Originally identified as a homolog of a viral oncogene (v-ets), the ETS family of transcription factors consists of over 25 proteins involved in regulating several biologic processes at the transcriptional level. The ETS DNA binding domain consists of a winged helix conformation and binds the DNA sequences 5′-GGA(A/T)-3′ with varying affinity depending on ETS phosphorylation status, epigenetic modifications, and various protein-protein interactions.1
ETS-1 is the founding member of the family of ETS transcription factors that play crucial roles in angiogenesis and hematopoiesis, and it is emerging as a mediator of vascular remodeling and kidney injury.2–4 Quantitative trait loci analysis in congenic rats previously identified Ets-1 as a candidate gene in a locus on the rat chromosome 8 linked to albuminuria.5 In this issue of the Journal of the American Society of Nephrology, Feng et al.6 show the significant contribution of kidney-specific ETS-1 on the pathogenesis of kidney injury in salt-sensitive hypertension. Using the Dahl salt-sensitive (SS) rat strain and Zinc-Finger Nuclease technology to mutate a single Ets-1 allele (ES), the authors showed that ETS-1 haploinsufficiency did not alter BP of the ES rats at baseline or those on low-salt diet. However, on a high-salt diet, the ES strain had attenuated rise in serum creatinine and albuminuria compared with the SS strain, despite similar BP at 1 week. Beyond the first week through the fourth week, the ES strain had lower rise in BP and abrogated glomerular injury and kidney fibrosis. Although this finding suggests that the lower serum creatinine and albuminuria may be independent of systemic BP early on, it raises the question of whether ETS-1 plays a direct role in glomerular hemodynamic and hyperfiltration in the early phase of high-salt exposure.
To address the possibility that ES rats had less renal injury because of lower systemic BP rather than a direct protective effect of ETS-1 haploinsufficiency, the authors took the elegant approach of renal crosstransplantation. Renal chimeric mice were generated using a salt-resistant rat strain (SS-13BN) as the recipient of kidneys from the SS, ES, or SS-13BN parental strain, all sharing identical MHC. No differences in baseline BP or albuminuria were observed among groups; however, when exposed to a high-salt diet, all three renal chimeric strains developed hypertension over a 2-week period. The SS renal chimeric rats had the highest systolic BP, diastolic BP, serum creatinine, urinary albumin concentration, and renal injury, whereas the ES renal chimeric rats had the lowest. Because the model is a bilateral native nephrectomy and one transplanted kidney model, these data suggest that the salt-resistant rat strain (SS-13BN) becomes salt sensitive with nephron mass reduction. Importantly, intrarenal ETS-1 significantly influences the severity of hypertension and kidney injury in the setting of high salt exposure and loss of nephron mass.
To address the underlying molecular mechanism through which the ETS-1 transcription factor influences kidney injury, the authors used mesangial cells to confirm the capacity of ETS-1 to bind to the promoter region of the monocyte chemoattractant protein 1 (MCP-1) gene previously identified in smooth muscle cells.7 Mesangial cells were activated by angiotensin II, and the ETS-1 transcription factor and associated cis-element were isolated and assayed by chromatin immunoprecipitation. Indeed, angiotensin II stimulation resulted in increased binding of ETS-1 to two of seven ETS-1 binding sites in the MCP-1 promoter. To validate this finding in vivo in the high-salt diet model, glomeruli were isolated from SS and ES rats 1 week after high salt exposure. Compared with glomeruli from SS rats, glomeruli from ES rats had significantly less ETS-1, MCP-1, and matrix metalloprotease 2 (MMP-2) protein expression. Although indirect, these data suggest that, when activated, ETS-1 promotes the expression of its target proteins MCP-1 and MMP-2 that are involved in leukocyte chemotaxis and extracellular remodeling. Because angiotensin II increases ETS-1 binding activity, of particular interest is the observation that ETS-1 haploinsufficiency is dependent on dietary salt intake. In this regard, no difference in ETS-1 protein level was observed between the SS and ES strains on low-salt diet and in a high renin state. The lower level of ETS-1 protein expression in ES rats was observed only during high salt intake. On the basis of the in vitro studies, it is plausible that local angiotensin II production may be increased in SS rats consuming a high-salt diet, thereby increasing ETS-1 binding activity. However, the exact factor that stimulates ETS-1 protein expression with increased dietary salt intake remains to be determined and may be highly relevant in the clinical context of salt consumption in CKD.
The finding by Feng et al.6 is consistent with previous report of glomerular production of MCP-1 (CCL2) in response to low-dose angiotensin II infusion8 and suggests that ETS-1 may mediate kidney injury in the Dahl SS rats by promoting the expression of chemotactic factor MCP-1 and enzymes involved in extracellular remodeling (MMP). MCP-1 is ubiquitously expressed and binds to the CCR2 receptor that is primarily present on monocytes and memory T cells. Previous studies have reported the deleterious function of monocytes and/or macrophages in glomerular diseases that could be ameliorated with monocyte/macrophage depletion.9,10 Administration of an antagonist to the CCR2b receptor subtype significantly reduced the number of macrophages in the renal cortex and ameliorated albuminuria and progression of hypertension in male Sprague–Dawley rats fed a high-salt diet and receiving angiotensin II.11 Thus, upregulation of ETS-1 may explain the observed increase in MCP-1/CCL2 expression and renal and glomerular monocyte/macrophage infiltration in the Dahl SS rats when exposed to a high-salt diet.12
MMPs are secreted zinc-dependent endopeptidases (some are membrane tethered) that proteolyze a variety of proteins, particularly components of the extracellular matrix. Secreted as inactive zymogens, MMPs are activated by other proteases (MMPs, trypsin, and plasmin) or interestingly, by oxidative stress. MMPs play an important role in glomerular homeostasis through their modulation of extracellular remodeling that is crucial for maintenance of the glomerular filtration barrier.13 The detrimental effect of MMPs on kidney health was illustrated by a study showing that MMP inhibitors significantly reduced glomerular injury, proteinuria, and mean arterial BP in salt-sensitive rats fed a high-salt diet.14 Genetic deletion of Mmp2 in salt-sensitive rats ameliorated hypertension and proteinuria compared with wild-type salt-sensitive rats.15 Combined, these data suggest that MMPs are crucial regulators of glomerular health and may be key mediators of renal injury with ETS-1 activation.
Additional studies are needed to provide more definitive support of Ets-1 as the causative gene in the quantitative trait loci on chromosome 8 identified for differences in susceptibility to albuminuria from high salt exposure in the Dahl SS and SHR rat strains. Is ETS-1 differentially expressed between the two strains? In other rodent models of salt-sensitive hypertension and kidney injury, is ETS-1 expression and/or activity upregulated, or is this phenomenon unique to the Dahl SS strain? Is ETS-1 expression altered in salt-resistant strains exposed to high-salt diet? Are there genetic variants in the human ETS-1 gene that are associated with kidney disease development?
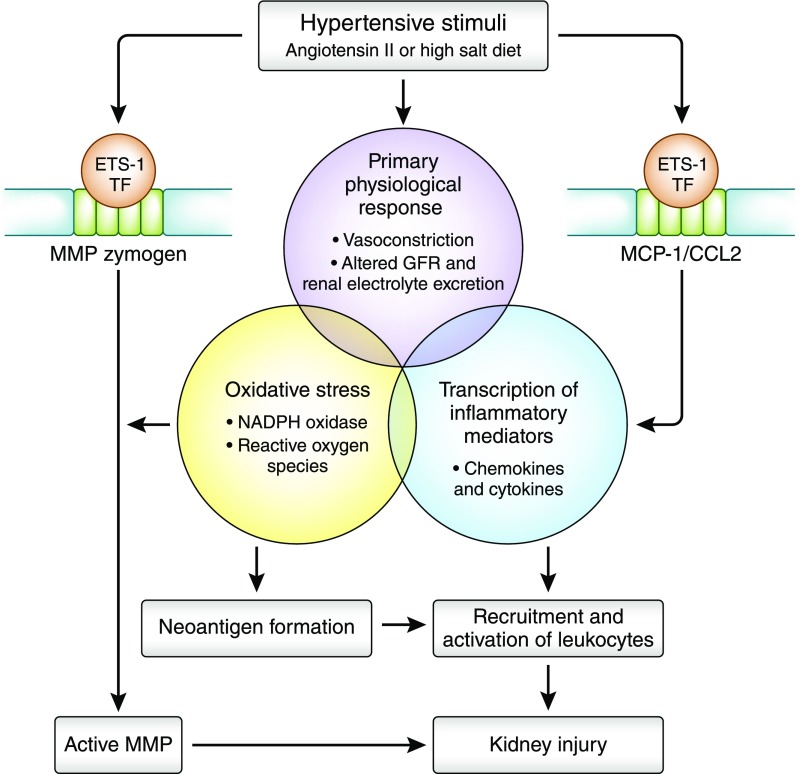
In summary, the findings of Feng et al.6 align with the emerging paradigm that the introduction of a hypertensive stimulus (such as angiotensin II or high-salt diet) initiates a sequential series of events that includes an inflammatory response and culminates with the maintenance of hypertension and end organ renal damage16 (Figure 1). The ETS-1 transcription factor in the kidney may be an important link between the hypertensive stimulus and the inflammatory response, and it provides a promising new therapeutic target in the treatment of hypertension-associated kidney disease.
Figure 1.
Potential involvement of ETS-1 in the emerging paradigm of the pathophysiology of hypertension-associated kidney injury. A hypertensive stimulus initiates a series of cellular and pathophysiologic events that culminate with end organ kidney injury. Through its role in initiating and/or enhancing the transcription of MCP-1 and MMPs, renal ETS-1 transcription factor (TF), when activated by hypertension or high-salt diet, contributes to glomerular damage by promoting glomerular remodeling and recruitment of leukocytes. NADPH, nicotinamide adenine dinucleotide phosphate.
Disclosures
None.
Acknowledgments
The research of T.H.L. is supported by National Institutes of Health grant R01 DK113632.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related article, “Haploinsufficiency of the Transcription Factor Ets-1 Is Renoprotective in Dahl Salt-Sensitive Rats,” on pages 3239–3250.
References
- 1.Sharrocks AD: The ETS-domain transcription factor family. Nat Rev Mol Cell Biol 2: 827–837, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Sato Y, Teruyama K, Nakano T, Oda N, Abe M, Tanaka K, Iwasaka-Yagi C: Role of transcription factors in angiogenesis: Ets-1 promotes angiogenesis as well as endothelial apoptosis. Ann N Y Acad Sci 947: 117–123, 2001 [PubMed] [Google Scholar]
- 3.Bartel FO, Higuchi T, Spyropoulos DD: Mouse models in the study of the Ets family of transcription factors. Oncogene 19: 6443–6454, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Tanaka H, Terada Y, Kobayashi T, Okado T, Inoshita S, Kuwahara M, Seth A, Sato Y, Sasaki S: Expression and function of Ets-1 during experimental acute renal failure in rats. J Am Soc Nephrol 15: 3083–3092, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Garrett MR, Joe B, Yerga-Woolwine S: Genetic linkage of urinary albumin excretion in Dahl salt-sensitive rats: Influence of dietary salt and confirmation using congenic strains. Physiol Genomics 25: 39–49, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Feng W, Chen B, Xing D, Li X, Fatima H, Jaimes EA, Sanders PW: Haploinsufficiency of the transcription factor Ets-1 is renoprotective in Dahl salt-sensitive rats. J Am Soc Nephrol 28: 3239–3250, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhan Y, Brown C, Maynard E, Anshelevich A, Ni W, Ho IC, Oettgen P: Ets-1 is a critical regulator of Ang II-mediated vascular inflammation and remodeling. J Clin Invest 115: 2508–2516, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruiz-Ortega M, Ruperez M, Lorenzo O, Esteban V, Blanco J, Mezzano S, Egido J: Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int Suppl 82: S12–S22, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Rovin BH, Schreiner GF: Cell-mediated immunity in glomerular disease. Annu Rev Med 42: 25–33, 1991 [DOI] [PubMed] [Google Scholar]
- 10.Duffield JS: Macrophages and immunologic inflammation of the kidney. Semin Nephrol 30: 234–254, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elmarakby AA, Quigley JE, Olearczyk JJ, Sridhar A, Cook AK, Inscho EW, Pollock DM, Imig JD: Chemokine receptor 2b inhibition provides renal protection in angiotensin II - salt hypertension. Hypertension 50: 1069–1076, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian N, Moore RS, Braddy S, Rose RA, Gu JW, Hughson MD, Manning Jr. RD: Interactions between oxidative stress and inflammation in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol 293: H3388–H3395, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Tan RJ, Liu Y: Matrix metalloproteinases in kidney homeostasis and diseases. Am J Physiol Renal Physiol 302: F1351–F1361, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams JM, Zhang J, North P, Lacy S, Yakes M, Dahly-Vernon A, Roman RJ: Evaluation of metalloprotease inhibitors on hypertension and diabetic nephropathy. Am J Physiol Renal Physiol 300: F983–F998, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams JM, Slaughter TN, Paige A, Chen CC, Fan F, Guerts AM, Jacob HJ, Roman RJ: The role of matrix metalloproteinase-2 during the development of hypertension-induced renal injury in Dahl salt-sensitive rats. Hypertension 60: A347, 2012 [Google Scholar]
- 16.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM: Inflammation, immunity, and hypertension. Hypertension 57: 132–140, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]