Abstract
Cancer stem cells are enriched in triple-negative breast cancer (TNBC) tumor tissues, which present strong capacities of proliferation and tumorigenicity. The present study detected the distribution of cancer stem cell markers cluster of differentiation (CD)44/CD24 and analyzed the clinical outcomes of different CD44/CD24 phenotypes in patients with TNBC. Multivariate Cox regression analyses were performed with regard to the prognostic value of cancer stem cell markers CD44/CD24, aldehyde dehydrogenase 1 and other baseline clinical characteristics, including tumor size, lymph node involved, adjuvant chemotherapy, Ki-67, breast cancer susceptibility gene 1, cellular tumor antigen p53, vimentin and basal-like status. The multivariate analyses showed that three of these factors, CD44/CD24 phenotype, basal-like status and number of lymph nodes involved, had an impact on overall survival. Furthermore, patients with CD44+/CD24− phenotype, basal-like tumors and ≥4 lymph nodes involved had a significantly worse prognosis. The expression of CD44 and CD24 was detected by double-staining immunohistochemistry, which can locate cancer stem cells individually. Overall, the present results indicated that CD44/CD24 status evaluated by double-staining immunohistochemistry constitutes an independent prognostic factor for TNBC.
Keywords: triple-negative breast cancer, cancer stem cells, CD44, CD24, prognosis
Introduction
Breast cancer is a highly histological heterogeneous disease comprised of several biologically different phenotypes (1). One of these subtypes, triple-negative breast cancer (TNBC), is defined by the lack of expression of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) in the tumor specimen, and comprises 10–20% of all diagnosed breast cancer cases (2,3). Patients diagnosed with TNBC normally have a younger age, higher grade and higher rate of cellular tumor antigen p53 (p53) mutation when compared with those of other breast cancer subtypes (4). Furthermore, due to the lack of hormone receptor and HER2 targets, patients with TNBC do not have access to targeted therapy or adjuvant endocrine treatment. Thus, they have a higher incidence of early local recurrence or distant organ metastases (5). Therefore, novel methods to improve the prognosis of TNBC are urgently required.
According to the cancer stem cell hypothesis (6), cancer stem cells are considered as the source of malignancy, invasion and metastasis. In preclinical study, the cancer stem cell subpopulation is defined by two key characteristics, namely, self-renewal and multi-directional differentiation (7). A previous study by Al-hajj et al demonstrated that as few as 100 epithelial-specific antigen (ESA+)/lineage (Lin−)/cluster of differentiation (CD)44+/CD24− breast cancer cells were able to serially reproduce tumors when transplanted into immunodeficient mice, whereas 200-fold more cells without these surface markers did not possess tumorigenic potential; i.e., these breast cancer stem cells had properties of self-renewal and longevity (8). In addition, the key cell surface markers that can isolate the cancer stem cell subpopulation are CD44+/CD24−, aldehyde dehydrogenase 1 (ALDH1) (9), ESA+, Lin−, bromodeoxyuridine (10) and side-population cell labeling (11). More recently, breast cancer stem cells have been isolated primarily by cell surface markers of CD44+/CD24− and ALDH1+ (12).
Previous findings have shown that the cancer stem cell markers CD44+/CD24− and ALDH1+ are more enriched in TNBC tumor tissues compared with those in other breast cancer subtypes, including luminal A, luminal B and HER2-enriched (13,14). However, the study of the role of cancer stem cells with regard to the survival of patients with TNBC remains inadequate. In the present study, the distribution of CD44/CD24 and ALDH1 expression was detected using double-staining or single-staining immunohistochemistry, and the clinical outcomes of different CD44/CD24 phenotypes and ALDH1 expression, as well as other clinical characteristics, were analyzed in patients with TNBC.
Patients and methods
Patients and tissue specimens
A cohort of 1,036 female patients with breast cancer who received breast surgery between Feb 2004 and December 2008 in the Cancer Hospital of Harbin Medical University (Harbin, China) were studied, and 145 eligible patients with TNBC were identified. Patients were aged between 28 and 76 years (median age, 50 years) at enrollment. Immunohistochemistry (ER <1%, PR <1% and HER2 <10%) of breast tumors was not positive by re-staining. Patients were excluded from this cohort if they had undergone radiotherapy, chemotherapy, targeted therapy or adjuvant endocrine treatment prior to surgery. Patients were also excluded if the histological specimens could not be collected or if they had not undergone active follow-up. In addition, the patients who had developed distant organ metastases or T4 tumors prior to surgery were not included in the present study. Consequently, the final study cohort consisted of 145 patients with stage I-Шa TNBC. For pathological staging, the Tumor-Node-Metastasis staging system in the seventh edition of the American Joint Committee on Cancer was used (15).
Detailed clinical data of patients were collected from hospital registries and medical records. Information about age at diagnosis (<40, 40–49, 50–59 and ≥60 years), menopausal status (premenopausal or postmenopausal), tumor size (<2, 2–5 and >5 cm), the number of lymph nodes involved (0, 1–3 and ≥4), pathology (intraductal carcinoma, invasive ductal carcinoma, invasive lobular carcinoma and other types), radiotherapy (yes or no), chemotherapy [no chemotherapy, derivative regimens of cyclophosphamide, adriamycin and fluorouracil (CAF), such as CAF, cyclophosphamide, epirubicin and fluorouracil (CEF), cyclophosphamide, pirarubicin and fluorouracil (CTF), or ifosfamide, epirubicin and fluorouracil (IEF), regimens including taxanes, such as paclitaxel or docetaxel and epirubicin (TE), docetaxel and cyclophosphamide (TC), or docetaxel, adriamycin and cyclophosphamide (TAC), and other regimens], dates of recurrences or metastases and dates of mortality were collected. Disease-free survival (DFS) was defined as the duration from the date of diagnosis to the appearance of a regional recurrence or distant metastasis. Overall survival (OS) was defined as the time from the date of diagnosis to the mortality of the patient. The current study was approved by the Harbin Medical University Medical Ethics Committee and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All patients provided written informed consent prior to inclusion in the study.
The suitable formalin-fixed, paraffin-embedded tissues of invasive tumors were consecutively retrieved from the Laboratory of Pathology, and 4-µm sections were cut and used for immunohistochemical staining of Ki-67, p53, androgen receptor (AR), vimentin, breast cancer susceptibility gene 1 (BRCA1), cytokeratin5/6 (CK5/6), epidermal growth factor receptor (EGFR), ALDH1, CD44 and CD24.
Immunohistochemical staining
Expression of Ki-67, p53, AR, vimentin, BRCA1, CK5/6, EGFR and ALDH1 was measured by immunohistochemical staining in formalin-fixed paraffin tissue sections using methods described previously (16,17). Sections were dewaxed in xylene, rehydrated through a graded ethanol series and rinsed in distilled water. Antigen retrieval was achieved by placing the glass slides in citrate (pH 6.0; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) or EDTA buffer (pH 9.0; Sigma-Aldrich; Merck KGaA) for 2 min under high pressure. Sections (4-µm) were stained with the following antibodies obtained from Abcam (Cambridge, UK): anti-Ki-67 (rabbit; polyclonal; catalog no., ab15580; dilution, 1:50), anti-p53 (rabbit; monoclonal; catalog no., ab179477; dilution, 1:400), anti-AR (rabbit; polyclonal; catalog no., ab74272; dilution, 1:50), anti-vimentin (mouse; monoclonal; catalog no., ab8978; dilution, 1:150), anti-BRCA1 (mouse; monoclonal; catalog no., ab16780; dilution, 1:150), anti-CK5/6 (mouse; monoclonal; catalog no., ab17133; dilution, 1:200), anti-EGFR (rabbit; polyclonal; catalog no., ab2430; dilution, 1:25) and anti-ALDH1 (rabbit; monoclonal; catalog no. ab52492; dilution, 1:250). The positive and negative controls were designed in each staining experiment.
Double-staining immunohistochemistry with antibodies for CD44 and CD24 was performed on all cases. The antibodies used were anti-CD24 mouse antibody (monoclonal; catalog no., ab31622; dilution, 1:500; Abcam) and anti-CD44 rabbit antibody (monoclonal; catalog no., ab51037; dilution, 1:100; Abcam). Antigen retrieval was performed under high pressure, and endogenous peroxidase vitality was blocked by incubating the glass slides according to the manufacturer's protocol. Following primary antibody application, the slides were incubated with MultiVision Polymer Cocktail (anti-rabbit/alkaline phosphatase and anti-mouse/horseradish peroxidase; Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China). CD44 was detected with Nitro-Blue-Tetrazolium working solutions and visualized as a brown stain, whereas CD24 was detected with 3-amino-9-ethylcarbozole and identified as a red stain. Sections were counterstained with hematoxylin, followed by mounting with aqueous ClearMount. A negative control of the primary antibody replaced by Tris phosphate-buffered saline and a positive control of a known CD24+/CD44+ tissue slice were used in each staining experiment.
Evaluation of staining
Staining results were assessed by two pathologists to determine the immunohistochemistry score independently. An average was used for any discrepant staining between cores from the same patient. Cytoplasmic staining for ALDH1 was graded as 0 if ≤10% of the cells were stained, 1+ when 10–25% of the cells were stained, 2+ when 26–50% of the cells were stained and 3+ when >50% of the cells were stained. Staining sections were visualized using an Axiophot microscope (Carl Zeiss AG, Oberkochen, Germany; magnification, ×400). Five fields of view were randomly selected for immunohistochemical scoring. The expression of Ki-67, p53, AR, vimentin, BRCA1, CK5/6 and EGFR were evaluated as previously described (17–21). CD44 was identified as brown mainly in the membranous staining and CD24 was identified as red mainly in the cytoplasmic and membranous staining. The frequencies of CD44+/CD24− tumor cells were determined as the percentage of cells with brown color staining without much interference from red coloration, whereas CD44−/CD24+ tumor cells were characterized as the cells with intense red staining with absence of brown coloration. CD44+/CD24+ cells were cells with brown membranous staining and red cytoplasmic staining. CD44−/CD24− cells exhibited no staining. A tumor was categorized with the CD44+/CD24− phenotype only when the proportion of CD44+/CD24− cells was >10%, and the CD44−/CD24+ and CD44+/CD24+ phenotypes were categorized in the same way. Tumors were defined as exhibiting the CD44−/CD24− phenotype when none of the three types of positive cells (CD44+/CD24−, CD44−/CD24+ and CD44+/CD24+ cells) reached a proportion of >10%. In addition, if two (12.4%) or three (6.9%) cell types reached a proportion of >10% in one field of view, they were categorized in accordance with the predominant cell type.
Statistical analysis
Associations between CD44/CD24 status and clinical characteristics were assessed using Fisher's exact test and the χ2 test for categorical variables. The Kaplan-Meier method was used to calculate DFS and OS curves, and the log-rank test was performed to assess changes in the relative risk of events according to prognostic factors in univariate analysis. In addition, the Cox proportional hazards regression model was used for multivariate analyses to estimate prognostic value [hazard ratios (HRs) and 95% confidence intervals (CIs)] for the two main study outcomes, DFS and OS. All tests were two-sided and P<0.05 was considered to indicate a statistically significant difference. Statistical analyses were performed using SPSS version 19.0 (IBM SPSS, Armonk, NY, USA).
Results
Baseline clinical characteristics
A total of 145 patients were enrolled in the present study with a median follow-up time of 76 months, and their baseline clinical characteristics are listed in Table I. The median times for DFS and OS were 67 and 71 months, respectively. A total of 39 patients (26.9%) had suffered recurrence or metastasis and 35 patients (24.1%) had succumbed by the end of follow-up. Overall, lymph node metastasis was recorded in 51.7% of cases, of which 23.4% had 1–3 lymph nodes involved and 28.3% had ≥4 lymph nodes involved. The predominant chemotherapy regimens included derivative regimens of CAF, such as CAF, CEF, CTF or IEF (n=53), and regimens including taxanes, such as TE, TC or TAC (n=43), which accounted for 96 (80.7%) of the 119 patients who underwent adjuvant chemotherapy.
Table I.
Baseline clinical characteristics of triple-negative breast cancer.
| Characteristics | Patients, n (%) |
|---|---|
| Age at diagnosis, years | |
| <40 | 19 (13.1) |
| 40–49 | 53 (36.6) |
| 50–59 | 48 (33.1) |
| ≥60 | 25 (17.2) |
| Menopausal status | |
| Premenopausal | 71 (49.0) |
| Postmenopausal | 74 (51.0) |
| Tumor size, cm | |
| ≤2 | 37 (25.5) |
| 2–5 | 96 (66.2) |
| >5 | 12 (8.3) |
| Number of lymph nodes involved | |
| 0 | 70 (48.3) |
| 1–3 | 34 (23.4) |
| ≥4 | 41 (28.3) |
| Pathology | |
| Intraductal carcinoma | 4 (2.8) |
| Invasive ductal carcinoma | 132 (91.0) |
| Invasive lobular carcinoma | 4 (2.8) |
| Other types | 5 (3.4) |
| Radiotherapy | |
| No | 125 (86.2) |
| Yes | 20 (13.8) |
| Chemotherapy | |
| No chemotherapy | 26 (17.9) |
| Derivative regimens of CAF | 53 (36.6) |
| (CAF, CEF, CTF or IEF) | |
| Regimens including taxanes | 43 (29.7) |
| (TE, TC or TAC) | |
| Other regimens | 23 (15.9) |
CAF, cyclophosphamide, adriamycin and fluorouracil; CEF, cyclophosphamide, epirubicin and fluorouracil; CTF, cyclophosphamide, pirarubicin and fluorouracil; IEF, ifosfamide, epirubicin and fluorouracil; TE, paclitaxel or docetaxel and epirubicin; TC, docetaxel and cyclophosphamide; TAC, docetaxel, adriamycin and cyclophosphamide.
Immunohistochemical expression of the biomarkers
As shown in Table II, 92.4% of cases exhibited a Ki-67 proliferative index >0%, of which 26.9% exhibited an index of 1–10%, 24.8% an index of 11–50% and 40.7% an index of 51–100%. The p53 and BRCA1 mutation carriers accounted for 37.2 and 24.1%, respectively. Immunophenotyping showed that AR was expressed in 11.7% (17/145) of the tumors, and vimentin in 44.1% (64/145). In this group, EGFR and CK5/6 expression was observed in 15.2 and 31.7% of 145 TNBCs, respectively. In addition, 57 tumors (39.3%) were classified as basal-like subtype, and 52 tumors (35.9%) were grade 3+ for ALDH1 staining (Fig. 1).
Table II.
Overview of the biomarkers of triple-negative breast cancer.
| Characteristics | Patients, n (%) |
|---|---|
| Ki-67 expression, % | |
| 0 | 11 (7.6) |
| 1–10 | 39 (26.9) |
| 11–50 | 36 (24.8) |
| 51–100 | 59 (40.7) |
| p53 | |
| Negative | 91 (62.8) |
| Positive | 54 (37.2) |
| Androgen receptor | |
| Negative | 128 (88.3) |
| Positive | 17 (11.7) |
| Vimentin | |
| Negative | 81 (55.9) |
| Positive | 64 (44.1) |
| Breast cancer susceptibility gene 1 | |
| Negative | 110 (75.9) |
| Positive | 35 (24.1) |
| EGFR | |
| Negative | 123 (84.8) |
| Positive | 22 (15.2) |
| CK5/6 | |
| Negative | 99 (68.3) |
| Positive | 46 (31.7) |
| Basal-like | |
| EGFR and CK5/6 negative | 88 (60.7) |
| EGFR or CK5/6 positive | 57 (39.3) |
| Aldehyde dehydrogenase 1 | |
| Grade 0 | 45 (31.0) |
| Grade 1+ | 20 (13.8) |
| Grade 2+ | 28 (19.3) |
| Grade 3+ | 52 (35.9) |
EGFR, epidermal growth factor receptor; CK5/6, cytokeratin 5/6.
Figure 1.
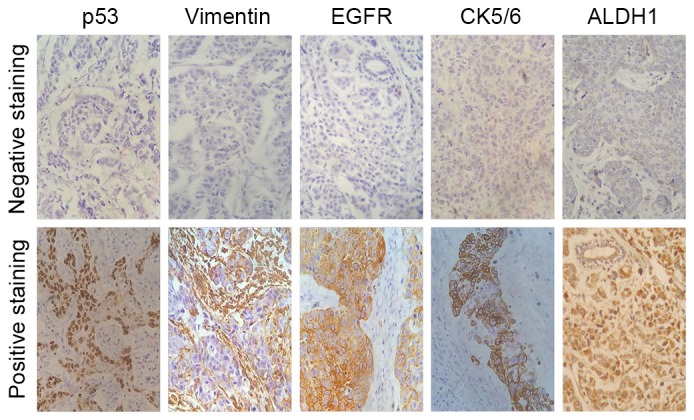
Immunohistochemical staining of p53, vimentin, EGFR, CK5/6 and ALDH1 in triple-negative breast cancer tumor tissues. Magnification, ×400. EGFR, epidermal growth factor receptor; ALDH1, aldehyde dehydrogenase 1; CK5/6, cytokeratin 5/6.
Immunohistochemical expression of CD44 and CD24
The presence of CD44 and CD24 antigens was analyzed in human breast cancer tissues using double-staining immunohistochemistry. The CD44 and CD24 expression was successfully determined in 145 cases. Fig. 2 shows representative staining patterns of various breast tumors. CD44 was identified as brown mainly in the membranous staining and CD24 was identified as red mainly in the cytoplasmic and membranous staining. In addition, to investigate the association between CD44/CD24 status and patient survival, tumors were classified according to the percentage of cells with different CD44 and CD24 expression, which resulted in four phenotypic groups: CD44−/CD24− (43.4%; 63/145), CD44+/CD24− (30.3%; 44/145), CD44+/CD24+ (3.4%; 5/145) and CD44−/CD24+ (22.8%; 33/145) (Table III). According to the classification, it was inferred that the CD44−/CD24− and CD44+/CD24− phenotypes accounted for the majority of TNBC cases. In the survival analyses, patients with the CD44+/CD24− phenotype had a significantly worse prognosis (P=0.005).
Figure 2.
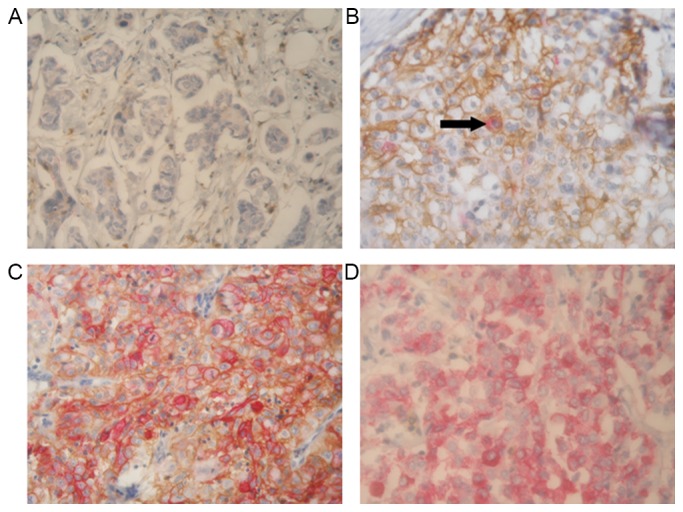
Representative immunohistochemical double-staining patterns of four CD44/CD24 phenotypes in triple-negative breast cancer tissues. CD44 (brown) exhibited homogenous membranous distribution, and CD24 (red) showed membranous and cytoplasmic immunoreactivity. (A) A tumor representing the CD44−/CD24− phenotype. (B) The predominant cells are CD44+/CD24− cells, only a few cells are CD44+/CD24+ (black arrow). (C) A tumor tissue considered as representing the CD44+/CD24+ phenotype. (D) Almost all cells in this tumor are CD44−/CD24+ cells. Magnification, ×400. CD, cluster of differentiation.
Table III.
Number and percentage of the different CD44/CD24 phenotypes.
| Characteristics | Patients, n (%) |
|---|---|
| CD44/CD24 status | |
| CD44−/CD24− | 63 (43.4) |
| CD44+/CD24− | 44 (30.3) |
| CD44+/CD24+ | 5 (3.4) |
| CD44−/CD24+ | 33 (22.8) |
CD, cluster of differentiation.
Association between CD44/CD24 phenotypes and other clinical characteristics
The distribution of different CD44/CD24 phenotypes (CD44−/CD24−, CD44+/CD24−, CD44+/CD24+ and CD44−/CD24+) was calculated in Table IV. The χ2 test identified the following factors to be associated with CD44/CD24 status: Pathology, AR status and vimentin. All four phenotypes appeared to be mainly invasive ductal carcinoma. In addition, the CD44−/CD24− phenotype contained a comparable amount of invasive lobular carcinoma (P=0.011). The CD44+/CD24− phenotype exhibited increased AR expression (25%) compared with the other three groups (P=0.022). CD44−/CD24+ tumors mostly expressed vimentin protein (75.8%; P<0.0001). In addition, CD44+/CD24− cases were more commonly scored as ALDH1 staining grade 3+ tumors when compared with CD44−/CD24+ cases, however, this difference was not statistically significant (50.0 vs. 30.3%, respectively; P=0.378).
Table IV.
Correlation of CD44/CD24 status with pathology, androgen receptor and vimentin.
| CD44/CD24 status, n (%) | |||||
|---|---|---|---|---|---|
| Characteristics | CD44−/CD24− | CD44+/CD24− | CD44+/CD24+ | CD44−/CD24+ | P-value |
| Pathology | 0.011 | ||||
| Intraductal carcinoma | 0 (0.0) | 4 (9.1) | 0 (0.0) | 0 (0.0) | |
| Invasive ductal carcinoma | 61 (96.8) | 37 (84.1) | 4 (80.0) | 30 (90.9) | |
| Invasive lobular carcinoma | 2 (3.2) | 1 (2.3) | 1 (20.0) | 0 (0.0) | |
| Other types | 0 (0.0) | 2 (4.5) | 0 (0.0) | 3 (9.1) | |
| Androgen receptor | 0.022 | ||||
| Negative | 59 (93.7) | 33 (75.0) | 5 (100.0) | 31 (93.9) | |
| Positive | 4 (6.3) | 11 (25.0) | 0 (0.0) | 2 (6.1) | |
| Vimentin | 0.000 | ||||
| Negative | 38 (60.3) | 32 (72.7) | 3 (60.0) | 8 (24.2) | |
| Positive | 25 (39.7) | 12 (27.3) | 2 (40.0) | 25 (75.8) | |
| Aldehyde dehydrogenase 1 | 0.378 | ||||
| Grade 0 | 23 (36.5) | 8 (18.2) | 2 (40.0) | 12 (36.4) | |
| Grade 1+ | 7 (11.1) | 6 (13.6) | 1 (20.0) | 6 (18.2) | |
| Grade 2+ | 14 (22.2) | 8 (18.2) | 1 (20.0) | 5 (15.2) | |
| Grade 3+ | 19 (30.2) | 22 (50.0) | 1 (20.0) | 10 (30.3) | |
CD, cluster of differentiation.
DFS analysis
For the prognostic evaluations, adjusted multivariable Cox regression analyses were performed and listed in Table V. In the analysis of CD44/CD24 status, patients with the CD44+/CD24− subtype possessed a slightly increased risk of recurrence or metastasis compared with patients with CD44−/CD24− phenotype, adjusting for confounders. The estimated HR for CD44+/CD24− subtype in CD44/CD24 status was 2.38 (95% CI, 0.90–6.33; P=0.081). No association was observed between the CD44+/CD24+ phenotype and DFS, nor between CD44−/CD24+ status and DFS, with estimated HRs being 0.38 (95% CI, 0.03–4.44; P=0.438) and 0.59 (95% CI, 0.18–1.98; P=0.393), respectively.
Table V.
Multivariate cox-regression analyses of prognosis factors with DFS and OS.
| DFS | OS | |||||
|---|---|---|---|---|---|---|
| Prognosis factors | HR | 95% CI | P-valuea | HR | 95% CI | P-valuea |
| Number of lymph nodes involved | ||||||
| 0 | 1.00 | 1.00 | ||||
| 1–3 | 0.96 | 0.28–3.27 | 0.949 | 1.75 | 0.54–5.71 | 0.353 |
| ≥4 | 5.79 | 1.73–19.32 | 0.004 | 12.90 | 3.57–46.59 | 0.000 |
| Chemotherapy | ||||||
| No chemotherapy | 1.00 | 1.00 | ||||
| Derivative regimens of CAF | 6.00 | 1.26–28.46 | 0.024 | 1.36 | 0.31–5.92 | 0.681 |
| (CAF, CEF, CTF or IEF) | ||||||
| Regimens including taxanes | 11.11 | 2.61–47.39 | 0.001 | 1.84 | 0.54–6.20 | 0.328 |
| (TE, TC or TAC) | ||||||
| Other regimens | 2.18 | 0.46–10.44 | 0.329 | 0.28 | 0.05–1.64 | 0.158 |
| Vimentin | ||||||
| Negative | 1.00 | 1.00 | ||||
| Positive | 4.15 | 1.53–11.28 | 0.005 | 2.88 | 0.96–8.61 | 0.059 |
| Basal-like | ||||||
| EGFR and CK5/6 negative | 1.00 | 1.00 | ||||
| EGFR or CK5/6 positive | 1.96 | 0.78–4.93 | 0.151 | 3.17 | 1.28–7.82 | 0.013 |
| Aldehyde dehydrogenase 1 | ||||||
| Grade 0 | 1.00 | 1.00 | ||||
| Grade 1+ | 0.61 | 0.14–2.58 | 0.605 | 0.57 | 0.11–2.87 | 0.492 |
| Grade 2+ | 0.33 | 0.09–1.23 | 0.329 | 0.44 | 0.10–1.94 | 0.279 |
| Grade 3+ | 1.30 | 0.42–4.06 | 1.298 | 1.40 | 0.44–4.43 | 0.571 |
| CD44/CD24 status | ||||||
| CD44−/CD24− | 1.00 | 1.00 | ||||
| CD44+/CD24− | 2.38 | 0.90–6.33 | 0.081 | 4.38 | 1.57–12.18 | 0.005 |
| CD44+/CD24+ | 0.38 | 0.03–4.44 | 0.438 | 0.89 | 0.09–8.99 | 0.920 |
| CD44−/CD24+ | 0.59 | 0.18–1.98 | 0.393 | 1.24 | 0.38–4.01 | 0.722 |
DFS, disease-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; EGFR, epidermal growth factor receptor; CD, cluster of differentiation; CK5/6, cytokeratin 5/6; CAF, cyclophosphamide, adriamycin and fluorouracil; CEF, cyclophosphamide, epirubicin and fluorouracil; CTF, cyclophosphamide, pirarubicin and fluorouracil; IEF, ifosfamide, epirubicin and fluorouracil; TE, paclitaxel or docetaxel and epirubicin; TC, docetaxel and cyclophosphamide; TAC, docetaxel, adriamycin and cyclophosphamide.
P<0.05 was considered to indicate a statistically significant difference.
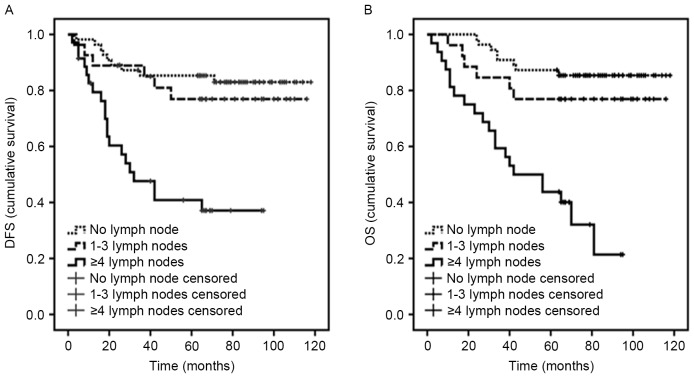
Notably, lymph node metastasis was associated with DFS in multivariable analysis (P=0.004; Fig. 3A). In the cohort, it was observed that patients with derivative regimens of CAF had a poor prognosis (P=0.024), while regimens including taxanes were associated with a shorter DFS time (P=0.001). Subsequently, additional analyses of data revealed that the majority of patients with ≥4 lymph nodes received regimens including taxanes. In addition, it was observed that patients with vimentin-positive tumors had a poor prognosis (P=0.005).
Figure 3.
Prognostic value of the number of lymph nodes involved in triple-negative breast cancer. Kaplan-Meier curves of estimated (A) DFS and (B) OS with regard to lymph node involvement. DFS, disease-free survival; OS, overall-survival.
No significant associations were observed between DFS and tumor size, pathology, radiotherapy, Ki-67, p53, AR, BRCA1, basal-like or ALDH1.
OS analysis
The number of lymph nodes involved was strongly associated with OS in multivariable analyses, with an estimated HR for ≥4 lymph nodes of 12.90 (P<0.0001; Fig. 3B).
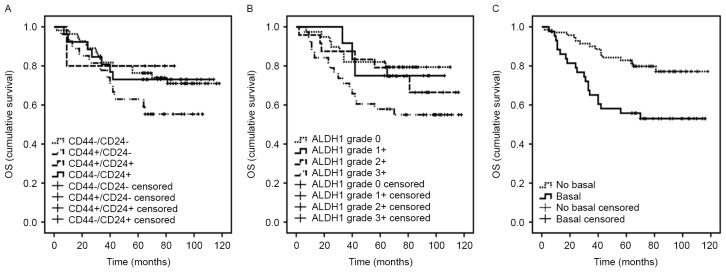
The roles of CD44/CD24 status and ALDH1 were assessed using multivariable Cox regression analyses in OS (Table V). Notably, for patients with a CD44+/CD24− phenotype, risk of mortality was statistically significantly increased compared with patients with CD44−/CD24− phenotype (P=0.005; Fig. 4A). The estimated HR for CD44+/CD24− subtype in CD44/CD24 status was 4.38 (95% CI, 1.57–12.18). Furthermore, no association was observed between CD44+/CD24+ phenotype and OS, with the estimated HR being 0.89 (95% CI, 0.09–8.99; P=0.920). Although the HR was increased for the CD44−/CD24+ phenotype compared with the CD44−/CD24− subtype, this difference was not statistically significant (HR, 1.24; 95% CI, 0.38–4.01; P=0.722). In this adjusted multivariable analysis, no significant association was observed between ALDH1 and OS in the TNBC cohort. However, the Kaplan-Meier curves of different levels of ALDH1 did separate and the survival of patients with ALDH1 grade 3+ was inferior to those of patients with grade 0, 1+ or 2+ (P=0.571; Fig. 4B). In general, ALDH1 overexpression could be a disadvantage for the survival of patients.
Figure 4.
Prognostic value of the main clinical and tissue characteristics in triple-negative breast cancer. Kaplan-Meier curves of estimated OS for (A) CD44/CD24 status (B) ALDH1 and (C) basal-like. OS, overall survival; CD, cluster of differentiation; ALDH1, aldehyde dehydrogenase 1.
In addition, there was a trend toward an inferior OS for patients with basal-like tumors vs. non-basal-like tumors (P=0.013; Fig. 4C) (Table V). Similarly, no significant difference was observed between OS and clinical characteristics, including tumor size, pathology, radiotherapy, chemotherapy, Ki-67, p53, AR, vimentin or BRCA1.
Discussion
Breast carcinoma is recognized as a heterogeneous disease and presents with distinct histopathological features and clinical behaviors, and a variety of outcomes (22). TNBC is a unique type of breast cancer with a poor prognosis (2). Cancer stem cells are more abundant in TNBC tumor tissues and perform important roles in the recurrence and metastasis of TNBC (6,14). Although several studies have shown that large percentages of CD44+/CD24− cells or ALDH1+ cells remain in TNBC (18,23–25), the importance of the role of these cancer stem cell subpopulations with regard to the prognosis of patients has not been clearly understood. The present study investigated the association between CD44/CD24 phenotypes and the ALDH1 expression and survival of patients with TNBC following surgical therapy.
Previously, a number of studies (13,26) demonstrated that the expression of TNBC antigens, including C-X-C chemokine receptor type 4 and octamer-binding transcription factor 4, are most prevalent in CD44+/CD24− cells, and may promote the epithelial-mesenchymal transition of the CD44+/CD24− phenotype, facilitating breast cancer recurrence or metastasis. In addition, breast cancer cell lines with a prevalence of CD44+/CD24− cells have a higher potential than others to invade Matrigel in vitro and a high metastatic ability in vivo in the lymph node microenvironment and in distant metastasis (27). Furthermore, in a cohort of 50 patients with TNBC, Idowu et al (24) reported that the tumor tissues with CD44+/CD24− were more likely to have a high Ki-67 proliferation index and be associated with a poor clinical outcome. Other breast cancer specimens in which single staining detected the expression of CD44 and CD24, it was clarified that the tumor tissues with the CD44+/CD24− subtype had a higher median vascular density compared with that in those tissues with the CD44−/CD24− phenotype; OS analysis of this study showed that patients with the CD44+/CD24− phenotype may have an unfavorable prognosis (23).
In the present study, the expression of CD24 and CD44 was examined using immunohistochemical double-staining. Results certified that the double-staining pattern did not interfere with the accurate expression of CD44 and CD24, and it could also locate cells with different expression profiles individually. The percentages of four types of cancer cells (CD44−/CD24−, CD44+/CD24−, CD44+/CD24+ and CD44−/CD24+) in tumors were counted following double-staining immunohistochemistry, and each tumor was defined by the presence of the predominant cell type. No association was observed between DFS and the CD44+/CD24− phenotype, however, OS analysis showed that patients with the CD44+/CD24− phenotype experienced a significantly shorter survival time compared with patients with other phenotypes. The CD44+/CD24− cell population has been associated with a poor prognosis (28), although not in all relevant studies. In a similar study (29), Ezrin and CD44 protein co-expression, which was detected with immunofluorescence double-staining, was shown to be associated with a poor disease-specific survival time in 726 breast cancer patients. In addition, according to the present data from Kaplan-Meier curves of estimated DFS and OS, the CD44−/CD24+ phenotype in TNBC may also be associated with a trend for an increased risk of recurrence or mortality when compared with the CD44−/CD24− phenotype, although this did not reach statistical significance. A previous study from Ahmed et al (30) demonstrated that the CD44−/CD24+ phenotype was significantly associated with shorter metastasis-free survival time and decreased 10-year breast cancer survival rate. In the study by Mylona et al (31), the CD44−/CD24+ phenotype emerged as a poor prognostic indicator, at least within the group of grade 2 tumors.
Furthermore, ALDH1 is another key cell surface marker isolating the cancer stem cell subpopulation. Charafe-Jauffret et al (32) found that the aggressive and metastatic behaviors of inflammatory breast cancer are mediated by ALDH1+ breast cancer stem cells in mouse xenograft models. In another study involving 577 breast carcinomas, the expression of ALDH1, as defined by immunohistochemistry staining, was significantly associated with a poor clinical outcome (9). In the present study, DFS and OS analyses showed that the survival curve of ALDH1 grade 3+ was inferior to those of grade 0, 1+ or 2+, although the analysis did not reach statistical significance.
In our OS analyses, patients with a basal-like subtype have been more likely to experience shorter OS times. The basal-like breast cancer, which is mostly comprised of TNBCs, is positive for CK5/6 and/or EGFR, and is particularly common in BRCA1 hereditary tumors (17). Moestue et al (33) observed that basal-like xenografts exhibited significantly higher phosphatidylinositol 3-kinase pathway activity than luminal-like xenografts in animal models of breast cancer; i.e., basal-like tumors exhibited worse prognoses compared with luminal-like tumors.
In the present cohort, the involvement of ≥4 lymph nodes has been associated with an increased risk of recurrence and mortality in patients with TNBC. In a cohort of 1,711 patients with the TNBC subtype, Hernandez-Aya et al (16) found that when comparing node-negative and node-positive patients, there was a significant difference in relapse-free survival.
In summary, the present study reported that the CD44/CD24 phenotype evaluated by double staining immunohistochemistry constitutes an independent prognostic factor for TNBC. Patients with TNBC with the CD44+/CD24− phenotype exhibit a significantly worse prognosis. Additional studies are required to investigate the molecular mechanism of the aggressive behaviors of cancer stem cells and guide clinical treatment for TNBC.
Acknowledgements
The authors are grateful for the excellent technical assistance of the pathologists in the Cancer Hospital of Harbin Medical University and for the assistance provided for the collection of follow-up data by the staff in the Department of Records. The study was supported by the National Natural Science Foundation of China (grant no. 81270034), the Chunhui Program from Chinese Ministry of Education (grant no. Z2012075) and the Yuweihan Academician Prominent Youth Foundation from Harbin Medical University (grant no. 2011-2013).
Glossary
Abbreviations
- TNBC
triple-negative breast cancer
- ER
estrogen receptor
- PR
progesterone receptor
- HER2
human epidermal growth factor receptor 2
- ESA
epithelial-specific antigen
- Lin
lineage
- ALDH1
aldehyde dehydrogenase 1
- CAF
cyclophosphamide, adriamycin and fluorouracil
- CEF
cyclophosphamide, epirubicin and fluorouracil
- CTF
cyclophosphamide, pirarubicin and fluorouracil
- IEF
ifosfamide, epirubicin and fluorouracil
- TE
paclitaxel or docetaxel and epirubicin
- TC
docetaxel and cyclophosphamide
- TAC
docetaxel, adriamycin and cyclophosphamide
- DFS
disease-free survival
- OS
overall survival
- AR
androgen receptor
- BRCA1
breast cancer susceptibility gene 1
- CK5/6
cytokeratin5/6
- EGFR
epidermal growth factor receptor
- HR
hazard ratio
References
- 1.Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N Engl J Med. 2009;360:790–800. doi: 10.1056/NEJMra0801289. [DOI] [PubMed] [Google Scholar]
- 2.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: A population-based study from the California Cancer Registry. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 3.Reis-Filho JS, Tutt AN. Triple negative tumours: A critical review. Histopathology. 2008;52:108–118. doi: 10.1111/j.1365-2559.2007.02889.x. [DOI] [PubMed] [Google Scholar]
- 4.Anders C, Carey LA. Understanding and treating triple-negative breast cancer. Oncology (Williston Park) 2008;22:1233–1239. [PMC free article] [PubMed] [Google Scholar]
- 5.Lee E, McKean-Cowdin R, Ma H, Spicer DV, Van Den Berg D, Bernstein L, Ursin G. Characteristics of triple-negative breast cancer in patients with a BRCA1 mutation: Results from a population-based study of young women. J Clin Oncol. 2011;29:4373–4380. doi: 10.1200/JCO.2010.33.6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 7.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells-perspectives on current status and future directions: AACR workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 8.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 11.Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2− cancer cells are similarly tumorigenic. Cancer Res. 2005;65:6207–6219. doi: 10.1158/0008-5472.CAN-05-0592. [DOI] [PubMed] [Google Scholar]
- 12.Lin L, Hutzen B, Lee HF, Peng Z, Wang W, Zhao C, Lin HJ, Sun D, Li PK, Li C, et al. Evaluation of STAT3 signaling in ALDH+ and ALDH+/CD44+/CD24− subpopulations of breast cancer cells. PLoS One. 2013;8:e82821. doi: 10.1371/journal.pone.0082821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honeth G, Bendahl PO, Ringnér M, Saal LH, Gruvberger-Saal SK, Lövgren K, Grabau D, Fernö M, Borg A, Hegardt C. The CD44+/CD24− phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 2008;10:R53. doi: 10.1186/bcr2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ricardo S, Vieira AF, Gerhard R, Leitão D, Pinto R, Cameselle-Teijeiro JF, Milanezi F, Schmitt F, Paredes J. Breast cancer stem cell markers CD44, CD24 and ALDH1: Expression distribution within intrinsic molecular subtype. J Clin Pathol. 2011;64:937–946. doi: 10.1136/jcp.2011.090456. [DOI] [PubMed] [Google Scholar]
- 15.Sinn HP, Helmchen B, Wittekind CH. TNM classification of breast cancer: Changes and comments on the 7th edition. Pathologe. 2010;31:361–366. doi: 10.1007/s00292-010-1307-0. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez-Aya LF, Chavez-Macgregor M, Lei X, Meric-Bernstam F, Buchholz TA, Hsu L, Sahin AA, Do KA, Valero V, Hortobagyi GN, Gonzalez-Angulo AM. Nodal status and clinical outcomes in a large cohort of patients with triple-negative breast cancer. J Clin Oncol. 2011;29:2628–2634. doi: 10.1200/JCO.2010.32.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler L, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 18.Resetkova E, Reis-Filho JS, Jain RK, Mehta R, Thorat MA, Nakshatri H, Badve S. Prognostic impact of ALDH1 in breast cancer: A story of stem cells and tumor microenvironment. Breast Cancer Res Treat. 2010;123:97–108. doi: 10.1007/s10549-009-0619-3. [DOI] [PubMed] [Google Scholar]
- 19.Abdel-Fatah TM, Arora A, Alsubhi N, Agarwal D, Moseley PM, Perry C, Doherty R, Chan SY, Green AR, Rakha E, et al. Clinicopathological significance of ATM-Chk2 expression in sporadic breast cancers: A comprehensive analysis in large cohorts. Neoplasia. 2014;16:982–991. doi: 10.1016/j.neo.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JG, Wu R. Erlotinib-cisplatin combination inhibits growth and angiogenesis through c-MYC and HIF-1α in EGFR-mutated lung cancer in vitro and in vivo. Neoplasia. 2015;17:190–200. doi: 10.1016/j.neo.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bane A, Viloria-Petit A, Pinnaduwage D, Mulligan AM, O'Malley FP, Andrulis IL. Clinical-pathologic significance of cancer stem cell marker expression in familial breast cancers. Breast Cancer Res Treat. 2013;140:195–205. doi: 10.1007/s10549-013-2591-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacquemier J, Ginestier C, Rougemont J, Bardou VJ, Charafe-Jauffret E, Geneix J, Adélaïde J, Koki A, Houvenaeghel G, Hassoun J, et al. Protein expression profiling identifies subclasses of breast cancer and predicts prognosis. Cancer Res. 2005;65:767–779. [PubMed] [Google Scholar]
- 23.Giatromanolaki A, Sivridis E, Fiska A, Koukourakis MI. The CD44+/CD24− phenotype relates to ‘triple-negative’ state and unfavorable prognosis in breast cancer patients. Med Oncol. 2011;28:745–752. doi: 10.1007/s12032-010-9530-3. [DOI] [PubMed] [Google Scholar]
- 24.Idowu MO, Kmieciak M, Dumur C, Burton RS, Grimes MM, Powers CN, Manjili MH. CD44(+)/CD24(−/low) cancer stem/progenitor cells are more abundant in triple-negative invasive breast carcinoma phenotype and are associated with poor outcome. Hum Pathol. 2012;43:364–373. doi: 10.1016/j.humpath.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Nalwoga H, Arnes JB, Wabinga H, Akslen LA. Expression of aldehyde dehydrogenase 1 (ALDH1) is associated with basal-like markers and features of aggressive tumours in African breast cancer. Br J Cancer. 2010;102:369–375. doi: 10.1038/sj.bjc.6605488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang D, Lu P, Zhang H, Luo M, Zhang X, Wei X, Gao J, Zhao Z, Liu C. Oct-4 and Nanog promote the epithelial-mesenchymal transition of breast cancer stem cells and are associated with poor prognosis in breast cancer patients. Oncotarget. 2014;5:10803–10815. doi: 10.18632/oncotarget.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pandit TS, Kennette W, Mackenzie L, Zhang G, Al-Katib W, Andrews J, Vantyghem SA, Ormond DG, Allan AL, Rodenhiser DI, et al. Lymphatic metastasis of breast cancer cells is associated with differential gene expression profiles that predict cancer stem cell-like properties and the ability to survive, establish and grow in a foreign environment. Int J Oncol. 2009;35:297–308. [PubMed] [Google Scholar]
- 28.Meyer MJ, Fleming JM, Ali MA, Pesesky MW, Ginsburg E, Vonderhaar BK. Dynamic regulation of CD24 and the invasive, CD44posCD24neg phenotype in breast cancer cell lines. Breast Cancer Res. 2009;11:R82. doi: 10.1186/bcr2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma L, Jiang T. Clinical implications of Ezrin and CD44 co-expression in breast cancer. Oncol Rep. 2013;30:1899–1905. doi: 10.3892/or.2013.2641. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed MA, Aleskandarany MA, Rakha EA, Moustafa RZ, Benhasouna A, Nolan C, Green AR, Ilyas M, Ellis IO. A CD44−/CD24+ phenotype is a poor prognostic marker in early invasive breast cancer. Breast Cancer Res Treat. 2012;133:979–995. doi: 10.1007/s10549-011-1865-8. [DOI] [PubMed] [Google Scholar]
- 31.Mylona E, Giannopoulou I, Fasomytakis E, Nomikos A, Magkou C, Bakarakos P, Nakopoulou L. The clinicopathologic and prognostic significance of CD44+/CD24 (−/low) and CD44−/CD24+ tumor cells in invasive breast carcinomas. Hum Pathol. 2008;39:1096–1102. doi: 10.1016/j.humpath.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Charafe-Jauffret E, Ginestier C, Iovino F, Tarpin C, Diebel M, Esterni B, Houvenaeghel G, Extra JM, Bertucci F, Jacquemier J, et al. Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin Cancer Res. 2010;16:45–55. doi: 10.1158/1078-0432.CCR-09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moestue SA, Dam CG, Gorad SS, Kristian A, Bofin A, Mælandsmo GM, Engebråten O, Gribbestad IS, Bjørkøy G. Metabolic biomarkers for response to PI3K inhibition in basal-like breast cancer. Breast Cancer Res. 2013;15:R16. doi: 10.1186/bcr3391. [DOI] [PMC free article] [PubMed] [Google Scholar]