Abstract
Background:
Fatigue is the most common side effect of cancer treatment with chemotherapy and/or radiation therapy, selected biologic response modifiers. The main purpose of this study is to evaluate the effects of aerobic exercise on cancer-related fatigue in patients of the solid tumor after chemotherapy and radiotherapy.
Methods:
After screening for cancer-related fatigue, 34 patients fulfilled the inclusive criteria and were assigned into two groups (n = 17 recruited in the intervention group and n = 17 in control group). The intervention group received aerobic exercise program which included treadmill walking with low to moderate intensity (50%–70% of maximum heart rate), for 20–40 min/day for 5 days/week. Control group were taught stretching exercises of hamstrings, gastrocnemius, and soleus (to be done at home) and were encouraged to remain active. Outcome measures such as brief fatigue inventory (BFI), 6-min walk test, and functional assessment of cancer therapy-general (FACT-G) were taken at baseline and after 6-weeks.
Results:
The data were analyzed using the Wilcoxon matched-pairs signed rank test for within group and Mann–Whitney U-test for between group comparisons. The results of this study showed that there was a significant reduction in cancer-related fatigue BFI score (P < 0.0001,), also there was significant improvement in the physical performance as in 6-min walk distance (P < 0.0001) and quality of life, FACT-G score (P = 0.0001).
Conclusion:
Aerobic exercise for 6 weeks has beneficial effects on cancer-related fatigue in patients with solid tumor after chemotherapy and/or radiotherapy.
Keywords: Aerobic exercise, cancer, fatigue
INTRODUCTION
Cancer patients commonly report having one or more cancer-related symptoms that impact their quality of life (QOL) and activities of daily living.[1] One of the most commonly reported symptoms is fatigue.[2] It is the most common side effect of cancer treatment with chemotherapy, radiation therapy, or selected biologic response modifiers.[3] Cancer-related fatigue (CRF) is a reported side effect of all types of cancer treatment.[4] It has been estimated that between 70% and 100% of cancer patients will experience CRF.[5] It has not been linked to specific types of cancer. Although fatigue may be a presenting symptom, CRF occurs more commonly during treatment, with 30% continuing to experience fatigue after treatment is completed.[6] Cancer treatment-related fatigue generally improves after therapy is completed, but some level of fatigue may persist for months or years following treatment. Research indicates that for at least a subset of patients, fatigue may be a significant issue long into survivorship.[7,8] The etiology of CRF most likely involves the dysregulation of several interrelated physiological, biochemical, and psychological systems. Factors related to the development of CRF include serotonin dysregulation, hypothalamic-pituitary-adrenocortical axis dysfunction, circadian rhythm disruption, muscle metabolism/adenosine triphosphate (ATP) dysregulation, and cytokine dysregulation. Comorbid conditions such as anemia, cachexia, and depression have an incremental effect on CRF.[9]
CRF has a profound impact on patients' lives. Indeed, CRF has been rated as more troublesome and to have a greater negative impact on patients' daily activities and QOL than other cancer-related symptoms, including pain, depression, and nausea.[10]
For the management of CRF both pharmacologic and nonpharmacological interventions is available. Pharmacological intervention includes psychostimulants, corticosteroids, and exercises (e.g., individually tailored walking, cycling, or swimming programs), and modification of activity (e.g., naps during morning and early afternoon), assessment of sleep patterns, stress management and cognitive therapies, and adequate nutrition and hydration are nonpharmacological methods of dealing with fatigue.[11]
National Comprehensive Cancer Network (NCCN) suggest treating any identifiable conditions – for example, anemia or insomnia. If these treatments do not work, or if no potentially causative factor can be identified, the guidelines suggest a variety of possible approaches. One of the recommended nonpharmacological approaches is increased activity. Activity and exercise may reduce both the physical effects and the psychological stress connected with cancer treatments, improve mood, and reduce anxiety and fear in patients.[4]
CRF frequently results from muscle metabolism/ATP dysregulation caused by cancer treatment. The muscle cells obtain energy for work through two metabolic pathways. In the first one, carbohydrates and fats are completely oxidized to water and carbon dioxide in the mitochondria; the energy obtained is stored in the cells as ATP. This process can only be carried out in the presence of oxygen and is therefore called aerobic. When the oxygen supply is reduced, the cells produce energy through the second metabolic pathway, called anaerobic glycolysis. In this process, glucose is incompletely metabolized, resulting in the production of ATP and lactic acid. The effects of physical activity are not limited to better cardiovascular or muscular function. Indeed, the improvement of physical performance can increase the feeling of control, independence, and self-esteem of patients; this improved self-confidence can result in better social interaction and a reduction in anxiety and fear. Therefore, physical activity also can result in secondary benefits, such as an improved mood state.[12] There is also some evidence suggesting that physical activity can have an effect on the immune system.[13,14]
The American College of Sports Medicine defines aerobic exercise as “any activity that uses large muscle groups, can be maintained continuously, and is rhythmic in nature.” There is a literature on the effects of exercise on cancer rehabilitation, especially for breast cancer patients, on whom the majority of research has been conducted. Only a limited number of formal scientific studies to see the benefits of aerobic exercise on CRF in patients with solid tumor after chemotherapy and/or radiotherapy have been carried out. This study tries to look for the actual benefits of aerobic exercise on fatigue, physical performance, and QOL.
METHODS
Participants
Patients both male and female with cancer-related fatigue after chemotherapy and/or radiotherapy between ages 35 and 70 years and willing to participate were included in the study. Patients with metastatic bone disease, Hemoglobin concentration <8–10 mg/dl, Platelet concentrations <20,000 mg/dl, Neutrophil counts <0.5 × 10 9 μl, Fever >37.8°C (100°F), cardiopulmonary disease, musculoskeletal disorders, uncontrolled hypertension, diabetes mellitus, and mental illness[15] were excluded from the study.
Procedure
Ethical clearance was taken from the institutional ethical committee, all patients who had undergone chemotherapy and/or radiotherapy, CRF was carefully reviewed by an oncologist before initiating exercise testing. The evaluation included a detailed history and physical examination to identify any medical problems which would limit participation in exercise. Screening of CRF was performed according to recommended algorithm of the NCCN. Each patient was screened for the presence and severity of fatigue. The rating was done on a 0–10 scale, with 0 being “no fatigue” and 10 “the worst fatigue imaginable.” A score of 1–3 indicated the presence of mild fatigue that did not require clinical intervention so were advised for general strategies to manage fatigue, i.e., energy conservation. The scores of 4–6 and 7–10 indicated moderate and severe fatigue, respectively, and needed clinical intervention.[2] A total of 55 patients underwent screening for the presence and severity of fatigue. After finding suitability as per inclusion and exclusion criteria, 34 candidates were selected and requested to participate in the study. They were informed in detail about the study and a written informed consent was taken. Patients were randomly included (using chit method) in the groups, 17 each in both the interventional group A in the control group B for 6 weeks.
Intervention group A received aerobic exercise program which comprised of a warm up period, aerobic exercise period (conditioning period) and a cool down period.
The warm up period included 10-min of mild total body movement and exercises, including walking (light-rate of perceived exertion on Borg scale). The aerobic exercise period[16] included low to moderate intensity exercise and the intensity was determined based on maximum heart rate (HR) and rate of perceived exertion. Aerobic training was performed at 50%–70% of maximum HR (maximum HR was considered to be 220 − age), so for the lower limit of HR training range (low target HR = HR maximum × 0.50) and for the upper limit of HR training range (high target HR = HR maximum × 0.70) and rate of perceived exertion 11–13 on the Borg scale. Duration of exercise was 20–40 min/day and the frequency was 5 days/week.[16] Aerobic exercise program included walking on a treadmill and following an interval training pattern. During the 1st week, exercise duration was a 3-min bout 5 times during the week. Exercise duration was increased weekly and the number of training bouts reduced (a 5-min bout 5 times in the 2nd week, an 8-min bout three times in the third, a 10-min bout 3 times in the fourth, a 15-min bout 2 times in the fifth and 30–35 min without interruption in the 6th week).[17] The cool down period lasted for 5–10 min, it included walking and hamstrings, calves stretching. The vital measures such as HR and blood pressure was monitored before patient left the department.
Control group B were instructed for self-stretching exercise of hamstrings and calves at home and were encouraged to remain active but not instructed about aerobic exercise.[18]
Outcome measures
The outcome measures included were fatigue, physical performance, and QOL at baseline and after 6 weeks. Cancer-related fatigue was measured with the brief fatigue inventory (BFI) which is patient-report instrument with established reliability and validity commonly used in studies of CRF. The BFI allows for the rapid assessment of fatigue in cancer patients and identifies those patients with severe fatigue as well as its impact on their ability to function over the previous 24 h. The BFI consists of nine items, measured on 0–10 numeric rating scales. Three items in the BFI ask the patients to rate the severity of their fatigue at its “worst,” “usual,” and “now” during normal waking hours, with 0 being “no fatigue and 10 being “fatigue as bad as you can imagine.” Six items assesses the amount that fatigue has interfered with different aspects of the patient's life during the past 24 h. The interference items include general activity, mood, walking ability, normal work (includes both work outside the home and housework), relations with other people, and enjoyment of life. The interference items are measured on a 0–10 scale, with 0 being “does not interfere and 10 being “completely interferes”[19] and English or Gujarati version of BFI was used.
The physical function was assessed using the 6-min walk test (6MWT) which assesses the submaximal level of functional capacity as most activities of daily living are performed at submaximal levels of exertion, so 6-min walk distance (6MWD) reflects the functional exercise level for daily physical activities. The 6MWT should be performed indoors, along a long, flat, straight, enclosed corridor with a hard surface that is seldom traveled. The walking course should be 30 m in length. This test measures the distance that a patient can quickly walk on a flat, hard surface in 6 min. It evaluates the global and integrated responses of all the systems involved during exercise, including the pulmonary and cardiovascular systems, systemic circulation, peripheral circulation, blood, neuromuscular units, and muscle metabolism.[20]
The QOL was assessed using the functional assessment of cancer therapy-general (FACT-G). This questionnaire is one of the most widely used instruments for QOL assessment of patients with cancer. The FACT-G questionnaire is a self-reported instrument that measures multidimensional QOL using a total of 29 items in the following five specific life domains: (1) Physical well-being (7 items), (2) social well-being (7 items), (3) emotional well-being (6 items), and (4) functional well-being (7 items). The patients were asked to indicate how true each statement was for them during the past week on a 5-point ordinal scale where 0 indicates not at all; 1, a little bit; 2, somewhat; 3, quite a bit; and 4, very much. The score of each domain (possible range, 0–28 for physical well-being, 0–28 for social well-being, 0–24 for emotional well-being, 0–28 for functional well-being, higher scores indicate better QOL and the total score of all domains (possible range, 0–108) were computed for each assessment and English or Hindi version of FACT-G was used.[21]
RESULTS
Data analyses
No differences were found in baseline sociodemographic and medical characteristics between the 34 patients who were included then divided equally into intervention group A and control group B. As Shown in Figure 1. out of 34 patients, 17 patients included in the intervention group, Five patients dropped out of the study and in the control group out of 17 patients four were dropped out. Hence, the intervention group made up of a total 12 patients and control group consisted of 13 patients. The statistical analyses were performed using GraphPad Prism statistical software, version 0.6 (GraphPad Software, Inc.). Table 1 shows the characteristics of patients.
Figure 1.
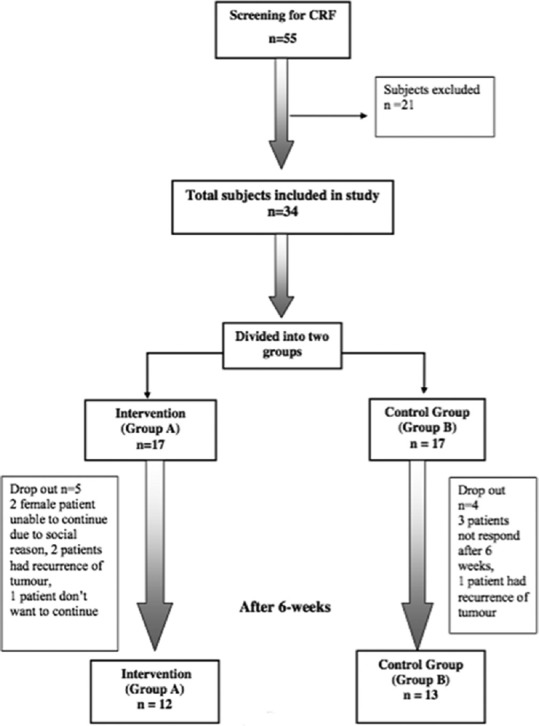
Flow Chart
Table 1.
Characteristics of patients
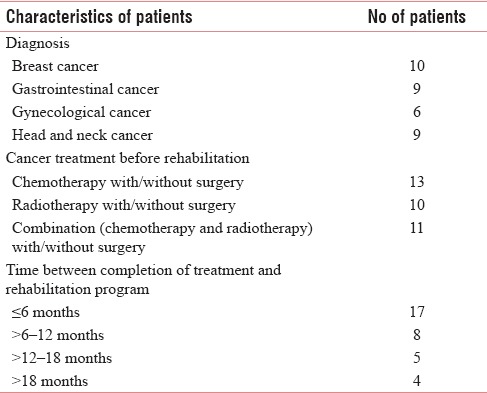
Table 2 shows the mean age and gender distribution of the 25 patients participated in the study. The mean age of intervention group and control group was 49.08 (±6.59) and 50.62 (±5.66), respectively, and intervention group A had six males and six females and the control group B had eight males and five females.
Table 2.
Demographic data

Table 3 and Figures 2–4 shows mean changes in BFI, FACT-G score and 6MWD in both group A and B in reference to pre- and post-treatment given to the patients. Here, Wilcoxon matched-pairs signed rank test was used for analysis and if the value of P < 0.05 was considered statistically significant.
Table 3.
Outcome measures at baseline and after 6 weeks intervention within a groups
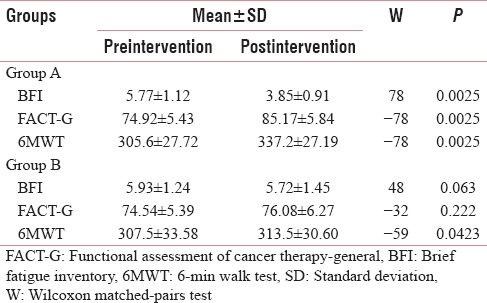
Figure 2.
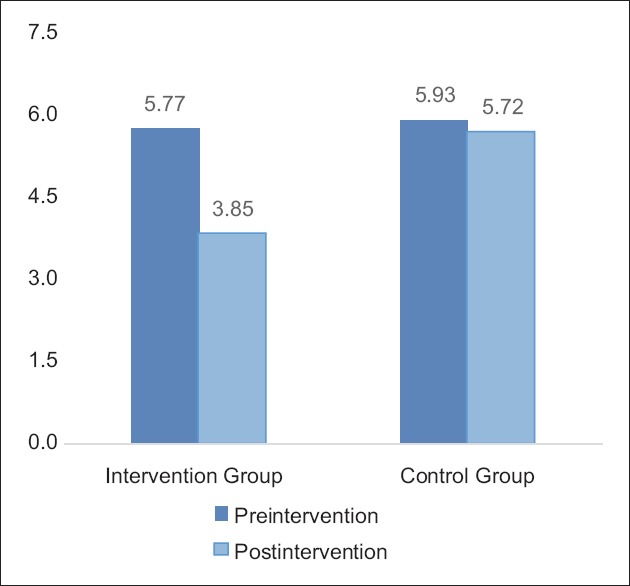
Graph: Mean changes in brief fatigue inventory score in Groups A and B
Figure 4.
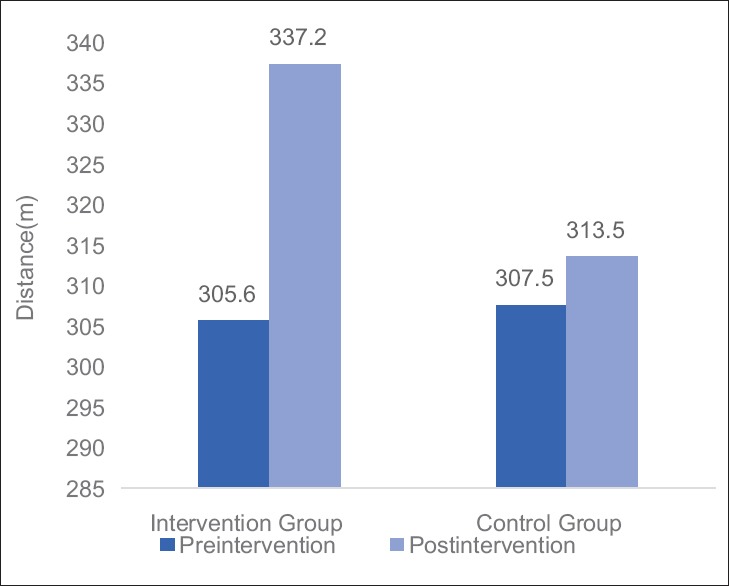
Graph: Mean changes in 6-min walk distance in Groups A and B
Figure 3.
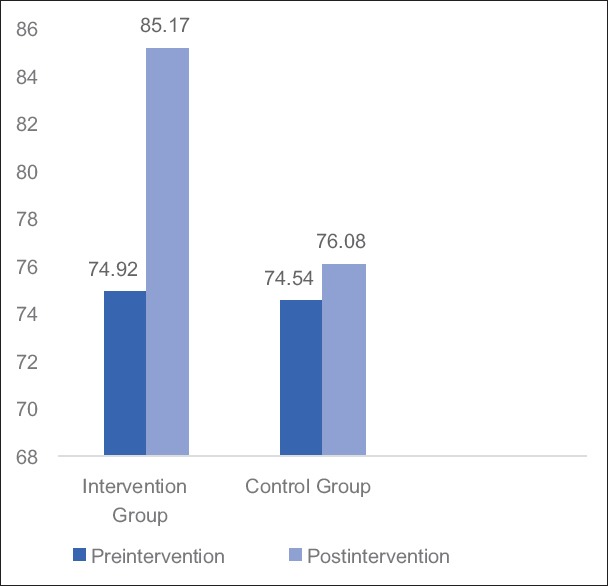
Graph: Mean changes in functional assessment of cancer therapy-general score in Groups A and B.
Table 4 and Figures 5–7 shows the statistical significant mean difference of pre- and post-value of BFI, FACT-G score and 6MWD in Group A versus Group B. Here, Mann–Whitney U-test, U = 0.50, 7.00,1.50 for BFI, FACT-G, and 6MWD, respectively, P < 0.0001 was found to be statistically significant.
Table 4.
Mean changes in brief fatigue inventory, functional assessment of cancer therapy-general and 6-min walk distance score difference between Groups A and Group B
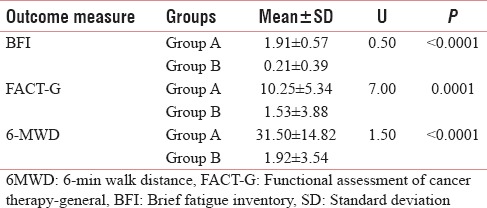
Figure 5.
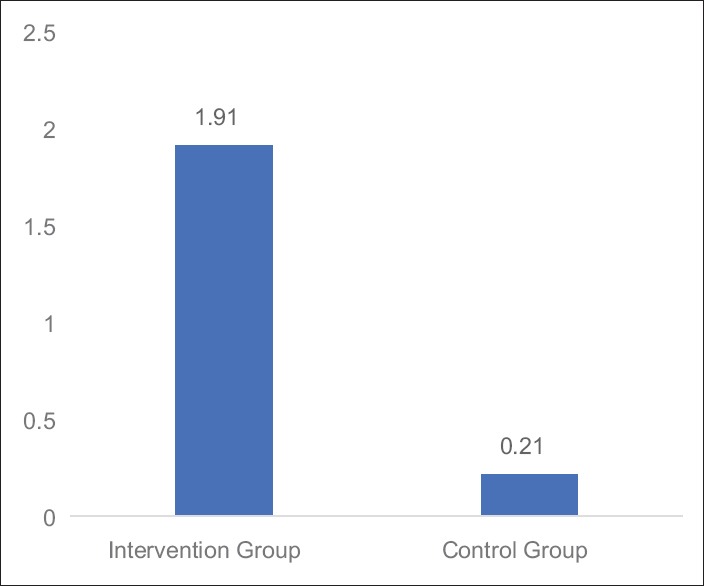
Graph: Mean changes in brief fatigue inventory score difference between Groups A and B
Figure 7.
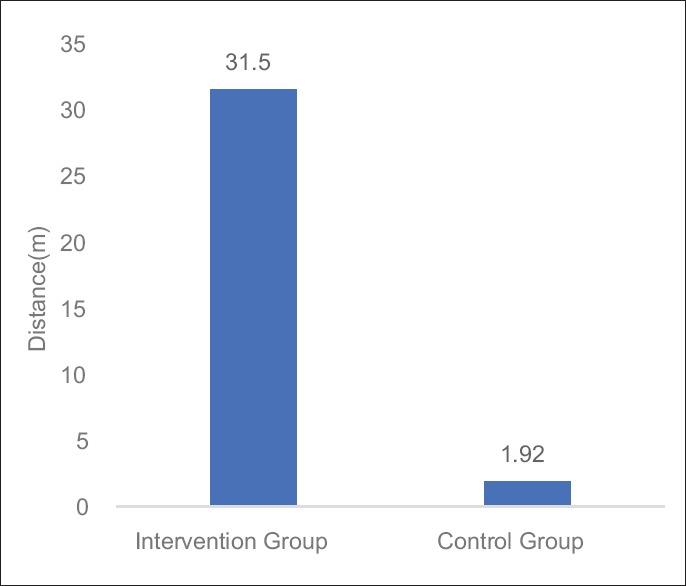
Graph: Mean changes in 6-min walk distance difference between Groups A and B
Figure 6.
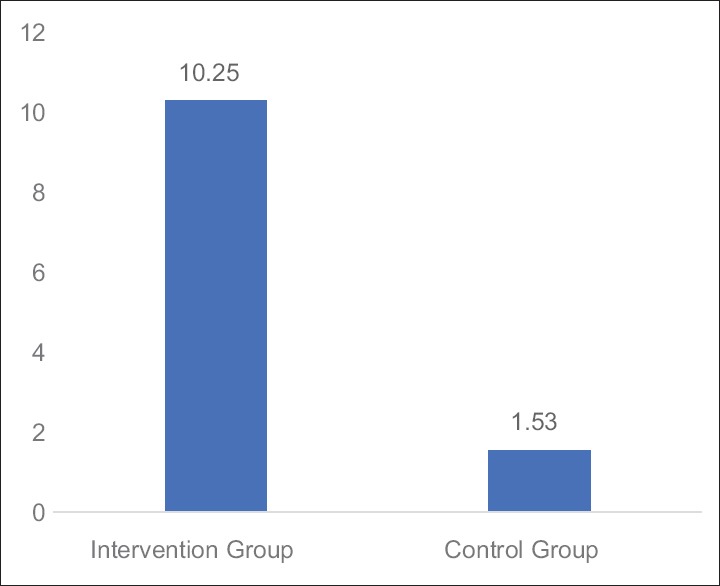
Graph: Mean changes in functional assessment of cancer therapy-general score difference between Groups A and B
DISCUSSION
The present study was designed to evaluate the effectiveness of aerobic exercises for patients with CRF as well as for the physical performance and QOL. The outcome measures analyzed were fatigue, physical performance, and QOL. The results of the present study showed that there was significant reduction in the CRF (BFI score [P < 0.0001]), significant improvement in the physical performance as (6 MWD [P < 0.0001]) and for QOL (FACT-G score [P = 0.0001]).
This study suggests that the beneficial effects on fatigue could be attributed to the intervention. Biological mechanisms evidence suggests that exercise may serve as a protective mechanism against the detrimental effects of proinflammatory cytokines by balancing the ratio of proinflammatory and anti-inflammatory cytokines. This protective mechanism may theoretically explain the positive effect of exercise in persons with cancer, and thus, it is included in the proposed theoretical model. Psychobehavioral mechanisms related to CRF that are theoretically sensitive to physical exercise include psychological distress and sleep disturbances. Functional Mechanisms evidence suggests that exercise improves physical functioning and functional capacity in persons with cancer.[22]
The results of this study showed that patients reported statistically significant reductions in CRF after completion of the aerobic exercise. These findings support previous research by confirming the safety of and showing benefits from aerobic exercise during radiation treatments.[23] In a Cochrane Database of Systematic Reviews by Cramp F, Byron-Daniel J. exercise for the management of CRF in adults. Benefits of exercise on fatigue were observed for interventions delivered during or postadjuvant cancer therapy. Aerobic exercise significantly reduced fatigue, but resistance training and alternative forms of exercise failed to reach significance. The authors concluded that aerobic exercise can be considered as beneficial for individuals with CRF during and postcancer therapy, specifically those with solid tumors.[24]
There was statistical significant improvement in the physical performance as in 6MWD. In this study, we have furnished result by evidence of aerobic exercise may be useful in preventing the loss of physical performance in cancer patients after myelotoxic chemotherapy.[25] Decreases in fatigue were found to be predominantly associated with beneficial changes in physical parameters such as a decrease in physical symptoms and an improvement in perceived physical (role) functioning.[12] Minimizing loss of physical function during treatment and regaining it afterward, are important for patients in terms of facilitating activities of daily living. Prolonged inactivity following surgery and adjuvant therapy exacerbates physical debilitation leading to increased fatigue with even minor exertion. Graded exercise for cancer patients exercise interventions have therefore been recommended for breaking the vicious cycle that can develop between inactivity, physical deconditioning, and fatigue.[26] Drouin et al. assessed physical function through peak aerobic capacity measurements and found a significant increase in the aerobic exercise group and a nonsignificant decrease in the placebo stretching group.[18] For a minimal distance to be clinically significant is at least 54 m for the 6MWT,[27] but in our study, 6MWD was clinically insignificant.
CRF is a major contributor to perceived overall QOL in cancer patients. The finding of the study was that aerobic exercise as an intervention significantly improved QOL (FACT-G). Evidence suggests that physical exercise enhances QOL during cancer treatment and survivorship.[28] Schwartz et al. suggest that the positive effect of physical exercise on QOL may be mediated by the positive effect of physical exercise on CRF, indicating that CRF may be inversely correlated with QOL.[29]
This study was focused to examine the effect of aerobic exercise on cancer-related fatigue. This study revealed the improvement in CRF in intervention group A and also better improvement in physical function and QOL.
Limitation of the study
The study had a small number of sample size, long-term follow up after 6-weeks of treatment was not carried out.
CONCLUSION
Fatigue continues to be a problem for cancer patients for many years after cancer treatment. The results of this study showed that intervention was more powerful in reducing fatigue. CRF is distinctly different from simple fatigue in individuals who are healthy. CRF is not necessarily alleviated by rest and sleep and its symptoms are disproportionate to a person's level of actual physical exertion. The results of the present study indicates a decrease in the nature and intensity of fatigue with aerobic exercise for 6 weeks in patients with solid tumor after chemotherapy and/or radiotherapy. The effects includes a significant reduction in cancer-related fatigue and also significantly improved physical function as seen with improved 6MWD. Furthermore, there was a significant improvement in the QOL of these patients postaerobic exercise.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Crom DB, Hinds PS, Gattuso JS, Tyc V, Hudson MM. Creating the basis for a breast health program for female survivors of hodgkin disease using a participatory research approach. Oncol Nurs Forum. 2005;32:1131–41. doi: 10.1188/05.ONF.1131-1141. [DOI] [PubMed] [Google Scholar]
- 2.NCCN Clinical Practice Guidelines in Oncology. Cancer-related fatigue. J Natl Compr Canc Netw. 2012;8:904–31. doi: 10.6004/jnccn.2010.0067. [DOI] [PubMed] [Google Scholar]
- 3.Prue G, Rankin J, Allen J, Gracey J, Cramp F. Cancer-related fatigue: A critical appraisal. Eur J Cancer. 2006;42:846–63. doi: 10.1016/j.ejca.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 4.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefriend W, Galvao DA, Pinto BM, et al. American college of sports medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–26. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 5.Curt GA, Breitbart W, Cella D, Groopman JE, Horning SJ, Itri LM, et al. Impact of cancer-related fatigue on the lives of patients: New findings from the fatigue coalition. Oncologist. 2000;5:353–60. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 6.Jereczek-Fossa BA, Marsiglia HR, Orecchia R. Radiotherapy-related fatigue. Crit Rev Oncol Hematol. 2002;41:317–25. doi: 10.1016/s1040-8428(01)00143-3. [DOI] [PubMed] [Google Scholar]
- 7.Baker F, Denniston M, Smith T, West MM. Adult cancer survivors: How are they faring? Cancer. 2005;104:2565–76. doi: 10.1002/cncr.21488. [DOI] [PubMed] [Google Scholar]
- 8.Bower JE, Ganz PA, Desmond KA, Bernaards C, Rowland JH, Meyerowitz BE, et al. Fatigue in long-term breast carcinoma survivors: A longitudinal investigation. Cancer. 2006;106:751–8. doi: 10.1002/cncr.21671. [DOI] [PubMed] [Google Scholar]
- 9.Ryan JL, Carroll JK, Ryan EP, Mustian KM, Fiscella K, Morrow GR, et al. Mechanisms of cancer-related fatigue. Oncologist. 2007;12(Suppl 1):22–34. doi: 10.1634/theoncologist.12-S1-22. [DOI] [PubMed] [Google Scholar]
- 10.Stone P, Richardson A, Ream E, Smith AG, Kerr DJ, Kearney N, et al. Cancer-related fatigue: Inevitable, unimportant and untreatable? results of a multi-centre patient survey. cancer fatigue forum. Ann Oncol. 2000;11:971–5. doi: 10.1023/a:1008318932641. [DOI] [PubMed] [Google Scholar]
- 11.Berger A. Treating fatigue in cancer patients. Oncologist. 2003;8(Suppl 1):10–4. doi: 10.1634/theoncologist.8-suppl_1-10. [DOI] [PubMed] [Google Scholar]
- 12.van Weert E, Hoekstra-Weebers J, Otter R, Postema K, Sanderman R, van der Schans C, et al. Cancer-related fatigue: Predictors and effects of rehabilitation. Oncologist. 2006;11:184–96. doi: 10.1634/theoncologist.11-2-184. [DOI] [PubMed] [Google Scholar]
- 13.Mackinnon LT. Future directions in exercise and immunology: Regulation and integration. Int J Sports Med. 1998;19(Suppl 3):S205–9. doi: 10.1055/s-2007-971993. [DOI] [PubMed] [Google Scholar]
- 14.Nash MS. Exercise and immunology. Med Sci Sports Exerc. 1994;26:125–7. doi: 10.1249/00005768-199402000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Mock V, Dow KH, Meares CJ, Grimm PM, Dienemann JA, Haisfield-Wolfe ME, et al. Effects of exercise on fatigue, physical functioning, and emotional distress during radiation therapy for breast cancer. Oncol Nurs Forum. 1997;24:991–1000. [PubMed] [Google Scholar]
- 16.ACSM's Exercise Management for Persons with Chronic Diseases and Disabilities. 3rd ed. Champaign, Ill: Human Kinetics; 2002. American College of Sports Medicine. [Google Scholar]
- 17.Dimeo F, Rumberger BG, Keul J. Aerobic exercise as therapy for cancer fatigue. Med Sci Sports Exerc. 1998;30:475–8. doi: 10.1097/00005768-199804000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Drouin JS, Armstrong H, Krause S, Orr J, Birk TJ, Hryniuk WM, et al. Effects of aerobic exercise training on peak aerobic capacity, fatigue, and psychological factors during radiation for breast cancer. Rehabil Oncol. 2005;23:11–7. [Google Scholar]
- 19.Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, et al. The rapid assessment of fatigue severity in cancer patients: Use of the brief fatigue inventory. Cancer. 1999;85:1186–96. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 20.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 21.Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The functional assessment of cancer therapy scale: Development and validation of the general measure. J Clin Oncol. 1993;11:570–9. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 22.Al-Majid S, Gray DP. A biobehavioral model for the study of exercise interventions in cancer-related fatigue. Biol Res Nurs. 2009;10:381–91. doi: 10.1177/1099800408324431. [DOI] [PubMed] [Google Scholar]
- 23.Windsor PM, Nicol KF, Potter J. A randomized, controlled trial of aerobic exercise for treatment-related fatigue in men receiving radical external beam radiotherapy for localized prostate carcinoma. Cancer. 2004;101:550–7. doi: 10.1002/cncr.20378. [DOI] [PubMed] [Google Scholar]
- 24.Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2012;11:CD006145. doi: 10.1002/14651858.CD006145.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dimeo F, Fetscher S, Lange W, Mertelsmann R, Keul J. Effects of aerobic exercise on the physical performance and incidence of treatment-related complications after high-dose chemotherapy. Blood. 1997;90:3390–4. [PubMed] [Google Scholar]
- 26.Dimeo FC. Effects of exercise on cancer-related fatigue. Cancer. 2001;92:1689–93. doi: 10.1002/1097-0142(20010915)92:6+<1689::aid-cncr1498>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 27.Redelmeier DA, Bayoumi AM, Goldstein RS, Guyatt GH. Interpreting small differences in functional status: The six minute walk test in chronic lung disease patients. Am J Respir Crit Care Med. 1997;155:1278–82. doi: 10.1164/ajrccm.155.4.9105067. [DOI] [PubMed] [Google Scholar]
- 28.Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS, et al. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: Cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21:1660–8. doi: 10.1200/JCO.2003.04.093. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz AL. Fatigue mediates the effects of exercise on quality of life. Qual Life Res. 1999;8:529–38. doi: 10.1023/a:1008978611274. [DOI] [PubMed] [Google Scholar]


