Abstract
Co-stimulatory molecule B7 homolog 3 protein (B7-H3) has been described as an important tumor antigen in various human tumors. The exact role of B7-H3 in tumor progression and its receptor are still ambiguous. The phenotype and the function of tumor-associated macrophages (TAMs) in human solid tumors are complicated and could contribute to the shaping of the tumor microenvironment. In the present study, B7-H3 expression and lymphocyte infiltration were investigated by immunohistochemistry in 117 colorectal carcinoma (CRC) patients. B7-H3 expression was positively associated with the infiltrating density of macrophage in CRC tissues, and B7-H3 expression and the infiltrating density of macrophages were negatively associated with the overall survival rate of patients. The putative B7-H3 receptor was found on activated monocytes and macrophages, indicating the direct function of B7-H3 signal on macrophages. Additional results revealed that during the differentiation of TAMs, B7-H3 promoted the polarization of type 2 macrophages (M2s) and switch of the M1 phenotype to the M2 phenotype. Thus, B7-H3 signaling promotes M2 differentiation via the putative receptor on monocytes and macrophages. Targeting the manipulation of TAMs through the B7-H3 pathway may be valuable for the development of novel immunotherapeutic strategies against human CRC.
Keywords: B7-H3, colorectal carcinoma, tumor-associated macrophage, differentiation
Introduction
When tumors emerge in the human body, the host immune surveillance should recognize tumor-specific antigens and eliminate the tumor through the tumor-specific T cell response (1). However, the response is ineffective due to an imbalance of co-stimulatory molecules in the tumor microenvironment, which could determine the functional maturation of T cells (2,3). Co-stimulatory members of the B7 family has seven known members: B7.1 (CD80), B7.2 (CD86), B7-H3, B7-H4, inducible costimulator ligand (ICOS-L), programmed death-1 ligand and programmed death-2 ligand, which bind to receptors on lymphocytes that to exert both inhibitory and stimulatory effects on T cell activation (4). These members were found to be abnormally expressed in various human malignancies in previous years, and were considered crucial targets in cancer immunotherapy (5). B7 homolog 3 protein (B7-H3), also termed cluster of differentiation (CD)276, is one of B7 family members (6). B7-H3 expression is found on activated T, natural killer and antigen-presenting cells (6,7), but hardly found in normal organ tissues. The function of B7-H3 remains a paradox. Both stimulatory and inhibitory effects were observed in T cell activation and antitumor immune response by different studies (8). Diverse associations between the expression of B7-H3 in tumor tissues and clinical parameters have also been reported (8). The contrasting results revealed that there may be two or more receptors for B7-H3 with opposing functions, which remain unknown (9). The mechanism of B7-H3 in the tumor microenvironment remains unclear, and the regulation of T cell activation, resistance of drug-induced apoptosis and enhancement of metastasis were all considered as probable mechanisms (10). However, the exact role of B7-H3 in the tumor microenvironment remains unclear.
Tumor-associated macrophages (TAMs) are a major leukocyte population in tumor tissues, and are involved in tumor growth, invasion and metastasis (11–21). TAM infiltration has been demonstrated to be associated with a poor prognosis and outcome in numerous human malignancies (13–15). Therefore, the manipulation of TAMs may be extremely valuable in antitumor therapy. Macrophages are highly heterogeneous cells that are induced into distinct functional states under various circumstances. The type 1 macrophage (M1) phenotype is induced by lipopolysaccharide (LPS) and interferon-γ, and exhibits the ability to kill pathogens and tumor cells. The M2 phenotype is induced by interleukin (IL)-4, IL-13 and IL-10, and is capable of suppressing inflammation and promoting tissue repair, tumor growth and angiogenesis (16–18). TAMs generally show polarized M2 phenotypes and elicit similar function in the tumor microenvironment, including promotion of angiogenesis, matrix remodeling and suppression of antitumor immunity (19–21). The tumor microenvironment is hypothesized to educate TAMs towards an M2 polarization, but the mechanisms underlying this are not fully understood.
Colorectal carcinoma (CRC) is one of the most frequent malignancies occurring in human beings, and ranks as the third most common cancer and the fourth leading cause of cancer-associated mortality worldwide (22). In China, CRC has been reported to have an increasing incidence due to changes of diets and lifestyles (23). Various therapeutic strategies, including surgery, chemotherapy, radiotherapy and immunotherapy, are used to treat patients with CRC, but the therapies may lead to different outcomes. Therefore, key factors that regulate tumor progression and antitumor immunity are required in the individualized therapy of human CRC. A previous study has demonstrated the clinical significance and regulation of B7-H3 expression in CRC patients (10). In the present study, we investigated the immune cells that infiltrated in CRC, and revealed the mechanism of B7-H3 signaling in the regulation of immune cells in the tumor microenvironment.
Materials and methods
Patients
In total, 117 CRC patients who underwent surgery between January 2003 and December 2003 at the Department of Gastrointestinal Surgery (the Fourth Affiliated Hospital, Suzhou University, Wuxi, China) were enrolled in the present study. No patient received pre-operative chemotherapy or radiotherapy, and all specimens were identified as CRC under hematoxylin and eosin staining. The paraffin-embedded blocks of tumor tissues were obtained from the archival collections at the Department of Pathology (the Fourth Affiliated Hospital, Suzhou University), and the survival data of patients were collected by the end of December 2008. Fresh resected CRC tissues were obtained from the First Affiliated Hospital, Suzhou University (Suzhou, China), and were used for the purification of TAMs immediately subsequent to surgery. The present study was approved by the Medical Ethics Committee (the Fourth Affiliated Hospital, Soochow University) and all patients provided informed consent.
Immunohistochemical staining and evaluation
Immunohistochemistry was performed using the Dako Envision™ (Agilent Technologies, Inc., Santa Clara, CA, USA), according to the manufacturer's instructions and as previously described (24). Mouse anti-human B7-H3 [clone no. 21D4; established and characterized in our institute (Soochow University) (24)], and CD8 and CD68 (cat. nos. MAB-0021 and Kit-0026 ready for use; Fuzhou Maxim Biotech Co., Ltd., Fuzhou, China) monoclonal antibodies were used to stain for B7-H3 expression, CD8+ T cell infiltration and CD68+ macrophage infiltration, respectively. The staining was evaluated as previously described (24). The B7-H3 immunostaining densities were assessed according to the H-score method described by Hammes et al (25). Assessment of the infiltration densities of CD8+ T cells and CD68+ macrophages was performed in both the tumor stroma and tumor nest. Tumor-infiltrating lymphocytes (TILs) in the tumor stroma were examined and categorized according to the density as follows: Grade 0, sparse; grade 1, moderate infiltration; grade 2, abundant infiltration; and grade 3, massive infiltration. The group consisting of grades 0 and 1 infiltration was defined as the low infiltration group, and the group consisting of grades 2 and 3 infiltration was defined as the high infiltration group. TILs in the tumor nest were counted and recorded using Image-Pro Plus 6.0 (Olympus, Tokyo, Japan) under high power field (×200 magnification). The results from the five areas were averaged and used in the statistical analysis. The median value of all sections was set as the cut-off value, as described in our previous study (26), to categorize the low and high infiltration densities in the tumor nest. The total density of lymphocyte infiltration was determined by the summary evaluation of both the tumor nest and stroma.
Purification of monocytes from peripheral blood mononuclear cells (PBMCs) and TAMs obtained from CRC tissue
PBMCs were isolated by Ficoll-Hypaque gradient centrifugation from the peripheral blood of healthy donors (Suzhou Central Blood Bank, Suzhou, China). Monocytes were purified with a CD14-positive selection kit (Stemcell Technologies, Inc., Vancouver, BC, Canada). The purity of the monocyte preparation was 95%. The isolated monocytes were then incubated with LPS (1 mg/ml) for up to 48 h for putative B7-H3 receptor detection.
TAMs were isolated from fresh resected CRC tissues. Tumor specimens were gently minced over a wire mesh screen, and then digested with collagenase IV (1 mg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 37°C for 1 h on a shaking platform to obtain a cell suspension. TAMs were then insolated with the CD14-positive selection kit.
Putative B7-H3 receptor detection
Stimulated monocytes, induced human monocyte THP-1 cells (American Type Culture Collection, Manassas, VA, USA) stimulated with 1 mg/ml phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich; Merck KGaA) for 48 h, and purified CRC TAMs were firstly incubated with human AB serum (10 ml/105 cells) (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 4°C for 30 min to block the Fc receptor (FcR). The cells were then stained with biotinylated hB7-H3 Ig (100 ng/ml) or biotinylated human IgG (100 ng/ml) (both from R&D Systems, Inc., Minneapolis, MN, USA) as the control, then the putative B7-H3 receptor was analyzed using flow cytometer and Diva software (version 6.1.2; BD Biosciences, San Jose, CA, USA).
Tumor cell culture supernatants
For tumor supernatant (TSN) collection, 5×106 human CRC SW480 cells (Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai, China) were seeded to the flask for 1–2 day growth at 37°C. When cells reached 70–80% confluence, fresh medium was added for another 24 h growth. The culture medium was harvested by centrifugation (1,500 × g for 10 min at 4°C), and filtration, and used as a stimulus.
Differentiation and polarization of macrophage cells with B7-H3
Purified human peripheral blood monocytes were used for the induction of macrophages. In total, 5×106 cells were cultured in the conditioned medium containing 10 ng/ml PMA for macrophage differentiation. After 1 day, the cells were thoroughly washed with PBS 3 times to remove the remaining PMA, and then re-seeded to the plates overnight. To polarize macrophages, 30% TSN was added to complete RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) for macrophage culture for 7 days. hB7-H3 Ig or control human Ig (5 ìg/ml; R&D Systems, Inc.) was added during the polarization of cells. The cells and supernatant were then collected for further study.
Macrophage phenotype and cytokine secretion analysis
The induced macrophages from different culture conditions were collected and firstly incubated with human AB serum to block the FcR, and then stained with phycoerythrin-labeled anti-CD206 antibody (cat. no. 321105; BioLegend, Inc., San Diego, CA, USA) and fluorescein isothiocyanate-labeled anti-human leukocyte antigen-antigen D related antibody (cat. no. IM0463U, HLA-DR; Beckman Coulter, Inc., Brea, CA, USA) (1 µg/105 cells), the cells was ultimately analyzed by FACScan to determine the macrophage phenotype. The culture supernatant of different groups was collected by centrifugation (1,500 × g for 10 min at 4°C). ELISA was performed to detect the concentration of cytokine IL-10, IL-12p70 and TNF-α using the Human IL-10 Quantikine ELISA kit (cat. no. D1000B), Human IL-12p70 Quantikine ELISA kit (cat. no. D1200), Human TNF-α Quantikine ELISA kit (cat. no. DTA00C; R&D Systems, Inc.). Reverse transcription-polymerase chain reaction (RT-PCR) was performed to analyze inducible nitric oxide synthase (iNOS) RNA expression. RNA was extracted from the induced macrophages using TRIzol reagent (Takara Bio, Inc., Shiga, Japan) and converted into cDNA with an oligo(dT) primer using the PrimeScript First Strand cDNA Synthesis kit at 42°C for 1 h (Takara Bio, Inc.). The sequences of the primers for iNOS were as follows: Forward, 5′-TCCGAGGCAAACAGCACATTCA-3′ and reverse, 5′-GGGTTGGGGGTGTGGTGATGT-3′. The primers were synthesized by Invitrogen (Thermo Fisher Scientific, Inc.) and the PCR reaction conditions for amplification of DNA were as follows: Initial denaturation at 98°C for 2 min, followed by 30 cycles of annealing commencing at 65°C and ending at 55°C for 15 sec and extension at 68°C for 30 sec.
Statistical analysis
B7-H3 expression and the densities of immune cells in association with the postoperative prognosis of CRC patients were examined by log-rank survival analysis. The association between B7-H3 expression and densities of immune cell infiltration were analyzed by χ2. The comparison of cytokine secretion from induced macrophages was analyzed using Student's t-test. All statistical tests were two tailed. All statistical analyses were performed using GraphPad Prism 5.0 software (GraphPad Software, Inc., San Diego, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
B7-H3 expression and infiltrated macrophage density were associated with the survival of CRC patients
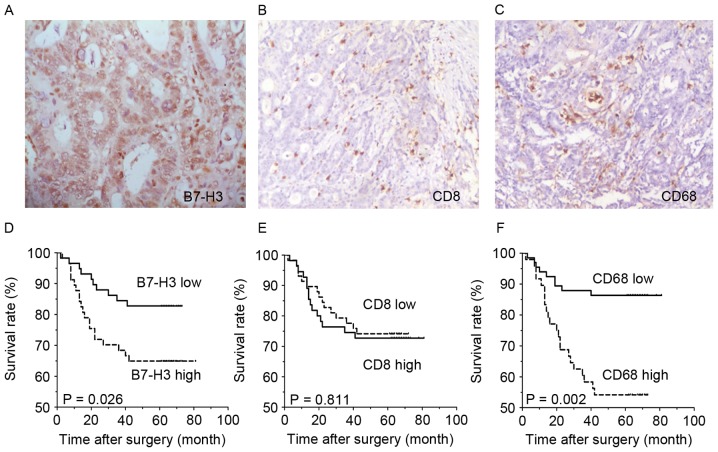
Immunohistochemical staining showed that B7-H3 expression was present in tumor cells of CRC tissues (Fig. 1A), and was located in the cell membrane, cytoplasm and nucleus. B7-H3 expression of 117 patients was categorized into two major subgroups, according to the H-score of immunohistochemical staining, as described in previous studies (24,26). In total, 113 out of 117 (96.6%) CRC tissues showed positive B7-H3 immunohistochemical staining, and 59 cases with high expression and 58 cases with low expression. CD8-labeled effective T cells and CD68-labeled macrophages were also found to have infiltrated all CRC tissues (Fig. 1B and C), both in the tumor nest and stroma. The infiltration density was also categorized into two major subgroups (Table I), according to the criterion in previous studies (24,26). Survival analysis demonstrated that the overall survival rate of the subgroup with low B7-H3 expression was significantly improved compared with the high B7-H3 expression subgroup (P=0.0262; Fig. 1D). Additionally, the density of infiltrating CD68+ macrophages was negatively associated with CRC survival. The survival rate of patients with a low infiltrating density of was improved compared with the patients with a high infiltration density of macrophages (P=0.002: Fig. 1F), while the infiltration density of CD8+ T cells was not significantly associated with the overall survival rate of CRC patients.
Figure 1.
B7-H3 expression and CD68+ macrophage infiltration were associated with the survival of CRC patients. (A) B7-H3 expression (magnification, ×200), (B) CD8+ T cell infiltration (magnification, ×100) and (C) CD68+ macrophage infiltration (magnification, ×100) infiltration were found in CRC tissue. Kaplan-Meier survival curves showed that (D) B7-H3 expression level was associated with the overall survival rate of the patients, but (E) CD8+ T cell infiltration was not associated with the survival rate. (F) Infiltrated CD68+ macrophage density was associated with the overall survival rate of the patients. CD, cluster of differentiation; CRC, colorectal cancer; B7-H3, B7 homolog 3 protein.
Table I.
Association between B7-H3 expression and lymphocytes infiltration in colorectal cancer tissue.
| B7-H3 expression | ||||
|---|---|---|---|---|
| Infiltration | Cases, n | Low, n (%) | High, n (%) | P-value |
| CD8+ T cells | ||||
| Tumor stroma | 58 (49.6) | 59 (50.4) | 0.268 | |
| Low density | 55 | 24 (43.6) | 31 (56.4) | |
| High density | 62 | 34 (54.8) | 28 (45.2) | |
| Tumor nest | 0.095 | |||
| Low density | 52 | 21 (40.4) | 31 (59.6) | |
| High density | 65 | 37 (56.9) | 28 (43.1) | |
| CD68+ TAMs | ||||
| Tumor stroma | 58 (49.6) | 59 (50.4) | 0.039 | |
| Low density | 69 | 40 (58.0) | 29 (42.0) | |
| High density | 48 | 18 (37.5) | 30 (62.5) | |
| Tumor nest | 0.005 | |||
| Low density | 61 | 38 (62.3) | 23 (37.7) | |
| High density | 56 | 20 (35.7) | 36 (64.3) | |
CD, cluster of differentiation; B7-H3, B7 homolog 3 protein; TAMs, tumor-associated macrophages.
B7-H3 expression was associated with the density of infiltrating CD68+ macrophages, but not infiltrating CD8+ T cells
The mechanism of how the B7-H3 pathway regulates the immune response remains unknown; therefore, the association between B7-H3 expression and density of infiltrated lymphocytes was analyzed. As the statistical analysis shows in Table I, the level of cancer cell expression of B7-H3 was found to be positively associated with the density of infiltrated macrophages in both the tumor nest and tumor stroma (P=0.039 and P=0.005, respectively; Table I). The subgroups with increased B7-H3 expression contained more cases with increased density of macrophage infiltration. No significant association was found between B7-H3 expression and the infiltrating density of CD8+ T cells (Table I).
B7-H3 putative receptor could be detected on activated monocytes and TAMs of CRC
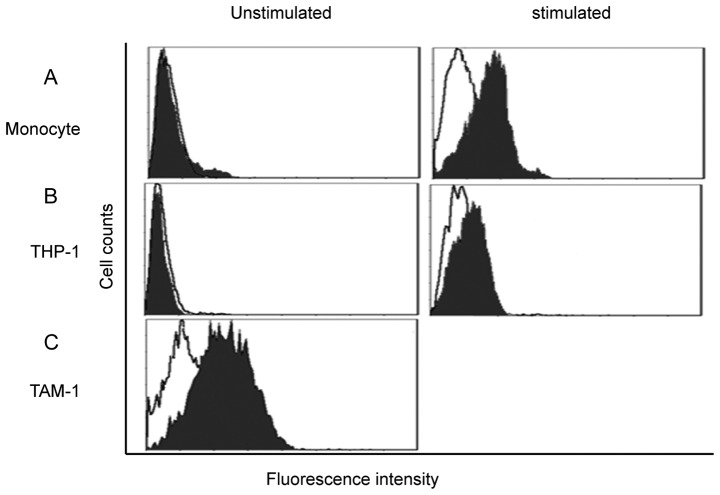
PBMC-derived monocytes were isolated from the peripheral blood of healthy donors; the purification reached 95%. FACScan showed that unstimulated monocytes had extremely low expression of the B7-H3 receptor. Subsequent to 48 h of LPS stimulation, the B7-H3 receptor was detected on the monocytes (Fig. 2A). THP-1 cells were also investigated; PMA was used for macrophage induction. Similarly, the putative B7-H3 receptor was not detected on THP-1 cells, but the expression was increased subsequent to PMA induction, which indicates that the putative B7-H3 receptor was expressed by macrophages (Fig. 2B). TAMs were obtained directly from the CRC tissue, and FACScan showed that B7-H3 receptor was highly expressed by TAMs (Fig. 2C).
Figure 2.
B7-H3 receptor was found on activated monocytes and macrophages. (A) PBMC-derived monocytes, (B) THP-1 cells and (C) TAMs were isolated from CRC tissues were investigated for B7-H3 putative receptor by flow cytometry. (A and B) The B7-H3 receptor was not detected on monocytes and THP-1 cells, but was found to be expressed after activation by stimulus. (C) The B7-H3 receptor was found to be highly expressed on CRC TAMs. CRC, colorectal cancer; B7-H3, B7 homolog 3 protein; PBMC, peripheral blood mononuclear cells; TAM, tumor-associated macrophages. The figure presents overlaps of B7-H3 Ig staining and control Ig staining.
B7-H3 signal could promote the M2-like macrophage polarization induced by TSN
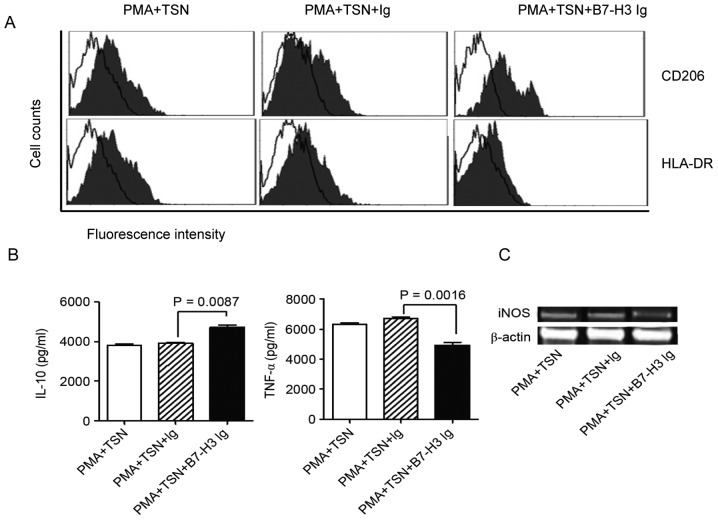
Based on the results of B7-H3 receptor detection, monocytes purified from the PBMCs of healthy donors were assessed. Monocytes were induced into macrophages by PMA stimulation and then polarized by TSN with or without B7-H3. Surface molecules and cytokine secretion were analyzed to determine the phenotype of polarized macrophages. The results show that compared to TSN polarization only, the combination of B7-H3 and TSN promoted CD206 expression and inhibited the HLA-DA expression on macrophages (Fig. 3A). This indicated a M2 macrophage phenotype. ELISA showed that the IL-10 concentration was increased when adding B7-H3 into the TSN polarization (P=0.0087), but TNF-α concentration was significantly decreased meanwhile (Fig. 3B) (P=0.0016). RT-PCR results indicated that iNOS RNA expression was evidently inhibited by B7-H3 combined with TSN polarization compared with the TSN polarization alone (Fig. 3C). All these results suggest that B7-H3 signaling may promote the polarization of M2-like macrophages induced by TSN.
Figure 3.
The phenotype and cytokine secretion of polarized macrophages. PBMC-derived monocytes were used for macrophage induction and differentiation. PMA was used for the induction of macrophages, and TSN and B7-H3 were used for macrophage polarization. (A) Surface molecules (CD206 and HLA-DR) were examined by flow cytometry to identify the phenotype of macrophages. The figure presents overlaps of CD206/HLA-DR staining and control Ig staining. (B) Cytokines (IL-10 and TNF-α) were detected by ELISA, and (C) iNOS RNA expression was detected by reverse transcription-polymerase chain reaction to analyze the function of macrophages. All results together indicated that B7-H3 could promote M2 macrophage differentiation. CRC, colorectal cancer; B7-H3, B7 homolog 3 protein; PBMCs, peripheral blood mononuclear cells; TAMs, tumor-associated macrophages; PMA, phorbol 12-myristate 13-acetate; TSN, tumor supernatant; CD, cluster of differentiation; HLA-DR, human leukocyte antigen-antigen D related; IL-10, interleukin; TNF-α, tumor necrosis factor-α; iNOS, inducible nitric oxide synthase.
Discussion
In our previous study, B7-H3 expression in CRC tissues and its clinical significance were investigated (24). In the present study, the prognostic value of the B7-H3 expression in CRC was further examined, and it was found that the overall survival rate of patients with lower B7-H3 expression was improved compared with those with increased B7-H3 expression. This finding confirmed that B7-H3 may be a useful indicator for the prognostic prediction of human CRC. The mechanism of B7-H3 signal involved in tumor progression remains unclear. B7 family members have important roles in the regulation of the tumor immune response and tumor suppressor microenvironment. The overexpression of B7-H3 and other B7 family molecules, and certain immune cells, contribute to the shaping of the tumor microenvironment (27); therefore, the present study investigated the association between B7-H3 expression and tumor-associated immune cells.
Effective immune cells, particularly T cells and macrophages, are immune effector cells of the antitumor immune response. CD8+ T cells and CD68+ macrophages were hypothesized to play important roles in the host immune response against tumors. While previous studies have shown that the characteristics of these cells, particularly the cytolytic and regulatory nature of effective immune cells were transformed when existing in different microenvironments (1,27). To determine the roles of immune cells in CRC, the present study detected the infiltration density of infiltrated CD8+ T cells and CD68+ macrophages and examined the prognostic value of as well. As showed in Fig. 1, we found that the infiltrating densities of CD8+ T cell had no association with the survival rate of CRC patients, while the infiltrating densities of CD68+ macrophage was significantly correlated to the patients survival rate. Patients with a low macrophage infiltration density had a significantly improved survival rate compared with patients with a high macrophage infiltration density. The immune function of CD4+ and CD8+ T cells were inhibited in certain diseases, including tumors and infections (28). The tumor-driven exhaustion of CD8+ T cells was associated with certain B7 family members, including PD-1 and BTLA (29). Dysfunction of CD8+ T cells leads to a failed antitumor immune response, and a high density of CD8+ T cells in esophageal tumor tissue does not result in improved outcomes for patients (26). TAMs are heterogeneous in response to environmental signals and generally exhibit similarities with polarized M2 macrophages (11,12). Increased TAM infiltration has been demonstrated to be associated with poorer prognosis in breast and ovarian cancer (30,31). The present results identified in patients with CRC were consistent with those of previous studies (10,24), and identified that the macrophages that infiltrate CRC tissues may have a harmful role in tumor immune response. The association between B7-H3 expression and expression of CD8+ T cells and CD68+ macrophages was then investigated. As shown in Table I, the B7-H3 expression level was not associated with CD8+ T cell infiltration density, but was positively associated with the CD68+ macrophage infiltration density, indicating that the B7-H3 signal was involved in the regulation of TAMs.
As a tumor-associated antigen, B7-H3 has dual function in tumor progression, which requires additional investigation to clarify the mechanism of B7-H3 signaling in the tumor microenvironment. The unknown counter-receptor is the key to illustrate the regulation mechanism of the B7-H3 function (9,10). Thus, the identification of the B7-H3 receptor may be attempted on tumor-associated cells. According to the aforementioned results, B7-H3 expression was associated with macrophage infiltration. Subsequently, the expression of the putative receptor of B7-H3 on the monocytes and macrophages was then investigated. The B7-H3 receptor was detected on monocytes isolated from PBMCs and THP-1 cells. LPS was then used to activate monocytes, and PMA was used to induce THP-1 cells into macrophages. The B7-H3 receptor was detected on the activated monocytes and induced THP-1 cells, which demonstrated that the expression of the putative B7-H3 receptor could be induced by activated monocytes and macrophages. TAMs in tumor tissues are a group of activated macrophages. TAMs were isolated from CRC tissue, and the B7-H3 receptor was highly expressed by TAMs. The expression of the putative B7-H3 receptor on macrophages indicated that the B7-H3 signal may participate in the regulation of TAMs. TAMs are the major component of tumor inflammatory infiltration. It has been identified that TAMs are heterogeneous, plastic cells with different functions and cytokine production in response to various signals in the tumor microenvironment (16,18).
Studies of numerous human tumors showed that TAMs generally exhibit M2 phenotypes, and also function as the immunosuppressive cell subset that promotes tumor growth, migration and metastasis. Thus, identifying the key factors that modulate TAM differentiation is crucial for inhibiting TAM-mediated promotion of tumor growth. To assess the role of B7-H3 in macrophage differentiation in the tumor microenvironment, TSN of CRC cells was prepared and used for the induction and polarization of macrophages. Monocytes were induced by PMA and polarized by TSN with or without human B7-H3 Ig. As shown in Fig. 3, when induced and polarized with the TSN and B7-H3 combination, the surface marker CD206 was increased and HLA-DR was decreased compared to TSN use only, exhibiting a M2 phenotype. Meanwhile, IL-10 secretion was increase and TNF-α secretion was decreased, also suggesting a M2 character. NO produce is important feature of M1, which could be reflected by iNOS RNA expression (16,18). The iNOS RNA expression was evidently inhibited by co-culture with B7-H3 Ig and TSN compared with TSN use only, which indicated the switch between M1 and M2. Overall, the results from the present study support an important role of B7-H3 in M2 macrophage polarization in the tumor microenvironment. How to manipulate the differentiation and phenotypical switch of TAMs through the B7-H3 signal requires additional investigation.
In conclusion, the present study indicates that B7-H3 expression and TAM density in tumor tissue are valuable prognostic indicators in patients with CRC. The B7-H3 signal could promote M2 macrophage differentiation via the putative receptor on activated monocytes and macrophages. Thus, targeting the manipulation of TAMs through the B7-H3 pathway may be valuable for the development of a new strategy for antitumor therapy in CRC.
Acknowledgements
The authors thank Dr Yuyu Wu (Department of Pathology, The Fourth People's Hospital of Wuxi, Wuxi, China) for her suggestions and technical assistance. This study was supported by the National Natural Science Foundation of China (grant nos. 31100634, 81201600, 81301960, 81372375 and 81502042), National Natural Science Foundation of Jiangsu (grant nos. BK2012538 and BK20140171) and Science and Technology plan of Suzhou (grant no. SYS201523).
References
- 1.Finn OJ. Immuno-oncology: Understanding the function and dysfunction of the immune system in cancer. Ann Oncol. 2012;23(Suppl 8):viii6–viii9. doi: 10.1093/annonc/mds256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marabelle A, Kohrt H, Caux C, Levy R. Intratumoral immunization: A new paradigm for cancer therapy. Clin Cancer Res. 2014;20:1747–1756. doi: 10.1158/1078-0432.CCR-13-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turtle CJ, Hudecek M, Jensen MC, Riddell SR. Engineered T cells for anti cancer therapy. Curr Opin Immunol. 2012;24:633–639. doi: 10.1016/j.coi.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poirier N, Blancho G, Vanhove B. A more selective costimulatory blockade of the CD28-B7 pathway. Transpl Int. 2011;24:2–11. doi: 10.1111/j.1432-2277.2010.01176.x. [DOI] [PubMed] [Google Scholar]
- 5.Maj T, Wei S, Welling T, Zou W. T cells and costimulation in cancer. Cancer J. 2013;19:473–482. doi: 10.1097/PPO.0000000000000002. [DOI] [PubMed] [Google Scholar]
- 6.Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K, Chen L. B7-H3: A costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2:269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 7.Suh WK, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, Duncan GS, Bukczynski J, Plyte S, Elia A, et al. The B7 family member B7-H3 preferentially down regulates T helper type 1-mediated immune responses. Nat Immunol. 2003;4:899–906. doi: 10.1038/ni967. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Kang FB, Shan BE. B7-H3-mediated tumor immunology: Friend or foe? Int J Cancer. 2014;134:2764–2771. doi: 10.1002/ijc.28474. [DOI] [PubMed] [Google Scholar]
- 9.Hashiguchi M, Kobori H, Ritprajak P, Kamimura Y, Kozono H, Azuma M. Triggering receptor expressed on myeloid cell-like transcript 2 (TLT-2) is a counter-receptor for B7-H3 and enhances T cell responses. Proc Natl Acad Sci USA. 2008;105:10495–10500. doi: 10.1073/pnas.0802423105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loos M, Hedderich DM, Friess H, Kleeff J. B7-h3 and its role in antitumor immunity. Clin Dev Immunol. 2010;2010:683875. doi: 10.1155/2010/683875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riabov V, Gudima A, Wang N, Mickley A, Orekhov A, Kzhyshkowska J. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front Physiol. 2014;5:75. doi: 10.3389/fphys.2014.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 13.Bingle L, Brown NJ, Lewis CE. The role of tumor-associated macrophages in tumor progression: Implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 14.Karnevi E, Andersson R, Rosendahl AH. Tumour-educated macrophages display a mixed polarisation and enhance pancreatic cancer cell invasion. Immunol Cell Biol. 2014;92:543–552. doi: 10.1038/icb.2014.22. [DOI] [PubMed] [Google Scholar]
- 15.Zhou W, Bao S. Reciprocal supportive interplay between glioblastoma and tumor-associated macrophages. Cancers (Basel) 2014;6:723–740. doi: 10.3390/cancers6020723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 17.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagemann T, Wilson J, Burke F, Kulbe H, Li NF, Plüddemann A, Charles K, Gordon S, Balkwill FR. Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. J Immunol. 2006;176:5023–5032. doi: 10.4049/jimmunol.176.8.5023. [DOI] [PubMed] [Google Scholar]
- 20.Sica A, Schioppa T, Mantovani A, Allavena P. Tumor-associated macrophages are a distinct M2 polarised population promoting tumor progression: Potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717–727. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Mantovani A, Allavena P, Sica A. Tumor-associated macrophages as a prototypic type II polarised phagocyte population: Role in tumor progression. Eur J Cancer. 2004;40:1660–1667. doi: 10.1016/j.ejca.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 22.Weitz J, Koch M, Debus J, Höhler T, Galle PR, Büchler MW. Colorectal cancer. Lancet. 2005;365:153–165. doi: 10.1016/S0140-6736(05)17706-X. [DOI] [PubMed] [Google Scholar]
- 23.Dong ZW QY, Li LD, Chen YD, Wang RT, Lei TH. Report of Chinese cancer control strategy. Chin Cancer. 2002;11:250–60. [Google Scholar]
- 24.Sun J, Chen LJ, Zhang GB, Jiang JT, Zhu M, Tan Y, Wang HT, Lu BF, Zhang XG. Clinical significance and regulation of the costimulatory molecule B7-H3 in human colorectal carcinoma. Cancer Immunol Immunother. 2010;59:1163–1171. doi: 10.1007/s00262-010-0841-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammes LS, Tekmal RR, Naud P, Edelweiss MI, Kirma N, Valente PT, Syrjänen KJ, Cunha-Filho JS. Up-regulation of VEGF, c-fms and COX-2 expression correlates with severity of cervical cancer precursor (CIN) lesions and invasive disease. Gynecol Oncol. 2008;110:445–451. doi: 10.1016/j.ygyno.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 26.Chen LJ, Sun J, Wu HY, Zhou SM, Tan Y, Tan M, Shan BE, Lu BF, Zhang XG. B7-H4 expression associates with cancer progression and predicts patient's survival in human esophageal squamous cell carcinoma. Cancer Immunol Immunother. 2011;60:1047–1055. doi: 10.1007/s00262-011-1017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flies DB, Chen L. Modulation of immune response by B7 family molecules in tumor microenvironments. Immunol Invest. 2006;35:395–418. doi: 10.1080/08820130600755017. [DOI] [PubMed] [Google Scholar]
- 28.Schietinger A, Greenberg PD. Tolerance and exhaustion: Defining mechanisms of T cell dysfunction. Trends Immunol. 2014;35:51–60. doi: 10.1016/j.it.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fourcade J, Sun Z, Pagliano O, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Olive D, Kuchroo V, Zarour HM. CD8(+) T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through up-regulation of the inhibitory receptors BTLA and PD-1. Cancer Res. 2012;72:887–896. doi: 10.1158/0008-5472.CAN-11-2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leek RD, Harris AL. Tumor-associated macrophages in breast cancer. J Mammary Gland Biol Neoplasia. 2002;7:177–189. doi: 10.1023/A:1020304003704. [DOI] [PubMed] [Google Scholar]
- 31.Hagemann T, Wilson J, Burke F, Kulbe H, Li NF, Plüddemann A, Charles K, Gordon S, Balkwill FR. Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. J Immunol. 2006;176:5023–5032. doi: 10.4049/jimmunol.176.8.5023. [DOI] [PubMed] [Google Scholar]