Abstract
Background
The efficacy of psychosocial intervention has been proven in treatment of diabetic patients with depression in some studies. This meta-analysis was conducted to explore the efficacy as well as additional effects of this method during diabetic management in patients with type 2 diabetes mellitus (T2DM) and comorbid depression.
Methods
Electronic databases were searched from March 2000 to March 2017 for randomized controlled trials (RCTs) studying the effects of psychosocial intervention on T2DM patients with depression. There was no language limitation. Outcome measurements were symptoms of depression and anxiety, as well as glycemic control. A random effects model was conducted.
Results
In total, 31 RCTs composed of 2,616 patients were eligible for this analysis. The psychosocial intervention was effective for depression symptoms with pooled standardized mean difference (SMD) of −1.50 (95% CI =−1.83, −1.18) and anxiety symptoms with SMD of −1.18 (95% CI =−1.50, −0.85). Meanwhile, the additional effects indicated a better improvement of glycemic control, including the fasting blood-glucose with SMD of −0.93 (95% CI =−1.15, −0.71), 2-hour postprandial plasma glucose with SMD of −0.84 (95% CI =−1.13, −0.56), and hemoglobin A1c with SMD of −0.81 (95% CI =−1.10, −0.53).
Conclusion
These results demonstrate that the psychosocial intervention is very effective in treating T2DM patients with depression.
Keywords: psychosocial intervention, type 2 diabetes mellitus, depression, meta-analysis
Introduction
In 2015 alone, diabetes mellitus has claimed over 1.5 million lives worldwide. As a highly prevalent metabolic disease, diabetes mellitus has been the sixth leading cause of death in the world, especially in developing countries.1 Among these diabetic patients, approximately 90% have type 2 diabetes mellitus (T2DM).2 Treating a patient with T2DM entails a complicated and comprehensive strategy. This includes strict diet control, moderate physical exercise, hypoglycemic drug intake, blood glucose monitoring, and treatment education.3 Managing both diabetes and the economic burden that follows could lead patients to anxiety, helplessness, and depression.4 Therefore, managing diabetes also involves patients’ emotional health.5
Depression is a common comorbidity of both type 1 diabetes and T2DM. In the United States, 28% of women and 18% of men with diabetes suffered from symptoms of depression.6 Compared with non-DM patients, the incidence of depression is two times higher in patients with T2DM.7 Poor mental health and harmful medical outcomes are prevalent in patients with diabetes and comorbid depression. Previous studies reported that diabetes patients with depression had lower quality of life, worse glycemic control, poor adherence to self-care practices, and disease- related complications.8–11 Moreover, depression due to diabetes is often associated with mortality.12 Therefore, it is important to treat depression during the diabetes management.
Currently, it is still very difficult to treat and/or prevent T2DM, even though we and other researchers have conducted much research.13–19 Pharmacotherapy is still the first-line treatment for depression, but a significant percentage of patients have little to no response to these treatments. Moreover, the side effects of antidepressants are likely to cause poor adherence for T2DM patients with depression.20 Researchers found that patients had a preference toward psychosocial interventions.21 A previous systematic review revealed that psychosocial intervention might be effective for diabetes patients with depression.22 However, consistent evidence is still lacking on the effect of psychosocial intervention in treating depression in T2DM patients. Additionally, considering the poor diabetes outcomes caused by comorbid depression, it is also ideal to explore the efficacy of this method with regard to glycemic control. Therefore, this meta-analysis was conducted to investigate the effect of psychosocial intervention on patients with T2DM and comorbid depression.
Methods
Literature research
Firstly, potential studies were searched for in the international databases (PubMed, CCTR, Cochrane Library, Web of Science, MEDLINE, PsycINFO, and Embase), two Chinese databases (CNKI and CBM-disc), and relevant websites from March 2000 to March 2017. The used search terms included “diabetes”, “mental intervention”, “mellitus”, “depress*”, “psychological”, “mental health”, “psychosocial state”, “psychosocial intervention”, “interpersonal therapy”, “problem solving therapy”, “behavioral therapy”, and “cognitive behavioral therapy”. To mitigate language bias, no language restriction was used. Conference summaries and the reference documents of the included studies were also researched.
Inclusion/exclusion criteria
The studies which met the following inclusion criteria were selected for the subsequent analysis: i) patients over 18 years with T2DM and comorbid depression; ii) randomized controlled trials (RCTs) with patients randomly assigned to the control and intervention groups; iii) all patients received conventional treatment for T2DM, but patients in the intervention group also received psychosocial intervention; iv) the depressive symptoms were assessed using the Montgomery–Åsberg Depression Rating Scale (MADRS), Self-Rating Depression Scale (SDS), or Hamilton Depression Rating Scale (HDRS); v) acute treatment phase (≤16 weeks). Meanwhile, studies that met any of the following criteria were excluded: i) case reports, retrospective studies, duplicate studies, and reviews; ii) patients with psychiatric diseases, other mental illnesses, other forms of diabetes besides type 2, malignant tumors, and severe physical illness; iii) patients with “narrow” or secondary depression diagnoses, such as post-partum depression, subthreshold depression, and vascular depression. All patients provided written informed consent, and all clinical trials were reviewed and approved by the Ethical Committee.
Outcome indexes
The changes in depressive symptoms were assessed and quantified by the established depression questionnaires (HDRS or SDS) and were used as the primary outcome. The anxiety symptoms were assessed by self-rating anxiety scale (SAS) or Hamilton Anxiety Scale (HAMA) and were used as the secondary outcome. The data about glycemic control, such as fasting plasma-glucose (FPG), 2-hour postprandial plasma glucose (2hPPG), and hemoglobin A1c (HbA1c), were also measured at baseline and post-intervention. These data were also used as the secondary outcome. The time-point that was given in the original study was preferred as the study endpoint.
Data extraction
Two reviewers independently reviewed the potential studies according to the aforementioned inclusion/exclusion criteria, and identified the included studies. Relevant data from these included studies were extracted and saved in the Cochrane data extraction template. The data extraction procedure was also independently completed by two reviewers. Any disagreement during the reviewing and extracting process was resolved by group discussion. The following data were retrieved from the included studies: i) demographic data, including age, sex ratio, disease duration, education level; ii) data about depressive and anxiety symptoms, including HDRS, SDS, HAMA, and SAS score at baseline and post-intervention; iii) data about glycemic control at baseline and post-intervention; iv) method of psychosocial intervention and conventional treatment.
Quality assessment
Two reviewers independently assessed the bias risk in the individual studies according to the Cochrane Handbook for Systematic Reviews of Intervention. The bias risk was assessed using the following six items: i) randomization used; ii) allocation concealment; iii) outcome blinding assessment; iv) incomplete outcome data addressed; v) free of selective reporting; vi) baseline matched.
Statistical analysis
RevMan 5.0 software was used to carry out the meta-analysis. The standardized mean difference (SMD) was calculated for the randomized studies. For SMD, an effect size of 0.2–0.5 was considered small, 0.5–0.8 was considered moderate, and above 0.8 was considered large.2 The effect size and the corresponding 95% CIs were calculated for the primary and secondary outcomes. Mantel-Haenszel random-effects model was selected, as it was assumed that the included RCTs probably had the diverse true treatment effects.23 Moreover, this model was more appropriate when heterogeneity existed. The heterogeneity was assessed by the I2 (>50%) and Q test (p<0.10).24 Subgroup analysis or sensitivity analysis were performed when appropriate. This meta-analysis was conducted according to the predetermined protocol and the recommendations of Sacks et al.25
Results
Search results
The search was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. First, 338 potentially relevant studies were obtained. Then, 307 studies were excluded due to the following reasons: i) duplicates (n=42); ii) not relevant to T2DM and comorbid depression (n=191); iii) not about psychosocial intervention (n=64); and iv) compared the psychosocial intervention with other antidepressants (n=10). Finally, 31 RCTs were included to perform meta-analysis (Figure 1).26–56
Figure 1.
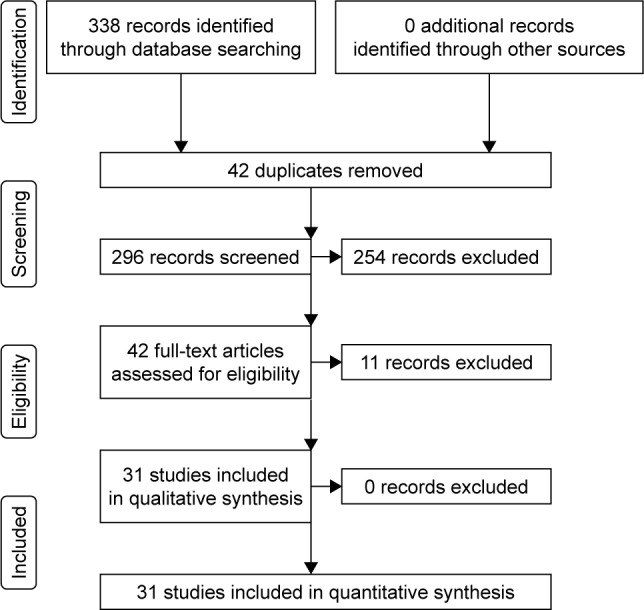
Workflow of literature search.
Study characteristics
There were 2,616 patients with T2DM and comorbid depression in these included studies. The average age of patients in the intervention group was approximately 43 years. Five of these studies were written in English, and 26 studies were written in Chinese. The treatment time ranged from 2 to 16 weeks. The average age of patients in most studies was between 50 and 70 years old. There were 28 studies that assessed the depressive symptoms of patients at the end of trial. The detailed information was described in Tables 1 and 2.
Table 1.
Clinical characteristics of the included randomized controlled trials
| Study | Control
|
Intervention
|
Criteria | Time | Conventional treatment | Psychological intervention | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (y) | M/F | Duration (y) | Age (y) | M/F | Duration (y) | |||||
| Du et al,26 2005 | 53.5 (10.2) | 15/15 | 5.5 (3.2) | 52.5 (11.2) | 14/16 | 5.2 (3.8) | SAS ≥50, SDS ≥50 | 4 w | Diet, exercise, drugs | Group psychotherapy and individual psychotherapy |
| Qiu et al,27 2005 | 71.6 (3.9) | 12/16 | NA | 71.2 (4.3) | 10/18 | NA | SDS ≥50 | 8 w | Diet, exercise, insulin therapy | NA |
| Ma et al,28 2006 | 44.7 (11.4) | 18/15 | 4.5 (3.6) | 44.1 (10.9) | 20/14 | 4.7 (3.3) | SAS ≥50, SDS ≥50 | 6 w | Gliclazide, 80 mg, three times/day | Group psychotherapy and individual psychotherapy |
| Sun et al,29 2006 | 52.7 (8.5) | 8/12 | 7.4 (5.5) | 54.1 (9.5) | 11/10 | 6.7 (5.0) | HDRS-17>17 | 8 w | Diet, drugs | Cognitive therapy and supportive diabetes education |
| Lei et al,30 2008 | 57.3 (10.6) | 28/32 | NA | 57.3 (10.6) | 28/32 | NA | SDS ≥50 | 8 w | Diet, exercise, insulin therapy | Health education and psychological counseling |
| Wu et al,31 2008 | 52.4 (11.2) | 104/96 | 6.3 (4.2) | 52.4 (11.2) | 104/96 | 6.3 (4.2) | SDS ≥50 | 4 w | Drugs | Humanistic therapy, cognitive and behavior intervention |
| Jiang,33 2011 | 50.7 (11.4) | 26/28 | 4.5 (3.6) | 51.1 (10.9) | 25/29 | 4.7 (3.3) | HDRS-24>17 | 6 w | NA | Relaxation therapy, cognitive and behavior intervention |
| Lu et al,34 2011 | 56 (5) | 36/24 | NA | 56 (5) | 36/24 | NA | HDRS-24>17 | 8 w | Hypoglycemic agent | Health education and psychological counseling |
| Wang et al,35 2011 | 56.8 (3.5) | 21/15 | 5.2 (1.4) | 56.5 (4.0) | 20/16 | 5.0 (1.5) | SAS ≥50, SDS ≥50 | 8 w | NA | Health education and psychological counseling |
| Xu et al,36 2011 | 64.0 (7.8) | 58/78 | NA | 64.0 (7.8) | 58/78 | NA | SAS ≥50, SDS ≥50 | 5 w | Insulin therapy | Group psychotherapy |
| Chen et al,38 2012 | 57.1 (5.9) | 30/37 | 9.8 (3.2) | 58.1 (6.2) | 21/31 | 9.3 (2.9) | HDRS-24>20 | 4 w | NA | Cognitive restructuring and psychological counseling |
| Qin et al,40 2012 | NA | 19/11 | NA | NA | 35/25 | NA | SDS >40, HDRS >17 | 12 w | Metformin, insulin therapy | Health education and psychological counseling |
| Gao et al,41 2013 | 46.1 (18.2) | NA | 9.3 (4.2) | 46.1 (18.2) | NA | 9.3 (4.2) | SAS ≥40, SDS ≥50 | 2 w | Hypoglycemic agent | Group psychotherapy |
| Gao et al,42 2013 | 64.9 (6.8) | 19/16 | 8.4 (2.1) | 65.4 (7.1) | 20/17 | 8.7 (2.3) | HDRS ≥18, HAMA ≥14 | 8 w | Hypoglycemic agent or insulin | Cognitive and behavior intervention |
| Lan et al,44 2013 | 60–83 | 23/19 | NA | 60–86 | 24/18 | NA | SAS ≥50, SDS ≥50 | 12 w | NA | Group psychotherapy and individual psychotherapy |
| Li,45 2013 | 60–83 | 23/12 | NA | 60–82 | 24/11 | NA | SAS ≥50, SDS ≥50 | 8 w | NA | Cognitive and behavior intervention |
| Li et al,46 2013 | 35.4 (4.5) | 24/17 | NA | 35.2 (4.8) | 22/19 | NA | HDRS ≥20 | 6 w | Hypoglycemic agent | Group psychotherapy, cognitive and behavior intervention |
| Sang,47 2013 | 62.9 (8.1) | 8/12 | NA | 61.3 (7.1) | 10/10 | NA | SDS ≥50 | 4 w | Hypoglycemic agent | Health education and group psychotherapy |
| Wang,49 2013 | 65.8 (8.3) | 17/13 | 10.2 (3.5) | 66.2 (7.5) | 16/14 | 9.8 (4.3) | HDRS >8 | 6 w | Drugs | Psychological counseling and behavior intervention |
| Zhang et al,32 2013 | 51.3 (4.9) | 17/19 | NA | 51.3 (4.9) | 17/19 | NA | SAS ≥40, SDS ≥40 | 12 w | Diet, exercise, hypoglycemic agent | NA |
| Li and Hu,53 2014 | 61.2 (12.1) | 75/45 | 5.5 (3.9) | 62.2 (11.3) | 80/40 | 5.6 (4.5) | HDRS ≥18 | 8 w | NA | Psychological support, cognitive and behavior intervention |
| Li et al,54 2014 | 31–88 | NA | 6.8 (3.9) | 31–88 | NA | 6.8 (3.9) | SAS ≥50, SDS ≥50 | 6 w | NA | Group psychotherapy and individual psychotherapy |
| Wang et al,48 2009 | 52.6 (6.9) | 32/28 | NA | 53.5 (6.5) | 31/29 | NA | HDRS ≥20, HAMA ≥14 | 2 w | Insulin therapy | Health education and psychological counseling |
| Zhang et al,50 2013 | 51.8 (3.7) | 19/12 | 3.2 (0.7) | 52.4 (3.2) | 16/15 | 3.3 (0.6) | HDRS ≥18 | 12 w | NA | Group psychotherapy and individual psychotherapy |
| Du,39 2012 | 59.8 (6.6) | 30/32 | NA | 59.8 (6.6) | 30/32 | NA | HDRS ≥18 | 9 w | Drugs | Group psychotherapy |
| Hu,43 2013 | 60.9 (7.9) | 30/30 | 8.9 (1.8) | 62.7 (8.7) | 33/27 | 9.4 (2.1) | HDRS ≥18 | 8 w | Drugs | Group psychotherapy |
| Penckofer et al,37 2012 | 54.0 (8.4) | 0/36 | 10.0 (6.5) | 54.8 (8.8) | 0/38 | 10.5 (8.2) | CES-D ≥16 | 12 w | NA | SWEEP Program |
| Sharif et al,52 2014 | 50–60 | 3/26 | NA | 50–60 | 8/21 | NA | DSM-IV criteria | 8 w | Anti-diabetic drugs | Group psychotherapy |
| Zheng et al,55 2015 | 61 (7) | 27/30 | 8.1 (4.5) | 62 (6) | 27/28 | 8.2 (3.8) | DSM-IV criteria | 12 w | Diet, exercise, drugs | 24 Move Shadow Boxing and psychosomatic relaxation |
| Huang et al,56 2016 | 57.8 (10.4) | 13/17 | 3.8 (1.5) | 55.1 (10.4) | 16/15 | 3.7 (1.8) | CES-D ≥16 | 12 w | Diet, exercise, drugs | MET and cognitive behavioral therapy |
| Safren et al,51 2014 | 58.3 (7.4) | 22/20 | NA | 55.4 (8.7) | 22/23 | NA | DSM-IV criteria | 16 w | Hypoglycemic agent, insulin | Cognitive behavioral therapy |
Abbreviations: M, male; F, female; NA, not available; y, year; SDS, Self-Rating Depression Scale; SAS, Self-Rating Anxiety Scale; HDRS, Hamilton Depression Rating Scale; HAMA, Hamilton Anxiety Scale; CES-D, epidemiologic studies depression scale; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, 4th Edition; w, week; SWEEP, Study of Women’s Emotions and Evaluation of a Psychoeducational; MET, motivation enhancement therapy.
Table 2.
Depressive symptoms and blood glucose of the patients at baseline
| Study | Control
|
Intervention
|
||||||
|---|---|---|---|---|---|---|---|---|
| Depression, scores (SD) | FPG, mmol/L (SD) | 2hPPG, mmol/L (SD) | HbA1c (%) | Depression, scores (SD) | FPG, mmol/L (SD) | 2hPPG, mmol/L (SD) | HbA1c (%) | |
| Du et al,26 2005 | 55.9 (10.2) SDS | 11.3 (3.6) | 17.9 (5.1) | 9.2 (1.8) | 55.7 (9.9) SDS | 11.1 (3.4) | 18.3 (4.1) | 9.3 (1.7) |
| Qiu et al,27 2005 | NA | 8.7 (1.3) | 12.6 (0.9) | 8.5 (2.4) | NA | 8.7 (1.4) | 12.7 (0.9) | 8.5 (2.3) |
| Ma et al,28 2006 | 53.7 (11.0) SDS | 11.3 (3.3) | 17.8 (5.2) | 9.2 (1.7) | 53.7 (10.0) SDS | 11.2 (3.2) | 18.5 (4.1) | 9.1 (1.6) |
| Sun et al,29 2006 | 25.2 (5.9) HDRS | NA | NA | 8.5 (1.3) | 25.1 (6.2) HDRS | NA | NA | 8.4 (1.3) |
| Lei et al,30 2008 | 56.7 (10.9) SDS | 8.7 (2.6) | 13.7 (4.4) | 9.6 (1.8) | 56.9 (9.9) SDS | 8.6 (2.2) | 13.6 (4.5) | 9.7 (1.6) |
| Wu et al,31 2008 | 57.2 (10.3) SDS | 12.1 (2.3) | 17.2 (6.5) | NA | 56.6 (10.3) SDS | 11.4 (4.3) | 18.7 (4.5) | NA |
| Jiang,33 2011 | 33.5 (4.7) HDRS | 12.1 (3.5) | 17.9 (6.2) | 9.2 (1.7) | 31.2 (7.8) HDRS | 11.2 (3.7) | 18.3 (5.7) | 9.1 (1.6) |
| Lu et al,34 2011 | 21.8 (3.0) HDRS | 9.0 (2.7) | NA | 7.9 (3.8) | 22.4 (2.8) HDRS | 10.0 (2.2) | NA | 8.5 (3.2) |
| Wang et al,35 2011 | 62.8 (4.2) SDS | NA | NA | NA | 62.5 (3.9) SDS | NA | NA | NA |
| Xu et al,36 2011 | 56.0 (10.4) SDS | 11.6 (1.7) | 15.9 (2.7) | 9.2 (1.6) | 56.1 (10.2) SDS | 11.3 (1.8) | 15.5 (2.5) | 8.9 (1.7) |
| Chen et al,38 2012 | 32.9 (9.1) HDRS | 7.9 (2.4) | NA | NA | 34.2 (8.4) HDRS | 8.2 (3.7) | NA | NA |
| Qin et al,40 2012 | 49 (8) SDS 21 (7) HDRS |
NA | NA | NA | 48 (7) SDS 30 (7) HDRS |
NA | NA | NA |
| Gao et al,41 2013 | 57.3 (9.2) SDS | 13.1 (2.5) | 18.3 (5.2) | NA | 57.6 (9.1) SDS | 11.6 (3.2) | 18.2 (4.6) | NA |
| Gao et al,42 2013 | 23.2 (4.3) HDRS | NA | NA | NA | 22.9 (3.6) HDRS | NA | NA | NA |
| Lan et al,44 2013 | NA | NA | NA | 8.2 (1.1) | NA | NA | NA | 8.2 (1.2) |
| Li,45 2013 | NA | NA | NA | 8.2 (1.2) | NA | NA | NA | 8.2 (1.2) |
| Li et al,46 2013 | 31.4 (5.7) HDRS | 10.4 (3.3) | 17.3 (2.9) | 8.9 (2.3) | 31.8 (5.4) HDRS | 10.4 (2.8) | 16.8 (3.3) | 9.5 (2.1) |
| Sang et al,47 2013 | 58.8 (7.1) SDS | 9.8 (2.5) | 14.3 (2.7) | NA | 56.2 (7.4) SDS | 10.2 (2.2) | 13.9 (2.7) | NA |
| Wang,49 2013 | 16.5 (4.1) HDRS | 7.2 (0.8) | 11.3 (1.1) | NA | 16.6 (4.3) HDRS | 7.1 (0.8) | 11.2 (1.0) | NA |
| Zhang et al,32 2013 | 51.9 (6.6) SDS | 10.2 (1.4) | 16.3 (2.5) | 8.3 (1.5) | 50.6 (6.3) SDS | 10.0 (1.6) | 16.2 (2.4) | 8.6 (2.0) |
| Li and Hu,53 2014 | 32.2 (3.5) HDRS | 13.1 (2.9) | 16.7 (5.9) | 8.7 (1.2) | 30.2 (7.1) HDRS | 10.5 (3.5) | 17.2 (5.1) | 9.0 (1.5) |
| Li et al,54 2014 | 50.4 (4.3) SDS | 10.4 (2.0) | 15.6 (3.2) | 9.3 (1.5) | 51.2 (3.3) SDS | 10.9 (2.3) | 15.7 (2.1) | 9.9 (1.8) |
| Wang et al,48 2009 | 55.7 (7.8) SDS 25.2 (3.3) HDRS |
12.4 (1.4) | NA | NA | 55.2 (8.2) SDS 25.1 (3.1) HDRS |
13.1 (1.7) | NA | NA |
| Zhang et al,50 2013 | 23.4 (1.9) HDRS | 8.9 (0.5) | 11.5 (0.9) | 7.9 (0.5) | 23.6 (1.8) HDRS | 9 (0.5) | 11.6 (0.8) | 8.2 (0.6) |
| Du,39 2012 | 21.6 (3.4) HDRS | 8.9 (1.3) | 12.9 (0.9) | 8.5 (2.3) | 21.3 (3.2) HDRS | 8.9 (1.4) | 12.7 (0.9) | 8.5 (2.4) |
| Hu,43 2013 | 23.9 (4.6) HDRS | 9.3 (3.6) | 13.7 (3.8) | 8.5 (1.6) | 24.5 (4.2) HDRS | 9.2 (3.2) | 13.4 (3.1) | 8.6 (1.7) |
| Penckofer et al,37 2012 | 28.9 (9.5) CES-D | 9.4 (4.2) | 7.9 (2.0) | NA | 27.7 (9.3) CES-D | 9.2 (3.9) | 7.8 (1.8) | NA |
| Sharif et al,52 2014 | 16.9 (5.4) BDI | NA | NA | 8.9 (1.3) | 18.2 (5.9) BDI | NA | NA | 9.3 (1.3) |
| Zheng et al,55 2015 | 54.3 (9.2) SDS | NA | NA | 7.4 (1.6) | 53.2 (8.5) SDS | NA | NA | 7.5 (1.5) |
| Huang et al,56 2016 | 22.0 (3.4) CES-D | 8.7 (3.0) | NA | 7.8 (1.9) | 21.8 (5.7) CES-D | 9.4 (3.2) | NA | 7.7 (1.4) |
| Safren et al,51 2014 | 23.3 (7.2) MADRS | NA | NA | 8.7 (1.4) | 25.6 (8.9) MADRS | NA | NA | 8.8 (1.8) |
Abbreviations: FPG, fasting plasma-glucose; 2hPPG, 2-hour postprandial plasma glucose; HbA1c, hemoglobin A1c; SD, standardized difference; SDS, Self-Rating Depression Scale; HDRS, Hamilton Depression Rating Scale; NA, not available; CES-D, epidemiologic studies depression scale; BDI, Beck’s depression inventory; MADRS, Montgomery–Åsberg Depression Rating Scale.
Study quality
Although all of the included studies had conducted randomization, the allocation concealment was only mentioned in 32.2% (10/31) of studies. Because the blinding of psychological intervention is almost impossible in a clinical trial, all of the included clinical studies were open-label. Studies from which patients withdrew reported the incomplete data, and performed intention-to-treat analysis. Similar baseline characteristics were reported in all studies. The act of randomization could lower the selection bias.2 Thus, these included studies might be free of selection bias.
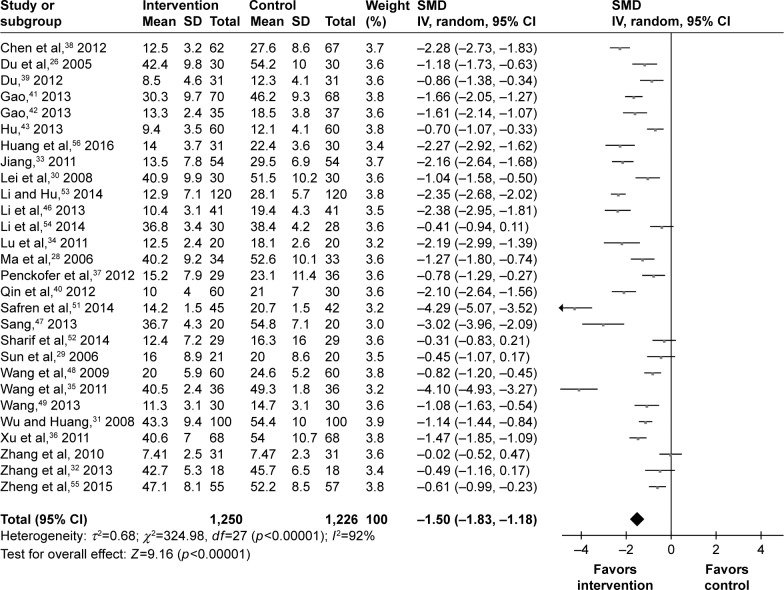
Depressive symptoms
In total, 28 studies assessed the depressive symptoms of 2,476 T2DM patients. The SMD was calculated for these studies: one study had no effect of −0.02, four studies had a small effect of −0.31, −0.41, −0.45, and −0.49, three studies had a moderate effect of −0.61, −0.70, and −0.78, and 20 studies had a large effect of above −0.80. Finally, the pooled SMD was −1.50 (95% CI =−1.83, −1.18) for the random-effects model (Figure 2), which indicated a large effect of psychosocial intervention on the depressive symptoms of T2DM patients. Meanwhile, the results of meta-regression analysis showed that the efficacy had a negligible relationship with the baseline depression scores. Among these studies, 13 studies used the HDRS to assess the depressive symptoms of 1,226 T2DM patients, and 13 studies used the SDS to assess the depressive symptoms of 1,189 T2DM patients. Therefore, subgroup analysis was conducted according to the different depression rating scale. The pooled SMD of studies using SDS was −1.37 (95% CI =−1.76, −0.97), and the pooled SMD of studies using HDRS was −1.46 (95% CI =−1.92, −1.00).
Figure 2.
Meta-analysis of depressive symptoms after treatment.
Abbreviation: SMD, standardized mean difference.
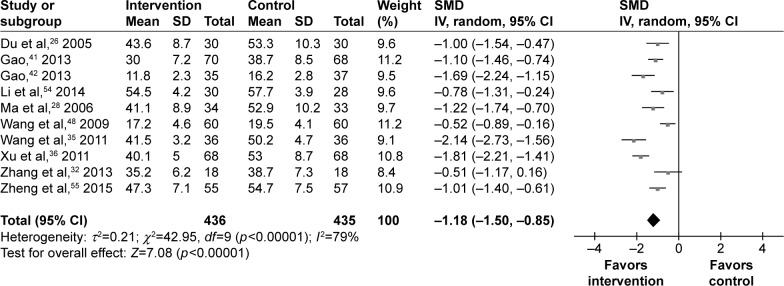
Anxiety symptoms
In total, eight studies used the SAS and two studies used HAMA to assess the anxiety symptoms of 871 T2DM patients. The SMD calculated for these studies was: three studies had a moderate effect of −0.51, −0.52, and −0.78, and seven studies had a large effect of above −0.80. Finally, the pooled SMD was −1.18 (95% CI =−1.50, −0.85) for the random-effects model (Figure 3), which indicated a large effect of psychosocial intervention on the anxiety symptoms of T2DM patients. Sensitivity analysis was performed by excluding the two studies using HAMA. The new SMD of −1.21 (95% CI =−1.54, −0.87) had no significant change compared to the original effect-size estimate. Meanwhile, the results of meta-regression analysis showed that efficacy had a negligible relationship with the baseline anxiety scores.
Figure 3.
Meta-analysis of anxiety symptoms after treatment.
Abbreviation: SMD, standardized mean difference.
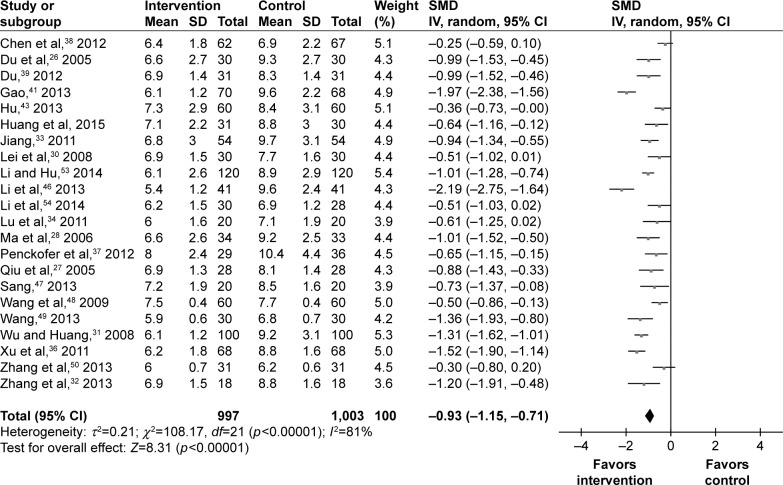
Glycemic control
The values of FPG at the end of trial were available for 22 studies. Among the 2,000 T2DM patients, there were 997 T2DM patients receiving psychosocial intervention. The SMD was calculated for these studies: four studies had a small effect, four studies had a moderate effect, and 12 studies had a large effect. Finally, the pooled SMD was −0.93 (95% CI =−1.15, −0.71) for the random-effects model (Figure 4), which indicated a large effect of psychosocial intervention on the FPG of T2DM patients.
Figure 4.
Meta-analysis of FPG after treatment.
Abbreviations: FPG, fasting plasma-glucose; SMD, standardized mean difference.
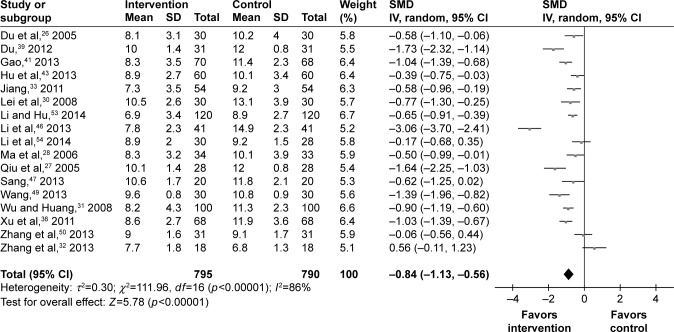
The values of 2hPPG at the end of trial were available for 17 studies. Among the 1,585 T2DM patients, there were 795 T2DM patients receiving psychosocial intervention. The SMD was calculated for these studies: three studies had no effect, two studies had a small effect, five studies had a moderate effect, and seven studies had a large effect. Finally, the pooled SMD was −0.84 (95% CI =−1.13, −0.56) for the random-effects model (Figure 5), which indicated a large effect of psychosocial intervention on the 2hPPG of T2DM patients.
Figure 5.
Meta-analysis of 2hPPG after treatment.
Abbreviations: 2hPPG, 2-hour postprandial plasma glucose; SMD, standardized mean difference.
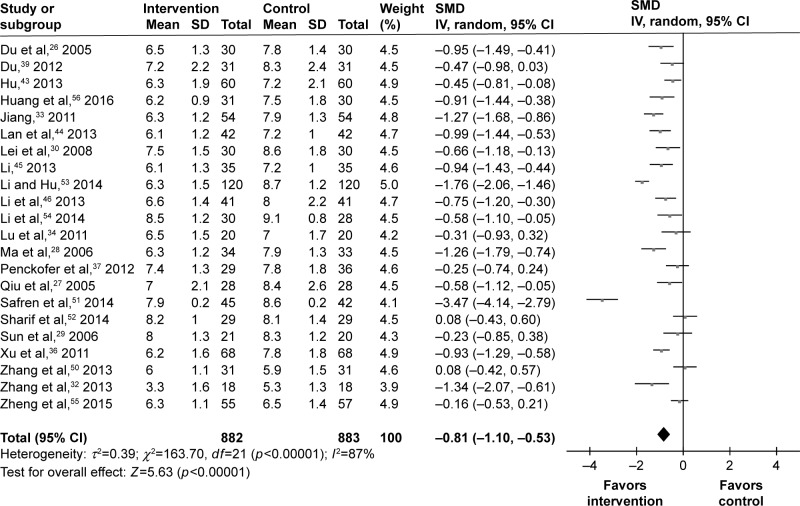
The values of HbA1c at the end of trial were available for 22 studies. Among the 1,765 T2DM patients, there were 882 T2DM patients receiving psychosocial intervention. The SMD was calculated for these studies: one study had no effect, four studies had a small effect, four studies had a moderate effect, and eight studies had a large effect. Finally, the pooled SMD was −0.81 (95% CI =−1.10, −0.53) for the random-effects model (Figure 6), which indicated a large effect of psychosocial intervention on the HbA1c of T2DM patients.
Figure 6.
Meta-analysis of HbA1c after treatment.
Abbreviations: HbA1c, hemoglobin A1c; SMD, standardized mean difference.
Discussion
This meta-analysis was based on 31 studies. The 2,616 T2DM patients with depression were randomly assigned to either receiving psychosocial intervention during diabetes management or not. The results demonstrated that the addition of psychosocial intervention could improve the depressive (SMD =−1.50, 95% CI =−1.83, −1.18) and anxiety (SMD =−1.18, 95% CI =−1.50, −0.85) symptoms of T2DM patients. Moreover, this method could also produce a significant effect on glycemic control. After treatment with psychosocial intervention, there was a significant improvement in the mean score of FPG (SMD =−0.93, 95% CI =−1.15, −0.71), 2hPPG (SMD =−0.84, 95% CI =−1.13, −0.56), and HbA1c (SMD =−0.81, 95% CI =−1.10, −0.53). These results demonstrated that the psychosocial intervention was very effective in treatment of T2DM patients with depression.
Heterogeneity is common because of the difference of included studies, methodological or clinical heterogeneity.57 Heterogeneity existed in this meta-analysis. In order to find the source of heterogeneity, subgroup analysis was conducted according to the different depression rating scales, treatment time, age, DM duration, and mean score of depression at baseline. Meanwhile, sensitivity analysis was conducted by excluding studies with unbalanced sex ratios. However, heterogeneity still existed in our outcomes. Although the detail of the psychosocial intervention in the different studies might be different, this method, on the whole, was about conversation between patients and doctors. Thus, the heterogeneity might not be induced by the methodological or clinical heterogeneity. Actually, the SMD of these included studies ranged from −0.02 to −4.29, which indicated that the heterogeneity was likely to come from the diverse true treatment effects. Therefore, in this meta-analysis, to obtain a robust conclusion in the presence of heterogeneity, we used the random-effects model in this study.
Kok et al conducted a systematic review to assess the effect of psychosocial interventions on patients with diabetes and comorbid depression.2 But they could not determine whether or not this method had a good effect on diabetes patients with depression. Meanwhile, several limitations existed in this study: i) only studies written in English were included; ii) the included studies recruited patients with type 1 or T2DM; iii) the relatively small number of included studies, and only five of the included studies were RCTs. Also, due to the disparate intervention types, DM duration and delivery method of the included studies, they did not conduct a meta-analysis. But these limitations were also the general problems for study of meta-analysis.58 Here, 31 RCTs written in English and Chinese were included to do meta-analysis. The obtained results determined that this method could be very effective in treating T2DM patients with depression.
Limitations of this meta-analysis should be mentioned here: i) most of the included RCTs were conducted in China, which possibly limited the generalizability of our conclusion; ii) all of the included studies recruited patients with T2DM only, thus, whether our conclusion is appropriate for patients with type 1 diabetes needs to be clarified by future studies; iii) although the total number of the included T2DM patients with depression was more than 2,500, only eleven studies recruited more than 100 patients; iv) only the effects of psychosocial intervention in acute treatment phase were assessed; v) heterogeneity, probably caused by the diverse true treatment effects of the included studies, existed; vi) many included studies did not provide details of conventional therapy, so there is no way to analyze the confounding effect of conventional therapy. Therefore, future large-scale RCTs with follow-up assessments after the intervention are still needed to further investigate the effects of psychosocial intervention on treating T2DM patients with depression.
Conclusion
These results obtained by the meta-analysis of 31 RCTs determined that the psychosocial intervention was effective in improving depressive and anxiety symptoms of T2DM patients with depression. Moreover, the addition of psychosocial intervention during diabetes management could improve glycemic control in those patients. However, future studies are still needed to verify and support our conclusion.
Acknowledgments
The project was supported by Cultivation Project of Chongqing Youth Medical High-end Reserve Talents and Technology Foresight and System Innovation of Chongqing Science and Technology Commission for Wuquan Deng and Yu Ma. Our sincere gratitude is extended to Jian-jun Chen and Qian-ping Wei from Chongqing Medical University for their efforts in extracting data and proofreading the manuscript. We also thank Dr He Zhou and Joel George for editing and proofreading the manuscript.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.World Health Organisation [webpage on the Internet] The top 10 causes of death. [Accessed October 10, 2017]. [cited January 2017]. Available from: http://www.who.int/mediacentre/factsheets/fs310/en/
- 2.Kok JL, Williams A, Zhao L. Psychosocial interventions for people with diabetes and co-morbid depression. A systematic review. Int J Nurs Stud. 2015;52(10):1625–1639. doi: 10.1016/j.ijnurstu.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Al-Khawaldeh OA, Al-Hassan MA, Froelicher ES. Self-efficacy, self-management, and glycemic control in adults with type 2 diabetes mellitus. J Diabetes Complications. 2012;26(1):10–16. doi: 10.1016/j.jdiacomp.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Fisher EB, Thorpe CT, Devellis BM, Devellis RF. Healthy coping, negative emotions, and diabetes management: a systematic review and appraisal. Diabetes Educ. 2007;33(6):1080–1103. doi: 10.1177/0145721707309808. [DOI] [PubMed] [Google Scholar]
- 5.Katon WJ. The comorbidity of diabetes mellitus and depression. Am J Med. 2008;121(11 Suppl 2):S8–S15. doi: 10.1016/j.amjmed.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with Type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 2006;23(11):1165–1173. doi: 10.1111/j.1464-5491.2006.01943.x. [DOI] [PubMed] [Google Scholar]
- 7.Roy T, Lloyd CE. Epidemiology of depression and diabetes: a systematic review. J Affect Disord. 2012;142(Suppl):S8–S21. doi: 10.1016/S0165-0327(12)70004-6. [DOI] [PubMed] [Google Scholar]
- 8.Baumeister H, Hutter N, Bengel J, Härter M. Quality of life in medically ill persons with comorbid mental disorders: a systematic review and meta-analysis. Psychother Psychosom. 2011;80(5):275–286. doi: 10.1159/000323404. [DOI] [PubMed] [Google Scholar]
- 9.Zhou H, Sabbagh M, Wyman R, et al. Instrumented trail-Making task to differentiate persons with no cognitive impairment, amnestic mild cognitive impairment, and alzheimer disease: a proof of concept study. Gerontology. 2017;63(2):189–200. doi: 10.1159/000452309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez JS, Peyrot M, McCarl LA, et al. Depression and diabetes treatment nonadherence: a meta-analysis. Diabetes Care. 2008;31(12):2398–2403. doi: 10.2337/dc08-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez JS, Vileikyte L, Ulbrecht JS, et al. Depression predicts first but not recurrent diabetic foot ulcers. Diabetologia. 2010;53(10):2241–2248. doi: 10.1007/s00125-010-1821-x. [DOI] [PubMed] [Google Scholar]
- 12.Katon W, Fan M, Unützer J, Taylor J, Pincus H, Schoenbaum M. Depression and diabetes: a potentially lethal combination. J Gen Intern Med. 2008;23(10):1571–1575. doi: 10.1007/s11606-008-0731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen JJ, Zhou CJ, Liu Z, et al. Divergent urinary metabolic phenotypes between major depressive disorder and bipolar disorder identified by a combined GC-MS and NMR spectroscopic metabonomic approach. J Proteome Res. 2015;14(8):3382–3389. doi: 10.1021/acs.jproteome.5b00434. [DOI] [PubMed] [Google Scholar]
- 14.Chen JJ, Zhao LB, Liu YY, Fan SH, Xie P. Comparative efficacy and acceptability of electroconvulsive therapy versus repetitive transcranial magnetic stimulation for major depression: a systematic review and multiple-treatments meta-analysis. Behav Brain Res. 2016;320:30–36. doi: 10.1016/j.bbr.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 15.Xie J, Chen J, Wei Q. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a meta-analysis of stimulus parameter effects. Neurol Res. 2013;35(10):1084–1091. doi: 10.1179/1743132813Y.0000000245. [DOI] [PubMed] [Google Scholar]
- 16.Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373(9665):746–758. doi: 10.1016/S0140-6736(09)60046-5. [DOI] [PubMed] [Google Scholar]
- 17.Chen JJ, Zeng BH, Li WW, et al. Effects of gut microbiota on the microRNA and mRNA expression in the hippocampus of mice. Behav Brain Res. 2017;322(Pt A):34–41. doi: 10.1016/j.bbr.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 18.Eitan R, Lerer B. Nonpharmacological, somatic treatments of depression: electroconvulsive therapy and novel brain stimulation modalities. Dialogues Clin Neurosci. 2006;8(2):241–258. doi: 10.31887/DCNS.2006.8.2/reitan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen JJ, Zhou CJ, Zheng P, et al. Differential urinary metabolites related with the severity of major depressive disorder. Behav Brain Res. 2017;332:280–287. doi: 10.1016/j.bbr.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Rizzo M, Creed F, Goldberg D, Meader N, Pilling S. A systematic review of non-pharmacological treatments for depression in people with chronic physical health problems. J Psychosom Res. 2011;71(1):18–27. doi: 10.1016/j.jpsychores.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 21.van Schaik DJ, Klijn AF, van Hout HP, et al. Patients’ preferences in the treatment of depressive disorder in primary care. Gen Hosp Psychiatry. 2004;26(3):184–189. doi: 10.1016/j.genhosppsych.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Baumeister H, Hutter N, Bengel J. Psychosocial and pharmacological interventions for depression in patients with diabetes mellitus and depression. Cochrane Database Syst Rev. 2012;12:CD008381. doi: 10.1002/14651858.CD008381.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. doi: 10.1136/bmj.d549. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sacks HS, Berrier J, Reitman D, Ancona-Berk VA, Chalmers TC. Meta-analyses of randomized controlled trials. N Engl J Med. 1987;316(8):450–455. doi: 10.1056/NEJM198702193160806. [DOI] [PubMed] [Google Scholar]
- 26.Du W, Zhang Q, Zhang Z. Effects of psycho-intervention on patients with type 2 diabetes accompanying depression. Journal of Clinical Psychosomatic Diseases. 2005;11(4):341–342. Chinese. [Google Scholar]
- 27.Qiu Z, Wang D, Ma H. Effect of antidepressant and psycho-intervention for depression in elderly patients with type 2 diabetes. Chinese Journal of Rehabilitation Medicine. 2005;20(6):456–457. Chinese. [Google Scholar]
- 28.Ma Z, Li M, Wang C. Effects of comprehensive psycho-intervention on life quality and carbohydrate metabolism in patients with type 2 diabetes mellitus. Chinese Journal of Clinical Rehabilitation. 2006;10(30):15–17. Chinese. [Google Scholar]
- 29.Sun Q, Duan W, Chen K, et al. Effect of paroxetine associated with psychological intervention on depression in type 2 diabetic patients. Chinese Journal of General Practice. 2006;9(10):805–806. Chinese. [Google Scholar]
- 30.Lei M, Huang Q, Yu C. Effect of psychosocial intervention on depression in type 2 diabetic patients. Journal of Clinical and Experimental Medicine. 2008;7(5):95–96. Chinese. [Google Scholar]
- 31.Wu G, Huang P. Effect of psychosocial intervention on treatment of diabetes. China Modern Doctor. 2008;46(10):62–63. Chinese. [Google Scholar]
- 32.Zhang Y, Zuo C, Cai L. Observation on the effect of psychotherapy on the diabetic patients with depression. Chinese Journal of Diabetes. 2013;21(9):801–802. Chinese. [Google Scholar]
- 33.Jiang H. Effects of systemic psychosocial intervention on symptom and life quality of type 2 diabetes with depression. China Journal of Health Psychology. 2011;19(1):34–36. Chinese. [Google Scholar]
- 34.Lu L, Chen N, Shi W, et al. Effect of Deanxit combined with psychosocial intervention on the diabetic patients with depression. Hebei Medical Journal. 2011;33(23):3587–3588. Chinese. [Google Scholar]
- 35.Wang F, Jiang H, Liu H. Study on influence of psychological intervention on SDS, SAS Score and CRP, Hcy levels of patients with diabetes mellitus and depression. Practical Preventive Medicine. 2011;18(9):1789–1790. Chinese. [Google Scholar]
- 36.Xu P, Li C, Shen W. Effect of group interpersonal psychotherapy in elderly diabetic combined with depression and anxiety symptoms. Journal of Psychiatry. 2011;24(2):115–117. Chinese. [Google Scholar]
- 37.Penckofer SM, Ferrans C, Mumby P, et al. A psychoeducational intervention (SWEEP) for depressed women with diabetes. Ann Behav Med. 2012;44(2):192–206. doi: 10.1007/s12160-012-9377-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen X, Zhao Y, Yu X, et al. Effect of psychosocial intervention on type 2 diabetes in patients with hypertension and depression. Chinese Journal of Aesthetic Medicine. 2012;21(12):594–595. Chinese. [Google Scholar]
- 39.Du W. Efficacy of group interpersonal psychotherapy for depression in patients with diabetes. China Higher Medical Education. 2012;2:141–142. Chinese. [Google Scholar]
- 40.Qin Y, Gong Y, Li H, et al. Effect of psychosocial intervention on type 2 diabetes patients with depression. China Practical Medicine. 2012;7(16):257–258. Chinese. [Google Scholar]
- 41.Gao D. Effects of psychosocial intervention in diabetic women combined with anxiety and depression symptoms. Chinese Maternal and Child Health. 2013;28:2904–2905. Chinese. [Google Scholar]
- 42.Gao W, Wang J. Influence of escitalopram combined with mental intervention on symptoms of depression and anxiety and compliance rate of blood sugar of diabetics. China Modern Doctor. 2013;51(27):145–147. Chinese. [Google Scholar]
- 43.Hu C. The efficacy of group interpersonal psychotherapy on diabetic patients with depression. China Journal of Health Psychology. 2013;21(8):1229–1230. Chinese. [Google Scholar]
- 44.Lan Y, Liu L, Lu X. Observation on the effect of psychosocial intervention on elderly type 2 diabetic patients with depression. Jilin Medicine. 2013;34(6):1191–1193. Chinese. [Google Scholar]
- 45.Li Q. The efficacy of psychosocial intervention on elderly type 2 diabetic patients with depression. Global Traditional Chinese Medicine. 2013;6(2):201–202. Chinese. [Google Scholar]
- 46.Li X, Wu T, Lu J. Effect of Sertraline combined with psychological intervention on the treatment of diabetic depression. Clinical Education of General Practice. 2013;11(4):405–407. Chinese. [Google Scholar]
- 47.Sang Y. Effect of psychosocial intervention on depression in patients with diabetes mellitus. Qilu Nursing Journal. 2013;19(1):109–110. Chinese. [Google Scholar]
- 48.Wang S, Shi Y, Xu Y, et al. The effect of mental intervention combined with insulin injection on newly-diagnosed type 2 diabetic patients with depression symptoms. China Journal of Health Psychology. 2009;17(4):471–473. Chinese. [Google Scholar]
- 49.Wang C. Analysis on the improvement effect of psychosocial intervention on depressive symptoms in patients with diabetes. Medical Journal of Chinese People’s Health. 2013;25(18):24–25. Chinese. [Google Scholar]
- 50.Zhang J, Deng Y, Deng F, et al. The clinical observation of Deanxit combined with psychosocial intervention on diabetes patients with depression. Journal of Practical Diabetology. 2013;9(6):56–57. Chinese. [Google Scholar]
- 51.Safren AS, Gonzalez JS, Wexler DJ, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in patients with uncontrolled type 2 diabetes. Diabetes Care. 2014;37(3):625–633. doi: 10.2337/dc13-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharif F, Masoudi M, Ghanizadeh A, Dabbaghmanesh MH, Ghaem H, Masoumi S. The effect of cognitive-behavioral group therapy on depressive symptoms in people with type 2 diabetes: a randomized controlled clinical trial. Iran J Nurs Midwifery Res. 2014;19(5):529–536. [PMC free article] [PubMed] [Google Scholar]
- 53.Li W, Hu B. Influence of psychosocial intervention on glucose metabolism and quality of life of type 2 diabetes patients with depression. Chinese Journal of Gerontology. 2014;34:3140–3141. Chinese. [Google Scholar]
- 54.Li Y, Yu Z, Cao L, et al. Influence of antidepressant treatment and psychological intervention on blood glucose control of T2DM patient associated with emotional disorder. Journal of Modern Medicine Health. 2014;30(6):814–815. Chinese. [Google Scholar]
- 55.Zheng Y, Zhou Y, Lai Q. Effects of twenty-four move shadow boxing combine with psychosomatic relaxation on depression and anxiety in patients with type-2 diabetes. Psychiatr Danub. 2015;27(2):174–179. [PubMed] [Google Scholar]
- 56.Huang CY, Lai HL, Chen CI, et al. Effects of motivational enhancement therapy plus cognitive behaviour therapy on depressive symptoms and health-related quality of life in adults with type II diabetes mellitus: a randomised controlled trial. Qual Life Res. 2016;25(5):1275–1283. doi: 10.1007/s11136-015-1165-6. [DOI] [PubMed] [Google Scholar]
- 57.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 58.Hunter JE, Schmidt FL. Methods of meta-analysis: Correcting error and bias in research findings. Evaluation & Program Planning. 2006;29(3):236–237. [Google Scholar]