versus tacrolimus regime, % | 39 | 11 | <0.001 |
| Calcineurin inhibitor coefficient of variation | 39.6±13.5 | 33.7±13.3 | 0.01 |
| HLA-A whole-antigen mismatch | 1.0±0.7 | 1.1±0.8 | 0.17 |
| HLA-B whole-antigen mismatch | 1.2±0.6 | 1.2±0.7 | 0.52 |
| HLA-C whole-antigen mismatch | 0.8±0.8 | 1.1±0.8 | 0.12 |
| HLA-DRβ1 whole-antigen mismatch | 1.4±0.5 | 1.2±0.7 | 0.14 |
| HLA-DRβ1/3/4/5 whole-antigen mismatch | 2.4±0.9 | 2.1±1.3 | 0.18 |
| HLA-DQβ1 whole-antigen mismatch | 1.2±0.5 | 1.1±0.7 | 0.25 |
| HLA-DQα1/β1 whole-antigen mismatch | 2.3±0.9 | 2.2±1.4 | 0.54 |
| HLA-DRβ1/3/4/5 eplet mismatch | 14.1±7.3 | 11.0±9.2 | 0.001 |
| HLA-DQα1/β1 eplet mismatch | 17.5±8.1 | 13.0±10.4 | 0.002 |
| Episodes of TCMR greater than or equal to borderline in 0–12 mo | 1.4±1.4 | 0.6±1.1 | <0.001 |
| Episodes of TCMR≥Banff 1A in 0–12 mo | 0.6±0.8 | 0.2±0.5 | <0.001 |
Class II HLA Eplet Mismatch Correlates with dnDSA Development and Graft Survival
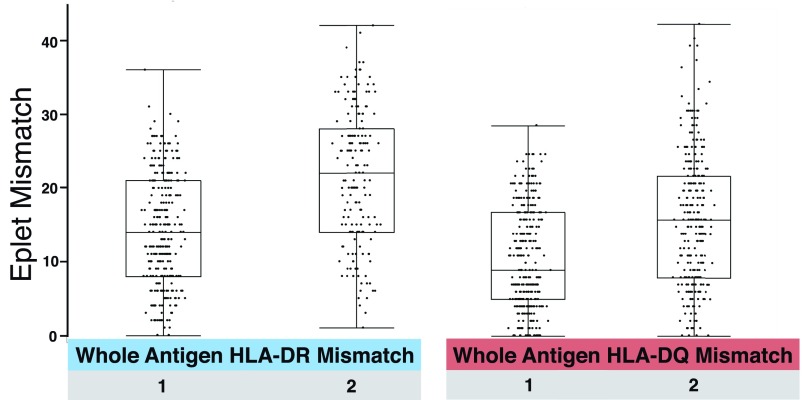
HLA-DR and -DQ antigen mismatches are associated with a broad range of eplet mismatches (Figure 2).
Figure 2.
Each HLA-DR and -DQ whole-antigen mismatch was associated with a broad range of eplet mismatches. Data points each represent one recipient's HLA-DR or DQ mismatch.
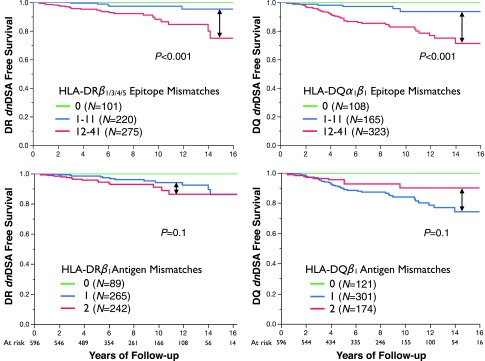
The median number of HLA-DR eplet mismatches was ten (range of 0–41). HLA-DR eplet mismatch was a significant predictor of HLA-DR dnDSA-free survival post-transplant (hazard ratio [HR], 2.50 per ten mismatches; 95% confidence interval [95% CI], 1.70 to 3.71; P<0.001). The HLA-DR eplet mismatch threshold that best correlated with HLA-DR dnDSA development was >11 mismatches (AUC=0.73, sensitivity =0.90, specificity =0.53) (Supplemental Figure 1). HLA-DR eplet mismatch also significantly correlated with graft failure (HR, 1.35 per ten mismatches; 95% CI, 1.01 to 1.81; P=0.05).
The median number of HLA-DQ eplet mismatches was 13 (range of 0–42). HLA-DQ eplet mismatch was significantly correlated with HLA-DQ dnDSA-free survival post-transplant (HR, 2.00 per ten mismatches; 95% CI, 1.54 to 2.61; P<0.001). The HLA-DQ eplet mismatch threshold that best correlated with HLA-DQ dnDSA development was >11 mismatches (AUC=0.72, sensitivity =0.94, specificity =0.45). HLA-DQ eplet mismatch also significantly correlated with graft failure (HR, 1.29 per ten mismatches; 95% CI, 1.01 to 1.67; P=0.05).
HLA-DR or -DQ eplet mismatch thresholds outperformed traditional whole-antigen HLA-DR or -DQ mismatch (zero, one, or two mismatches) to predict class II dnDSA-free survival post-transplant (Figure 3).
Figure 3.
HLA-DR or -DQ eplet mismatch thresholds outperformed traditional whole-antigen HLA-DR or -DQ mismatch (zero, one, or two mismatches) to predict Class II dnDSA-free survival post-transplant. HLA locus specific Kaplan-Meier dnDSA free survival curves shown stratified by eplet mismatch (top) or whole-antigen mismatch (bottom).
Defining Adequate Tacrolimus Immunosuppression to Avoid dnDSA Development
The majority (86%) of recipients were treated with tacrolimus, MMF, and prednisone. The analysis considered 50,011 tacrolimus trough levels (mean of 97 per recipient) after exclusion of levels drawn after dnDSA development. Tacrolimus trough-level CV was higher in recipients who developed HLA-DR/DQ dnDSA (39.6±13.5 versus 33.7±13.3; P=0.001).
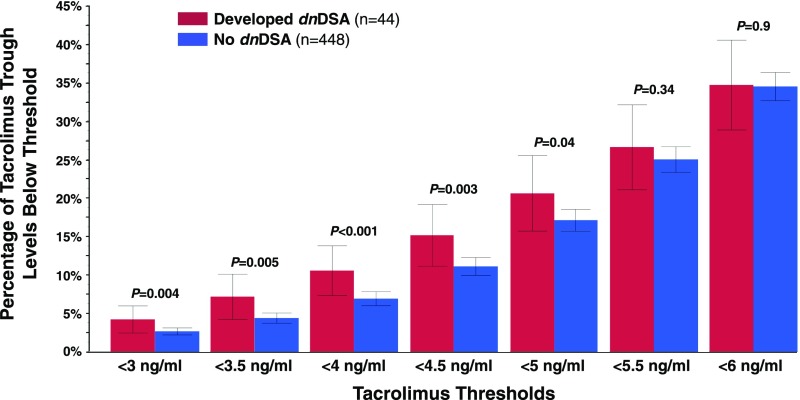
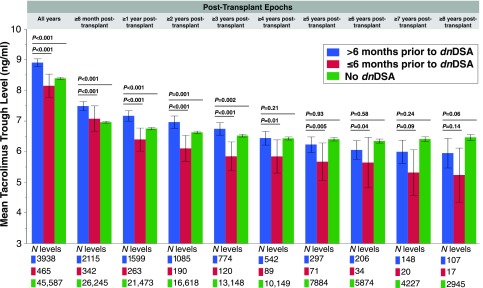
The lowest recorded tacrolimus trough levels for recipients who developed dnDSA were significantly less than those of recipients who did not (2.76±1.71 versus 3.42±1.45; P=0.01). Moreover, recipients who developed dnDSA had a significantly greater percentage of their tacrolimus trough levels below all thresholds <5 ng/ml compared with recipients who never developed dnDSA (Figure 4). Mean tacrolimus trough levels in the 6-month period before dnDSA development were significantly lower compared with tacrolimus trough levels in the same patients at all earlier time points (Figure 5). Furthermore, mean tacrolimus trough levels in the time period >6 months before dnDSA development were greater than or equal to those of recipients who never developed dnDSA.
Figure 4.
Recipients who developed dnDSA had a greater percentage of tacrolimus levels below thresholds of 5 ng/ml or less. For each tacrolimus threshold the percentage of trough levels measured below that threshold for each patient were analyzed. Values shown are the mean percentage below each threshold with 95% confidence intervals.
Figure 5.
In recipients who developed dnDSA, mean tacrolimus trough levels dropped significantly in the 6 months prior to dnDSA onset compared with their earlier trough levels. Mean tacrolimus levels in the six months prior to dnDSA onset were compared to all previous levels within distinct time epochs to show the consistency of association irrespective of the timing of dnDSA onset. Tacrolimus levels within the No dnDSA group are included for reference. Values represent the mean tacrolimus trough levels and their 95% confidence intervals.
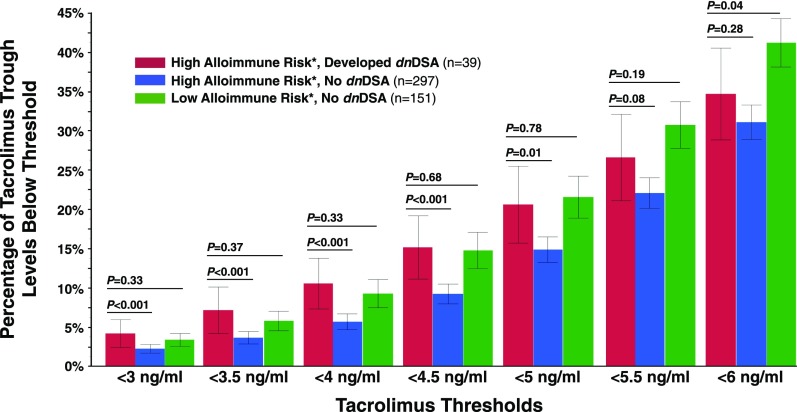
Recipients at high alloimmune risk (>11 HLA-DR or -DQ eplet mismatches) who developed dnDSA had a significantly greater percentage of tacrolimus trough levels <5 ng/ml compared with high-risk recipients who did not develop dnDSA (Figure 6). However, recipients with low alloimmune risk (≤11 HLA-DR and -DQ eplet mismatches) tolerated the same percentage of tacrolimus trough levels <5 ng/ml without developing dnDSA. Low-alloimmune risk recipients who developed dnDSA were rare (n=5), limiting comparisons. Finally, the proportions of tacrolimus trough levels below minimum thresholds of 3, 3.5, 4, and 4.5 ng/ml correlated with dnDSA development after adjusting for HLA-DR/DQ eplet mismatch when considered as a continuous variable rather than a threshold (Supplemental Table 1).
Figure 6.
Eplet mismatch modulates the effect of tacrolimus trough levels on the development of dnDSA. High risk: HLA-DR or -DQ eplet mismatch >11; low risk: HLA-DR and -DQ eplet mismatch ≤11. P values represent a comparison of high-risk patients who developed dnDSA with high-risk patients who did not develop dnDSA and a comparison of high-risk patients who developed dnDSA with low-risk patients who did not develop dnDSA. Values represent the mean percentages of tacrolimus trough levels below each threshold and their corresponding 95% confidence intervals.
After adjustment for HLA-DR/DQ eplet mismatch (odds ratio, 1.31; 95% CI, 1.10 to 1.56; P=0.002), the minimum recorded tacrolimus trough level correlated (odds ratio, 0.71 per 1 ng/ml; 95% CI, 0.57 to 0.88; P=0.001) with the risk for dnDSA development. Similarly, tacrolimus trough-level CV correlated (HR, 1.03; 95% CI, 1.00 to 1.05; P=0.02) with dnDSA development after adjustment for HLA-DR/DQ eplet mismatch (HR, 1.34; 95% CI, 1.14 to 1.58; P<0.001).
Independent Correlates of dnDSA Development and Antibody-Mediated Rejection
In a multivariate analysis, risk factors for class II dnDSA development in the entire cohort were younger recipient age, cyclosporin versus tacrolimus, nonadherence, and HLA-DR/DQ eplet mismatch (Table 2). In the subgroup (n=439) with one or more biopsies in the first post-transplant year, independent risk factors for dnDSA development were younger recipient age, nonadherence, HLA-DR/DQ eplet mismatch, and greater than or equal to borderline TCMR (Table 3). After restricting the analysis to those recipients on a tacrolimus regimen (n=492), the independent risk factors for dnDSA were younger recipient age, nonadherence, and HLA-DR/DQ eplet mismatch (Table 4). In all models evaluated, replacing traditional antigen mismatches with eplet mismatches resulted in improved model performance (Supplemental Table 2).
Table 2.
Multivariate correlates of dnDSA development: Total cohort
| Total Cohort | DR dnDSA n=596, 29 Events | DQ dnDSA n=596, 51 Events | DR or DQ dnDSA n=596, 66 Events | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Recipient age at transplant, yr | 0.97 (0.95 to 0.99) | 0.02 | 0.97 (0.95 to 0.98) | 0.002 | 0.97 (0.96 to 0.99) | 0.001 |
| Nonadherence | 3.07 (1.40 to 6.52) | <0.01 | 3.11 (1.71 to 5.58) | <0.001 | 3.09 (1.83 to 5.15) | <0.001 |
| Cyclosporin versus tacrolimus | 2.14 (0.93 to 4.70) | 0.07 | 1.97 (1.06 to 3.52) | 0.03 | 2.28 (1.35 to 3.78) | 0.002 |
| HLA-DRβ1/3/4/5 eplet mismatch/ten mismatches | 2.79 (1.84 to 4.27) | <0.001 | ||||
| HLA-DQα1/β1 eplet mismatch/ten mismatches | 2.00 (1.52 to 2.67) | <0.001 | ||||
| HLA-DRβ1/3/4/5 + HLA-DQα1/β1 eplet mismatch/ten mismatches | 1.37 (1.18 to 1.58) | <0.001 | ||||
Table 3.
Multivariate correlates of dnDSA development: Subset with histology 0–12 mo post-transplant
| Subset with Histology 0–12 mo Post-Transplant | DR dnDSA n=439, 29 Events | DQ dnDSA n=439, 48 Events | DR or DQ dnDSA n=439, 63 Events | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Recipient age at transplant, yr | 0.98 (0.96 to 1.00) | 0.09 | 0.97 (0.96 to 0.99) | 0.003 | 0.98 (0.96 to 0.99) | 0.003 |
| Nonadherence | 3.38 (1.53 to 7.28) | 0.003 | 3.78 (2.08 to 6.79) | <0.001 | 3.28 (1.93 to 5.51) | <0.001 |
| HLA-DRβ1/3/4/5 eplet mismatch/ten mismatches | 3.16 (1.96 to 5.24) | <0.001 | ||||
| HLA-DQα1/β1 eplet mismatch/ten mismatches | 1.46 (1.12 to 1.93) | <0.001 | ||||
| HLA-DRβ1/3/4/5 + HLA-DQα1/β1 eplet mismatch/ten mismatches | 1.34 (1.14 to 1.57) | 0.003 | ||||
| TCMR greater than or equal to borderline in 0–12 mo | 1.37 (1.08 to 1.69) | 0.01 | 1.31 (1.08 to 1.56) | 0.001 | 1.22 (1.03 to 1.43) | 0.02 |
Table 4.
Multivariate correlates of dnDSA development: Subset treated with tacrolimus regime
| Subset Treated with Tacrolimus Regime | DR dnDSA n=492, 19 Events | DQ dnDSA n=492, 34 Events | DR or DQ dnDSA n=492, 44 Events | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Recipient age at transplant, yr | 0.95 (0.91 to 0.98) | 0.002 | 0.95 (0.93 to 0.98) | <0.001 | 0.96 (0.94 to 0.97) | <0.001 |
| Nonadherence | 4.42 (1.74 to 11.41) | 0.002 | 3.59 (1.73 to 7.43) | 0.001 | 4.30 (2.29 to 8.08) | <0.001 |
| HLA-DRβ1/3/4/5 eplet mismatch/ten mismatches | 2.70 (1.64 to 4.53) | <0.001 | ||||
| HLA-DQα1/β1 eplet mismatch/ten mismatches | 2.24 (1.56 to 3.29) | <0.001 | ||||
| HLA-DRβ1/3/4/5 + HLA-DQα1/β1 eplet mismatch/ten mismatches | 1.34 (1.13 to 1.60) | 0.001 | ||||
In a multivariate analysis, risk factors for antibody-mediated rejection-free survival were younger recipient age (HR, 0.97 per year; 95% CI, 0.95 to 0.98; P=0.001), nonadherence (HR, 5.18; 95% CI, 2.7 to 9.9; P<0.001), and HLA-DR/DQ eplet mismatch (HR, 1.27 per ten mismatches; 95% CI, 1.1 to 1.5; P=0.01).
Independent Correlates of Allograft Loss
Fifty-seven recipients suffered allograft loss during the study period, and the causes were acute/chronic rejection (n=38), recurrent disease (n=12), acute tubular necrosis (n=3), chronic obstruction (n=2), BK nephropathy (n=1), and severe pyelonephritis (n=1). In the dnDSA group, 24 of 26 (92%) allografts lost were due to chronic active antibody-mediated rejection. Whether considering the entire study population or the cohort treated with a tacrolimus regimen, the best multivariate model of allograft survival included delayed graft function (HR, 2.32; 95% CI, 1.24 to 4.13; P=0.001), nonadherence (HR, 3.61; 95% CI, 2.04 to 6.36; P<0.001), and HLA-DR or -DQ dnDSA development (HR, 2.94; 95% CI, 1.66 to 5.15; P=0.003) (Supplemental Table 3).
Discussion
This study validates that HLA-DR and/or -DQ eplet mismatches are independent predictors of dnDSA development, antibody-mediated rejection, and allograft survival. Novel insights gained include the following. (1) Recipients treated with a tacrolimus regimen had significantly decreased risk of dnDSA development compared with those treated with a cyclosporin regimen after adjustment for other risk factors. (2) Tacrolimus trough levels <5 ng/ml had the greatest risk for dnDSA development, and (3) the effect of tacrolimus trough levels was modulated by the recipient’s baseline alloimmune risk as defined by their class II HLA-DR/DQ eplet mismatch. Each of these will be discussed in turn.
The relevance of HLA mismatch has been de-emphasized in recent years, leading to allocation largely driven by wait time. However, the advent of novel methods to assess HLA epitope mismatch has renewed interest in HLA compatibility. Using the current HLAMatchmaker software (updated version in 2016), this analysis found that an eplet mismatch threshold >11 predicted dnDSA development for HLA-DR or -DQ with sensitivity >90%. Importantly, HLA-DR/DQ eplet mismatch independently predicted dnDSA development, antibody-mediated rejection, and graft loss after adjustment for other risk factors (Tables 2–4). Thus, in the absence of pretransplant DSA, HLA-DR/DQ eplet mismatch represents a precise method of defining alloimmune risk. Using HLA-DR/DQ eplet mismatch to individualize immunosuppressive protocols and monitoring regimens may help prevent late dnDSA development—an outcome without a proven effective therapy. The challenge then is to determine the modulating effect of HLA-DR/DQ eplet mismatch on maintenance immunosuppression requirements.
Although tacrolimus has been approved by the Food and Drug Administration for kidney transplant since 1997, optimal target levels that prevent alloimmune events are still undefined.3 The ELITE-Symphony Study achieved mean tacrolimus trough levels ≥6.5 ng/ml throughout the first 12 months, with 75% of trough levels ranging between 4.1 and 9.5 ng/ml at 1 year.16 Despite only partial follow-up at 3 years (40% [162 of 401] of original cohort), recipients treated with tacrolimus who remained stable (mean GFR change =−1.9±13.8 ml/min from 12 to 36 months) had mean trough levels of 6.5±2.3 ng/ml at 3 years.17 Interestingly, the same tacrolimus trough level was achieved in those who did not develop class II dnDSA in this study (6.5±2.6 ng/ml) with much longer follow-up, whereas those who developed class II dnDSA had significantly lower levels in the 6 months immediately before dnDSA onset (Figure 5). Nevertheless, the lack of high-quality evidence to date constrained the 2017 COMMIT Group’s consensus recommendation to “aim for tacrolimus target trough levels of 5 to 10 ng/ml in the first year after transplantation,” with no recommendation for a long-term maintenance tacrolimus target trough level.18
The current interest of the transplant community is to withdraw or minimize tacrolimus therapy to limit long-term serious adverse events. However, early and late withdrawal strategies failed in two recent randomized, controlled trials (RCTs) largely due to rejection, dnDSA, or both shortly after tacrolimus cessation.7,8 Given the failure of tacrolimus withdrawal trials, the community is now shifting to define minimal tacrolimus target trough levels. In a recent RCT, Gatault et al.9 reported that a 50% reduction in the tacrolimus dose from 4 to 12 months post-transplant (12-month tacrolimus trough level =5.6±2.0 ng/ml) resulted in a higher incidence of rejection, subclinical inflammation, and dnDSA at 1 year. None of these studies used assessment of HLA eplet mismatch prospectively to define low-risk patients but instead, relied on clinical and histologic stability for enrolment. In retrospect, one of the aforementioned RCTs reported a higher HLA-DQ eplet mismatch load in patients withdrawn from tacrolimus who developed HLA-DQ dnDSA, suggesting that these events were predictable.7 Furthermore, it suggests that defining a lower tacrolimus target may need to take into account the modulating effect of class II eplet mismatch.
Identifying a lower tacrolimus target is challenging for a variety of reasons. (1) Levels decrease over time secondary to deliberate protocols or nonadherence; therefore, comparing events with controls in the same time period is critical. (2) Mean levels taken over many years do not capture brief low levels that may be clinically significant. (3) CV suggests nonadherence or variability in absorption/metabolism but does not necessarily equate with low levels. (4) Levels need to be understood in the context of an individual’s alloimmune risk. This study addressed these limitations and observed the following. (1) The risk for dnDSA development correlated with the proportion of tacrolimus trough levels <5 ng/ml (Figure 4). (2) dnDSA development was immediately preceded (≤6 months) by lower mean tacrolimus trough levels (Figure 5). (3) Recipients at high alloimmune risk (>11 HLA-DR or -DQ eplet mismatches) who developed HLA-DR/DQ dnDSA had a significantly greater percentage of tacrolimus levels <5 ng/ml compared with high-risk recipients who did not develop dnDSA (Figure 6). (4) Recipients with low alloimmune risk (≤11 HLA-DR and -DQ eplet mismatches) tolerated the same percentage of tacrolimus levels <5 ng/ml without developing dnDSA (Figure 6). Although an eplet mismatch >11 was the best threshold identified by ROC analysis, this same pattern is observed when other thresholds were analyzed (i.e., a higher proportion of low tacrolimus trough levels is tolerated by those with fewer mismatches). Thus, there seems to be a clear link between the degree of alloimmune risk as defined by HLA-DR/DQ eplet mismatch and the level of tacrolimus immunosuppression required to preclude dnDSA development.
Because of the relatively small sample size and the associated risk of type II error, risk quantification should be interpreted with caution and validated in a larger independent cohort. Histologic analysis ≤12 months post-transplant was only available in 441 of 598 (74%) of the cohort; nevertheless, 95% of the dnDSA and 88% of the graft losses occurred in these recipients. Mycophenloate mofetil levels were not routinely monitored. Although this analysis focuses on class II dnDSA, in our experience, isolated class I dnDSA is less pathogenic, with only one patient in this entire cohort suffering allograft failure after developing isolated class I dnDSA.
For clinicians seeking to minimize tacrolimus in the setting of infection (i.e., BK viremia) or to limit serious adverse effects, HLA-DR/DQ eplet mismatch provides a precise and individualized assessment of alloimmune risk to guide decision making. Conversely, in patients at high alloimmune risk, tacrolimus trough levels <5 ng/ml should not be targeted unless essential, in which case monitoring for dnDSA development would be advisable.
Concise Methods
Study Population
Approval was obtained from the IRB (H2011: 211), and it was in adherence with the Declaration of Helsinki. 654 adult and pediatric consecutive renal transplants between January of 1999 and January of 2015 were considered for inclusion. Patients with primary nonfunction (n=16) or pretransplant DSA (n=42) were excluded, leaving 596 recipients (adult n=541, pediatric n=55) for analysis. Median follow-up was 87 months (range of 18–210). Recipients who moved (n=21) or died with a functioning graft (n=82) were censored at last follow-up. Standard immunosuppression consisted of a CNI (tacrolimus [86%] or cyclosporin [14%]), MMF, and prednisone. Induction therapy with thymoglobulin (16%) or basiliximab (19%) was used in 35% of patients.
HLA Typing and Epitope Mismatch Identification
High-resolution class II HLA typing (HLA-DRβ1/3/4/5 and HLA-DQα1/β1) was performed using sequence-specific oligonucleotide probes or sequence-specific primer technology (LABType HD SSO, Micro SSP; One Lambda, Canoga Park, CA). HLAMatchmaker software (HLA DRDQDP Matching, version 2.0) was used to define class II eplet mismatches between donors and recipients.
HLA eplet identification is on the basis of two underlying principles. (1) The immune system recognizes and develops antibodies against nonself-antigens or more specifically, the epitopes on those antigens, while ignoring self-antigens/epitopes. (2) Epitope binding affinity is largely determined by a small number of polymorphic amino acids near the center of the epitope. Comparing the amino acid sequences between donor and recipient alleles allows for the identification and quantification of differences.19 Using HLAMatchmaker, only polymorphic amino acids are of interest, and only amino acids at or near the molecule’s surface accessible to antibody binding are considered. Patches of polymorphic amino acids on the surface are called eplets. HLAMatchmaker software (HLA DRDQDP Matching, version 2.0; http://www.epitopes.net) allows for the comparison of the number of eplet mismatches between donor and recipient HLA alleles. In the last year, the HLAMatchmaker software has been updated, resulting in a reduction in the total number of eplets (deletion of eplets with significant overlap on the molecular surface). Thus, previously determined HLA-DR and -DQ thresholds11 predicting locus-specific dnDSA development have changed.
Antibody Monitoring
Post-transplant serum samples were collected and stored at 0, 1, 2, 3, 6, 12, 18, and 24 months and then yearly or at the time of biopsy for graft dysfunction as routine clinical practice in our program since 1990. Since 2007, post-transplant surveillance for dnDSA was instituted for all patients with renal transplants. DSA screening was performed using FlowPRA beads representing HLA-A, -B, -Cw, -DR, -DQ, and -DP antigens (One Lambda). If the screening assay was positive, determination of HLA antibody specificities was performed using FlowPRA single-antigen class I and II beads (One Lambda) and analyzed according to the manufacturer’s recommendations. HLA antibody specificities were validated using LABScreen single-antigen beads (One Lambda) using a threshold mean fluorescence intensity ≥500 (mean fluorescence intensity ≥1000 initially or on a subsequent sample in 98% of patients).
Pretransplant, all patients had remote and immediate pretransplant sera screened by FlowPRA, and if they were positive, they were evaluated by FlowPRA single-antigen beads. Even if the FlowPRA screen was negative, patient sera were still evaluated by FlowPRA single-antigen beads if there was elevated risk of sensitization (e.g., pregnancy, history of transfusion). To rule out a DSA pretransplant, the mismatched donor antigens had to be represented on the single-antigen beads. If donor-specific antibodies were absent pretransplant, as determined by solid-phase assays and a negative flow crossmatch, and became detectable post-transplant, they were classified as dnDSA. Patients with dnDSA had banked post-transplant sera tested to determine the approximate timing of dnDSA onset by FlowPRA single-antigen beads. All patients continue to be prospectively tested for dnDSA according to the serum collection schedule outlined above to detect new dnDSA or assess the persistence of existing dnDSA.
Drug Monitoring
Tacrolimus and cyclosporin levels were measured by immune assay from 1999 to 2008 and mass spectroscopy subsequently. Tacrolimus trough targets (nanograms per milliliter) were 12±2 ng/ml in weeks 0–3, 10±2 ng/ml in weeks 3–12, 8–10 ng/ml in months 3–6, and then 5–8 ng/ml thereafter (Supplemental Figure 2). Cyclosporin trough targets (micrograms per milliliter) were 400 μg/ml for month 0 and then decreased by 25 μg/ml per month until at 150 μg/ml; then, they were 75–150 μg/ml thereafter. Tacrolimus trough levels ≥40 ng/ml and cyclosporin trough levels ≥1000 μg/ml were excluded, and levels below the tacrolimus detection limit (<2 ng/ml which occurred in 0.2% of levels) were considered zero. For calculation of tacrolimus means and CV, only recipients with five or more levels within the time period were analyzed. MMF target dose was 1 g twice per day as tolerated. Prednisone was tapered to 5 mg/d by 6 months post-transplant. Nonadherence was defined as patient admission of medication nonadherence or a recurring pattern of missed clinic appointments.
Clinical and Pathologic Monitoring
Study patients were followed at a single center in the adult or pediatric transplant clinic. Protocols beyond 6 months include serum creatinine measurement every 4–8 weeks and quarterly urine collections for proteinuria assessment. Six-month protocol biopsies were performed on all consenting patients. Renal biopsy was offered to all patients with newly detected dnDSA since January of 2008 as standard of care. Clinically indicated allograft biopsies were performed if proteinuria was ≥0.5 g/d or the serum creatinine rose ≥25% from baseline without a known cause. Clinical rejections were biopsy proven in 92%, including 100% of the clinical rejections preceding the onset of dnDSA in the dnDSA subgroup. Histology was evaluated using Banff criteria by a single experienced renal transplant pathologist (I.W.G.).20
Rejection Treatment
Recipients with dnDSA and/or acute rejection were treated by optimizing tacrolimus trough levels (8±2 ng/ml) and mycophenolate dose (2 g/d as tolerated). A steroid bolus with taper was given when clinical or subclinical acute rejection TCMR and/or antibody-mediated rejection was present on a biopsy. Occasionally, in patients with severe clinical TCMR, thymoglobulin was administered. For clinical antibody-mediated rejection, high-dose IVIG (2 g/kg) was given. Initially, recipients with subclinical antibody-mediated rejection received high-dose IVIG at the time of dnDSA detection; however, as previously reported, this was ineffective at resolving inflammation on a subsequent biopsy, and this practice was discontinued.21
Statistical Analyses
Comparisons between baseline predictors and clinical outcomes were done using paired t tests for parametric continuous variables and Wilcoxon rank tests for nonparametric data. Chi-squared or Fisher exact tests were used to test categorical variables. Comparisons across multiple groups were done using Kruskal–Wallis tests for nonparametric date and ANOVA for parametric variables. Survival analysis was done by the Kaplan–Meier method using the log rank test for significance. Cox proportional hazards model was used to evaluate predictors of graft loss and dnDSA-free survival. Akaike information criterion was calculated with Cox models to allow model comparisons within specific cohorts. Logistic regression was used when analyzing the proportion of minimum tacrolimus levels. Variables for multivariate regression were selected on the basis of bivariate screening, with P≤0.20 used to identify candidates for inclusion in the final model. The proportional hazard assumption was not violated (assessed by both Schoenfeld residuals and Harrell rho). Colinearity was assessed, and all variance inflation factors were less than three. Statistical software used was R (version 3.0.1) and JMP (version 12.2).
Disclosures
P.W.N. is a consultant for Astellas Pharma and Vitaeris Inc.
Supplementary Material
Acknowledgments
C.W. received funding from a Research Manitoba operating grant. P.W.N. is funded by the Canadian Institutes for Health Research and received salary support from the Flynn Family Chair in Renal Transplantation.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017030287/-/DCSupplemental.
References
- 1.El-Zoghby ZM, Stegall MD, Lager DJ, Kremers WK, Amer H, Gloor JM, Cosio FG: Identifying specific causes of kidney allograft loss. Am J Transplant 9: 527–535, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Wiebe C, Gibson IW, Blydt-Hansen TD, Pochinco D, Birk PE, Ho J, Karpinski M, Goldberg A, Storsley L, Rush DN, Nickerson PW: Rates and determinants of progression to graft failure in kidney allograft recipients with de novo donor-specific antibody. Am J Transplant 15: 2921–2930, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Leas BF, Uhl S, Sawinski DL, Trofe-Clark J, Tuteja S, Kaczmarek JL, Umscheid CA; ECRI Institute-Penn Medicine Evidence-based Practice Center : Calcineurin Inhibitors for Renal Transplant, Rockville, MD, Agency for Healthcare Research and Quality, 2016 [PubMed] [Google Scholar]
- 4.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group : KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 9[Suppl 3]: S1–S155, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Ekberg H, Tedesco-Silva H, Demirbas A, Vítko S, Nashan B, Gürkan A, Margreiter R, Hugo C, Grinyó JM, Frei U, Vanrenterghem Y, Daloze P, Halloran PF; ELITE-Symphony Study : Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 357: 2562–2575, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Hart A, Smith JM, Skeans MA, Gustafson SK, Stewart DE, Cherikh WS, Wainright JL, Kucheryavaya A, Woodbury M, Snyder JJ, Kasiske BL, Israni AK: OPTN/SRTR 2015 annual data report: Kidney. Am J Transplant 17[Suppl 1]: 21–116, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hricik DE, Formica RN, Nickerson P, Rush D, Fairchild RL, Poggio ED, Gibson IW, Wiebe C, Tinckam K, Bunnapradist S, Samaniego-Picota M, Brennan DC, Schröppel B, Gaber O, Armstrong B, Ikle D, Diop H, Bridges ND, Heeger PS; Clinical Trials in Organ Transplantation-09 Consortium : Adverse outcomes of tacrolimus withdrawal in immune-quiescent kidney transplant recipients. J Am Soc Nephrol 26: 3114–3122, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dugast E, Soulillou JP, Foucher Y, Papuchon E, Guerif P, Paul C, Riochet D, Chesneau M, Cesbron A, Renaudin K, Dantal J, Giral M, Brouard S: Failure of calcineurin inhibitor (tacrolimus) weaning randomized trial in long-term stable kidney transplant recipients. Am J Transplant 16: 3255–3261, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Gatault P, Kamar N, Büchler M, Colosio C, Bertrand D, Durrbach A, Albano L, Rivalan J, Le Meur Y, Essig M, Bouvier N, Legendre C, Moulin B, Heng AE, Weestel PF, Sayegh J, Charpentier B, Rostaing L, Thervet E, Lebranchu Y: Reduction of extended-release tacrolimus dose in low immunological risk kidney transplant recipients increases risk of rejection and appearance of Donor-Specific Antibodies: A randomized study. Am J Transplant 17: 1370–1379, 2017 [DOI] [PubMed] [Google Scholar]
- 10.Duquesnoy RJ, Askar M: HLAMatchmaker: A molecularly based algorithm for histocompatibility determination. V. Eplet matching for HLA-DR, HLA-DQ, and HLA-DP. Hum Immunol 68: 12–25, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiebe C, Pochinco D, Blydt-Hansen TD, Ho J, Birk PE, Karpinski M, Goldberg A, Storsley LJ, Gibson IW, Rush DN, Nickerson PW: Class II HLA epitope matching-A strategy to minimize de novo donor-specific antibody development and improve outcomes. Am J Transplant 13: 3114–3122, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Wiebe C, Nevins TE, Robiner WN, Thomas W, Matas AJ, Nickerson PW: The synergistic effect of class II HLA epitope-mismatch and nonadherence on acute rejection and graft survival. Am J Transplant 15: 2197–2202, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Sapir-Pichhadze R, Tinckam K, Quach K, Logan AG, Laupacis A, John R, Beyene J, Kim SJ: HLA-DR and -DQ eplet mismatches and transplant glomerulopathy: A nested case-control study. Am J Transplant 15: 137–148, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Kosmoliaptsis V, Mallon DH, Chen Y, Bolton EM, Bradley JA, Taylor CJ: Alloantibody responses after renal transplant failure can be better predicted by donor-recipient HLA amino acid sequence and physicochemical disparities than conventional HLA matching. Am J Transplant 16: 2139–2147, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiebe C, Nickerson P: Strategic use of epitope matching to improve outcomes. Transplantation 100: 2048–2052, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Ekberg H, Mamelok RD, Pearson TC, Vincenti F, Tedesco-Silva H, Daloze P: The challenge of achieving target drug concentrations in clinical trials: Experience from the Symphony study. Transplantation 87: 1360–1366, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Ekberg H, Bernasconi C, Tedesco-Silva H, Vítko S, Hugo C, Demirbas A, Acevedo RR, Grinyó J, Frei U, Vanrenterghem Y, Daloze P, Halloran P: Calcineurin inhibitor minimization in the Symphony study: Observational results 3 years after transplantation. Am J Transplant 9: 1876–1885, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Neuberger JM, Bechstein WO, Kuypers DR, Burra P, Citterio F, De Geest S, Duvoux C, Jardine AG, Kamar N, Krämer BK, Metselaar HJ, Nevens F, Pirenne J, Rodríguez-Perálvarez ML, Samuel D, Schneeberger S, Serón D, Trunečka P, Tisone G, van Gelder T: Practical recommendations for long-term management of modifiable risks in kidney and liver transplant recipients: A guidance report and clinical checklist by the consensus on managing modifiable risk in transplantation (COMMIT) group. Transplantation 101[4S Suppl 2]: S1–S56, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Duquesnoy RJ: A structurally based approach to determine HLA compatibility at the humoral immune level. Hum Immunol 67: 847–862, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, Castro MC, David DS, David-Neto E, Bagnasco SM, Cendales LC, Cornell LD, Demetris AJ, Drachenberg CB, Farver CF, Farris AB 3rd, Gibson IW, Kraus E, Liapis H, Loupy A, Nickeleit V, Randhawa P, Rodriguez ER, Rush D, Smith RN, Tan CD, Wallace WD, Mengel M; Banff meeting report writing committee : Banff 2013 meeting report: Inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant 14: 272–283, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Wiebe C, Gibson IW, Blydt-Hansen TD, Karpinski M, Ho J, Storsley LJ, Goldberg A, Birk PE, Rush DN, Nickerson PW: Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant 12: 1157–1167, 2012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.