Abstract
Jun activation domain-binding protein 1 (JAB1) has been shown to have multiple roles in tumorigenesis, including the degradation of tumor suppressor proteins such as p53, Smad7, Runx3 and the cyclin-dependent kinase inhibitor p27Kip1, and the activation of oncogenic transcription factors, such as c-Jun and hypoxia-inducible factor-1α. In addition, our previous study revealed that JAB1 positively regulates signal transducer and activator of transcription 3 (STAT3) DNA-binding activity in human colon cancer cells. In turn, the oncogenic transcription factor STAT3 positively regulates JAB1 expression, indicative of a positive feedback loop. Furthermore, high JAB1 expression is associated with a poor prognosis in numerous malignant carcinomas. However, the association between JAB1 expression and prognosis in colorectal cancer remains unclear. The aim of the present study was to elucidate the association between JAB1 and STAT3 expression and recurrence in colorectal cancer. In the present study, it was found that high JAB1 expression in primary colorectal cancer tissues is an independent predictor of recurrence following 5-fluorouracil (5-FU)-based adjuvant chemotherapy in colorectal cancer patients, and that high expression of both JAB1 and STAT3 in primary colorectal cancer tissues is associated with a lower recurrence-free survival rate following 5-FU-based adjuvant chemotherapy compared to high expression of only JAB1 or STAT3. Overall, these results suggest that JAB1 is a novel predictive marker of recurrence following 5-FU-based adjuvant chemotherapy in colorectal cancer patients, and that the JAB1-STAT3 activation loop may be a potential therapeutic target in recurrent colorectal cancer following 5-FU-based adjuvant chemotherapy.
Keywords: JAB1, STAT3, JAB1-STAT3 activation loop, recurrence, colorectal cancer, 5-FU-based adjuvant chemotherapy
Introduction
Jun activation domain-binding protein 1 (JAB1) was originally identified as a co-activator of c-Jun that stabilizes its DNA-binding through protein-protein interaction (1). Subsequently, JAB1 was found to interact with numerous other proteins, affecting protein stability and transcriptional activity of its interacting partner proteins, which are involved in the regulation of the cell cycle, signal transduction and DNA repair (2). JAB1 has an important role in tumorigenesis, inactivating tumor suppressor proteins and activating oncogenic transcription factors. JAB1 facilitates the translocation of tumor suppressor proteins, including p53 (3,4), Smad7 (5), Runx3 (6) and the cyclin-dependent kinase inhibitor p27Kip1 (7,8), from the nucleus to the cytoplasm, where they are subsequently degraded in the proteasome. JAB1 is also a transcriptional co-activator of c-Jun (1), hypoxia-inducible factor-1α (9,10) and signal transducer and activator of transcription 3 (STAT3) (11). JAB1 is positively regulated by oncogenic transcription factors STAT3 and β-catenin/TCF-4 (12,13). STAT3 positively regulates JAB1 expression through its binding to the JAB1 promoter (12), and HER2 increases JAB1 expression through the binding of β-catenin/TCF-4 to the JAB1 promoter in human breast cancer cells (13). These findings suggest that JAB1 is a target gene of STAT3 and β-catenin/TCF-4. Overall, with our recent findings that JAB1 positively regulates STAT3 DNA-binding activity in human colon cancer cells (11), the results of these studies suggest that the JAB1-STAT3 activation loop exists in human colorectal cancer cells. Furthermore, high JAB1 expression has been reported to be associated with poor prognosis in numerous malignant carcinomas, including ovarian cancer (14,15), oral squamous cell carcinoma (16), laryngeal squamous cell carcinoma (17), hepatocellular carcinoma (18), glioma (19), soft-tissue sarcoma (20), pancreatic cancer (21), esophageal squamous cell carcinoma (22), lung cancer (23) and non-Hodgkin's lymphoma (24). However, the association between JAB1 expression and prognosis in colorectal cancer remains largely unknown. The objectives of the present study were therefore to elucidate the associations between JAB1 and STAT3 expression and recurrence in colorectal cancer.
In the present study, it was found that high JAB1 expression in primary colorectal cancer tissues is an independent predictor of recurrence following 5-fluorouracil (5-FU)-based adjuvant chemotherapy in colorectal cancer patients, and high expression of both JAB1 and STAT3 in primary colorectal cancer tissues is associated with a lower recurrence-free survival rate compared to high expression of only JAB1 or STAT3.
Materials and methods
Patients and clinical samples
A total of 57 patients with colorectal cancer who underwent surgical treatment at Yamaguchi University Hospital (Ube, Yamaguchi, Japan), Yamaguchi Saiseikai Shimonoseki General Hospital (Shimonoseki, Yamaguchi, Japan) and Yamaguchi Rosai Hospital (Sanyo-Onoda, Yamaguchi, Japan) between April 2012 and December 2013 were enrolled in the present study. All patients had stage II or III colorectal cancer and were treated with FOLFOX, UFT/UZEL or Xeloda following curative surgical operation. Primary colorectal cancer tissues from 50 patients (age range, 48–84 years; 31 males and 19 females) were immediately taken from resected colorectal tissues and kept at −80°C until total RNA extraction, followed by reverse transcription-quantitative polymerase chain reaction (RT-qPCR). Additionally, 7 paired samples of primary colorectal cancer tissue and liver metastasis from 7 patients (age range, 63–81 years; 6 males and 1 female) who had undergone mainly 5-FU-based chemotherapy were formalin-fixed and paraffin-embedded immunohistochemical staining. Written informed consent was obtained from all patients, and approval was provided by Institutional Review Board of Yamaguchi University Hospital and the affiliated hospitals. The samples were used in accordance with the Declaration of Helsinki.
Regimens of adjuvant chemotherapy
The choice of adjuvant chemotherapy was made by the patient in consultation with the surgeon. UFT/LV was given in 5-week cycle consisting of 4 weeks of treatment and 1 week of rest. The cycle was repeated at least 5 times. The UFT dose was 300 mg/m2/day and LV dose 75 mg/day. Xeloda was administered at a dose of 1,250 mg/m2 twice a day for 14 days, followed by 7 days of rest. Standard care included a total of 8 cycles. Modified FOLFOX6 [a modified folinic acid, leucovorin (LV), 5-FU, and oxaliplatin (OX)] regimen was as follows; OX 85 mg/m2, LV 200 mg/m2, 5-FU bolus 400 mg/m2, 5-FU infusion 2,400 mg/m2 over 46 h. The treatment was repeated every 2 weeks. Standard care included a total of 12 cycles.
Immunohistochemical staining
Tissue specimens were fixed with 20% formalin for 3–5 days at room temperature and paraffin-embedded. Sections (3-µm thick) from the tissue specimens were deparaffinized with xylene at room temperature and rehydrated with graded ethanol. The tissue sections were then incubated with 3% hydrogen peroxide (H2O2) in methanol for 30 min to block endogenous peroxidase activity, followed by antigen retrieval at 95°C for 20 min in Dako Target Retrieval solution (Agilent Technologies, Inc., Santa Clara, CA, USA). Tissue sections were subsequently incubated in Dako Protein Block Serum-Free Ready-to-Use (Agilent Technologies, Inc.) for 30 min at room temperature to prevent non-specific binding, and were then incubated with anti-rabbit polyclonal JAB1 (FL-334) primary antibody (catalog no. sc-9074; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) diluted at 1:100 overnight at 4°C. After washing several times with phosphate-buffered saline, the sections were incubated with anti-rabbit immunoglobulin conjugated to horseradish peroxidase (EnVision+ HRP-labeled polymer anti-rabbit system; catalog no. K4002; Dako North America, Inc., Carpinteria, CA, USA) as a secondary antibody for 30 min at room temperature. The sections were treated with 0.2 mg/ml diaminobenzidine for 15 sec and counterstained in Mayer's hematoxylin for 30 sec. Images were captured from at least 10 randomly selected fields per sample using an All-in-One fluorescence microscope (magnification, ×40; KEYENCE, Osaka, Japan). Immunoreactivity was independently evaluated by two.
RT-qPCR
Resected primary colorectal cancer tissues were disrupted in RLT buffer (Qiagen, Valencia, CA, USA) and homogenized by shaking with stainless steel beads using a Mixer Mill MM300 (both from Qiagen). Total RNA was isolated using an RNeasy Mini kit (Qiagen), according to the manufacturer's protocol. Reverse transcription was performed using PrimeScript RT Master Mix (Perfect Real-Time; Takara Bio, Inc., Otsu, Japan). The template cDNAs were amplified using a QuantiTect SYBR-Green PCR kit (Qiagen) with the specific primers. The sequences of the primers were as follows: JAB1 forward, 5′-GCAGTGGTGATTGATCCAAC-3′ and reverse, 5′-GTCTGGTACTCAGAAGGTCC-3′; STAT3 forward, 5′-CACTACTAAAGTCAGGTTGCTGGTC-3′ and reverse, 5′-AACGTCCCCAGAGTCTTTGTC-3′; MCL1 forward, 5′-CACAGACGTTCTCGTAAGGAC-3′ and reverse, 5′-GATGCCACCTTCTAGGTCCTC-3′; cyclin D1 forward, 5′-CGAGAAGCTGTGCATCTACACC-3′ and reverse, 5′-TTCCACTTGAGCTTGTTCACC-3′; and GAPDH forward, 5′-TTGGTATCGTGGAAGGACTCA-3′ and reverse, 5′-TGTCATCATATTTGGCAGGTT−3′. The PCR thermocycling conditions were 95°C for 15 min, followed by 50 cycles of 95°C for 10 sec and 60°C for 30 sec. JAB1 and STAT3 expression was normalized to GAPDH expression. RT-qPCR was performed using LightCycler software version 3.5 (Roche Applied Science, Penzberg, Germany), and data were evaluated using the 2−ΔΔCq method (25).
Statistical analysis
Statistical analyses were performed using SPSS Statistics 20 for Windows (SPSS, Inc., Chicago, IL, USA). Differences between groups were analyzed using the paired t-test, Mann-Whitney U test or χ2 test, as appropriate. The association between mRNA expression levels was assessed using Pearson's correlation coefficient. Survival curves were generated using the Kaplan-Meier method and compared using the log-rank test. Receiver operating characteristic (ROC) curve analysis was used to determine the optimum cut-off values for predicting outcome. To determine the cut-off values for JAB1 and STAT3 expression, ROC curves were constructed by plotting all possible sensitivity/1-specificity pairs in the training set. The Cox proportional hazards regression model was used to identify the variables associated with recurrence-free survival. P<0.05 was considered to indicate a statistically significant difference.
Results
Association between JAB1 and STAT3 expression, and clinicopathological parameters in primary colorectal cancer tissues
To investigate JAB1 and STAT3 expression level in 50 primary colorectal cancer tissues, RT-qPCR was performed. ROC curve analysis was used to obtain the optimal cut-off values of JAB1 and STAT3 expression, and this was used to classify 50 primary colorectal cancer tissues into high or low expression group of JAB1 or STAT3. The association between JAB1 and STAT3 expression, and clinicopathological parameters was investigated. As shown in Table I, high JAB1 expression was not associated with any of the investigated clinicopathological parameters, including age, sex, tumor location, histological grade, invasion depth, lymphatic metastasis, lymphatic invasion, venous invasion and tumor-node-metastasis (TNM) stage. However, high STAT3 expression was significantly associated with advanced TNM stage (P=0.04).
Table I.
The association between JAB1 and STAT3 expression, and clinicopathological variables in primary colorectal cancer tissues.
| JAB1 expression | STAT3 expression | |||||
|---|---|---|---|---|---|---|
| Clinicopathological variables | Low | High | P-value | Low | High | P-value |
| Total | 33 | 17 | 33 | 17 | ||
| Gender | 0.23 | 0.23 | ||||
| Male | 20 | 11 | 20 | 11 | ||
| Female | 13 | 6 | 13 | 6 | ||
| Age (years) | 70.0±10.6 | 72.1±6.6 | 0.48 | 70.5±9.8 | 71.2±8.9 | 0.80 |
| Location | 0.84 | 0.84 | ||||
| Right | 13 | 8 | 13 | 8 | ||
| Left | 10 | 4 | 10 | 4 | ||
| Rectum | 10 | 5 | 10 | 5 | ||
| Histological grade | 0.77 | 0.77 | ||||
| Well | 3 | 2 | 4 | 1 | ||
| Moderate | 26 | 14 | 26 | 14 | ||
| Poor | 4 | 1 | 3 | 2 | ||
| Invasion depth | 0.40 | 0.40 | ||||
| T2 | 1 | 1 | 1 | 1 | ||
| T3 | 22 | 8 | 22 | 8 | ||
| T4 | 10 | 8 | 10 | 8 | ||
| Lymphatic metastasis | 0.87 | 0.05 | ||||
| + | 21 | 11 | 18 | 14 | ||
| − | 12 | 6 | 15 | 3 | ||
| Lymphatic invasion | 0.96 | 0.96 | ||||
| + | 30 | 16 | 30 | 16 | ||
| − | 3 | 1 | 3 | 1 | ||
| Venous invasion | 0.20 | 0.49 | ||||
| + | 15 | 11 | 16 | 10 | ||
| − | 18 | 6 | 17 | 7 | ||
| Stage | 0.13 | 0.04 | ||||
| 2a | 9 | 1 | 10 | 0 | ||
| 2b | 3 | 5 | 5 | 3 | ||
| 3a | 1 | 0 | 1 | 0 | ||
| 3b | 13 | 5 | 12 | 6 | ||
| 3c | 7 | 6 | 5 | 8 | ||
STAT3, signal transducer and activator of transcription 3; JAB1, Jun activation domain-binding protein 1.
Association between JAB1 and STAT3 expression in primary colorectal cancer tissues
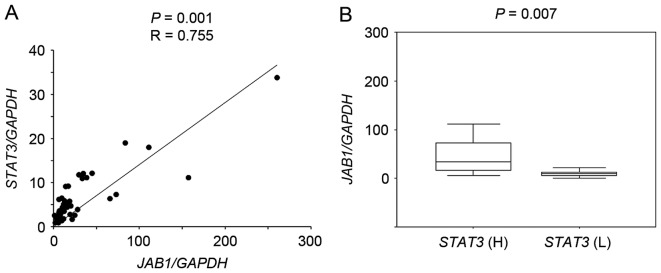
RT-qPCR followed by scatter plot analysis showed that JAB1 expression significantly correlated with STAT3 expression in primary colorectal cancer tissues (Fig. 1A, P=0.001, r=0.755), and JAB1 expression in tumors with high STAT3 expression was significantly increased compared with that in tumors with low STAT3 expression (Fig. 1B, P=0.007).
Figure 1.
Association between JAB1 and STAT3 expression in 50 primary colorectal cancer tissues. Reverse transcription-quantitative polymerase chain reaction was performed to determine the JAB1, STAT3 and GAPDH expression level. (A) The ratio of JAB1 or STAT3 expression to GAPDH expression was normalized to the lowest value and is presented as a scatter plot. (B) JAB1 expression in tumors with high or low expression of STAT3. STAT3 (H), high STAT3 expression group; STAT3 (L), low STAT3 expression group. JAB1, Jun activation domain-binding protein 1; STAT3, signal transducer and activator of transcription 3.
Expression of the STAT3 target genes MCL1 and cyclin D1 is associated with JAB1 expression
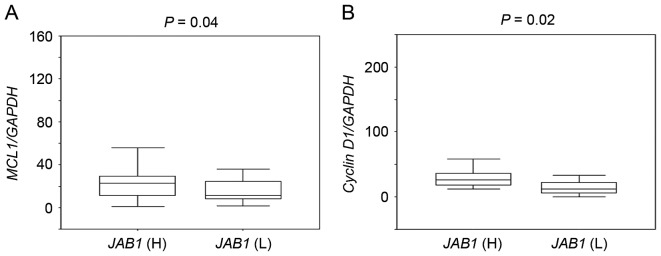
The association between JAB1 expression and the expression of the STAT3 target genes MCL1 and cyclin D1 was then investigated. MCL1 and cyclin D1 expression in tumors with high JAB1 expression was significantly higher than that in tumors with low JAB1 expression (Fig. 2, P=0.04 and P=0.02, respectively).
Figure 2.
Comparison of the expression of the signal transducer and activator of transcription 3 target genes MCL1 and cyclin D1 in primary colorectal cancer tissues with high or low expression of JAB1. (A) Comparison of MCL1 expression in tumors with high or low JAB1 expression. (B) Comparison of cyclin D1 expression in tumors with high or low JAB1 expression. The ratio of MCL1 or cyclin D1 expression to GAPDH expression was normalized to the lowest value. JAB1 (H), high JAB1 expression group; JAB1 (L), low JAB1 expression group. JAB1, Jun activation domain-binding protein 1.
High JAB1 expression in primary colorectal cancer tissues is associated with a lower recurrence-free survival rate following 5-FU-based adjuvant chemotherapy
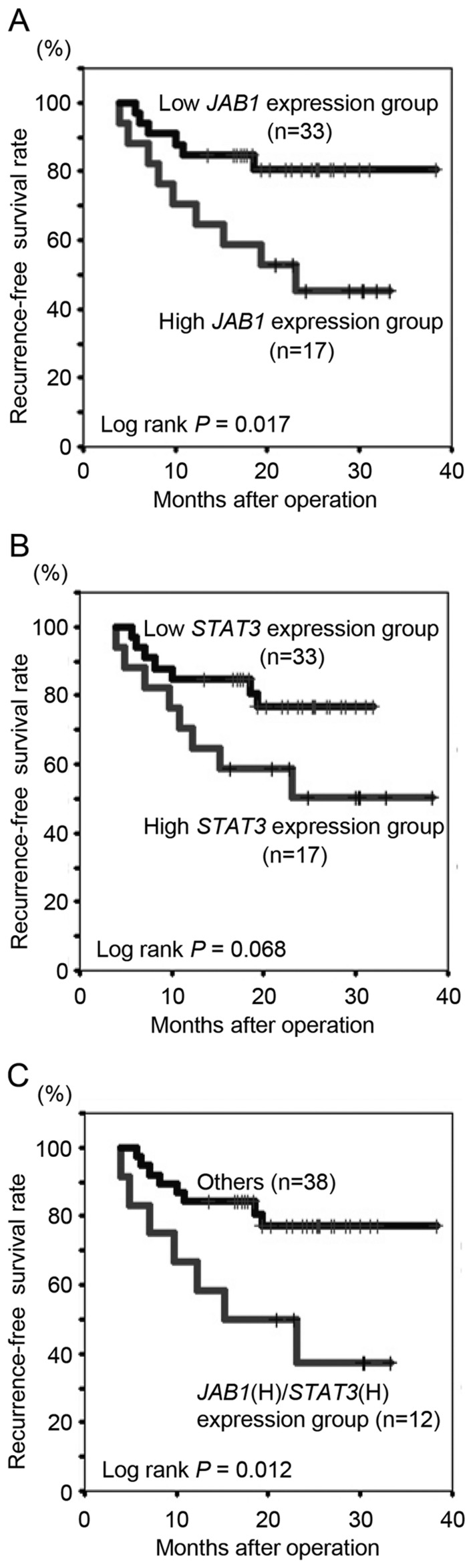
The present study determined whether clinicopathological variables, including JAB1 and STAT3 expression, in primary colorectal cancer tissues were associated with recurrence following 5-FU-based adjuvant chemotherapy. The median follow-up period for all patients was 21.4 months (range, 5.6–38.3 months). Histological grade (poor), invasion depth (T4) and JAB1 expression (high) were significantly associated with recurrence (Table II, P=0.03, 0.01 and 0.01, respectively). To further examine the associations between JAB1 and STAT3 expression and recurrence-free survival rate following 5-FU-based adjuvant chemotherapy, Kaplan-Meier analyses were performed. Patients with high JAB1 expression had a significantly decreased recurrence-free survival rate following 5-FU-based adjuvant chemotherapy compared to those with low JAB1 expression (Fig. 3A, P=0.017), whilst there was no significant difference in survival with respect to STAT3 expression (Fig. 3B, P=0.068). Patients with high expression of both JAB1 and STAT3 had a significantly decreased recurrence-free survival rate following 5-FU-based adjuvant chemotherapy compared to all other patients (Fig. 3C, P=0.012), and compared to patients with high expression of only JAB1 or STAT3.
Table II.
Association between recurrence following fluorouracil-based adjuvant chemotherapy and clinicopathological variables, including JAB1 and STAT3 expression, in primary colorectal cancer tissues.
| Clinicopathological variables | No recurrence | Recurrence | P-value |
|---|---|---|---|
| Total | 35 | 15 | |
| Gender | 0.98 | ||
| Male | 23 | 8 | |
| Female | 12 | 7 | |
| Age (years) | 71.2±8.3 | 69.6±11.7 | 0.60 |
| Location | 0.29 | ||
| Right | 14 | 7 | |
| Left | 12 | 2 | |
| Rectum | 9 | 6 | |
| Histological grade | 0.03 | ||
| Well | 3 | 2 | |
| Moderate | 31 | 9 | |
| Poor | 1 | 4 | |
| Invasion depth | 0.01 | ||
| T2 | 2 | 0 | |
| T3 | 26 | 4 | |
| T4 | 7 | 11 | |
| Lymphatic | 0.73 | ||
| metastasis | |||
| + | 21 | 11 | |
| − | 14 | 4 | |
| Lymphatic | 0.45 | ||
| invasion | |||
| + | 33 | 13 | |
| − | 2 | 2 | |
| Venous | 0.16 | ||
| invasion | |||
| + | 18 | 8 | |
| − | 17 | 7 | |
| Stage | 0.10 | ||
| 2a | 10 | 0 | |
| 2b | 4 | 4 | |
| 3a | 1 | 0 | |
| 3b | 13 | 5 | |
| 3c | 7 | 6 | |
| Oxaliplatin | 1.00 | ||
| + | 7 | 3 | |
| − | 28 | 12 | |
| JAB1 | 0.01 | ||
| High | 8 | 9 | |
| Low | 27 | 6 | |
| STAT3 | 0.06 | ||
| High | 9 | 8 | |
| Low | 26 | 7 |
STAT3, signal transducer and activator of transcription 3; JAB1, Jun activation domain-binding protein 1.
Figure 3.
Kaplan-Meier curves for recurrence-free survival following 5-FU-based adjuvant chemotherapy. (A) Recurrence-free survival curve following 5-FU-based adjuvant chemotherapy with respect to JAB1 expression. (B) Recurrence-free survival curve following 5-FU-based adjuvant chemotherapy with respect to STAT3 expression. (C) Recurrence-free survival curve following 5-FU-based adjuvant chemotherapy with respect to combined JAB1 and STAT3 expression. JAB1 (H)/STAT3 (H) expression group, high expression group of both JAB1 and STAT3. JAB1, Jun activation domain-binding protein 1; STAT3, signal transducer and activator of transcription 3; 5-FU, 5-fluorouracil.
Cox proportional hazard regression analysis was then performed to identify independent prognostic factors. The recurrence-free survival rate following 5-FU-based adjuvant chemotherapy was strongly associated with histological grade (poor), invasion depth (T3) and JAB1 expression (high) on univariate analysis (Table III, P=0.02, 0.01 and 0.03, respectively). In multivariate analysis, high JAB1 expression was the only independent predictor of recurrence-free survival following 5-FU-based adjuvant chemotherapy (Table III, P=0.04).
Table III.
Univariate analysis and multivariate analysis for clinicopathological variables affecting the recurrence-free survival rate following fluorouracil-based chemotherapy.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Clinicopathological variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Gender (male vs. female) | 1.55 (0.56–4.28) | 0.40 | ||
| Age (years) | 0.99 (0.94–1.04) | 0.63 | ||
| Location (rectum) | 1.83 (0.65–5.15) | 0.25 | ||
| Histological grade (poor) | 4.07 (1.29–12.9) | 0.02 | 3.10 (0.90–10.65) | 0.07 |
| Invasion depth (≥T3) | 4.10 (1.40–12.0) | 0.01 | 2.80 (0.89–8.87) | 0.08 |
| Lymphatic metastasis (+) | 1.66 (0.53–5.20) | 0.39 | ||
| Lymphatic invasion (+) | 0.45 (0.10–2.02) | 0.30 | ||
| Venous invasion (+) | 0.96 (0.35–2.65) | 0.94 | ||
| Stage (≥3a) | 1.85 (0.59–5.80) | 0.29 | ||
| JAB1 (high) | 3.27 (1.16–9.20) | 0.03 | 2.80 (1.07–8.78) | 0.04 |
| STAT3 (high) | 2.45 (0.90–6.86) | 0.08 | ||
HR, hazard ratio; CI, confidence interval; STAT3, signal transducer and activator of transcription 3; JAB1, Jun activation domain-binding protein 1.
JAB1 protein expression in primary colorectal cancer tissues and liver metastases following mainly 5-FU-based chemotherapy
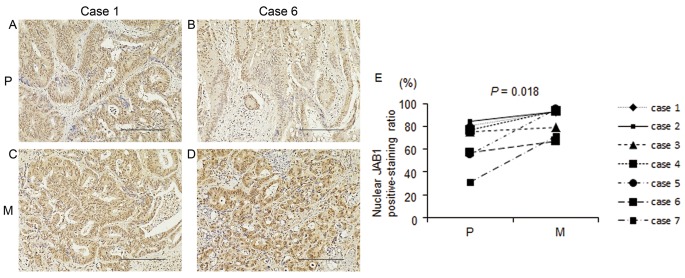
Seven paired samples of primary colorectal cancer tissue and liver metastasis from 7 patients who had undergone mainly 5-FU-based chemotherapy were prepared. Clinical variables, including the chemotherapy regimen and treatment effects, are shown in Table IV. Nuclear and cytoplasmic JAB1 protein expressions were detected in both primary colorectal cancer tissues and liver metastases. Nuclear and cytoplasmic JAB1 protein expressions in liver metastases appeared to be increased compared with those in primary colorectal cancer tissues (Fig. 4A-D), and the proportion of cells with positive nuclear JAB1 expression in liver metastases was significantly increased compared with that in primary colorectal cancer tissues (Fig. 4E, P=0.018).
Table IV.
Clinicopathological characteristics of colorectal patients with liver metastasis.
| Case no. | Regimen | Cycle | Effect | Invasion depth | Histological grade | Lymph invasion | Venous invasion |
|---|---|---|---|---|---|---|---|
| 1 | Pmab + FOLFOX | 6 | PR | SE | mod | 1 | 1 |
| 2 | Bmab + Xelox | 11 | PD | SS | mod | 1 | 0 |
| 3 | Bmab + FOLFIRI | 6 | SD | SS | mod | 1 | 1 |
| 4 | Pmab + FOLFOX | 6 | PR | SS | pap | 1 | 1 |
| 5 | Bmab + FOLFOX | 10 | SD | SS | mod | 2 | 2 |
| 6 | Bmab + Xeloda | 4 | PR | SS | mod | 0 | 0 |
| 7 | Bmab + FOLFIRI | 20 | SD | SS | mod | 1 | 2 |
Pmab, panitumumab; Bmab, bevacizumab; FOLFOX, folinic acid + fluorouracil + oxaliplatin; FOLFIRI, folinic acid + fluorouracil + irinotecan; Xelox, capecitabine + oxaliplatin; PR, partial response; PD, progressive disease; SD, stable disease; SE, serosa; SS, subserosa; mod, moderately differentiated carcinoma; pap, papillary adenocarcinoma.
Figure 4.
Immunohistochemical analysis of JAB1 expression in primary colorectal cancer tissues and liver metastases following mainly 5-FU-based chemotherapy. (A-D) Representative images of JAB1 staining in primary colorectal cancer tissues (A and B) and in liver metastases (C and D). Primary colorectal cancer tissue (A) and liver metastasis (C) are derived from the same patient (case 1). Primary colorectal cancer tissue (B) and liver metastasis (D) are also paired (case 6). Magnification, ×40. Scale bar, 200 µm. (E) The proportion of cells positive for nuclear JAB1 expression in primary colorectal cancer tissues and liver metastases following mainly 5-FU-based chemotherapy (n=7). P, primary colorectal cancer tissue; M, liver metastasis. JAB1, Jun activation domain-binding protein 1; 5-FU, 5-fluorouracil.
Discussion
It has been reported that JAB1 expression is transcriptionally regulated through STAT3 binding to the JAB1 promoter in human breast cancer cells (12). Furthermore, our previous study revealed that JAB1 positively regulates STAT3 DNA-binding activity in human colorectal cancer cells (11). Consistent with these findings, in the present study, it was found that JAB1 expression was increased in tumors with high STAT3 expression compared with that in tumors with low STAT3 expression, and that the STAT3 target genes MCL1 and cyclin D1 had increased expressed in tumors with high JAB1 expression compared to those with low JAB1 expression. These findings suggest that JAB1 cooperates with STAT3, and that the JAB1-STAT3 activation loop is present in human colorectal cancer cells. Notably, patients with tumors that highly expressed both JAB1 and STAT3 had a lower recurrence-free survival rate following 5-FU-based adjuvant chemotherapy than those patients with tumors that highly expressed only JAB1 or STAT3, suggesting that the JAB1-STAT3 activation loop has an important role in recurrence following 5-FU-based adjuvant chemotherapy. Furthermore, the present findings indicate that high JAB1 expression in primary colorectal cancer is a significant predictor of recurrence following this treatment. It was also found that the proportion of tumor cells with positive nuclear JAB1 expression was significantly increased in liver metastases following mainly 5-FU-based chemotherapy compared with that in primary colorectal cancer tissues. Notably, a recent study found that nuclear JAB1 expression was increased in recurrent nasopharyngeal carcinoma following radiotherapy compared with primary nasopharyngeal carcinoma (26). Together with the present findings, this suggests that nuclear JAB1 has a role in recurrence. Additional studies are required to elucidate the association between nuclear JAB1 and recurrence.
Overall, the present findings suggest that the JAB1-STAT3 activation loop can confer resistance to 5-FU-based adjuvant chemotherapy and is thus a potential therapeutic target in recurrent colorectal cancer following 5-FU-based adjuvant chemotherapy.
Acknowledgements
This study was supported by the Oncochishin Project from Yamaguchi University. The authors thank Dr K. Ueki (Yamaguchi Saiseikai Shimonoseki General Hospital, Shimonoseki, Yamaguchi, Japan) and Dr C. Kato (Yamaguchi Rosai Hospital, Sanyo-Onoda, Yamaguchi, Japan) for their assistance in acquiring samples and collecting information for colorectal cancer patients. The authors also thank Yukari Hironaka (Department of Surgery and Clinical Science, Yamaguchi University Graduate School of Medicine, Ube, Yamaguchi, Japan) for providing technical assistance.
References
- 1.Claret FX, Hibi M, Dhut S, Toda T, Karin M. A new group of conserved coactivators that increase the specificity of AP-1 transcription factors. Nature. 1996;383:453–457. doi: 10.1038/383453a0. [DOI] [PubMed] [Google Scholar]
- 2.Shackleford TJ, Claret FX. JAB1/CSN5: A new player in cell cycle control and cancer. Cell Div. 2010;5:26. doi: 10.1186/1747-1028-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh W, Lee EW, Sung YH, Yang MR, Ghim J, Lee HW, Song J. Jab1 induces the cytoplasmic localization and degradation of p53 in coordination with Hdm2. J Biol Chem. 2006;281:17457–17465. doi: 10.1074/jbc.M601857200. [DOI] [PubMed] [Google Scholar]
- 4.Zhang XC, Chen J, Su CH, Yang HY, Lee MH. Roles for CSN5 in control of p53/MDM2 activities. J Cell Biochem. 2008;103:1219–1230. doi: 10.1002/jcb.21504. [DOI] [PubMed] [Google Scholar]
- 5.Kim BC, Lee HJ, Park SH, Lee SR, Karpova TS, McNally JG, Felici A, Lee DK, Kim SJ. JAB1/CSN5, a component of the COP9 signalosome, regulates transforming growth factor beta signaling by binding to Smad7 and promoting its degradation. Mol Cell Biol. 2004;24:2251–2262. doi: 10.1128/MCB.24.6.2251-2262.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim JH, Choi JK, Cinghu S, Jang JW, Lee YS, Li YH, Goh YM, Chi XZ, Lee KS, Wee H, Bae SC. JAB1/CSN5 induces the cytoplasmic localization and degradation of RUNX3. J Cell Biochem. 2009;107:557–565. doi: 10.1002/jcb.22157. [DOI] [PubMed] [Google Scholar]
- 7.Tomoda K, Kubota Y, Kato J. Degradation of the cyclin-dependentkinase inhibitor p27Kip1 is instigated by Jab1. Nature. 1999;398:160–165. doi: 10.1038/18230. [DOI] [PubMed] [Google Scholar]
- 8.Tomoda K, Kubota Y, Arata Y, Mori S, Maeda M, Tanaka T, Yoshida M, Yoneda-Kato N, Kato JY. The cytoplasmic shuttling and subsequent degradation of p27Kip1 mediated by JAB1/CSN5 and the COP9 signalosome complex. J Biol Chem. 2002;277:2302–2310. doi: 10.1074/jbc.M104431200. [DOI] [PubMed] [Google Scholar]
- 9.Bae MK, Ahn MY, Jeong JW, Bae MH, Lee YM, Bae SK, Park JW, Kim KR, Kim KW. Jab1 interacts directly with HIF-1alpha and regulates its stability. J Biol Chem. 2002;277:9–12. doi: 10.1074/jbc.C100442200. [DOI] [PubMed] [Google Scholar]
- 10.Bemis L, Chan DA, Finkielstein CV, Qi L, Sutphin PD, Chen X, Stenmark K, Giaccia AJ, Zundel W. Distinct aerobic and hypoxic mechanisms of HIF-alpha regulation by CSN5. Genes Dev. 2004;18:739–744. doi: 10.1101/gad.1180104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishimoto A, Kugimiya N, Hosoyama T, Enoki T, Li TS, Hamano K. JAB1 regulates unphosphorylated STAT3 DNA-binding activity through protein-protein interaction in human colon cancer cells. Biochem Biophys Res Commun. 2013;438:513–518. doi: 10.1016/j.bbrc.2013.07.105. [DOI] [PubMed] [Google Scholar]
- 12.Shackleford TJ, Zhang Q, Tian L, Vu TT, Korapati AL, Baumgartner AM, Le XF, Liao WS, Claret FX. Stat3 and CCAAT/enhancer binding protein beta (C/EBP-beta) regulate Jab1/CSN5 expression in mammary carcinoma cells. Breast Cancer Res. 2011;13:R65. doi: 10.1186/bcr2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu MC, Chang HC, Hung WC. HER-2/neu transcriptionally activates Jab1 expression via the AKT/beta-catenin pathway in breast cancer cells. Endocr Relat Cancer. 2007;14:655–667. doi: 10.1677/ERC-07-0077. [DOI] [PubMed] [Google Scholar]
- 14.Sui L, Dong Y, Watanabe Y, Yamaguchi F, Sugimoto K, Tokuda M. Clinical significance of Skp2 expression, alone and combined with Jab1 and p27 in epithelial ovarian tumors. Oncol Rep. 2006;15:765–771. [PubMed] [Google Scholar]
- 15.Sui L, Dong Y, Ohno M, Watanabe Y, Sugimoto K, Tai Y, Tokuda M. Jab1 expression is associated with inverse expression of p27 (kip1) and poor prognosis in epithelial ovarian tumors. Clin Cancer Res. 2001;7:4130–4135. [PubMed] [Google Scholar]
- 16.Harada K, Kawashima Y, Yoshida H, Sato M. High expression of Jun activation domain-binding protein 1 (Jab1) is a strong prognostic marker in oral squamous cell carcinoma patients treated by UFT in combination with radiation. Anticancer Res. 2006;26:1615–1619. [PubMed] [Google Scholar]
- 17.Dong Y, Sui L, Watanabe Y, Yamaguchi F, Hatano N, Tokuda M. Prognostic significance of Jab1 expression in laryngeal squamous cell carcinomas. Clin Cancer Res. 2005;11:259–266. [PubMed] [Google Scholar]
- 18.Wang Y, Yu YN, Song S, Li TJ, Xiang JY, Zhang H, Lu MD, Ji F, Hu LQ. JAB1 and phospho-Ser10 p27 expression profile determine human hepatocellular carcinoma prognosis. J Cancer Res Clin Oncol. 2014;140:969–978. doi: 10.1007/s00432-014-1646-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He SM, Zhao ZW, Wang Y, Zhao JP, Wang L, Hou F, Gao GD. Potential role of Jun activation domain-binding protein 1 and phosphorylated p27 expression in prognosis of glioma. Brain Tumor Pathol. 2012;29:3–9. doi: 10.1007/s10014-011-0061-1. [DOI] [PubMed] [Google Scholar]
- 20.Sorbye SW, Kilvaer TK, Valkov A, Donnem T, Smeland E, Al-Shibli K, Bremnes RM, Busund LT. Prognostic impact of Jab1, p16, p21, p62, Ki67 and Skp2 in soft tissue sarcomas. PLoS One. 2012;7:e47068. doi: 10.1371/journal.pone.0047068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukumoto A, Ikeda N, Sho M, Tomoda K, Kanehiro H, Hisanaga M, Tsurui Y, Tsutsumi M, Kato JY, Nakajima Y. Prognostic significance of localized p27Kip1 and potential role of Jab1/CSN5 in pancreatic cancer. Oncol Rep. 2004;11:277–284. [PubMed] [Google Scholar]
- 22.Wang F, Wang Y, Yu X, Yang D, Wang Z, Lu C, Yuan Z, Xiao M, Shen A. Significance of Jab1 expression in human esophageal squamous cell carcinoma. J Clin Gastroenterol. 2009;43:520–526. doi: 10.1097/MCG.0b013e3181919245. [DOI] [PubMed] [Google Scholar]
- 23.Osoegawa A, Yoshino I, Kometani T, Yamaguchi M, Kameyama T, Yohena T, Maehara Y. Overexpression of Jun activation domain-binding protein 1 in nonsmall cell lung cancer and its significance in p27 expression and clinical features. Cancer. 2006;107:154–161. doi: 10.1002/cncr.21961. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Fei M, Cheng C, Zhang D, Lu J, He S, Zhao Y, Wang Y, Shen A. Jun activation domain-binding protein 1 negatively regulate p27 kip1 in non-Hodgkin's lymphomas. Cancer Biol Ther. 2008;7:460–467. doi: 10.4161/cbt.7.3.5456. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Xu T, Su B, Wang C, Wang S, Huang H, Pan Y, Wang D, Wei W, Claret FX, Yang H. Molecular markers to assess short-term disease local recurrence in nasopharyngeal carcinoma. Oncol Rep. 2015;33:1418–1426. doi: 10.3892/or.2015.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]