Abstract
Simvastatin is a low density lipoprotein-lowering drug that is widely used to prevent and treat cardiovascular disease by inhibiting the mevalonate pathway. Simvastatin also exhibits inhibitory effects on a number of types of cancer. In the present study, the effects of simvastatin on the activity of doxorubicin in the breast cancer MCF-7 cell line, and the mechanisms by which this interaction occurs were investigated. The effect of simvastatin and doxorubicin treatment, alone and in combination, on the growth of MCF-7 cells was evaluated by a sulforhodamine B and colony formation assay. To delineate the mechanisms of cell death, the following parameters were measured: Reactive oxygen species (ROS) production using the fluorescence probe dihydroethidium; caspase 3 activity by the fluorometry method; gene expression by quantitative polymerase chain reaction; and apoptotic- and proliferative-related protein levels by western blotting. MCF-7 cell proliferation was significantly suppressed by 24–48 h treatment with simvastatin alone. Doses of 10–50 µM simvastatin also enhanced the cytotoxicity of doxorubicin against MCF-7 cells in a dose-dependent manner, and decreased the colony-forming ability of MCF-7 cells. Simvastatin alone or in combination with doxorubicin significantly increased ROS levels. Combination treatment significantly decreased expression of the cell cycle regulatory protein Ras-related C3 botulinum toxin substrate 1 and numerous downstream proteins including cyclin-dependent kinase (Cdk) 2, Cdk4 and Cdk6. Additionally, simvastatin in combination with doxorubicin significantly induced expression of the cyclin-dependent kinase inhibitor p21, increased cytochrome c and caspase 3 expression and reduced cyclin D1 expression. In conclusion, simvastatin acts synergistically with the anticancer drug doxorubicin against MCF-7 cells, possibly through a downregulation of the cell cycle or induction of apoptosis. Although additional studies are required, simvastatin and doxorubicin combination may be a reasonable regimen for the treatment of breast cancer.
Keywords: simvastatin, doxorubicin, breast cancer, Ras-related C3 botulinum toxin substrate 1, caspase 3
Introduction
Breast cancer is the most common type of malignancy, and the leading cause of cancer-associated female mortality, in a number of countries (1,2) Chemotherapy with or without resection of the tumor is the only known treatment strategy for long-term survival, and survival is limited with standard chemotherapeutic options (3). Breast cancer cells exhibit intrinsic and acquired resistance to numerous anticancer drugs (4), this is a major problem for the effective treatment of breast cancer (5). Improved treatment protocols and alternative chemotherapeutic strategies are therefore required.
Statins competitively inhibit 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme in cholesterol biosynthesis (6), and are used to treat hyperlipidemia by reducing serum lipids such as cholesterol and triglycerides (7,8). This activity, combined with their protective effects on the blood vessels and heart, allows statins to be used in the treatment and prevention of cardiovascular events (9,10). In addition, statins perform roles in immune regulation (11), the inhibition of inflammation (12) and the modulation of angiogenesis (13). Statins also exhibit anticancer activities (14,15), decrease cellular proliferation (15–17) and induce apoptosis (15,18,19) in breast, colorectal, lung, prostate and pancreatic cancer (20). Notably, statins inhibit cancer cell growth in vivo and decrease metastasis at clinically therapeutic doses (21).
The suppression of HMG-CoA reductase results in the reduction of several important cholesterol intermediates, including mevalonate, geranylgeranyl pyrophosphate (GGPP) and farnesyl pyrophosphate (FPP) (22). These proteins are necessary for the post-translational modification of intracellular G-proteins, including Rho, ras-related C3 botulinum toxin substrate (Rac) and Ras, which regulate cellular mechanisms including cytoskeletal reorganization and cellular transformation, migration, invasion and proliferation (23). Statins activate cancer cell death, including apoptosis, via a reduction of GGPP and FPP levels. Additionally, Ras-related C3 botulinum toxin substrate 1 (RAC1) regulates and modulates several signaling pathways that control cellular proliferation (24), and direct inhibition of RAC1 activity or gene expression induces cell cycle arrest and apoptosis in breast cancer cells (25).
In clinical studies, the role of statins in cancer treatment remains debatable, and appears to be dependent on the molecular identity of the type of cancer. In order to better understand the interaction between statins and breast cancer cells, the ability of simvastatin to potentiate the doxorubicin-induced inhibition of cellular proliferation and apoptosis using the MCF-7 cancer cell line was investigated. It was hypothesized that simvastatin sensitizes MCF-7 cells to doxorubicin, and may be a viable strategy for improving the efficacy of other anticancer drugs against breast cancer.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum and other cell culture reagents were purchased from Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Simvastatin (cat. no. s6196), doxorubicin (cat. no. D1515), protease inhibitor cocktail (cat. no. P8340), dihydroethidium (DHE; cat. no. D7008), radioimmunoprecipitation assay (RIPA) lysis buffer (cat. no. R0278), sulforhodamine B (SRB; cat. no. s1402) and a caspase 3 activity assay kit (cat. no. CASP3F-1KT) were obtained from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). The primary antibodies against cyclin-dependent kinase inhibitor 1 (p21; cat. no. 2947), caspase 3 (cat. no. 9662), cytochrome c (cat. no. 4272), cyclin D1 (cat. no. 2922), β-actin (cat. no. 4967) and the secondary anti-rabbit immunoglobulin G horseradish peroxidase (HRP)-linked antibody (cat. no. 7074) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). iScript reverse transcription Supermix for reverse transcription quantitative polymerase chain reaction (RT-qPCR; cat. no. 170-8841) and SsoFast EvaGreen Supermix (cat. no. 172-5200) were supplied by Bio-Rad Laboratories, Inc. (Hercules, CA, USA).
Cell line and cell culture
The human breast cancer MCF-7 cell line was obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA) and maintained according to ATCC's recommendations at 37°C and 5% CO2 in DMEM medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin G and 100 µg/ml streptomycin. The DMEM media was renewed every 2–3 days, trypsinized with 0.25% trypsin-EDTA and subcultured in the same media.
Cell viability assay
The SRB assay was used to determine the effect of simvastatin and doxorubicin, alone and in combination, on the viability of MCF-7 cells. A 96-well plate was seeded with 1×104 MCF-7 cells/well and incubated for 24 h at 37°C. Subsequent to exposure to 0–100 µM simvastatin for 24–48 h, 0–10 µM doxorubicin for 24–48 h and in combination (cells were treated with 0–100 µM simvastatin with or without 1 µM doxorubicin for 24 h) at 37°C, the cultured cells were fixed with ice-cold 10% trichloroacetic acid and stained with 0.4% SRB for 30 min at room temperature. Excess dye was removed by rinsing several times with 1% acetic acid, and protein-bound dye was dissolved with 200 µl 10 mM Tris base solution for the determination of absorbance with a microplate reader with a filter wavelength of 540 nm.
Colony formation assay
Approximately 800 MCF-7 cells were seeded in 6-well plates and allowed to grow for 24 h at 37°C. The cells were then treated with 0–50 µM simvastatin and in combination of 0–50 µM simvastatin with or without 0.5 µM doxorubicin treatment for 24 h at 37°C. Following this, the cells were washed with PBS and fresh medium was added. The cells were then grown for another 14 days. Subsequently, the DMEM medium was discarded, the cells were washed with PBS buffer three times, fixed with 100% methanol at −20°C for 1 h, stained with 0.5% crystal violet in 100% methanol for 1 h at room temperature, washed with tap water, and the colonies were then viewed and captured using a digital camera (Nikon D3100, Nikon Corporation, Tokyo, Japan). Colonies containing >50 individual cells were counted using Image-Pro Plus software version 2.0 (Media Cybernetics, Inc., Rockville, MD, USA).
Reactive oxygen species (ROS) production assay
Intracellular ROS generation was measured using the cell-permeable fluorescent probe, DHE. Black 96-well plates were seeded with ~1×104 MCF-7 cells/well and incubated for 24 h at 37°C. The medium was discarded and the cells were washed with PBS. The cells were then treated with 0–50 µM of simvastatin alone, or 50 µM simvastatin in combination with 1 µM doxorubicin, for 90 min. The cells were then assessed for ROS production by incubation with 25 µM DHE in serum-free medium, in a 5% CO2 atmosphere, at 37°C, for 90 min, in the dark. The fluorescence intensity was measured at a 518 nm excitation and 605 nm emission wavelength on a fluorescence microplate reader. The data were expressed as the percentage of ROS relative to the untreated controls.
Caspase 3 activity assay
Caspase 3 activity was measured using fluorimetric assay kits (Sigma-Aldrich; Merck KGaA) according to the manufacturer's protocol. Subsequent to treatment with the test compounds for 24 h, the medium was removed, the cells were trypsinized and the cell pellet was lysed with cell lysis buffer on ice for 10 min. The lysed pellet was then centrifuged (10,000 × g, 4°C, 30 min), and protein concentrations were measured with Bradford's reagent (Bio-Rad Laboratories, Inc.), using albumin as a standard. Briefly, 50 µl of cell protein or the albumin standard was mixed with 200 µl Bradford reagent and incubated for 15 min at room temperature in the dark. The absorbance at 620 nm was measured with a spectrophotometer, and the protein concentration was calculated using a standard. A total of 5 µl cell lysates (0.5 mg/ml) were added to 195 µl of buffer containing an Ac-DEVD-7-amino-4-methylcoumarin (AMC)-conjugated substrate for caspase (Sigma-Aldrich; Merck KGaA). This was followed by 90 min incubation at 37°C in the dark. The concentration of the released AMC was calculated from the fluorescence intensity, which was read using a fluorescence plate reader with the excitation and emission wavelengths of 360 and 460 nm, respectively, and using AMC standard to calculate caspase 3 activity. Data were adjusted according to the protein content.
Gene expression assay
The MCF-7 cells were seeded in 6 well-plates and allowed to grow for 24 h. Cells were treated with the test compounds, and RNA was isolated using TRIzol® reagent according to the manufacturer's protocol (Sigma-Aldrich; Merck KGaA). Recovered RNA was quantified by using a spectrophotometer to measure the 260/280 nm absorbance ratio. Complementary DNA (cDNA; 1 µg) was prepared by reverse transcription of isolated RNA using the iScript Reverse Transcription Supermix for RT-qPCR. PCR amplification was performed using primers specific for RAC1, cdk2, cdk4 and cdk6, and using β-actin (ACTB) as an internal control. The PCR primer sequences were as follows: RAC1 (GenBank accession no. NM_018890) forward, 5′ATG-TCC-GTG-CAA-AGT-GGT-ATC3′ and reverse, 5′CTC-GGA-TCG-CTT-CGT-CAA-ACA3′; Cdk2 (GenBank accession no. NM_001798) forward, 5′CCA-GGA-GTT-ACT-TCT-ATG-CCT-GA3′ and reverse, 5′TTC-ATC-CAG-GGG-AGG-TAC-AAC3′; Cdk4 (GenBank accession no. NM_000075) forward, 5′ATG-GCT-ACC-TCT-CGA-TAT-GAG-C3′ and reverse, 5′CAT-TGG-GGA-CTC-TCA-CAC-TCT3′; Cdk6 (GenBank accession no. NM_001145306) forward, 5′GCT-GAC-CAG-CAG-TAC-GAA-TG3′ and reverse, 5′GCA-CAC-ATC-AAA-CAA-CCT-GAC-C3′; ACTB (GenBank accession no. NM_001101) forward, 5′CAT-GTA-CGT-TGC-TAT-CCA-GGC3′ and reverse, 5′CTC-CTT-AAT-GTC-ACG-CAC-GAT3′.
qPCR was carried out in a final reaction volume of 20 µl containing SYBR Green PCR Master mix (Bio-Rad Laboratories, Inc.), 0.5 µM of each target gene and the internal control. The expression of each gene was monitored using an Applied Biosystems® StepOne™ real-time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.) with the following conditions: Denaturation at 95°C for 3 min, then amplification by cycling 40 times at 95°C for 15 sec and 60°C for 30 sec. The differences in gene expression levels were calculated using the 2−ΔΔCq method for relative quantification (26), and expressed as the fold change relative to the untreated control. Data from 3 independent experiments were normalized to the expression of ACTB mRNA, which included on the same PCR array plate as the target genes.
Protein extraction and western blot analysis
The MCF-7 cells were lyzed with RIPA lysis buffer for 30 min on ice. The lysates were collected, and the protein concentrations were determined using Bradford's reagent, as described in the caspase 3 activity assay. A total of 20 µg protein was separated by 12% SDS-PAGE, and transferred to an Immobilon® polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA, USA). The blots were blocked for 2 h at room temperature with 5% (w/v) skimmed milk in Tris buffered saline containing 0.1% Tween-20 (TBST). The membrane was probed with each primary antibody at 4°C overnight (dilution, 1:1,000). Subsequent to washing with TBST, the blots were incubated with the HRP-conjugated secondary antibody for 2 h at room temperature (dilution, 1:2,500). The immunoactive bands were detected using an Enhanced Clarity™ Western enhanced chemiluminescence substrate (Bio-Rad Laboratories, Inc.). Images of the specific protein bands were captured and analyzed using the ImageQuant™ LAS-4000 and Image Gauge version 3.1 (GE Healthcare Life Sciences, Chalfont, UK).
Statistical analysis
Statistical comparison between the control and treatment groups was analyzed with an unpaired Student's t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of simvastatin and doxorubicin on MCF-7 cellular viability and colony formation efficacy
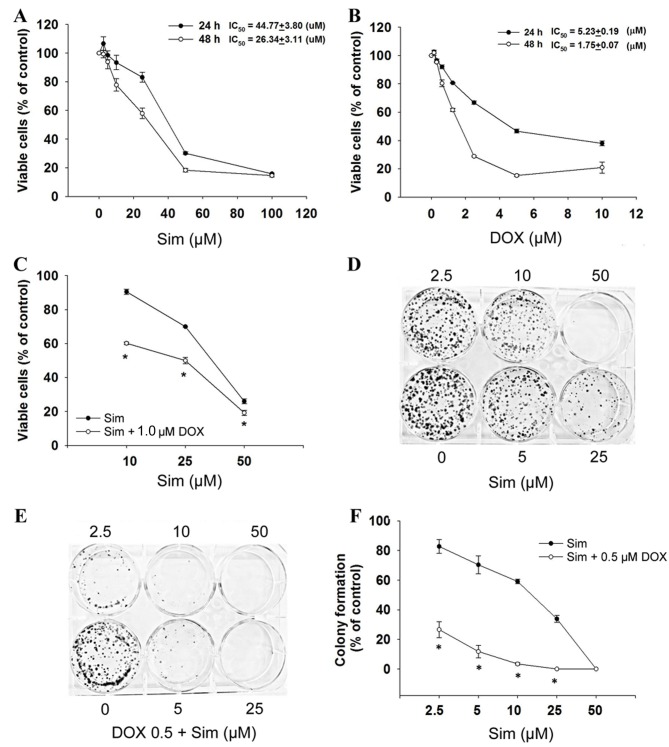
To evaluate the cytotoxicity of simvastatin with or without doxorubicin, the MCF-7 cells were exposed to simvastatin and doxorubicin and assessed for viability by the SRB assay. The results demonstrated that cell growth was inhibited after 24 and 48 h of treatment, with simvastatin IC50 values of 44.8±3.8 µM at 24 h and 26.3±3.1 µM at 48 h (P<0.05, Fig. 1A), and doxorubicin IC50 values of 5.2±0.2 µM at 24 h and 1.8±0.1 µM at 48 h (P<0.05, Fig. 1B) in a dose- and time-dependent manner. The combination of simvastatin and doxorubicin significantly enhanced cytotoxicity (Fig. 1C).
Figure 1.
Effects of sim and DOX on MCF-7 cell growth. (A) Cells were treated with 0–100 µM sim, (B) 0–10 µM DOX for 24–48 h or (C) 10–50 µM sim combined with 1 µM DOX for 24 h. Subsequent to treatment, cell numbers were determined by the sulforhodamine B assay. Results are presented as the percentage of the control. Cells were then grown in 6-well plates and treated with (D) 0–50 µM sim or (E and F) 0–50 µM sim with or without 0.5 µM DOX, for 24 h. After 14 days, cells were stained with 0.5% crystal violet, imaged and counted. Figures are representative of 3 independent experiments. The graph shows percentage colony formation relative to the control. Data are presented as the mean ± standard deviation calculated from 3 independent experiments. *P<0.05 vs. control. Sim, simvastatin; DOX, doxorubicin.
To determine the effect of simvastatin and doxorubicin on the longer-term viability and replicative potential of the MCF-7 cells, a colony formation assay was used. Treatment with simvastatin alone caused a dose-dependent decrease in the colony forming ability of the MCF-7 cells (Fig. 1D). When simvastatin and doxorubicin were used in combination, colony formation was significantly reduced compared with simvastatin treatment alone (Fig. 1E and F; P<0.05). These results indicated that simvastatin enhances the activity of doxorubicin in breast cancer cells, and prompted the investigation of the mechanism(s) by which this increase in activity occurs.
Effects of simvastatin and doxorubicin on RAC1 and downstream gene expression
To investigate whether simvastatin enhances the effects of doxorubicin on the cell cycle regulator RAC1, mRNA expression of RAC1, cdk2, cdk4 and cdk6 were measured. The treatment of cells with a combination of simvastatin and doxorubicin decreased RAC1 mRNA expression significantly more than treatment with simvastatin or doxorubicin alone (Fig. 2A; P<0.05). Cdk2 mRNA expression was reduced by simvastatin and doxorubicin individually; however, there was no additive effect when the two were used in combination (Fig. 2B; P<0.05). Notably, it was observed that simvastatin or doxorubicin treatment significantly reduced cdk4 and cdk6, and that the combination treatment exhibited an additive effect when compared with simvastatin or doxorubicin treatment alone (Fig. 2C and D; P<0.05).
Figure 2.
Effects of sim and DOX on the gene expression of (A) RAC1, (B) Cdk2, (C) Cdk4 and (D) Cdk6 in MCF-7 cells. Cells were treated with 50 µM sim and 1 µM of DOX, alone and in combination for 24 h, and total RNA was prepared and analyzed by quantitative polymerase chain reaction. Data are presented as the mean ± standard deviation, calculated from 3 independent experiments. *P<0.05 vs. untreated control group; #P<0.05 vs. DOX group; †P<0.05 vs. sim group. Sim, simvastatin; DOX, doxorubicin; RAC1, Ras-related C3 botulinum toxin substrate 1; Cdk, cyclin-dependent kinase.
Effects of simvastatin and doxorubicin on ROS and caspase 3 activity
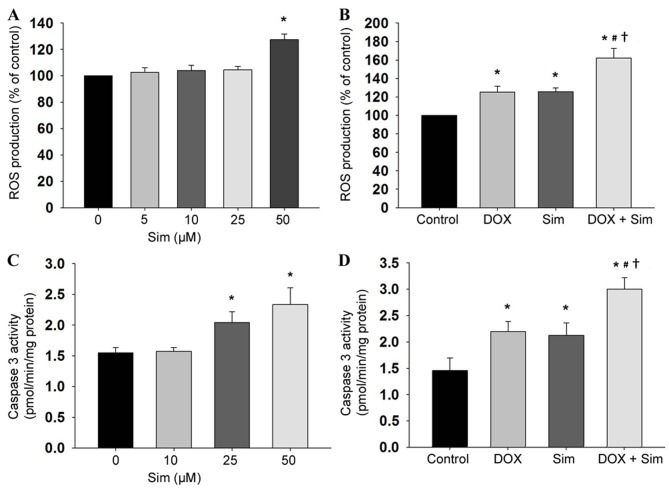
To establish the mechanism of action by which simvastatin sensitizes MCF-7 cells to doxorubicin, the intracellular accumulation of ROS was also monitored by the DHE-enhanced chemiluminescence method. Simvastatin induced intracellular ROS production in the MCF-7 cells (Fig. 3A, P<0.05), and cells treated with simvastatin in combination with doxorubicin demonstrated higher ROS production compared with those treated with simvastatin or doxorubicin alone (Fig. 3B, P<0.05). The involvement of mitochondria in simvastatin- and doxorubicin-induced cytotoxicity was investigated by measuring the extent of caspase 3 activity. The results revealed that treatment with simvastatin increased caspase 3 activity (Fig. 3C, P<0.05), and that the combination of simvastatin and doxorubicin increased caspase 3 activity, to a greater extent than treatment with simvastatin alone (Fig. 3D, P<0.05).
Figure 3.
Effects of sim and DOX on ROS production and caspase 3 activity in MCF-7 cells. Cells were seeded in black 96-well culture plates for 24 h. Cells were then treated with (A) 0–50 µM sim or (B) 50 µM sim in combination with 1 µM DOX for 90 min and assessed for ROS production using 25 µM dihydroethidium. Cells were seeded, and treated (C) with sim doses 0–50 µM or (D) sim with, or without, 1 µM DOX, for 24 h. The total cell lysates were assayed for caspase 3 activity using specific fluorogenic Ac-DEVD-7-amino-4-methylcoumarin substrates for caspase 3. Data are presented as the mean ± standard deviation, calculated from 3 independent experiments. *P<0.05 vs. untreated control group; #P<0.05 vs. DOX group; †P<0.05 vs. sim group. Sim, simvastatin; DOX, doxorubicin; ROS, reactive oxygen species.
Effects of simvastatin and doxorubicin on protein-related apoptosis
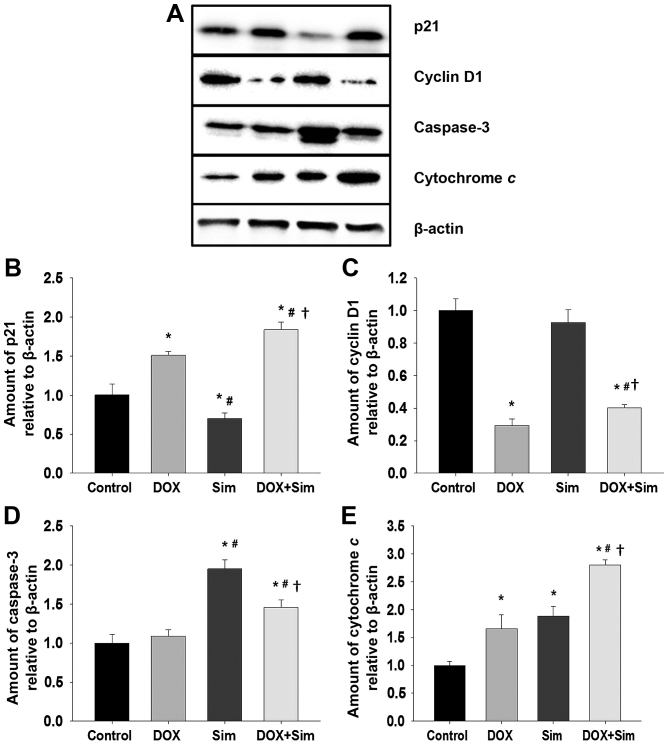
To understand how simvastatin may enhance doxorubicin cytotoxicity in MCF-7 cells, the level of proteins associated with cell survival and apoptosis, including p21, cyclin D1, caspase 3 and cytochrome c, were assessed with western blotting (Fig. 4A-E).
Figure 4.
Effects of sim and DOX on the level of proteins associated with growth and apoptosis. MCF-7 cells were treated with 50 µM sim or 1 µM DOX, individually or combined. Whole cell extracts were prepared and subjected to western blot analysis. (A) The western blot was used to compare the relative protein expression of (B) p21, (C) cyclin D1, (D) caspase 3 and (E) cytochrome c. The levels of each target protein were normalized to the loading control, β-actin. Data is displayed as the mean of 3 independent experiments ± standard error of the mean. *P<0.05 vs. untreated control group; #P<0.05 vs. DOX group; †P<0.05 vs. sim group. Sim, simvastatin; DOX, doxorubicin.
The results revealed that the level of p21 was significantly altered in all treatment groups; p21 was increased following treatment with doxorubicin, decreased following treatment with simvastatin and increased by the combination of doxorubicin with simvastatin, to a greater extent than treatment with doxorubicin or simvastatin alone (Fig. 4B; P<0.05). Cyclin D1 levels did not change significantly following treatment with simvastatin; however, treatment with doxorubicin or the combination significantly suppressed cyclin D1 levels (Fig. 4C; P<0.05). Caspase 3 levels were not significantly altered by treatment with doxorubicin alone; however, they were significantly increased following treatment with simvastatin or the combination treatment (Fig. 4D; P<0.05) Cytochrome c protein levels were significantly increased in all treatment groups. The doxorubicin and simvastatin combination had a significantly greater effect on cytochrome c levels than doxorubicin or simvastatin alone (Fig. 4E; P<0.05).
The combination of doxorubicin with simvastatin stimulated a significant increase in p21, cytochrome c and caspase 3 protein levels, and a significant reduction in cyclin D1 protein level. It was also associated with a marked increase in MCF-7 cell death, confirming the potentiating effect of simvastatin upon doxorubicin treatment of breast cancer cells.
Discussion
Simvastatin is one of the most frequently prescribed drugs due to the efficacy and low toxicity when used to treat hyperlipidemia (27). Previously, statins have been identified to reduce proliferation and induce apoptosis in several cancer cells (28). In the present study, the mechanisms by which simvastatin reduces the rates of cell proliferation and increases the rate of cell death in the doxorubicin-treated breast cancer line MCF-7 cell line were investigated. The results suggest that simvastatin potentiation of doxorubicin-induced cell death is accompanied by a suppression of the cell cycle regulator protein RAC1 signaling pathway. The enhanced cytotoxicity of the combination treatment is possibly due to an induction in intracellular ROS formation, leading to increased levels of p21, cytochrome c, and caspase 3 and decreased cyclin D1 levels. This may be associated with the mechanism for MCF-7 breast cancer cell sensitization to anticancer drugs and the induction of cell death. Therefore, simvastatin may potentially be used in the prevention and treatment of breast cancer.
Statins or HMG-CoA reductase inhibitors are drugs commonly used for the treatment of hypercholesterolemia. Additionally, statins exhibit a number of effects on cancer cells (29) including inhibition of cancer cell growth, metastasis and invasion, angiogenesis and the induction of apoptosis. By inhibiting the mevalonic acid pathway, statins may reduce the levels of the isoprenoid intermediates FPP and GGPP (22). These intermediates are critical for post-translational modification of the intracellular G-proteins, including Rho, Rac, and Ras, which regulate the signal transduction of several proteins. These proteins, in turn, are essential for the gene transcription involved in cellular proliferation, differentiation and apoptosis (29). RAC1 is overexpressed in a number of tumors and serves a critical role in cytoskeleton reorganization, cell migration and cell survival (24). An overexpression of RAC1 is associated with the progression, including the metastasis and staging, of human breast cancer (30).
A role for RAC1 in the activation of extracellular signal-regulated kinases (ERK) 1/2 and phosphoinositide 3-kinase/protein kinase B pro-survival signaling was also identified and demonstrated to promote cell survival (30,31). The direct suppression of RAC1 activity induces apoptosis and cell cycle arrest in breast cancer cells (25). In the present study, simvastatin significantly inhibited breast cancer cell proliferation with IC50 values in the low micromolar range. This result is concomitant with the data of previous studies (16,18). Simvastatin stimulates cell cycle arrest and apoptosis in a number of cancer cell types (32) via the intracellular signaling mechanisms of RAC1 and the associated downstream pathway. Cell cycle progression is controlled by Cdk activity (33). The cyclin D1-Cdk4/6 complex also promotes G1 phase cell-cycle progression by modulating the Cdk inhibitor p21 (34,35). RAC1 suppression may inhibit the cyclin-Cdk complex, leading to the activation of p21 and inhibition of cellular proliferation.
The results of the present study indicate that treatment with simvastatin or doxorubicin alone significantly inhibits RAC1, Cdk4 and Cdk6 mRNA expression after 24 h, whilst combination of the two drugs results in increased activity. Cdk2 is also inhibited by treatment with simvastatin or doxorubicin alone, but in combination this inhibitory effect is not greater compared with that observed with doxorubicin alone. Similar to these observations, a previous study revealed that a blockade of RAC1 activity induces cell cycle arrest or apoptosis in breast cancer cells (25).
In addition to inhibiting the proliferation of MCF-7 cells, the ability of simvastatin to induce apoptosis has been demonstrated. Simvastatin-induced apoptosis was characterized by increased levels of caspase 3, cytochrome c and intracellular ROS. Several studies have revealed that ROS are key signaling molecules in mammalian cells. An accumulation of ROS is directly correlated with mitochondrial dysfunction and promotion of cell apoptosis (36). The results of the present study suggest that simvastatin induced ROS production in a concentration-dependent manner. In combination simvastatin and doxorubicin generated even larger quantities of ROS, a result indicative of an additive effect. This is potentially a key reason why MCF-7 cell apoptosis is induced by simvastatin and doxorubicin. The observation of the present study that a combination of simvastatin and doxorubicin increased cytochrome c protein expression and caspase 3 activity more compared with each drug individually is consistent with the hypothesis that simvastatin sensitizes MCF-7 cells.
The present study identified that simvastatin inhibited MCF-7 cell proliferation and colony formation in a dose-dependent manner and, notably, that it enhanced the activity of doxorubicin. The effect on cell cycle progression was also investigated in the present study by measuring p21 and cyclin D1 protein expression. Simvastatin and doxorubicin treatment resulted in an increase in p21, and a decrease in cyclin D1 expression level. These data suggest that simvastatin enhances doxorubicin-induced cancer cell death by inhibiting cell cycle progression (37).
In conclusion, the present study has demonstrated that simvastatin enhances doxorubicin cytotoxicity towards MCF-7 cells through an inhibition of the RAC1 pathway and induction of caspase- and cytochrome c-dependent apoptosis in a process involving oxidative stress. These data also reveal that the Cdk inhibitor p21 is activated in the process of simvastatin-induced cell death, leading to an inhibition of cell cycle progression. These results contribute to the current understanding of the molecular mechanisms of simvastatin, and provide a basis for future studies seeking to validate the mevalonate pathway as a novel therapeutic target. The inclusion of statins in anticancer treatment regimens may potentially reduce the quantity of anticancer drugs required to achieve therapeutic effects and thereby reduce the side effects associated with cancer treatment.
Acknowledgements
The present study was supported by the Office of the Higher Education Commission (grant no., 2558A10962234), a grant from the Mahasarakham University (MSU) Faculty of Medicine, a Mahasarakham University 2016 Thailand Research Fund (Grant no. TRG5780254) and the National Research Council of Thailand (Grant no. 2559A10902073). The authors would like to thank Dr. Tim Cushnie (Faculty of Medicine, Mahasarakham University, Thailand) for language-editing the manuscript.
References
- 1.Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, Mandelblatt JS, Yakovlev AY, Habbema JD, Feuer EJ. Cancer Intervention and Surveillance Modeling Network (CISNET) Collaborators: Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Pagani O, Senkus E, Wood W, Colleoni M, Cufer T, Kyriakides S, Costa A, Winer EP, Cardoso F. ESO-MBC Task Force: International guidelines for management of metastatic breast cancer: Can metastatic breast cancer be cured? J Natl Cancer Inst. 2010;102:456–463. doi: 10.1093/jnci/djq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Driscoll L, Clynes M. Biomarkers and multiple drug resistance in breast cancer. Curr Cancer Drug Targets. 2006;6:365–384. doi: 10.2174/156800906777723958. [DOI] [PubMed] [Google Scholar]
- 5.Coley HM. Mechanisms and strategies to overcome chemotherapy resistance in metastatic breast cancer. Cancer Treat Rev. 2008;34:378–390. doi: 10.1016/j.ctrv.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Istvan ES, Deisenhofer J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science. 2001;292:1160–1164. doi: 10.1126/science.1059344. [DOI] [PubMed] [Google Scholar]
- 7.Hoeg JM, Brewer HB., Jr 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors in the treatment of hypercholesterolemia. JAMA. 1987;258:3532–3536. doi: 10.1001/jama.1987.03400240064025. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe Y, Ito T, Shiomi M, Tsujita Y, Kuroda M, Arai M, Fukami M, Tamura A. Preventive effect of pravastatin sodium, a potent inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase, on coronary atherosclerosis and xanthoma in WHHL rabbits. Biochim Biophys Acta. 1988;960:294–302. doi: 10.1016/0005-2760(88)90037-9. [DOI] [PubMed] [Google Scholar]
- 9.Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, Langendorfer A, Stein EA, Kruyer W, Gotto AM., Jr Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: Results of AFCAPS/TexCAPS. Air Force/Texas coronary atherosclerosis prevention study. JAMA. 1998;279:1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 10.Khush KK, Waters DD. Effects of statin therapy on the development and progression of heart failure: Mechanisms and clinical trials. J Card Fail. 2006;12:664–674. doi: 10.1016/j.cardfail.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Qi XF, Kim DH, Yoon YS, Li JH, Jin D, Teng YC, Kim SK, Lee KJ. Fluvastatin inhibits expression of the chemokine MDC/CCL22 induced by interferon-gamma in HaCaT cells, a human keratinocyte cell line. Br J Pharmacol. 2009;157:1441–1450. doi: 10.1111/j.1476-5381.2009.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blum A, Shamburek R. The pleiotropic effects of statins on endothelial function, vascular inflammation, immunomodulation and thrombogenesis. Atherosclerosis. 2009;203:325–330. doi: 10.1016/j.atherosclerosis.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 13.Elewa HF, El-Remessy AB, Somanath PR, Fagan SC. Diverse effects of statins on angiogenesis: New therapeutic avenues. Pharmacotherapy. 2010;30:169–176. doi: 10.1592/phco.30.2.169. [DOI] [PubMed] [Google Scholar]
- 14.Cai JP, Chen W, Hou X, Liang LJ, Hao XY, Yin XY. Simvastatin enhances the chemotherapeutic efficacy of S-1 against bile duct cancer: E2F-1/TS downregulation might be the mechanism. Anticancer Drugs. 2013;24:1020–1029. doi: 10.1097/CAD.0b013e328364f935. [DOI] [PubMed] [Google Scholar]
- 15.Kamigaki M, Sasaki T, Serikawa M, Inoue M, Kobayashi K, Itsuki H, Minami T, Yukutake M, Okazaki A, Ishigaki T, et al. Statins induce apoptosis and inhibit proliferation in cholangiocarcinoma cells. Int J Oncol. 2011;39:561–568. doi: 10.3892/ijo.2011.1087. [DOI] [PubMed] [Google Scholar]
- 16.Shen Y, Du Y, Zhang Y, Pan Y. Synergistic effects of combined treatment with simvastatin and exemestane on MCF-7 human breast cancer cells. Mol Med Rep. 2015;12:456–462. doi: 10.3892/mmr.2015.3406. [DOI] [PubMed] [Google Scholar]
- 17.Spampanato C, De Maria S, Sarnataro M, Giordano E, Zanfardino M, Baiano S, Cartenì M, Morelli F. Simvastatin inhibits cancer cell growth by inducing apoptosis correlated to activation of Bax and down-regulation of BCL-2 gene expression. Int J Oncol. 2012;40:935–941. doi: 10.3892/ijo.2011.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller T, Yang F, Wise CE, Meng F, Priester S, Munshi MK, Guerrier M, Dostal DE, Glaser SS. Simvastatin stimulates apoptosis in cholangiocarcinoma by inhibition of Rac1 activity. Dig Liver Dis. 2011;43:395–403. doi: 10.1016/j.dld.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Werner M, Sacher J, Hohenegger M. Mutual amplification of apoptosis by statin-induced mitochondrial stress and doxorubicin toxicity in human rhabdomyosarcoma cells. Br J Pharmacol. 2004;143:715–724. doi: 10.1038/sj.bjp.0705928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sassano A, Platanias LC. Statins in tumor suppression. Cancer Lett. 2008;260:11–19. doi: 10.1016/j.canlet.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 21.Kusama T, Mukai M, Iwasaki T, Tatsuta M, Matsumoto Y, Akedo H, Inoue M, Nakamura H. 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitors reduce human pancreatic cancer cell invasion and metastasis. Gastroenterology. 2002;122:308–317. doi: 10.1053/gast.2002.31093. [DOI] [PubMed] [Google Scholar]
- 22.Wong WW, Dimitroulakos J, Minden MD, Penn LZ. HMG-CoA reductase inhibitors and the malignant cell: The statin family of drugs as triggers of tumor-specific apoptosis. Leukemia. 2002;16:508–519. doi: 10.1038/sj.leu.2402476. [DOI] [PubMed] [Google Scholar]
- 23.Jaffe AB, Hall A. Rho GTPases: Biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 24.Bosco EE, Mulloy JC, Zheng Y. Rac1 GTPase: A “Rac” of all trades. Cell Mol Life Sci. 2009;66:370–374. doi: 10.1007/s00018-008-8552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshida T, Zhang Y, Rosado Rivera LA, Chen J, Khan T, Moon SY, Zhang B. Blockade of Rac1 activity induces G1 cell cycle arrest or apoptosis in breast cancer cells through downregulation of cyclin D1, survivin, and X-linked inhibitor of apoptosis protein. Mol Cancer Ther. 2010;9:1657–1668. doi: 10.1158/1535-7163.MCT-09-0906. [DOI] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 28.Martirosyan A, Clendening JW, Goard CA, Penn LZ. Lovastatin induces apoptosis of ovarian cancer cells and synergizes with doxorubicin: Potential therapeutic relevance. BMC Cancer. 2010;10:103. doi: 10.1186/1471-2407-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hindler K, Cleeland CS, Rivera E, Collard CD. The role of statins in cancer therapy. Oncologist. 2006;11:306–315. doi: 10.1634/theoncologist.11-3-306. [DOI] [PubMed] [Google Scholar]
- 30.Schnelzer A, Prechtel D, Knaus U, Dehne K, Gerhard M, Graeff H, Harbeck N, Schmitt M, Lengyel E. Rac1 in human breast cancer: Overexpression, mutation analysis, and characterization of a new isoform, Rac1b. Oncogene. 2000;19:3013–3020. doi: 10.1038/sj.onc.1203621. [DOI] [PubMed] [Google Scholar]
- 31.Eblen ST, Slack JK, Weber MJ, Catling AD. Rac-PAK signaling stimulates extracellular signal-regulated kinase (ERK) activation by regulating formation of MEK1-ERK complexes. Mol Cell Biol. 2002;22:6023–6033. doi: 10.1128/MCB.22.17.6023-6033.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saito A, Saito N, Mol W, Furukawa H, Tsutsumida A, Oyama A, Sekido M, Sasaki S, Yamamoto Y. Simvastatin inhibits growth via apoptosis and the induction of cell cycle arrest in human melanoma cells. Melanoma Res. 2008;18:85–94. doi: 10.1097/CMR.0b013e3282f60097. [DOI] [PubMed] [Google Scholar]
- 33.Sherr CJ, Roberts JM. CDK inhibitors: Positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 34.Fournier AK, Campbell LE, Castagnino P, Liu WF, Chung BM, Weaver VM, Chen CS, Assoian RK. Rac-dependent cyclin D1 gene expression regulated by cadherin- and integrin-mediated adhesion. J Cell Sci. 2008;121:226–233. doi: 10.1242/jcs.017012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gartel AL, Tyner AL. The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer Ther. 2002;1:639–649. [PubMed] [Google Scholar]
- 36.D'Autréaux B, Toledano MB. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 37.Sadeghi-Aliabadi H, Minaiyan M, Dabestan A. Cytotoxic evaluation of doxorubicin in combination with simvastatin against human cancer cells. Res Pharm Sci. 2010;5:127–133. [PMC free article] [PubMed] [Google Scholar]