Abstract
Acute promyelocytic leukemia (APL) is characterized by a specific chromosomal translation, resulting in a fusion gene that affects the differentiation, proliferation and apoptosis of APL cells. Epigallocatechin-3-gallate (EGCG), a catechin, exhibits numerous biological functions, including antitumor activities. Previous studies have reported that EGCG induces apoptosis in NB4 cells. However, the molecular mechanism underlying EGCG-induced apoptosis remains unclear. The present study aimed to determine the molecular basis of EGCG-induced apoptosis in NB4 cells. EGCG treatment significantly inhibited the viability of NB4 cells in a dose-dependent manner. In addition, EGCG treatment induced apoptosis and increased the levels of (Bcl-2-like protein 4) Bax protein expression. Moreover, EGCG treatment was able to increase phosphorylated (p)-p38α mitogen-activated protein kinase (MAPK) and Src homology 1 domain-containing protein tyrosine phosphatase (SHP-1) expression. Pretreatment with PD169316 (a p38 MAPK inhibitor) partially blocked EGCG-induced apoptosis and inhibited EGCG-mediated Bax expression. Similarly, pretreatment with NSC87877, an inhibitor of SHP-1, partially blocked EGCG-induced apoptosis and inhibited EGCG-mediated increases in p-p38α MAPK and Bax expression. Therefore, the results of the present study indicate that EGCG is able to induce apoptosis in NB4 cells via the SHP-1-p38αMAPK-Bax cascade.
Keywords: leukemia, apoptosis, epigallocatechin-3-gallate, Src homology 1 domain-containing protein tyrosine phosphatase, p38α mitogen-activated protein kinase, Bcl-2-like protein 4
Introduction
Acute promyelocytic leukemia (APL), a unique subtype of acute myeloid leukemia, is characterized by a translocation between chromosomes 15 and 17 that encodes the oncogenic fusion protein promyelocytic leukemia/retinoic acid receptor-α (PML/RARα) (1). PML-RARα has an essential role in the development of APL by interfering with target genes that control differentiation, proliferation and apoptosis of APL cells (2). Considerable success in treating APL has been achieved using all-trans retinoic acid (3) and arsenic trioxide (4) in clinical settings. However, the toxicity of these molecules and the prevalence of drug-resistant forms of APL limit the clinical application of these drugs (5). Therefore, novel therapeutics to treat APL are urgently required.
Src homology 1 domain-containing protein tyrosine phosphatase (SHP-1), also known as PTPN6 (6), consists of 17 exons and 16 introns and spans ~17 kb (7). SHP-1 controls the changes in the levels of intracellular phosphorylation, including JAK/STAT (8). SHP-1 exerts multiple biological functions through the alteration of several signaling pathways (9,10). A number of agonists and inhibitors of SHP-1 have been applied in clinical cancer therapies. For example, γ-tocotrienol (11) and regorafenib (12) have been used to treat breast tumors and colorectal cancer, respectively. Studies have reported that SHP-1 is highly expressed in normal hematopoietic cells (13) but weakly expressed in hematological malignancies, including Burkitt's lymphoma (14), APL (15) and chronic myeloid leukemia (16). Therefore, the present authors hypothesize that increases in SHP-1 expression may have notable roles in APL treatment.
Epigallocatechin-3-gallate (EGCG), a major constituent of green tea (17) induces cell death in AML (18) via cellular mechanisms that currently remain unclear. A previous report demonstrated that EGCG induced apoptosis in chronic myeloid leukemia cells by increasing SHP-1 expression and dephosphorylating the fusion protein breakpoint cluster region protein-tyrosine-protein kinase ABL1 (19). An interesting question is whether SHP-1 can be increased by EGCG in NB4 cells. Previous studies suggested that EGCG mediates increased SHP-1 expression, which subsequently activates the p38α mitogen-activated protein kinase (MAPK)-Bcl-2-like protein 4 (Bax) cascade via phosphorylation (20). The p38 MAPK signaling pathway has a notable role in differentiation, proliferation, apoptosis and invasion (21–23), and is also known to affect the development of APL (24).
Therefore, the present study hypothesize that EGCG induces apoptosis in NB4 cells by increasing SHP-1 expression and activating the p38α MAPK-Bax cascade.
Materials and methods
Materials
EGCG was purchased from MedChem Express (Monmouth Junction, NJ, USA). The p38MAPK inhibitor PD 169316 was purchased from MedChem Express. The SHP-1 inhibitor NSC87877 was purchased from Tocris Bioscience (Bristol, UK).
Cell culture
NB4 cells (Shanghai Institutes for Biological Sciences of the Chinese Academy of Sciences, Shanghai, China) were cultured in RPMI 1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 100 mg/ml penicillin and streptomycin (Beyotime Institute of Biotechnology, Haimen, China), in an incubator with 5% CO2 at 37°C.
Western blot analysis
The cells were collected and washed with chilled PBS for three times. Then, the cells were lysed using radioimmunoprecipitation assay buffer with protease and phosphatase inhibitors (Beyotime Institute of Biotechnology). Discarding the supernatant following centrifugation at 13,000 × g at 4°C for 30 min. Protein lysate concentrations were quantified using a bicinchoninic acid assay kit (Beyotime Institute of Biotechnology). Equal quantities (50 µg) of the protein were isolated by 10% SDS-PAGE and transferred to polyvinylidene fluoride membranes. The membranes were blocked with 5% skimmed milk suspended by Tris-Buffered Saline with 0.05% Tween-20 (TBST) for 2 h and then probed with anti-SHP-1 (1:1,000; cat. no. ab32559; Abcam, Cambridge, UK), anti-p38α MAPK (1:1,000; cat. no. 9218; Cell Signaling Technology, Inc., Danvers, MA, USA), anti-p-p38α MAPK (1:1,000; cat. no. 09-272; Merck KGaA, Darmstadt, Germany), Bax (1:1,000; cat. no. wl01637; Wanleibio Co., Ltd., Shanghai, China) and β-actin (1:500; cat. no. BM0627; Boster Biological Technology, Pleasanton, CA, USA) at 4°C overnight. Following washing with TBST, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies, goat anti-rabbit (cat. no. ZA-0448) and HRP-conjugated goat anti-mouse (cat. no. ZM-0491) IgG (1:1,000; Beijing Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China) for 1 h. The membranes were then washed with TBS and TBST and the protein levels were detected by an enhanced chemiluminescence kit (EMD Millipore, Billerica, MA, USA). β-actin (1:500; cat. no. BM0627; Boster Biological Technology) protein levels were used as a control to verify equal protein loading. Protein bands were visualized using the Quantity One Software version 4.5.2 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Cell viability assay
NB4 cells (1×104 cells/well) were seeded into wells of a 96-well plate. NB4 cells were pretreated with different concentrations of EGCG (0, 10, 20 or 30 µM), 10 µM PD169316 (MedChem Express, Monmouth Junction, NJ, USA) or 10 µM NSC87877 (Tocris Bioscience, Bristol, UK) and equal volumes of a solvent control (PBS) for 24 h. Cell viability was quantified using a Cell Counting kit-8 (CCK-8; 7Sea Biotech Co., Ltd., Shanghai, China). The cell number index was calculated at 450 nm using a spectrophotometer (Bio-Rad Laboratories, Inc.) as follows: Cell number index=(ABS of treated/ABS of blank)/(ABS of control/ABS of blank) ×100.
Flow cytometric analysis of apoptosis
NB4 cells (1×104 cells/well) were seeded into wells of a 6-well plate. NB4 cells were pretreated at 37°C with different concentrations of EGCG (0, 10, 20 or 30 µM), 10 µM PD169316 (MedChem Express) or 10 µM NSC87877 (Tocris Bioscience) and equal volumes of PBS for 24 h. Cells were then collected and washed using chilled PBS. Apoptosis was detected using propidium iodide and annexin V double labeling assay kit (Sigma-Aldrich; Merck KGaA) by a FACsorter (BD Biosciences, San Jose, CA, USA).
Statistical analysis
All results are expressed as the mean ± standard error. Statistical analysis was performed using SPSS (version 23.0; IBM Corp., Armonk, NY, USA). The data were analyzed using one-way analysis of variance. P<0.05 was considered to indicate a statistically significant difference.
Results
EGCG induces apoptosis in NB4 cells
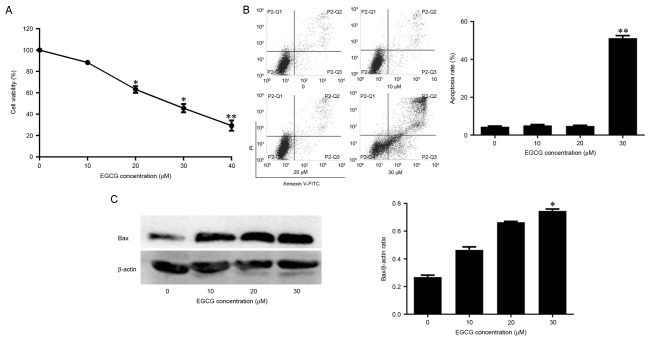
CCK-8 assay was used to detect the effect of EGCG on the viability of NB4 cells. Cell viability was negatively associated with EGCG concentration (Fig. 1A). Whether EGCG-induced cell death was associated with apoptosis was also assessed. In normal cells, the phosphatidylserine (PS) groups in the plasma membrane are directed towards the inside of the cell. However, the PS groups are exposed to the environment upon apoptosis (25). The propidium iodide and annexin-V double-labeling assay indicated that this observed cell death was due to apoptosis. The apoptotic rates of cells exposed to 10 or 20 µM EGCG did not differ significantly from the apoptotic rate of the control group. However, the apoptotic rate of cells exposed to 30 µM EGCG was significantly higher compared with the control group (Fig. 1B). The levels of the apoptosis-associated protein Bax were also quantified in EGCG-treated cells. Bax expression increased with EGCG in a dose-dependent manner (Fig. 1C). Therefore, these data suggest that EGCG was able to induce apoptosis in NB4 cells.
Figure 1.
Effects of EGCG on apoptosis and the level of Bax protein expression in NB4 cells. (A) NB4 cells were treated with 10, 20, 30 or 40 µM EGCG for 24 h. Cell Counting kit-8 assay was used to assess the viability of NB4 cells. (B and C) NB4 cells were treated with 10, 20 or 30 µM EGCG for 24 h. Flow cytometric analysis was used to determine the apoptotic rate of NB4 cells. Western blot analysis was used to detect Bax protein expression. All experiments were performed in triplicate. *P<0.05, **P<0.01 vs. control group. EGCG, epigallocatechin-3-gallate; Bax, apoptosis regulator BAX; PI, propidium iodide; FITC, fluorescein isothiocyanate.
EGCG increases SHP-1 expression and levels of phosphorylated (p)-p38α MAPK
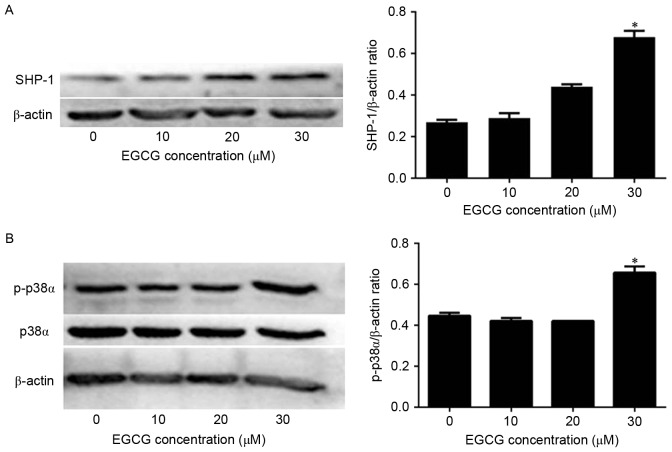
Next, the mechanism of EGCG-induced apoptosis in NB4 cells was investigated by quantifying SHP-1 expression via western blot analysis. SHP-1 protein levels were not affected when the cells were treated with 10 or 20 µM EGCG compared with the expression in untreated cells. However, SHP-1 expression was significantly increased when cells were treated with 30 µM EGCG compared with the expression in untreated cells (Fig. 2A). Since p38α MAPK acts downstream of SHP-1, the present authors hypothesized that p38α MAPK levels would also be affected by EGCG treatment. In fact, p38α MAPK expression was not affected by EGCG treatment (Fig. 2B). However, p-p38α MAPK levels were significantly increased when the cells were treated with 30 µM EGCG compared with the expression in untreated cells (Fig. 2B). Therefore, these data indicate that EGCG was able to increase SHP-1 expression and trigger the phosphorylation of p38α MAPK to p-p38α MAPK in NB4 cells.
Figure 2.
Effects of EGCG on the levels of SHP-1 and p-p38α protein expression in NB4 cells. NB4 cells were treated with 10, 20 and 30 µM EGCG for 24 h. (A) Western blot analysis was used to detect SHP-1 protein expression. (B) Western blot analysis was used to detect the levels of p38α and p-p38α protein. All experiments were performed in triplicate. *P<0.05 vs. control group. EGCG, epigallocatechin-3-gallate; SHP-1, Src homology region 2 domain-containing phosphatase-1; p38α, p38α mitogen activated protein kinase; p-, phosphorylated.
Inhibition of p38α MAPK partially blocks EGCG-induced NB4 cell apoptosis
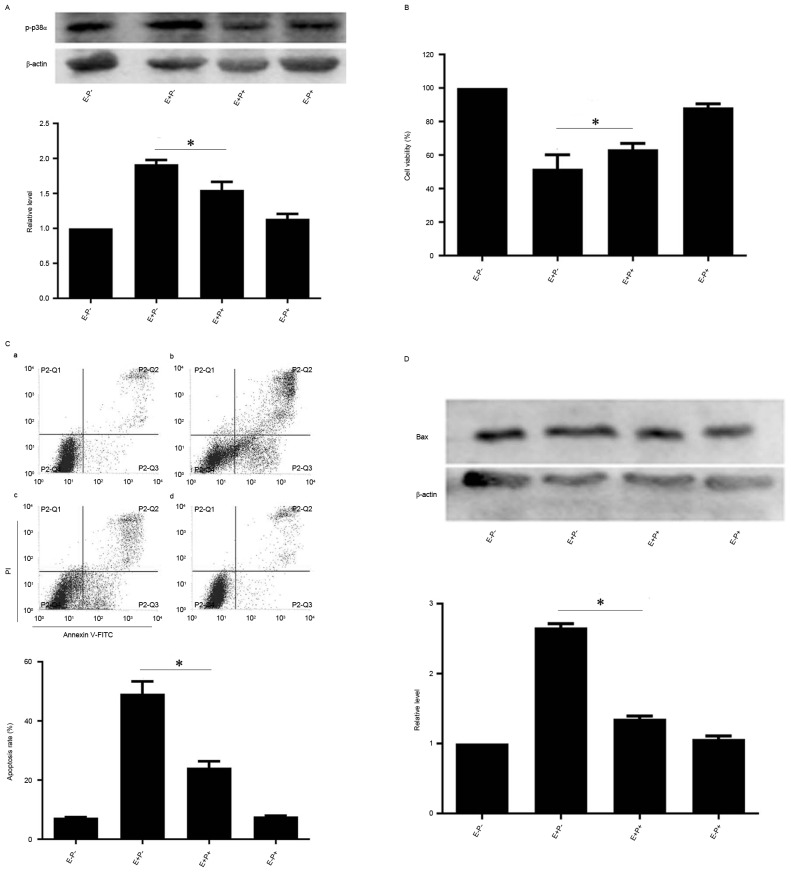
As p-p38α MAPK levels were increased by EGCG treatment, the role of p38α MAPK in EGCG-induced NB4 cell apoptosis was investigated further. NB4 cells were pretreated with PD169316, an inhibitor of p38 MAPK, and subsequently treated with EGCG. Protein levels of p38α MAPK were not markedly affected by the combined treatment compared with the levels in cells treated with EGCG alone, but p-p38α MAPK levels were reduced compared with the levels in cells treated with EGCG alone (Fig. 3A). However, the viability of NB4 cells when treated with PD169316 and EGCG increased compared with the viability of cells treated with EGCG alone (Fig. 3B). Since PD169316 treatment increased EGCG-induced viability of NB4 cells, whether PD169316 affected EGCG-induced apoptosis was assessed. The apoptotic rate of PD169316 inhibitor and EGCG-pretreated cells was reduced compared with the rate of the cells treated with EGCG alone (Fig. 3C). Protein levels of the apoptosis-associated protein Bax were also reduced in the PD169316 inhibitor and EGCG-pretreated cells in comparison with the cells treated with EGCG alone (Fig. 3D). Therefore, these data suggest that EGCG may induce apoptosis via p38α MAPK in NB4 cells.
Figure 3.
Effects of p38α inhibition on EGCG-mediated apoptosis and protein expression levels of Bax and p-p38α in NB4 cells. NB4 cells were pretreated with 10 µM PD169316 for 0.5 h and then treated with 30 µM EGCG for 24 h. (A and D) Western blot analysis was used to detect the protein expression level of p-p38α and Bax. (B) Cell-Counting Kit-8 assay was used to measure the cell viability of NB4 cells. (C) Flow cytometric analysis was used to determine apoptotic rate in NB4 cells. a, EGCG− PD169316−; b, EGCG+ PD169316−; c, EGCG+ PD169316+; d, EGCG− PD169316+. All experiments were performed in triplicate. *P<0.05 vs. control group. EGCG, epigallocatechin-3-gallate; Bax, Bcl-2-like protein 4; E, 30 µM EGCG. P, 10 µM PD169316; PI, propidium iodide; FITC, fluorescein isothiocyanate.
Inhibition of SHP-1 partially blocks EGCG-induced apoptosis and decreases levels of p-p38α MAPK and Bax
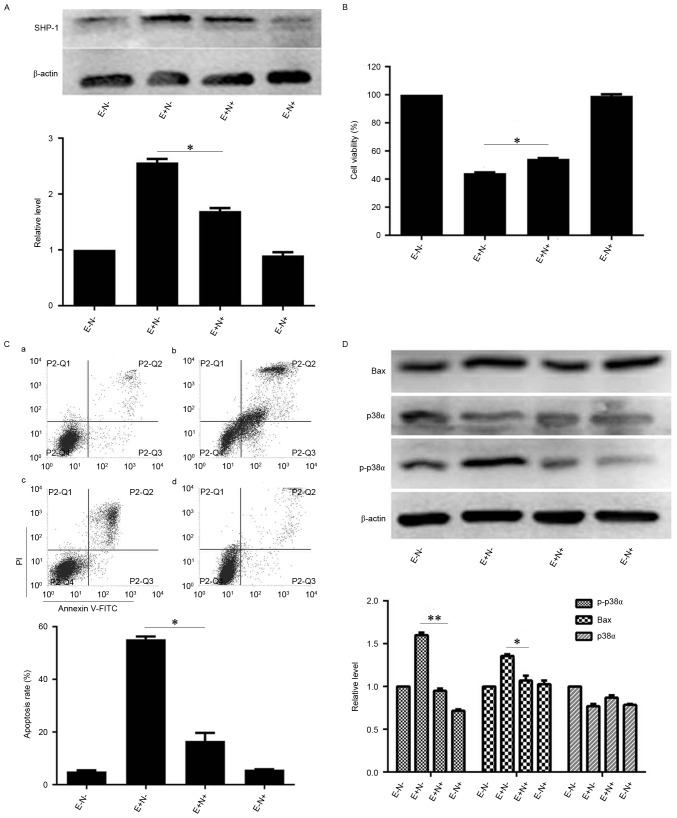
As the levels of SHP-1 were increased by EGCG treatment, the effect of SHP-1 inhibition on EGCG-induced NB4 apoptosis was subsequently investigated. When NB4 cells were pretreated with NSC87877 and subsequently treated with EGCG, SHP-1 protein levels were reduced compared with the levels in the cells treated with EGCG alone (Fig. 4A). Similarly, the viability of NSC87877 inhibitor and EGCG-pretreated NB4 cells increased (Fig. 4B). Notably, pretreatment with NSC87877 reduced the EGCG-induced apoptotic rate of NB4 cells, compared with the apoptotic rate of cells treated with EGCG alone (Fig. 4C). To investigate the potential mechanisms underlying changes in viability and apoptotic rates upon SHP-1 inhibition, the changes in expression of proteins associated with the apoptotic cascades were quantified.
Figure 4.
Effects of SHP-1 inhibition on EGCG-mediated apoptosis and expression levels of associated proteins in NB4 cells. NB4 cells were pretreated with 10 µM NSC87877 for 0.5 h and then treated with 30 µM EGCG for 24 h. (A) Western blot analysis was used to detect the expression level of SHP-1. (B) Cell Counting Kit-8 assay was used to measure the viability of NB4 cells. (C) Flow cytometric analysis was used to determine the apoptotic rate of NB4 cells. a, EGCG− NSC87877−; b, EGCG+ NSC87877−; c, EGCG+ NSC87877+; d, EGCG− NSC87877+. (D) p38α, p-p38α and Bax protein levels were assessed by western blot analysis. All experiments were performed in triplicate. *P<0.05, **P<0.01 vs. control group. EGCG, epigallocatechin-3-gallate; SHP-1, Src homology region 2 domain-containing phosphatase-1; p38α, p38α mitogen activated protein kinase; E, 30 µM EGCG. N, 10 µM NSC87877; p-, phosphorylated.
When the cells were pretreated with SHP-1 inhibitor and EGCG, the levels of p-p38α MAPK and Bax proteins were lower compared with the expression in the cells that were treated with EGCG alone. However, the expression level of p38α MAPK was unaffected by pretreatment with SHP-1 inhibitor (Fig. 4D). Therefore, these data suggest that SHP-1 may have an important role in EGCG-induced NB4 cell apoptosis.
Discussion
The purpose of the present study was to investigate whether EGCG induces apoptosis of NB4 cells through a SHP-1-p38α MAPK-Bax cascade. EGCG treatment increased the levels of p-p38α MAPK and Bax expression compared with control group, although p38α MAPK expression was unaffected. The observed increase in p-p38α MAPK and Bax expression was associated with the expression level of SHP-1. It was observed that the inhibition of p38α MAPK was able to reduce EGCG-induced apoptosis of NB4 cells. Additionally, inhibition of SHP-1 reduced EGCG-induced apoptosis of NB4 cells and EGCG-mediated increase in p-p38α MAPK and Bax expression.
EGCG, a catechin, has been demonstrated to exhibit antitumor activities in multiple studies on solid tumor (26,27) and leukemia (28,29) cells, particularly in APL cells (30). EGCG mediates its anti-leukemic activity primarily through the induction of apoptosis, which has been indicated by increased levels of Bax in this study (30). However, the exact mechanisms underlying antitumor activities in APL were unclear.
The present study reports, to the best of our knowledge for the first time, that SHP-1 expression is increased in NB4 cells treated with EGCG, suggesting that SHP-1 has a pivotal role in mediating the antitumor activity of EGCG. Inhibition of SHP-1 partially blocked EGCG-induced apoptosis and triggered a reduction in the levels of p-p38αMAPK and Bax. SHP-1 is known to be a key modulator of protein phosphorylation levels in cells, and protein phosphorylation has an important role in numerous biological functions, including the differentiation, apoptosis and invasion of cells (9,10). Therefore, on the basis of the findings detailed in the present study, the present authors hypothesize that SHP-1 contributes to EGCG-induced apoptosis by modifying the phosphorylation patterns of key apoptosis regulators, including p38α MAPK. Although a number of agonists and inhibitors of SHP-1 have been employed to treat solid tumors, these treatments are rarely used for leukemia (11,12). The finding that SHP-1 affects apoptosis in NB4 cells suggests that agonists and inhibitors of SHP-1, either alone or combination with EGCG, may also be used to treat leukemia in the future.
As SHP-1 has an important role in EGCG-induced apoptosis of NB4 cells, the downstream mechanism of SHP-1 was investigated. A number of studies indicated that p38α MAPK is a downstream target of SHP-1 (31,32). However, whether the p38α MAPK signaling pathway contributes to EGCG-induced NB4 cell apoptosis has not been demonstrated. In the present study, p38α MAPK was activated upon treatment with EGCG. In addition, pretreatment with PD169316 (p38α MAPK inhibitor) partially blocked EGCG-induced apoptosis of NB4 cells and decreased Bax expression. Therefore, the findings of the present study indicate that p38α MAPK activation was associated with apoptosis in EGCG-treated NB4 cells. However, whether p38α MAPK is activated directly by SHP-1 remains unclear. Further investigation is therefore required to understand the association between SHP-1 and p38α MAPK better.
In conclusion, the present study revealed the molecular mechanism underlying EGCG-induced apoptosis in NB4 cells. Although the effect of EGCG on APL cells had been studied previously, the findings of the present study indicate that EGCG-mediated apoptosis in NB4 cells is dependent on the SHP-1-p38α MAPK-Bax cascade.
Acknowledgements
The present study was supported by grants from the National Natural Science Foundation of China (grant no. 81171658) and the Natural Science Foundation of Major Project of Chongqing of China (grant no. 2011BA5037).
References
- 1.de Thé H, Le Bras M, Lallemand-Breitenbach V. The cell biology of disease: Acute promyelocytic leukemia, arrsenic, and PML bodies. J Cell Biol. 2012;198:11–21. doi: 10.1083/jcb.201112044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laurenzana A, Pettersson F, Miller WH. Role of PML/RARAα in the pathogenesis of APL. Drug Discov Today Dis Mech. 2006;3:499–505. doi: 10.1016/j.ddmec.2006.11.008. [DOI] [Google Scholar]
- 3.Congleton J, MacDonald R, Yen A. Src inhibitors, PP2 and dasatinib, increase retinoic acid-induced association of Lyn and c-Raf (S259) and enhance MAPK-dependent differentiation of myeloid leukemia cells. Leukemia. 2012;26:1180–1188. doi: 10.1038/leu.2011.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang H, Li X, Wang L, Yu S, Xu Z, Gu Y, Pan Z, Li T, Hu M, Cui H, et al. MicroRNAs contribute to promyelocyte apoptosis in As2O3-treated APL cells. Cell Physiol Biochem. 2013;32:1818–1829. doi: 10.1159/000356615. [DOI] [PubMed] [Google Scholar]
- 5.Petrie K, Zelent A, Waxman S. Differentiation therapy of acute myeloid leukemia: Past, present and future. Curr Opin Hematol. 2009;16:84–91. doi: 10.1097/MOH.0b013e3283257aee. [DOI] [PubMed] [Google Scholar]
- 6.Neel BG, Gu H, Pao L. The “Shp”ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284–293. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- 7.Stocco DR, Shen SH. Human protein tyrosine phosphatase 1C (PTPN6) gene structure: Alternate promoter usage and exon skipping generate multiple transcripts. Genomics. 1995;27:165–173. doi: 10.1006/geno.1995.1020. [DOI] [PubMed] [Google Scholar]
- 8.Tseng CYLM, Su JC, Chang KC, Chu PY, Tai WT, Shiau CW, Chen KF. Novel sorafenib analogues induces apoptosis through SHP-1 dependent STAT3 inactivation in human breast cancer cells. Breast Cancer Res. 2013;15:R63. doi: 10.1186/bcr3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong G, You M, Ding L, Fan H, Liu F, Ren D, Hou Y. STING negatively regulates Double-Stranded DNA-activated JAK1-STAT1 signaling via SHP-1/2 in B cells. Mol Cells. 2015;38:441–451. doi: 10.14348/molcells.2015.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Jamal HA, Jusoh Mat SA, Hassan R, Johan MF. Enhancing SHP-1 expression with 5-azacytidine may inhibit STAT3 activation and confer sensitivity in lestaurtinib (CEP-701)-resistant FLT3-ITD positive acute myeloid leukemia. BMC Canner. 2015;15:869. doi: 10.1186/s12885-015-1695-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiong A, Yu W, Liu Y, Sanders BG, Kline K. Elimination of ALDH+ breast tumor initiating cells by docosahexanoic acid and/or gamma tocotrienol through SHP-1 inhibition of Stat3 signaling. Mol Carcinog. 2016;55:420–430. doi: 10.1002/mc.22291. [DOI] [PubMed] [Google Scholar]
- 12.Fan LC, Teng HW, Shiau CW, Lin H, Hung MH, Chen YL, Huang JW, Tai WT, Yu HC, Chen KF. SHP-1 is a target of regorafenib in colorectal cancer. Oncatarget. 2014;5:6243–6251. doi: 10.18632/oncotarget.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakase K, Cheng J, Zhu Q, Marasco WA. Mechanisms of SHP-1 P2 promoter regulation in hematopoietic cells and its silencing in HTLV-1-transformed T cells. J Leukoc Biol. 2009;85:165–174. doi: 10.1189/jlb.0608383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delibrias CC, Floettmann JE, Rowe M, Fearon DT. Downregulated expression of SHP-1 in Burkitt lymphomas and germinal center B lymphocytes. J Exp Med. 1997;186:1575–1583. doi: 10.1084/jem.186.9.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uesugi Y, Fuse I, Toba K, Kishi K, Furukawa T, Koike T, Aizawa Y. Involvement of SHP-1, a phosphotyrosine phosphatase, during myeloid cell differentiation in acute promyelocytic leukemia cell lines. Eur J Haematol. 1999;62:239–245. doi: 10.1111/j.1600-0609.1999.tb01753.x. [DOI] [PubMed] [Google Scholar]
- 16.Amin HM, Hoshino K, Yang H, Lin Q, Lai R, Garcia-Manero G. Decreased expression level of SH2 domain-containing protein tyrosine phosphatase-1 (Shp1) is associated with progression of chronic myeloid leukaemia. J Pathol. 2007;212:402–410. doi: 10.1002/path.2178. [DOI] [PubMed] [Google Scholar]
- 17.Nakazato T, Ito K, Miyakawa Y, Kinjo K, Yamada T, Hozumi N, Ikeda Y, Kizaki M. Catechin, a green tea component, rapidly induces apoptosis of myeloid leukemia cells via modulation of reactive oxygen species production in vitro andinhibits tumor growth in vivo. Haematologica. 2005;90:317–325. [PubMed] [Google Scholar]
- 18.Britschgi A, Simon HU, Tobler A, Fey MF, Tschan MP. Epigallocatechin-3-gallate induces cell death in acute myeloid leukaemia cells and supports all-trans retinoic acid-induced neutrophil differentiation via death-associated protein kinase 2. Br J Haematol. 2010;149:55–64. doi: 10.1111/j.1365-2141.2009.08040.x. [DOI] [PubMed] [Google Scholar]
- 19.Jung JH, Yun M, Choo EJ, Kim SH, Jeong MS, Jung DB, Lee H, Kim EO, Kato N, Kim B, et al. A derivative of epigallocatechin-3-gallate induces apoptosis via SHP-1-mediated suppression of BCR-ABL and STAT3 signalling in chronic myelogenous leukaemia. Br J Pharmacol. 2015;172:3565–3578. doi: 10.1111/bph.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geraldes P, Hiraoka-Yamamoto J, Matsumoto M, Clermont A, Leitges M, Marette A, Aiello LP, Kern TS, King GL. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med. 2009;15:1298–1306. doi: 10.1038/nm.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harsha Raj M, Yashaswini B, Rössler J, Salimath BP. Combinatorial treatment with anacardic acid followed by TRAIL augments induction of apoptosis in TRAIL resistant cancer cells by the regulation of p53, MAPK and NFκβ pathways. Apoptosis. 2016;21:578–593. doi: 10.1007/s10495-016-1223-8. [DOI] [PubMed] [Google Scholar]
- 22.Zhang JG, Yang S, Qiao J, Li T, Yang S, Hong Y. Macrophage migration inhibitory factor regulating the expression of VEGF-C through MAPK signal pathways in breast cancer MCF-7 cell. World J Surg Oncol. 2016;14:51. doi: 10.1186/s12957-016-0797-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodríguez-Berriguete G, Torrealba N, Fraile B, Paniagua R, Royuela M. Epidermal growth factor induces p38 MAPK-dependent G0/G1-to-S transition in prostate cancer cells upon androgen deprivation conditions. Growth Factor. 2016;34:5–10. doi: 10.3109/08977194.2015.1132712. [DOI] [PubMed] [Google Scholar]
- 24.Mandegary A, Hosseini R, Ghaffari SH, Alimoghaddam K, Rostami S, Ghavamzadeh A, Ghahremani MH. The expression of p38, ERK1 and Bax proteins has increased during the treatment of newly diagnosed acute promyelocytic leukemia with arsenic trioxide. Ann Oncol. 2010;21:1884–1890. doi: 10.1093/annonc/mdq034. [DOI] [PubMed] [Google Scholar]
- 25.Tyurin VA, Balasubramanian K, Winnica D, Tyurina YY, Vikulina AS, He RR, Kapralov AA, Macphee CH, Kagan VE. Oxidatively modified phosphatidylserines om the surface of apoptotic cells are essential phagocytic ‘eat-me’ signals: Cleavage and inhibition of phagocytosis by Lp-PLA2. Cell Death Differ. 2014;21:825–835. doi: 10.1038/cdd.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toden S, Tran HM, Tovar-Camargo OA, Okugawa Y, Goel A. Epigallocatechin-3-gallate targets cancer stem-like cells and enhances 5-fluorouracil chemosensitivity in colorectal cancer. Oncotarget. 2016;7:16158–16171. doi: 10.18632/oncotarget.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li M, Li JJ, Gu QH, An J, Cao LM, Yang HP, Hu CP. EGCG induces lunch canner A549 cells apoptosis by regulating Ku70 acetylation. Oncol Rep. 2016;35:2339–2347. doi: 10.3892/or.2016.4587. [DOI] [PubMed] [Google Scholar]
- 28.Tofolean IT, Ganea C, Ionescu D, Filippi A, Garaiman A, Goicea A, Gaman MA, Dimancea A, Baran I. Cellular determinants involving mitochondrial dysfunction, oxidative stress and apoptosis correlate with the synergic cytotoxicity of epigallocatechin-3-gallate and menadione in human leukemia Jurkat T cells. Pharmacol Res. 2016;103:300–317. doi: 10.1016/j.phrs.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 29.Vézina A, Chokor R, Annabi B. EGCG targeting efficacy of NF-κB downstream gene products is dictated by the monocytic/macrophagic differentiation status of promyelocytic leukemia cells. Cancer Immunol Immunother. 2012;16:2321–2331. doi: 10.1007/s00262-012-1301-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elbling L, Herbacek I, Weiss RM, Jantschitsch C, Micksche M, Gerner C, Pangratz H, Grusch M, Knasmüller S, Berger W. Hydrogen peroxide mediates EGCG-induced antioxidant protection in human keratinocytes. Free Radic Biol Med. 2010;49:1444–1452. doi: 10.1016/j.freeradbiomed.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Khan TH, Srivastava N, Srivastava A, Sareen A, Mathur RK, Chande AG, Musti KV, Roy S, Mukhopadhyaya R, Saha B. SHP-1 plays a crucial role in CD40 signaling reciprocity. J Immunol. 2014;193:3644–3653. doi: 10.4049/jimmunol.1400620. [DOI] [PubMed] [Google Scholar]
- 32.Chen YY, Hsieh CY, Jayakumar T, Lin KH, Chou DS, Lu WJ, Hsu MJ, Sheu JR. Andrographolide induces vascular smooth muscle cell apoptosis through a SHP-1-PP2A-p38MAPK-p53 cascade. Sci Rep. 2014;4:5651. doi: 10.1038/srep05651. [DOI] [PMC free article] [PubMed] [Google Scholar]