Abstract
Five-fluorouracil (5-FU) is a widely used chemotherapeutic agent for digestive system tumors; however, continuous use of 5-FU may cause severe side effects, including myelosuppression and immunosuppression. Our previous study revealed that calcineurin B subunit (CnB), an innovative genetic engineering antitumor protein, possesses tumor-suppressive effects with low toxicity. CnB can bind to and activate integrin αM on macrophages, subsequently promoting the expression, and secretion of TNF-related apoptosis-inducing ligand, a specific proapoptotic cytokine. In the present study, whether the combined use of CnB and 5-FU can reverse the myelosuppression, and immunosuppressive effects of 5-FU by reactivating the immune system thus increasing antitumor efficacy, was investigated. It was demonstrated that combined treatment of 5-FU and CnB led to increased tumor-suppressive effects, as indicated by reduced tumor volume and weight when compared with 5-FU or CnB treatment alone in a hepatoma xenograph model. In addition, it was demonstrated that combined treatment inhibited the proliferation of hepatoma cells. Notably, the addition of CnB to 5-FU-based therapy completely reversed the immunosuppressive effect of 5-FU. The spleen index and total number of white blood cells in the combination group were higher compared with that of the 5-FU alone group. Furthermore, pathological examinations indicated that CnB attenuated 5-FU-induced organ damage. Based on these findings, it is proposed that CnB may serve as a novel and promising drug candidate for the improvement of 5-FU-based chemotherapy.
Keywords: calcineurin B subunit, 5-fluorouracil, combination therapy, immunosuppression
Introduction
Chemotherapy is one of the most effective and widespread methods for the treatment of cancers (1). Five-fluorouracil might be the outstanding representative among these methods (2). Five-fluorouracil is an effective treatment for cancers of the colon, breast, stomach, head and neck and is particularly effective in the management of liver cancer (3,4). The mechanism of the cytotoxicity of 5-FU has been ascribed to the misincorporation of fluoronucleotides into RNA and DNA and to the inhibition of the nucleotide synthetic enzyme thymidylate synthase (5). When used as a monotherapy for hepatocellular carcinoma (HCC) patients, 5-FU results in improvements of median survival times by 14 weeks (6). However, 5-FU treatment results in numerous side effects due to its poor selectivity for tumor cells over normal cells; 5-FU not only effectively inhibits the proliferation of tumor cells but also kills normal cells. Clinically, 5-FU treatment leads to the dysfunction of organs that include the heart, liver and kidney. Treatment with 5-FU also induces myelosuppression and immunosuppression (7–10). Moreover, the administration of routine doses of 5-FU of patients is typically accompanied by diarrhea, nausea, vomiting, poor appetite and low blood counts (11). Therefore, it is essential to develop new drugs to prevent the unwanted side effects induced by 5-FU in cancer patients while simultaneously enhancing its efficacy against tumors (12).
Calcineurin (Cn) is the only Ca2+/calmodulin (CaM)-dependent serine/threonine protein phosphatase. Cn is a heterodimer composed of a 61-kDa catalytic subunit (CnA) and a 19-kDa regulatory subunit (CnB) (13,14). Recent research has shown that Cn is necessary for the inhibition of tumor outbreaks, and genetic and pharmacological suppression of the function of the calcineurin/nuclear factor of activated T cells (NFAT) promotes tumor formation in mouse skin (15). Cn inhibitors, such as cyclosporin A (CsA), might increase the risk of squamous cell carcinomas in organ transplant recipients to a greater extent than in the normal population (16). CnB was traditionally thought to regulate the phosphatase activity of the calcineurin A subunit (17,18). However, in recent years, it has been shown that CnB has functions that are independent of its role as the regulatory subunit of CnA. For example, CnB is indispensible for the positive selection of thymocytes; however, CnB is unnecessary for negative selection (18). CnB is also necessary for enabling centrioles to retain pricentriolar material (PCM) and to organize the interphase aster in Drosophila Melanogaster neuroblasts (19). Moreover, CnB can potentiate the activation of procaspase-3 by accelerating its proteolytic maturation (20). Previous work in our lab has revealed that intraperitoneal injection of CnB prolongs the survival of mice with H22 ascitic tumors and inhibits the growth of S180 sarcomas in a mouse xenograft model (21). The antitumor effect of CnB is closely related to its function in immune regulation because we also found that CnB can mature and activate dendritic cells and enhance antigen presentation and thus function as a novel adjuvant of cancer vaccines (22) and the Engerix-B® HBV vaccine (23). Additionally, CnB can activate macrophages by binding to integrinαM, and then promotes the expression and secretion of TNF-related apoptosis-inducing ligand (TRAIL), a specific pro-apoptotic cytokine (24,25). Further, synergistic interaction between CnB and IFN-γ enhances macrophage antitumor activity by polarize tumor associated macrophages to M1-phenotype (26). The toxicity of CnB has also been evaluated; acute toxicity experiments have indicated that mice can endure at least 50-fold the normal CnB dose (21,23). Because CnB is an innovative genetic engineering antitumor drug candidate with very low toxicity, and it can activate immune system so we were interested in determining whether combination therapies with CnB and clinical chemotherapeutic drugs, like 5-FU, could produce enhanced antitumor activity with reduced side effects on immune systems, for instance, myelosuppression and immunosuppressive.
Materials and methods
Ethics statement
The experimental procedures were approved by the Animal Ethics Committee of Beijing Normal University and were performed in strict accordance with institutional guidelines. All efforts were made to minimize the number of animals used and their suffering.
Materials
Recombinant human CnB protein was produced in our laboratory (the amino acid sequences of the human, mouse and rat CnB proteins are identical). Endotoxin was removed using Cellufine™ ETclean S endotoxin-removing beads (Chisso Corporation, Japan). The purity of CnB was greater than 98%, and LPS contamination was below 4 EU/mg (27). Anti-Ki-67 (a proliferation marker) and 5-FU were purchased from Canspec Scientific Instruments Corporation (Shanghai, China). All other reagents were of standard laboratory grade.
Animals and tumor transplantation
Specific pathogen-free female CD-1 (ICR) mice weighting 18–20 g were purchased from the Vital River Laboratories (Beijing, China) (28). All animals were housed in microisolator cages with autoclaved food and bedding to minimize exposure to viral and microbial pathogens, and all procedures were handled according to protocols approved by the Institutional Animal Care and Use Committee (23). Hepatoma 22 cells were obtained from H22 ascite-bearing mice, diluted with 0.9% normal saline (NS) solution to 5×106/ml and then transplanted s.c. (at 0.2 ml/mouse) via an injection syringe into the left armpits of the ICR mice with aseptic manipulations (29).
Drug preparation and treatment
CnB was suspended in endotoxin-free water, and 5-FU was diluted in 0.9% NaCl. For the combination therapy, CnB and 5-FU were simultaneously dissolved in endotoxin-free water. CD-1 (ICR) mice were randomly divided into 4 groups (10 mice in each group), and the different groups were injected i.p. with different drugs. The CnB group and the combination therapy group were administered CnB (5 mg/kg) doses three times (1 time/day) prior to S.C. tumor implantation to activate their immune systems. The 5-FU group was administered 15 mg/kg of 5-FU once every 2 days following S.C. tumor implantation. The day after the S.C. tumor implantation, interval dosing was initiated in the combination therapy group; only CnB (5 mg/kg) was administered the first day, and the combined drugs (5 mg/kgCnB+15 mg/kg5-FU) were injected on the following day.
Antitumor effects
The antitumor effects of the drugs were determined by calculating the volumes of the solid tumors and the tumor inhibition rates. Approximately 6 days after S.C. tumor implantation, nearly all of the solid tumors had become larger, and the smallest tumor was the size of a rice grain. Subsequently, the tumor volumes were recorded every two days. The long and short axes of each solid tumor were measured with calipers, and the tumor volumes were calculate using the formula 1/2 × length × width2 (30). After the tumors were harvested (as described in the next paragraph), the tumor weights were measured using electric scales. The tumor inhibition rates were calculated according to the following formula: (mean tumor weight of the control group-mean tumor weight of the drug treated group)/mean tumor weight of the control group ×100% (21).
Routine blood examinations
Approximately 15 days after the S.C. tumor implantations, when the average tumor volume of the negative control (NaCl) group exceeded 1000 mm3, anesthetize the mouse by exposure to 2–3% isoflurane, then blood from retro orbital plexus of each mouse was collected into an EDTA anticoagulation tube, and the numbers of platelets and white blood cells were measured using a blood testing instrument. Subsequently, the mice were sacrificed by cervical dislocation.
Splenic index
The spleens were harvested and weighed after the mice were sacrificed (as described in the preceding paragraph). The splenic index was calculated according to the following formula: organ weight (mg)/body weight(g).
Body weight
The mean body weights of each group were measured before and after the experiment. The ratio of body weight gain was calculated according to the following formula: (mean weight after the experiment-mean weight before the experiment)/mean weight before the experiment ×100%.
Histological and immunohistochemical tissue staining
The tumors and organs (liver and kidneys) were harvested, fixed in 10% formalin, embedded in paraffin and sliced into 4-µm-thick slices. The slices were stained with hematoxylin and eosin (H&E) according to routine methods. Once sufficiently dyed, each slice was observed using a conventional light microscope. Immunostaining was also performed by deparaffinizing and by rehydrating the 4-µm-thick sections. Then, 3% or 5% H2O2 was used to block the activity of endogenous peroxidases, and the sections were blocked with 5% serum. All sections were incubated with an antimouse Ki-67 monoclonal antibody (1:200) at 37°C for 90 min. After being washed 3 times with PBS (10 min/time), the sections were incubated with a secondary antibody at 37°C for 22 min and then washed three times with PBS (10 min/time) and visualized with 3,3′diaminobenzidine tetrahydrochloride (DAB) as a chromogen substrate. Finally, the tissue sections were lightly counterstained with hematoxylin, cleared and mounted.
Statistical analyses
The data are expressed as the means and standard deviations (SDs). All the data were analyzed using independent-sample t tests. P<0.05 was considered statistically significant. The statistical analyses were performed using Graphpad Prism Software.
Results
Combined CnB and 5-FU exerts an enhanced antitumor effect in hepatoma 22-transplanted mice
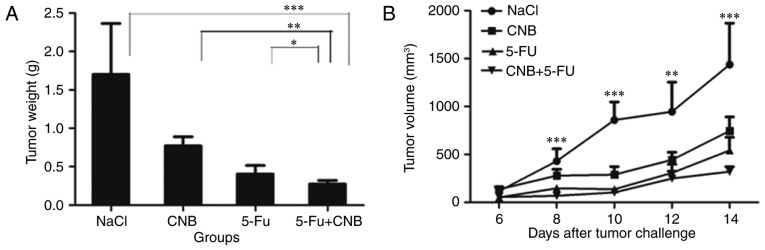
First, we sought to determine the antitumor activity of the combination therapy. Thus, we studied the effects of CnB in combination with 5-FU on tumor growth in these mice. The results are shown in Fig. 1. Fig. 1A shows that the mean volume of the tumors in the combination therapy group was much smaller than that in the negative control (NaCl) group (***P<0.001, **P<0.01). The antitumor effect observed in the combination group was also greater than those observed in the single-agent groups. In addition to observing the growth of the tumors by measuring their volume every two days, we also cut out the solid tumors and calculated the mean tumor weights for the different therapeutic groups. Fig. 1B shows that the tumor inhibiting effects were significantly enhanced when CnB and 5-FU were combined (*P<0.05 compared to 5-FU and **P<0.01 compared to CnB). The tumor inhibition rate of the combination therapeutic group was 83.60%, which was approximately 30% greater than that of the CnB group (54.60%) and also higher than that of the 5-FU group (76.20%). The tumor volume and tumor weight data strongly illustrate the enhanced antitumor effect of the CnB and 5-FU combination therapy.
Figure 1.
Reduction of tumor growth by different drugs. (A) Combination therapy significantly reduced the growth of H22 transplanted tumor cells compared to the control group (n=10, **P<0.01, and ***P<0.001 compared to the negative control (NaCl) group) and was more effective than CnB or 5-FU alone. (B) After the ICR mice were sacrificed, the solid hepatoma 22 tumors were cut out and measured. The mean tumor weight of the negative control (NaCl) group was 1.70 g. In the single drug-treated groups, the mean tumor weights were 0.77 g (CnB) and 0.40 g (5-FU). In the combination therapy group, the mean tumor weight was 0.27 g (n=10, ***P<0.001 compared to the negative control group, **P<0.01 compared to CnB alone, *P<0.05 compared to 5-FU alone).
Pathological examination of the tumor tissues reveals that the combination therapy leads to increased tumor cell death
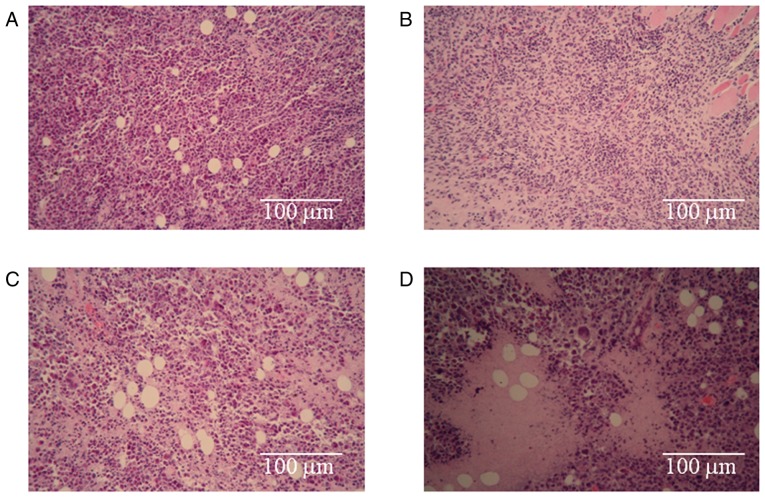
To investigate the morphologies the H22 cells treated with the different drugs, pathological examinations of the tumor tissues were performed. The results are shown in Fig. 2. The arrangement of the H22 cells in the negative control (NaCl) group (Fig. 2A) was tight, and a well-developed proliferative ability was present. The staining was bright, deep and consistent, and no necrotic areas were observed in this group. The most obvious observations in the CnB group (Fig. 2B) were the looseness of the tumor and the smaller sizes of the cells compared to those of the negative control (NaCl) group. Apparently necrotic areas were observed in the 5-FU treatment group (Fig. 2C), and necrotic cellular debris were scattered around. The necrotic area in the combination group (Fig. 2D) was much larger than that in the single-treatment 5-FU group, and necrotic cellular debris was also obvious in this group. These results prove that the treatments with CnB, 5-FU and the combination therapy led to the death of H22 tumor cells and that the death rate in the combination group was greater than those of the single-treated CnB and 5-FU groups.
Figure 2.
Photomicrographs of H22 tumor samples with H&E staining. (A) Negative control (NaCl) group, (B) CnB group, (C) 5-FU group, (D) 5-FU+CnB group. H&E staining, Original magnifications ×100.
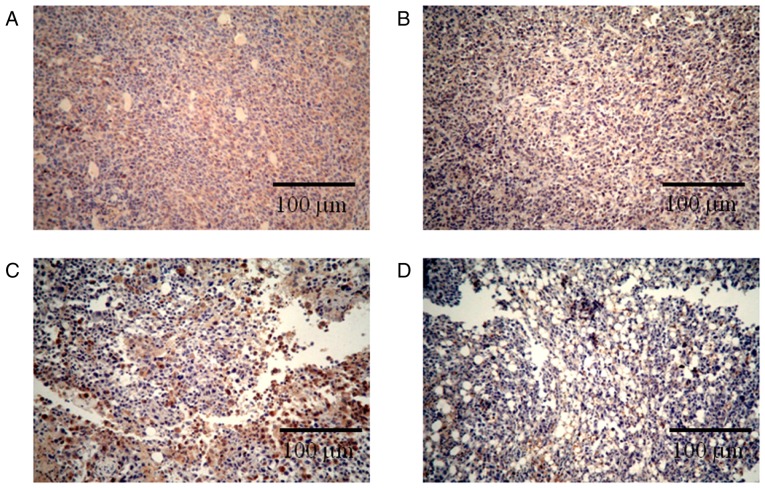
Immunohistochemical staining for Ki-67 revealed that the combination therapy enhanced the inhibition of tumor cell proliferation. Ki-67 is a nuclear protein that is only expressed during the active phases of the cell cycle and is a known proliferative and prognostic marker in both the laboratory and the clinic (31). Reduction in the expression of Ki-67 represent the anti-proliferation activities of different drugs (32,33). As seen in Fig. 3, the number of Ki-67-positive cells in the negative control (NaCl) group (Fig. 3A) was much higher than the numbers observed in the single-treated CnB and 5-FU groups (Fig. 3B, C); in the combination group, most of the cells did not express Ki-67 (Fig. 3D). These results indicate that the combination CnB and 5-FU therapy significantly inhibited the proliferation of H22 tumor cells and was more effective than either of the single treatments with CnB or 5-FU.
Figure 3.
Effects of the different drugs on the Ki-67 activity in the H22 transplanted tumors. (A-D) The expression of Ki-67 in the H22 transplanted tumors of the NaCl, CnB, 5-FU and combination groups, respectively. Brown indicates the Ki-67-positive nuclei, and blue indicates the Ki-67-negative nuclei. Magnification ×100.
CnB ameliorated the body weight loss induced by 5-Fu
The body weights of each therapeutic group were recorded before and after the experiments. Compared to the negative control (NaCl) group, 5-FU resulted in significant inhibition of the increase in body weight (weight gain was reduced by approximately 50% compared to the NaCl group) (Table I). In contrast, CnB treatment alone group exhibited a significant increase in body weight (35.6% greater than that of the NaCl group). When the H22-transplanted mice were treated with CnB and 5-FU together, the mean body weights exhibited significant growth compared to those observed in the 5-FU group (69.2%), which indicates that CnB greatly improved the 5-FU side effect of weight loss.
Table I.
CnB ameliorated the adverse effects of 5-FUa.
| Groups | Ratio of body weight gain (%) | Spleen index (mg/g) | Platelet (×109/l) | WBC (×109/l) |
|---|---|---|---|---|
| Nacl | 13.29 | 10.64±2.18 | 976.56±203.71 | 12.16±6.09 |
| CnB | 18.02 | 10.52±2.34 | 1001.27±269.14c* | 11.50±2.88e** |
| 5-FU | 6.48 | 9.01±1.88b* | 815.50±168.72d* | 6.56±1.59f** |
| CnB+5-FU | 10.97 | 11.09±1.71 | 1005.82±229.38 | 11.17±3.08 |
The results are presented are the means ± the standard deviations, n=10.
P<0.05 5-FU vs. 5-FU+CnB
P<0.05 CnB vs. 5-FU
P<0.05 5-FU vs. 5-FU+CnB
P<0.01 CnB vs. 5-FU
P<0.01 5-FU vs. 5-FU+CnB.
CnB reduced the toxicity of 5-Fu as indicated by organ weight
The spleen is the largest peripheral immune organ, is the site of residence of mature T and B lymphocyte cells and is the most important site of immune responses. The splenic index directly reflects immune function. As shown in Table I, the immunosuppressor 5-FU significantly reduced the splenic index. In contrast, the splenic indices of the CnB-alone and negative control (NaCl) groups were maintained at the same level. Furthermore, following the addition of CnB, the splenic index of the combination therapeutic group was significantly increased (*P<0.05). This result strongly indicates that CnB improved the immunosuppressive effect induced by 5-Fu.
CnB ameliorated the abnormalities in the hematological parameters
Clinically, some of the most apparent side effects of chemotherapeutic treatment are declines in hematological parameters. These declines might have a negative effect on patient immunity and can even lead to many complications. As seen in Table I, the hematological parameters of the 5-FU-treated group sharply dropped. The platelet numbers exhibited a 20% decrease compared to those observed in the negative control (NaCl) group. Furthermore, the WBC numbers of the 5-FU group were only half of those observed in the negative control (NaCl) group. However, CnB significantly ameliorated the abnormalities of the platelet and WBC numbers that were induced by 5-Fu (platelets: *P<0.05 CnB compared with 5-FU, WBC: **P<0.01 CnB compared with 5-FU). Following the addition of CnB, the platelet and WBC numbers reached levels that were similar to those observe in the negative control (NaCl) group. Significant differences were observed between the 5-FU group and the combination group (platelets: *P<0.05 5-FU compared to the 5-FU+ CnB group, WBC: **P<0.01 5-FU compared to the 5-FU+CnB group). These results indicated that CnB rescued the changes in the hematological parameters that were induced by 5-Fu.
CnB ameliorated the hepatotoxicity caused by 5-FU
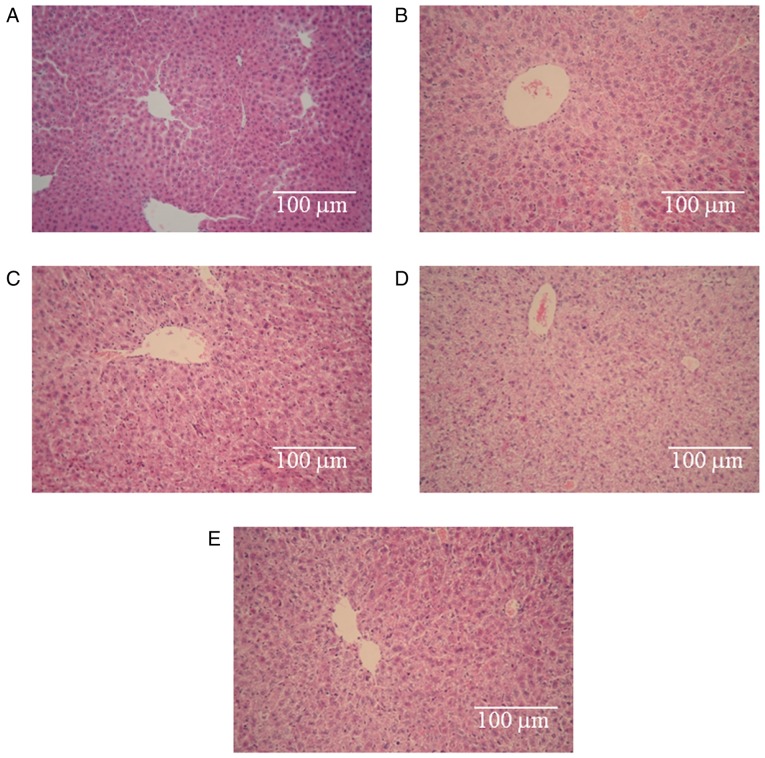
Light microscopy observations revealed that the control hepatic tissues exhibited normal large polygonal cells with prominent round nuclei, eosinophilic cytoplasm, a few spaced hepatic sinusoids arranged between the hepatic cords, with a fine arrangement of Kupffer cells (Fig. 4A) (34). Fig. 4B, C and E illustrate some degree of degeneration of the hepatic cords, loose cytoplasm and focal inflammatory cell infiltration. The degrees of damage observe in the three groups were identical. However, pronounced histopathological abnormalities were observed in the 5-FU group (Fig. 4D). These abnormalities involved the dissolution of the hepatic cords, which appeared as empty vacuoles aligned by strands of necrotic hepatocytes. The hepatotoxicity in this group was much greater than the hepatotoxicities observed in the other groups. Based on these results, we conclude that the hepatotoxicity induced by 5-FU was obvious, no hepatotoxicity was observed in the CnB group, and CnB ameliorated the hepatotoxicity caused by 5-FU.
Figure 4.
Histopathological effects of the different drugs in the mouse liver. (A) Histology of a normal control mouse liver. (B) Histology of a negative control (NaCl) mouse liver. (C-E) Histopathological changes observed in the mice treated with CnB, 5-FU, and CnB+5-FU, respectively. Magnification ×200.
Discussion
Clinically, combination therapy has become a general approach to cancer treatment (35). The goal of combination therapy is to augment the antitumor effects and to reduce the side effects and toxicities of drugs to the fullest extent possible.
Five-fluorouracil is widely used for a range of cancers. However, despite many efforts, systemic single-agent treatments have exhibited poor efficacy (36–39) and have only been able to achieve objective response rates of approximately 10% (40). Additionally, some patients discontinue therapy due to serious adverse effects (10,35,41). Although 5-FU in combination with other chemotherapeutic agents improves response rates and survival new therapeutic strategies are urgently required (5).
As an innovative genetic engineering antitumor protein, CnB exhibits efficacious antitumor activity and has low toxicity (21). Comparatively, CnB is easy to express and to purify (42), and the cost of CnB is lower than that of other protein drugs. Unlike some antitumor agents, that have to be extracted from Chinese herbs (43,44), CnB does not have any disadvantages for the environment.
Regarding the drawbacks of 5-FU and the merits of CnB, we speculate that combination therapy will result in good effects. Our results revealed that combination treatment of CnB and 5-Fu produced a significant augmentation of the antitumor effect in H22-transplanted ICR mice. When the mice were treated simultaneously with CnB and 5-FU, the tumor volumes were clearly reduced compared to those of the single-treated CnB and 5-FU groups. Moreover, the tumor inhibition rate of the combination group exceeded 80%, whereas these rates in the CnB and 5-FU single-agent groups were approximately 70 and 50%, respectively. Significant differences were observed between the combination group and single-agent groups (P<0.01). The pathological examinations of the tumor tissues from the negative control (NaCl) group revealed that the H22 cells grew vigorously in a compact arrangement, whereas different amounts of necrotic cellular debris were observed in the single-treated CnB and 5-FU groups. Furthermore, the necrotic areas in the combination group were much larger. Additionally, Ki-67 nuclear protein immunohistochemical staining further demonstrated a decline in the numbers of positive proliferation cells after animals were treated with combined CnB and 5-FU compared with the single-agent groups. These findings indicate that CnB effectively increased the ability of 5-FU to inhibit cell proliferation in tumors.
Adverse drug reactions are a major clinical problem, and a meta-analysis involving 1219 patients with colorectal cancer revealed that grade 3 to 4 toxicity is encountered in 31–34% of the patients who receive 5-FU and that 0.5% of these patients experience lethal toxicity (45,46). Recent data suggest that combination therapy not only significantly improves the quality of life of cancer patients (39) but might also improve their responses potentially their survival (47). Indeed, the clinical experience accumulated over the last 30 years of cancer management suggests that combinations of different agents offer the best possible therapeutic efficacies (10,48). In our experiment, the reduced side effects of combination therapy were evaluated by analyzing the body weights, immune organs indices, peripheral blood platelet and WBC cell numbers, and the pathologies of the livers of the mice. Our results revealed that 5-FU evidently reduced body weight gain, immune organ growth and the numbers of platelets and WBC cells. However, CnB ameliorated all of these adverse effects. Significant improvements were observed in the animals that were treated with both CnB and 5-FU (P<0.01). Moreover, H&E staining of the mouse livers revealed that CnB also improved the microstructural damage induced by 5-FU. We also performed pathological examinations of the kidneys; however, no significant differences were observed between the groups. We suggest that this lack of difference arose because liver is more sensitive to 5-FU than kidney and the toxicity of 5-FU in the blood is reduced upon arrival at the kidney due to previous detoxification in the liver.
In conclusion, our current study revealed that the combination of CnB and 5-FU had a more potent inhibitory effect on H22 tumor growth. Furthermore, the side effects were not increased by the combination therapy; rather the CnB significantly attenuated the toxicity induced by 5-FU. We speculate that the immune regulation function of CnB played an important role in this process that involved promoting the cytotoxic effects of immunocytes on tumor cells, aiding the repair of damaged tissues, and maintaining homeostasis in the bodies of the mice. The high efficiency and low toxicity of CnB makes it a promising novel antitumor drug.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China, the National Important Novel Medicine Research Project, the International Cooperation Project and the Fundamental Research Funds for the Central Universities.
References
- 1.Zhao T, Mao G, Zhang M, Zou Y, Feng W, Gu X, Zhu Y, Mao R, Yang L, Wu X. Enhanced antitumor and reduced toxicity effect of Schisanreae polysaccharide in 5-Fu treated Heps-bearing mice. Int J Biol Macromol. 2014;63:114–118. doi: 10.1016/j.ijbiomac.2013.10.037. [DOI] [PubMed] [Google Scholar]
- 2.Cao Z, Liao L, Chen X, Lan L, Hu H, Liu Z, Chen L, Huang S, Du J. Enhancement of antitumor activity of low-dose 5-fluorouracil by combination with Fuzheng-Yiliu granules in hepatoma 22 tumor-bearing mice. Integr Cancer Ther. 2013;12:174–181. doi: 10.1177/1534735412450514. [DOI] [PubMed] [Google Scholar]
- 3.Simonetti RG, Liberati A, Angiolini C, Pagliaro L. Treatment of hepatocellular carcinoma: A systematic review of randomized controlled trials. Ann Oncol. 1997;8:117–136. doi: 10.1023/A:1008285123736. [DOI] [PubMed] [Google Scholar]
- 4.Cheng MR, Li Q, Wan T, He B, Han J, Chen HX, Yang FX, Wang W, Xu HZ, Ye T, Zha BB. Galactosylated chitosan/5-fluorouracil nanoparticles inhibit mouse hepatic cancer growth and its side effects. World J Gastroenterol. 2012;18:6076–6087. doi: 10.3748/wjg.v18.i42.6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 6.Boucher E, Corbinais S, Brissot P, Boudjema K, Raoul JL. Treatment of hepatocellular carcinoma (HCC) with systemic chemotherapy combining epirubicin, cisplatinum and infusional 5-fluorouracil (ECF regimen) Cancer Chemother Pharmacol. 2002;50:305–308. doi: 10.1007/s00280-002-0503-x. [DOI] [PubMed] [Google Scholar]
- 7.Fan Y, Lu Y, Wang D, Liu J, Song X, Zhang W, Zhao X, Nguyen TL, Hu Y. Effect of epimedium polysaccharide-propolis flavone immunopotentiator on immunosuppression induced by cyclophosphamide in chickens. Cell Immunol. 2013;281:37–43. doi: 10.1016/j.cellimm.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Liao HF, Chen YJ, Yang YC. A novel polysaccharide of black soybean promotes myelopoiesis and reconstitutes bone marrow after 5-flurouracil- and irradiation-induced myelosuppression. Life Sci. 2005;77:400–413. doi: 10.1016/j.lfs.2004.10.080. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Wang Y, Liu X, Yuan Y, Yue T. Free radical scavenging and immunomodulatory activities of Ganoderma lucidum polysaccharides derivatives. Carbohydr Polym. 2013;91:33–38. doi: 10.1016/j.carbpol.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 10.Hou S, Kou G, Fan X, Wang H, Qian W, Zhang D, Li B, Dai J, Zhao J, Ma J, et al. Eradication of hepatoma and colon cancer in mice with Flt3L gene therapy in combination with 5-FU. Cancer Immunol Immunother. 2007;56:1605–1613. doi: 10.1007/s00262-007-0306-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park SH, Lee Y, Han SH, Kwon SY, Kwon OS, Kim SS, Kim JH, Park YH, Lee JN, Bang SM, et al. Systemic chemotherapy with doxorubicin, cisplatin and capecitabine for metastatic hepatocellular carcinoma. BMC Cancer. 2006;6:3. doi: 10.1186/1471-2407-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Wu B, Zhang X, Xu M, Chang H, Lu X, Ren X. Purification of a polysaccharide from Boschniakia rossica and its synergistic antitumor effect combined with 5-Fluorouracil. Carbohydr Polym. 2012;89:31–35. doi: 10.1016/j.carbpol.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 13.Crabtree GR. Generic signals and specific outcomes: Signaling through Ca2+, calcineurin, and NF-AT. Cell. 1999;96:611–614. doi: 10.1016/S0092-8674(00)80571-1. [DOI] [PubMed] [Google Scholar]
- 14.Manalan AS, Klee CB. Activation of calcineurin by limited proteolysis; Proc Natl Acad Sci USA; 1983; pp. 4291–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu X, Nguyen BC, Dziunycz P, Chang S, Brooks Y, Lefort K, Hofbauer GF, Dotto GP. Opposing roles for calcineurin and ATF3 in squamous skin cancer. Nature. 2010;465:368–372. doi: 10.1038/nature08996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003;348:1681–1691. doi: 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]
- 17.Heit JJ, Apelqvist AA, Gu X, Winslow MM, Neilson JR, Crabtree GR, Kim SK. Calcineurin/NFAT signalling regulates pancreatic beta-cell growth and function. Nature. 2006;443:345–349. doi: 10.1038/nature05097. [DOI] [PubMed] [Google Scholar]
- 18.Neilson JR, Winslow MM, Hur EM, Crabtree GR. Calcineurin B1 is essential for positive but not negative selection during thymocyte development. Immunity. 2004;20:255–266. doi: 10.1016/S1074-7613(04)00052-4. [DOI] [PubMed] [Google Scholar]
- 19.Januschke J, Reina J, Llamazares S, Bertran T, Rossi F, Roig J, Gonzalez C. Centrobin controls mother-daughter centriole asymmetry in Drosophila neuroblasts. Nat Cell Biol. 2013;15:241–248. doi: 10.1038/ncb2671. [DOI] [PubMed] [Google Scholar]
- 20.Saeki M, Irie Y, Ni L, Itsuki Y, Terao Y, Kawabata S, Kamisaki Y. Calcineurin potentiates the activation of procaspase-3 by accelerating its proteolytic maturation. J Biol Chem. 2007;282:11786–11794. doi: 10.1074/jbc.M609347200. [DOI] [PubMed] [Google Scholar]
- 21.Wei Q, Lian ML, Jing FZ, Zhang N, Yan MS, Chen Y, Gao QS. Studies of calcineurin B subunit from genetic engineering for use in medicine. Drug Dev Res. 2002;56:40–43. doi: 10.1002/ddr.10051. [DOI] [Google Scholar]
- 22.Li J, Guo J, Su Z, Hu M, Liu W, Wei Q. Calcineurin subunit B activates dendritic cells and acts as a cancer vaccine adjuvant. Int Immunol. 2011;23:327–334. doi: 10.1093/intimm/dxr008. [DOI] [PubMed] [Google Scholar]
- 23.Hu M, Su Z, Yin Y, Li J, Wei Q. Calcineurin B subunit triggers innate immunity and acts as a novel Engerix-B HBV vaccine adjuvant. Vaccine. 2012;30:4719–4727. doi: 10.1016/j.vaccine.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 24.Su Z, Xin S, Xu L, Cheng J, Guo J, Li L, Wei Q. The calcineurin B subunit induces TNF-related apoptosis-inducing ligand (TRAIL) expression via CD11b-NF-κB pathway in RAW264. 7 macrophages. Biochem Biophys Res Commun. 2012;417:777–783. doi: 10.1016/j.bbrc.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 25.Liu L, Su Z, Xin S, Cheng J, Li J, Xu L, Wei Q. The calcineurin B subunit (CnB) is a new ligand of integrin αM that mediates CnB-induced Apo2L/TRAIL expression in macrophages. J Immunol. 2012;188:238–247. doi: 10.4049/jimmunol.1102029. [DOI] [PubMed] [Google Scholar]
- 26.Su Z, Yang R, Zhang W, Xu L, Zhong Y, Yin Y, Cen J, DeWitt JP, Wei Q. The synergistic interaction between the calcineurin B subunit and IFN-γ enhances macrophage antitumor activity. Cell Death Dis. 2015;6:e1740. doi: 10.1038/cddis.2015.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su Z, Xin S, Li J, Guo J, Long X, Cheng J, Wei Q. A new function for the calcineurin b subunit: Antiplatelet aggregation and anticoagulation. IUBMB Life. 2011;63:1037–1044. doi: 10.1002/iub.562. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Li L, Huang Y, Wei Q. Calcineurin subunit B upregulates β-interferon production by phosphorylation of interferon regulatory factor 3 via Toll-like receptor 4. Cancer Sci. 2012;103:515–521. doi: 10.1111/j.1349-7006.2011.02160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jia L, Xu B, Guo W, Ge ZM, Li RT, Cui JR. Enhanced antitumor effect of TM208 in combination with 5 fluorouracil in H22 transplanted mice. J Chine Pharmace Sci. 2011;20:615–626. [Google Scholar]
- 30.Rolny C, Mazzone M, Tugues S, Laoui D, Johansson I, Coulon C, Squadrito ML, Segura I, Li X, Knevels E, et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell. 2011;19:31–44. doi: 10.1016/j.ccr.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Antoon JW, White MD, Slaughter EM, Driver JL, Khalili HS, Elliott S, Smith CD, Burw ME, Beckman BS. Targeting NFκB mediated breast cancer chemoresistance through selective inhibition of sphingosine kinase-2. Cancer Biol Ther. 2011;11:678–689. doi: 10.4161/cbt.11.7.14903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hosaka N, Ichikawa Y, Ishikawa T, Nagashima Y, Kunisaki C, Takahashi M, Moriwaki Y, Akiyama H, Yamaguchi S, Ota M, et al. Correlation of immunohistochemical p53 labeling index with inhibition rate in chemosensitivity test in gastric and colon cancer. Anticancer Res. 2000;21:229–235. [PubMed] [Google Scholar]
- 33.Scholzen T, Gerdes J. The Ki-67 protein: From the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 34.El-Sayyad HI, Ismail MF, Shalaby F, Abou-El-Magd R, Gaur RL, Fernando A, Raj MH, Ouhtit A. Histopathological effects of cisplatin, doxorubicin and 5-flurouracil (5-FU) on the liver of male albino rats. Int J Biol Sci. 2009;5:466–473. doi: 10.7150/ijbs.5.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmoll HJ, Arnold D. Update on capecitabine in colorectal cancer. Oncologist. 2006;11:1003–1009. doi: 10.1634/theoncologist.11-9-1003. [DOI] [PubMed] [Google Scholar]
- 36.Hejna M, Zielinski CC. Nonsurgical management of gallbladder cancer: Cytotoxic treatment and radiotherapy. Expert Rev Anticancer Ther. 2001;1:291–300. doi: 10.1586/14737140.1.2.291. [DOI] [PubMed] [Google Scholar]
- 37.Knox JJ, Hedley D, Oza A, Siu LL, Pond GR, Moore MJ. Gemcitabine concurrent with continuous infusional 5-fluorouracil in advanced biliary cancers: A review of the Princess Margaret Hospital experience. Ann Oncol. 2004;15:770–774. doi: 10.1093/annonc/mdh172. [DOI] [PubMed] [Google Scholar]
- 38.Todoroki T. Chemotherapy for gallbladder carcinoma-a surgeon's perspective. Hepatogastroenterology. 2000;47:948–955. [PubMed] [Google Scholar]
- 39.Harrop R, Drury N, Shingler W, Chikoti P, Redchenko I, Carroll MW, Kingsman SM, Naylor S, Griffiths R, Steven N, Hawkins RE. Vaccination of colorectal cancer patients with TroVax given alongside chemotherapy (5-fluorouracil, leukovorin and irinotecan) is safe and induces potent immune responses. Cancer Immunol Immunother. 2008;57:977–986. doi: 10.1007/s00262-007-0428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobayashi K, Tsuji A, Morita S, Horimi T, Shirasaka T, Kanematsu T. A phase II study of LFP therapy (5-FU (5-fluorourasil) continuous infusion (CVI) and Low-dose consecutive (Cisplatin) CDDP) in advanced biliary tract carcinoma. BMC Cancer. 2006;6:121. doi: 10.1186/1471-2407-6-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nowak AK, Chow PK, Findlay M. Systemic therapy for advanced hepatocellular carcinoma: A review. Eur J Cancer. 2004;40:1474–1484. doi: 10.1016/j.ejca.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 42.Wei Q, Lee EY. Expression and reconstitution of calcineurin A and B subunits. Biochem Mol Biol Int. 1997;41:169–177. doi: 10.1080/15216549700201171. [DOI] [PubMed] [Google Scholar]
- 43.Shoemaker M, Hamilton B, Dairkee SH, Cohen I, Campbell MJ. In vitro anticancer activity of twelve Chinese medicinal herbs. Phytother Res. 2005;19:649–651. doi: 10.1002/ptr.1702. [DOI] [PubMed] [Google Scholar]
- 44.Tang W, Hemm I, Bertram B. Recent development of antitumor agents from Chinese herbal medicines; part I. Low molecular compounds. Planta Med. 2003;69:97–108. doi: 10.1055/s-2003-38494. [DOI] [PubMed] [Google Scholar]
- 45.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. Jama. 1998;279:1200–1205. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 46.van Kuilenburg AB, Meinsma R, Zonnenberg BA, Zoetekouw L, Baas F, Matsuda K, Tamaki N, van Gennip AH. Dihydropyrimidinase deficiency and severe 5-fluorouracil toxicity. Clin Cancer Res. 2003;9:4363–4367. [PubMed] [Google Scholar]
- 47.Kornek G, Raderer M, Schüll B, Fiebiger W, Gedlicka C, Lenauer A, Depisch D, Schneeweiss B, Lang F, Scheithauer W. Effective combination chemotherapy with paclitaxel and cisplatin with or without human granulocyte colony-stimulating factor and/or erythropoietin in patients with advanced gastric cancer. Br J Cancer. 2002;86:1858–1863. doi: 10.1038/sj.bjc.6600345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liljenfeldt L, Gkirtzimanaki K, Vyrla D, Svensson E, Loskog AS, Eliopoulos AG. Enhanced therapeutic anti-tumor immunity induced by co-administration of 5-fluorouracil and adenovirus expressing CD40 ligand. Cancer Immunol Immunother. 2014;63:273–282. doi: 10.1007/s00262-013-1507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]